Extracellular Oxygen Sensors Based on PtTFPP and Four-Arm Block Copolymers
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Synthesis
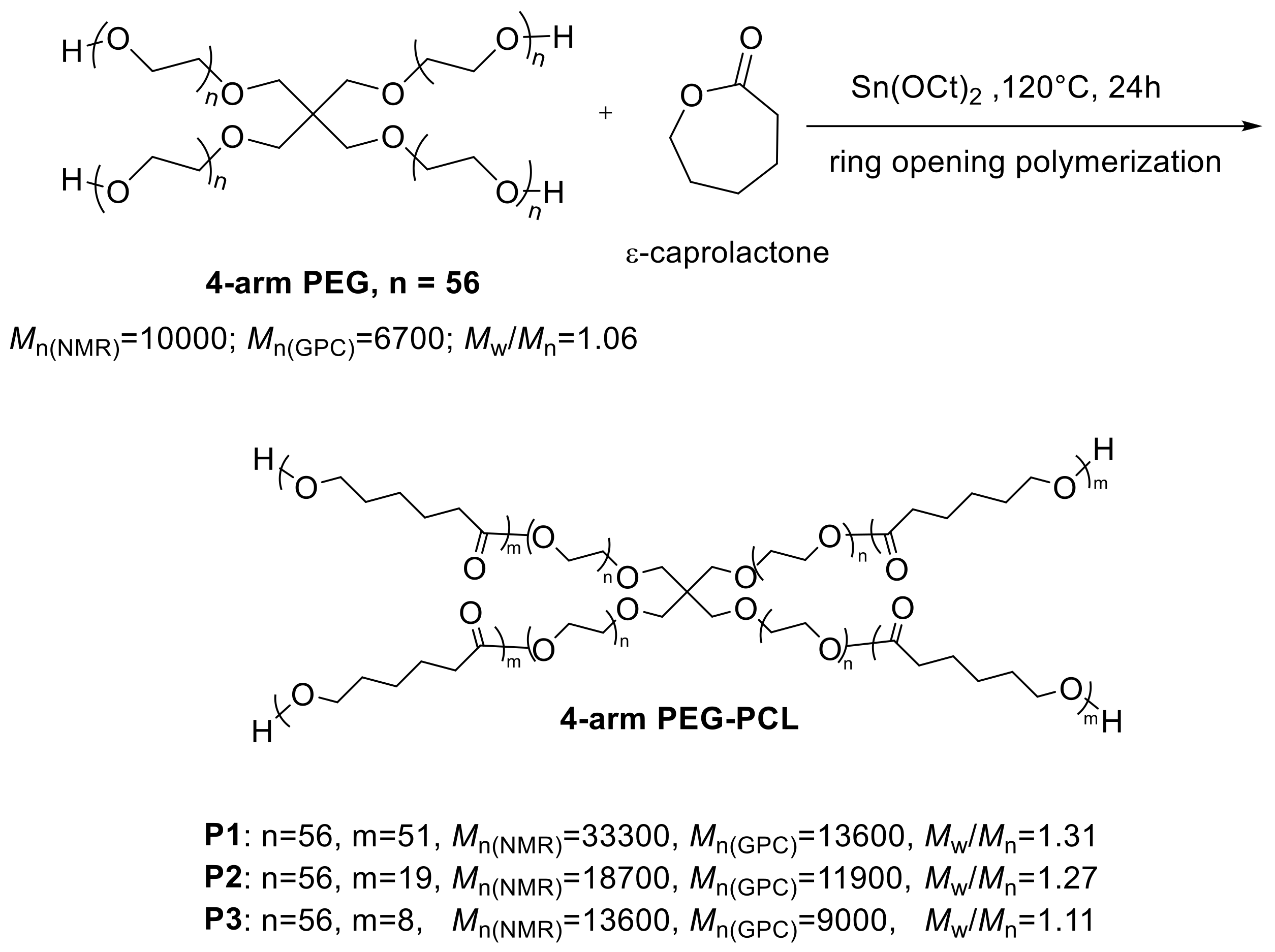
2.3.1. Synthesis of P1
2.3.2. Synthesis of P2
2.3.3. Synthesis of P3
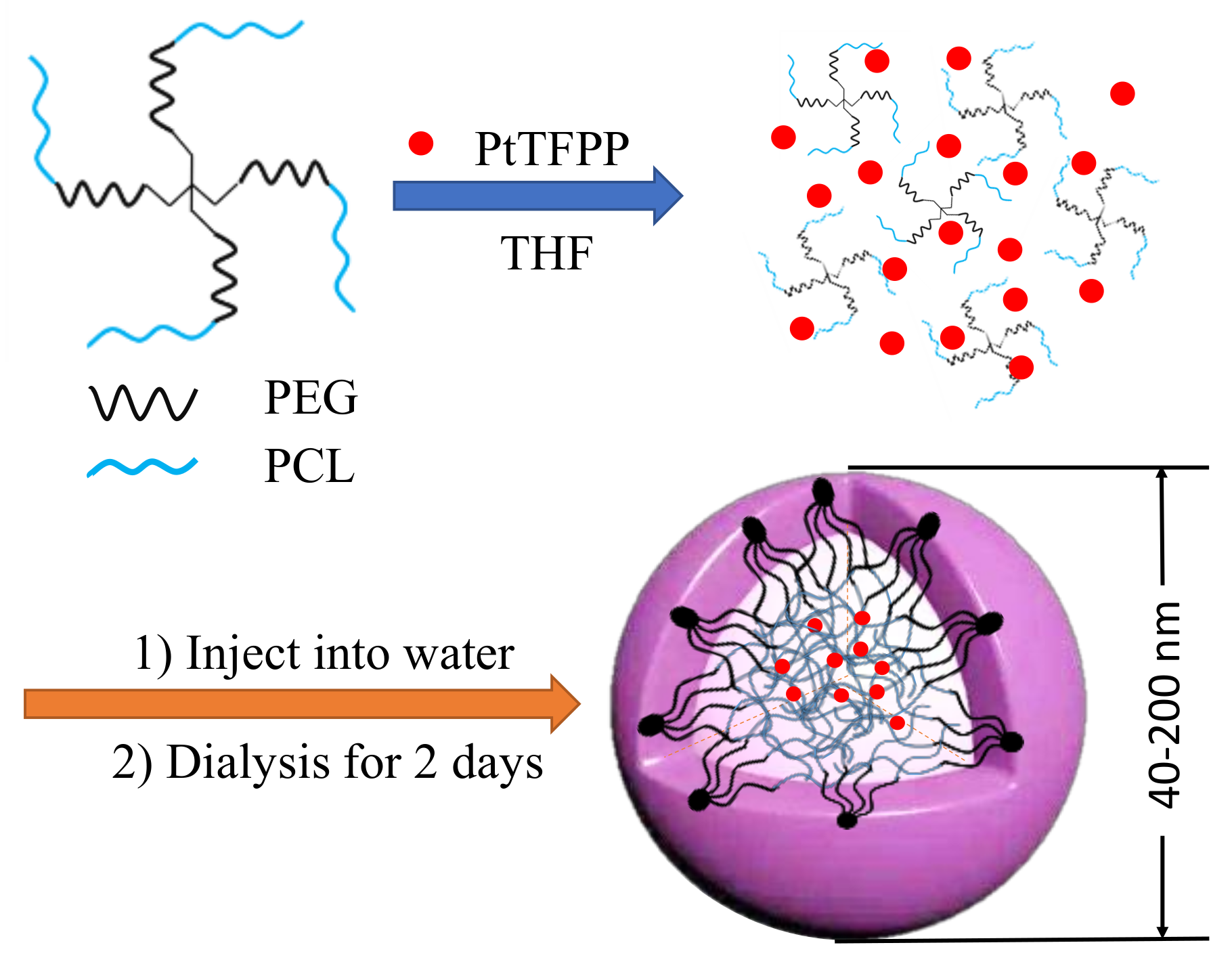
2.4. Preparation of Micelles
2.5. Oxygen Sensing Performance
2.6. Critical Micelle Concentration (CMC) Determination
2.7. Determination of Quantum Yields
2.8. Response Time Measurements
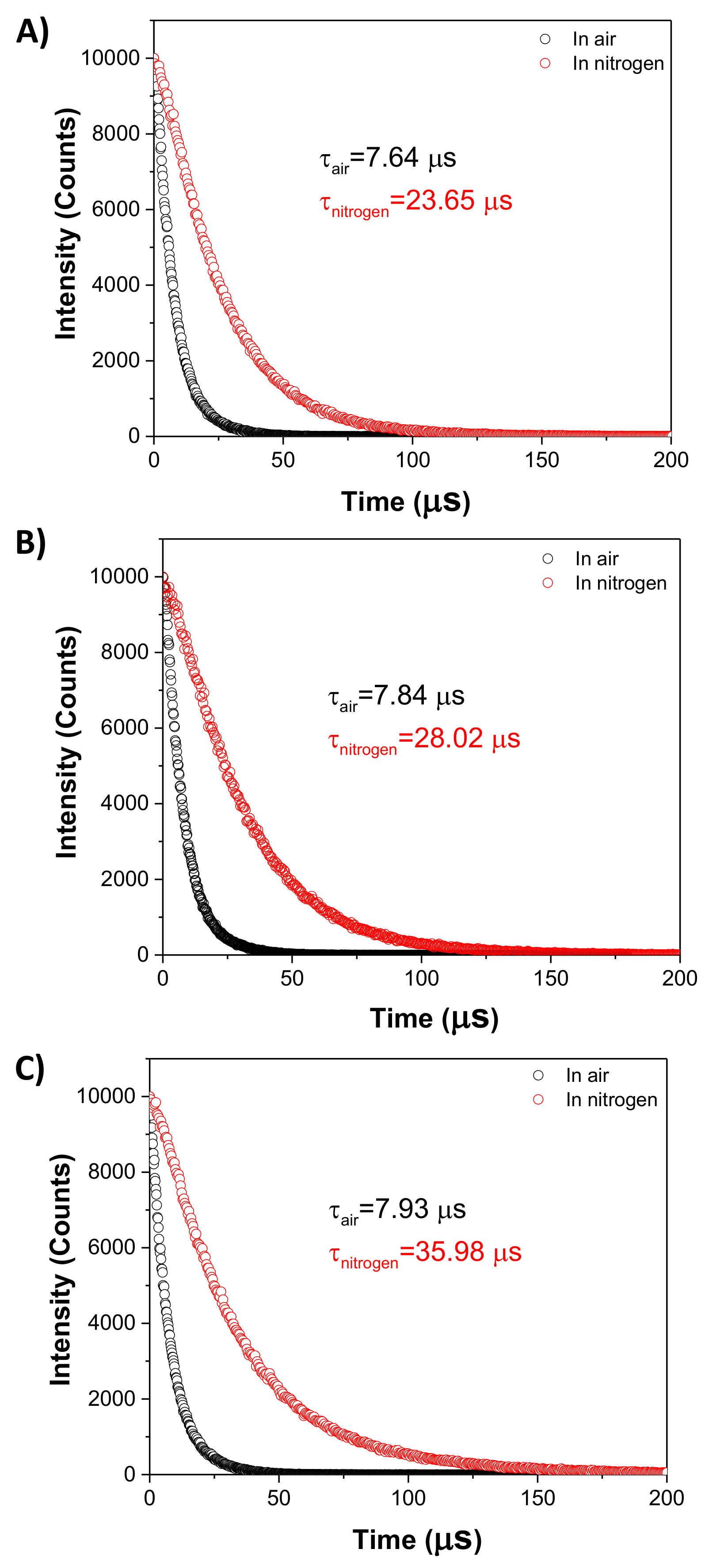
2.9. Lifetime Determination
2.10. Micelles’ Stability
2.11. Culture of E. coli for Extracellular Sensing
2.12. Culture of Mammalian Cells for Extracellular Sensing
2.13. Oxygen Respiration Experiments
3. Results and Discussion
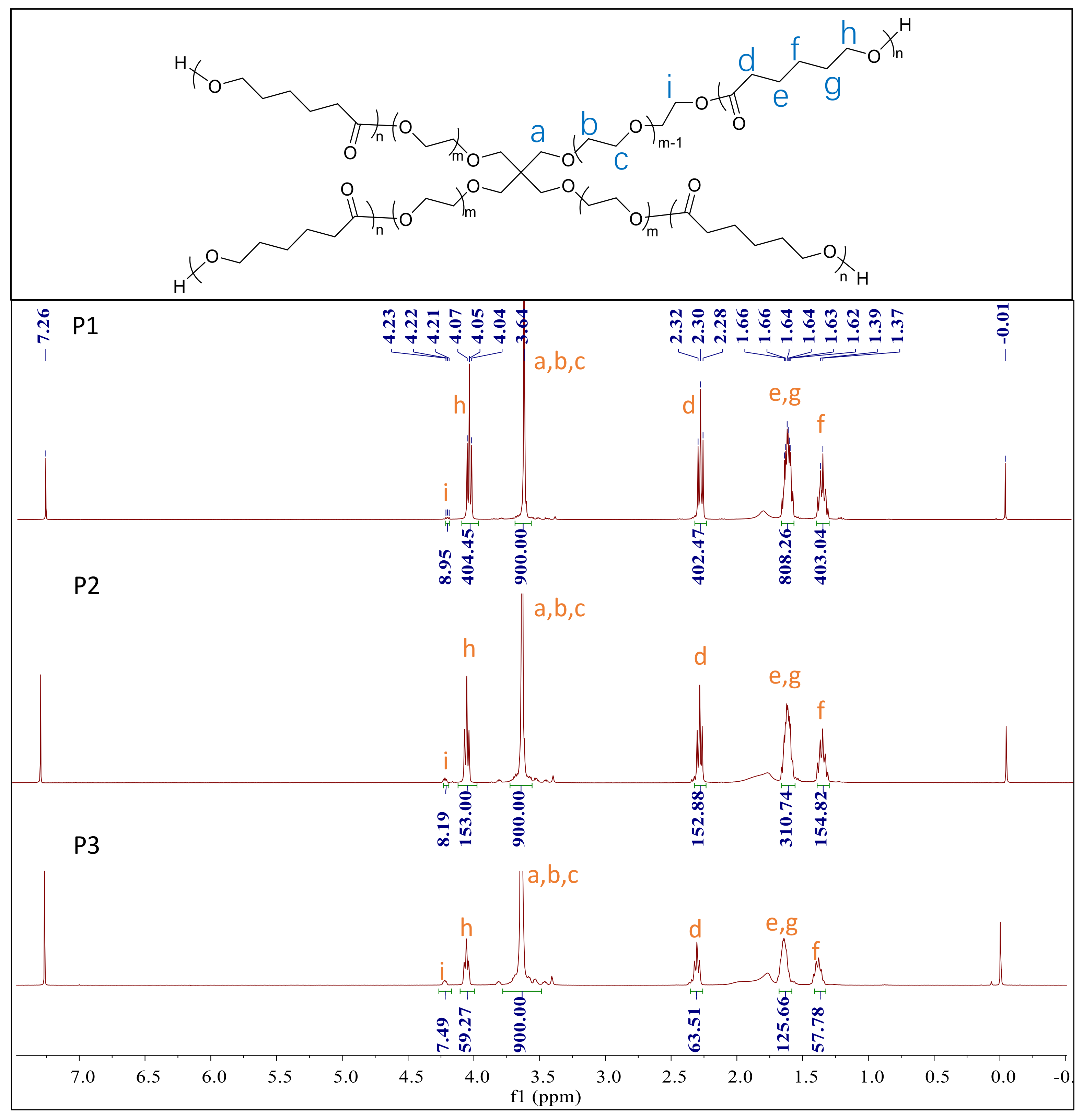
3.1. Synthesis and Structure Characterization of the Polymers
3.2. Micelle Preparation and Characterization
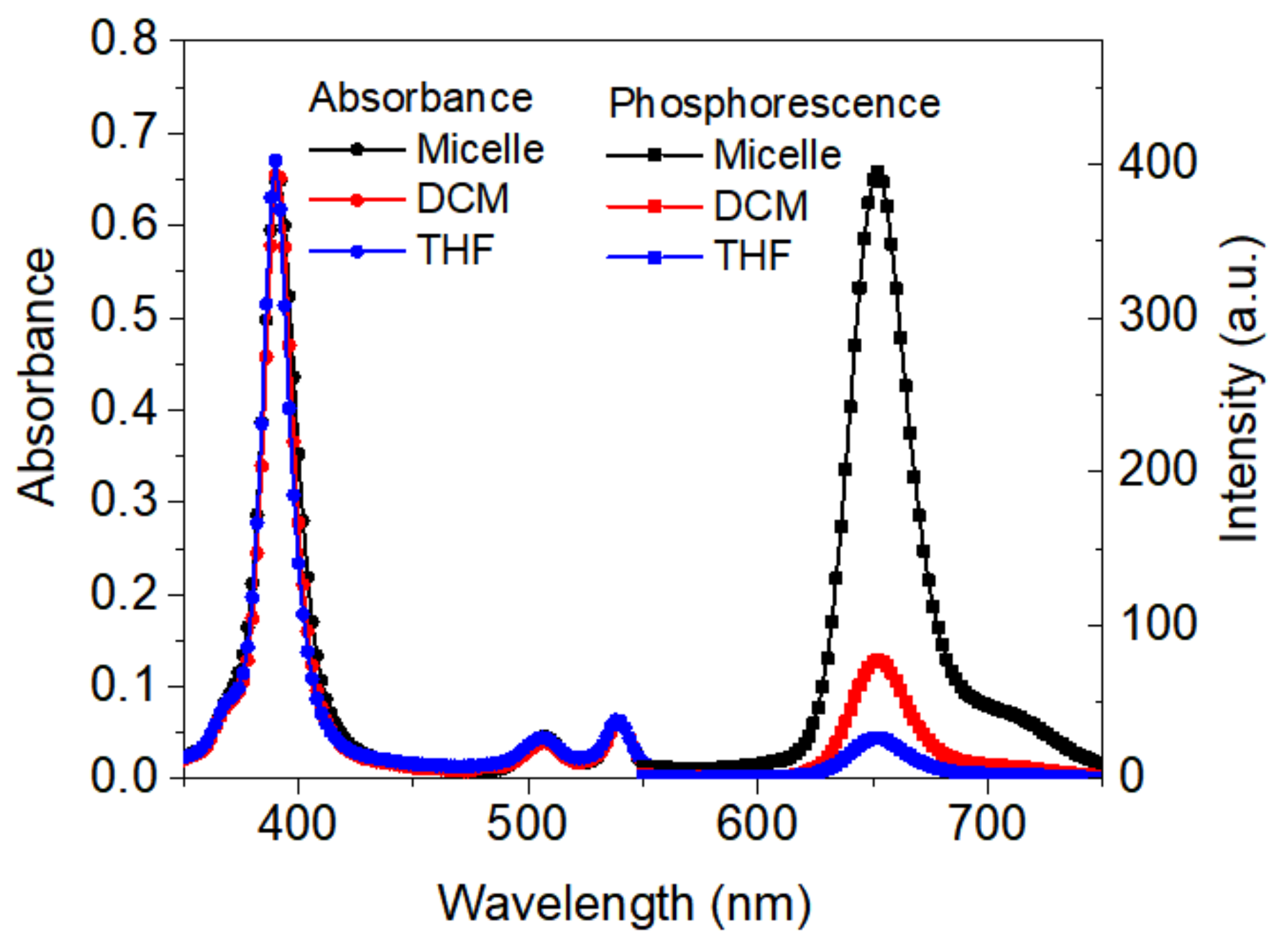
3.3. Photophysical Properties
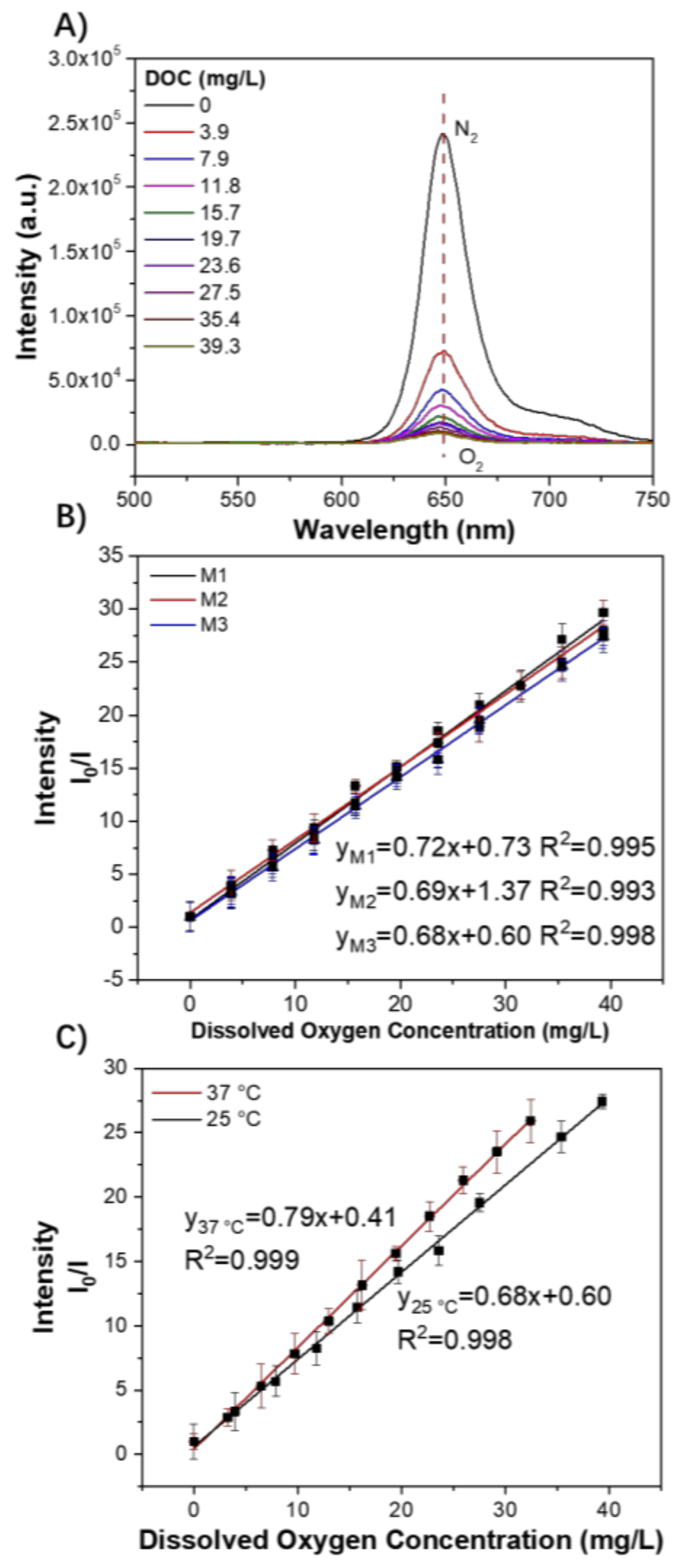
3.4. Oxygen Sensing Properties
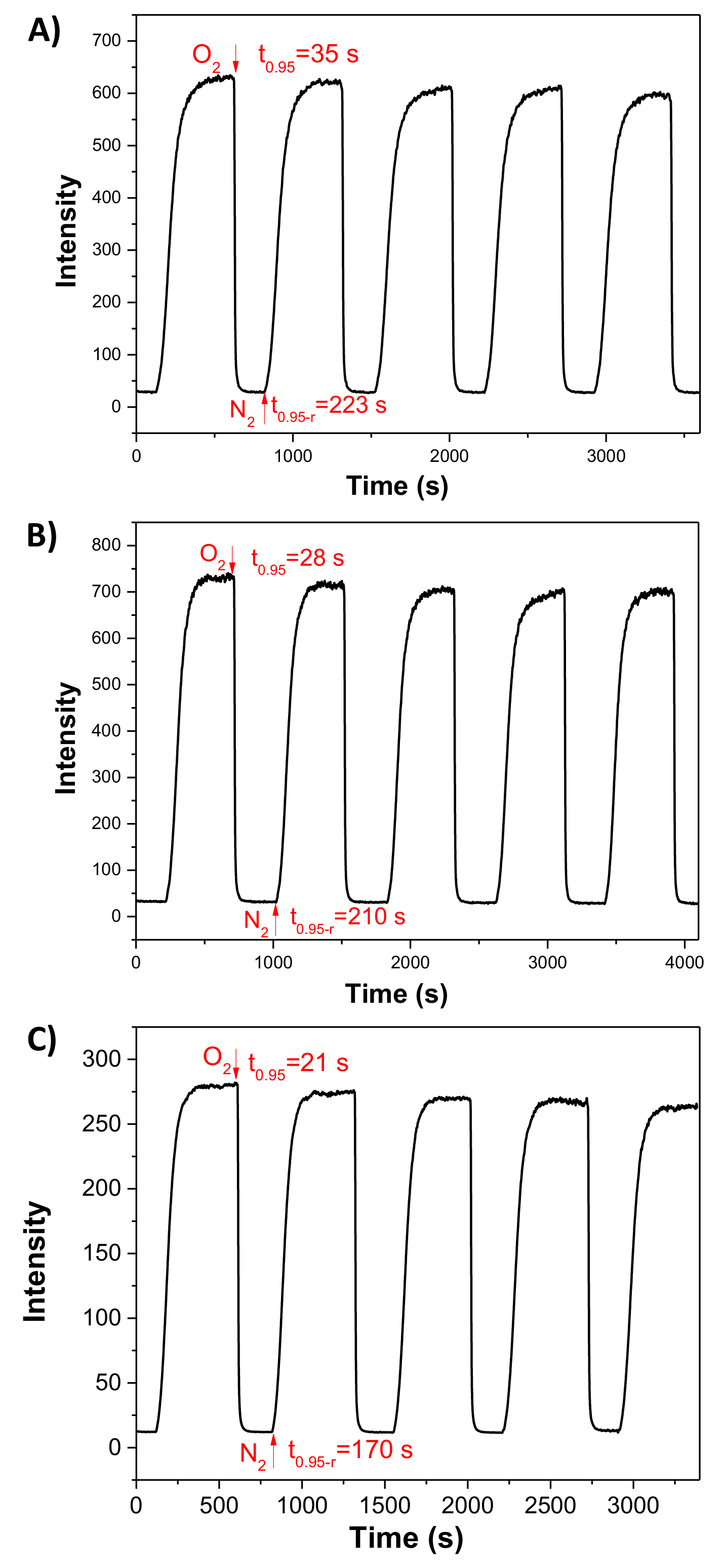
3.5. Response Time and Reversibility
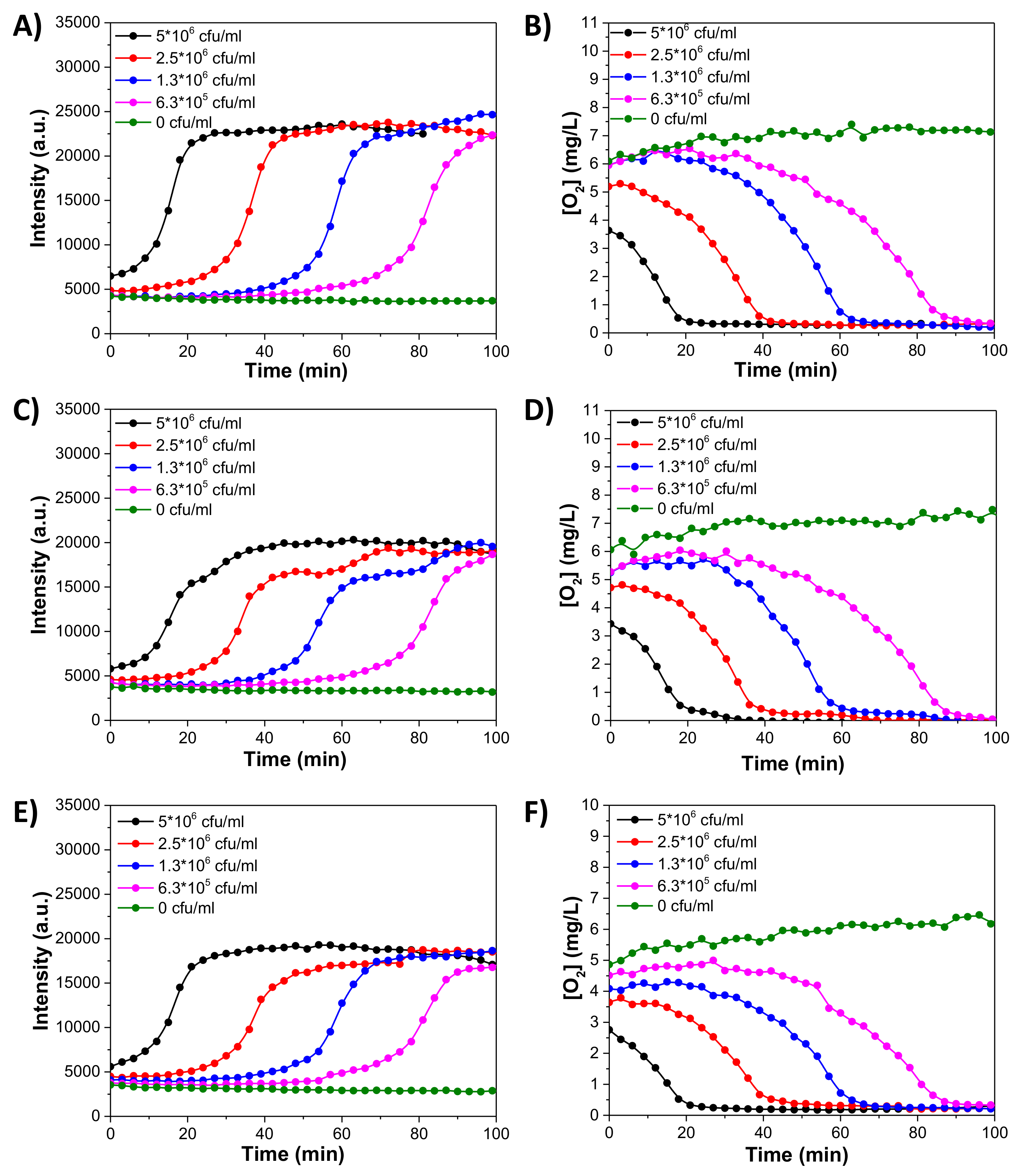
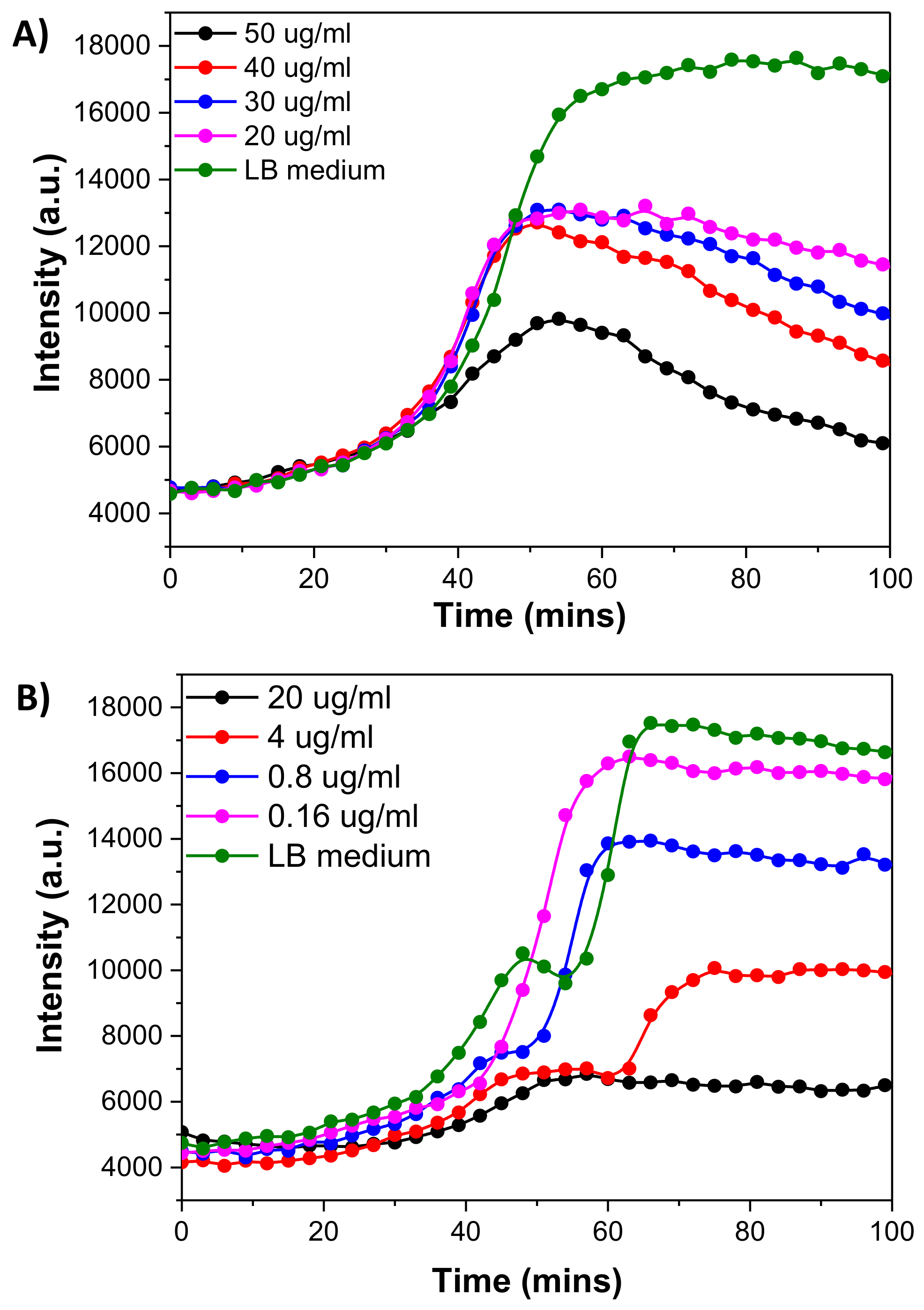
3.6. Monitoring of Oxygen Consumption of E. coli
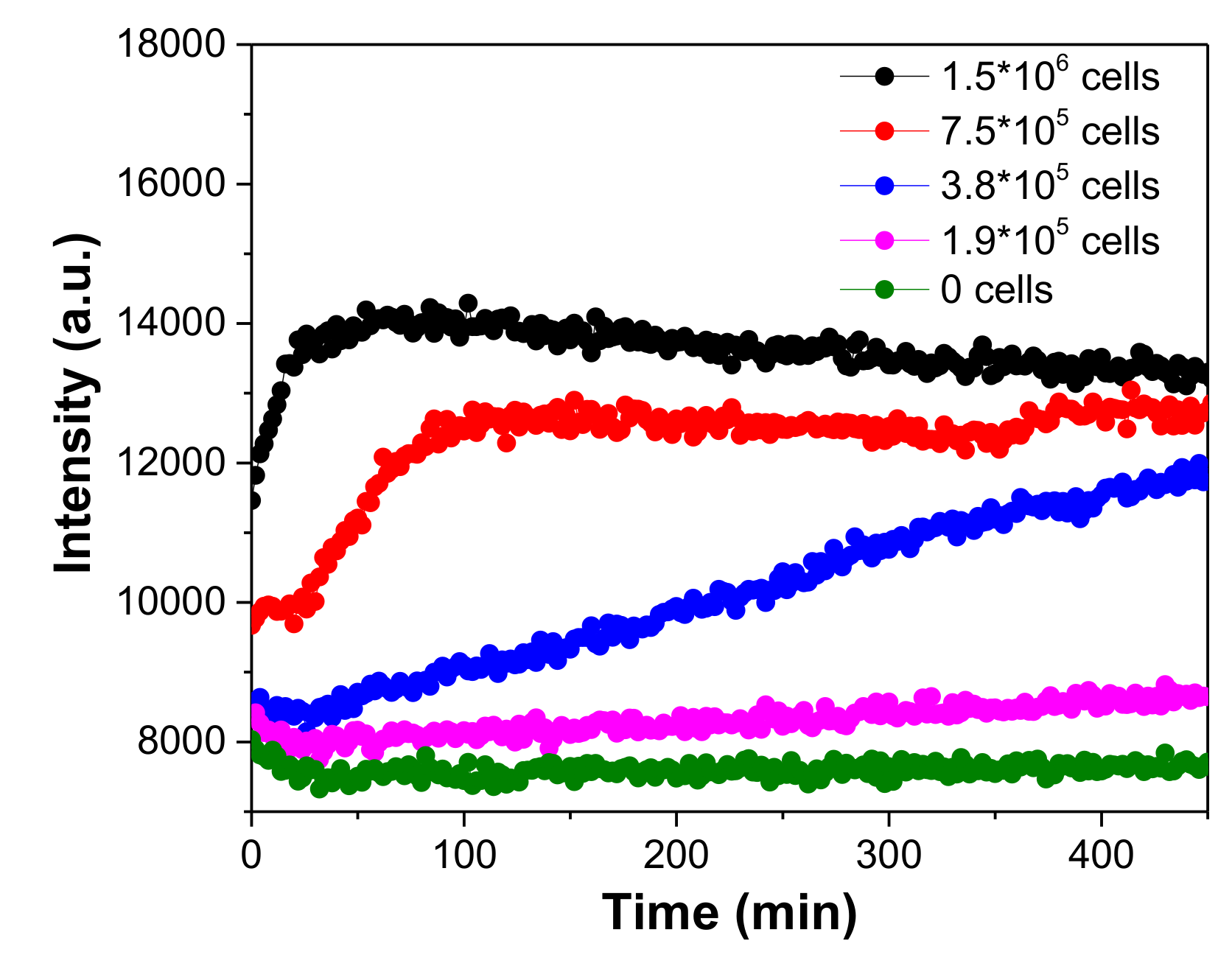
3.7. Monitoring Oxygen Consumption of Romas Cells
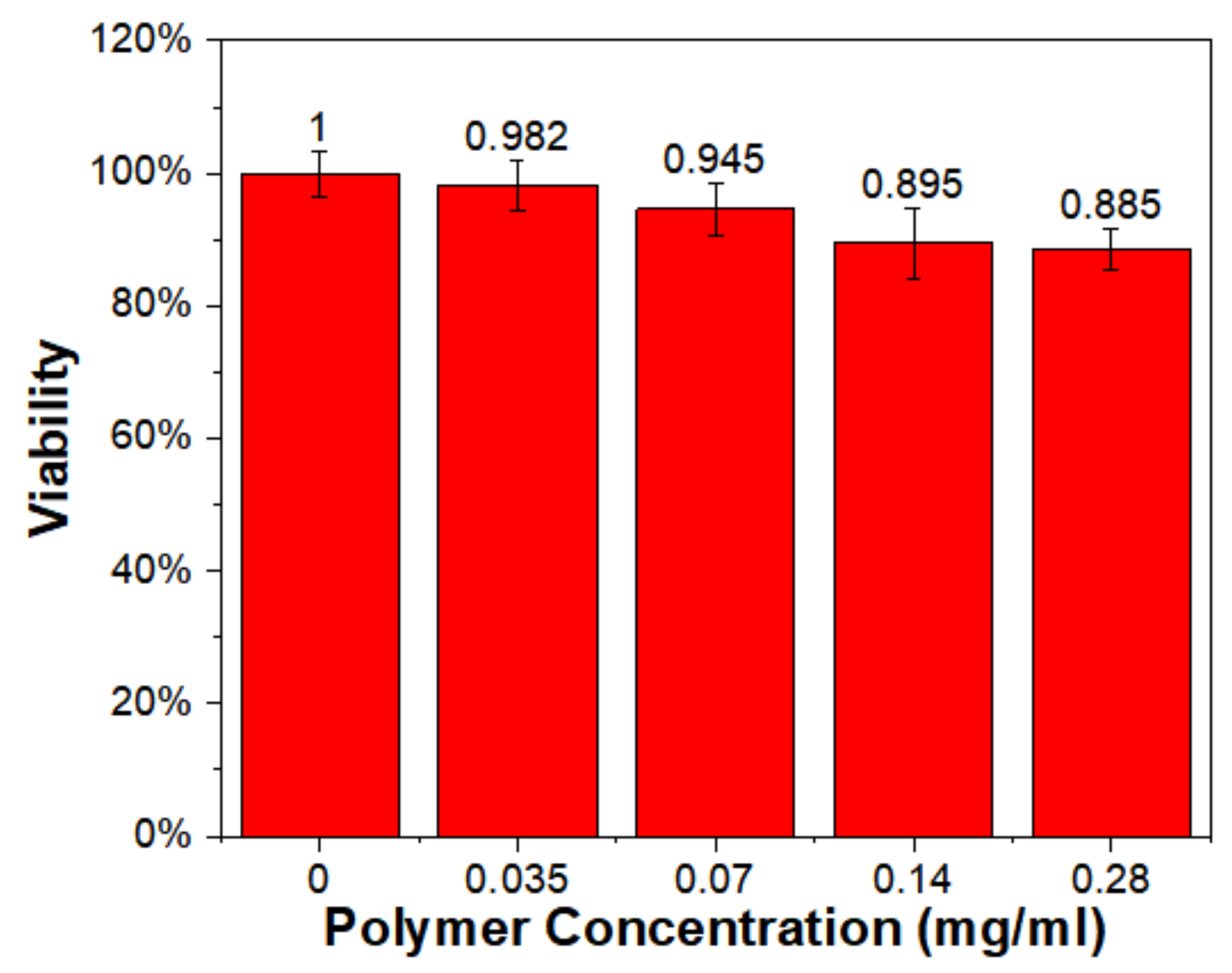
3.8. Cell Viability Test
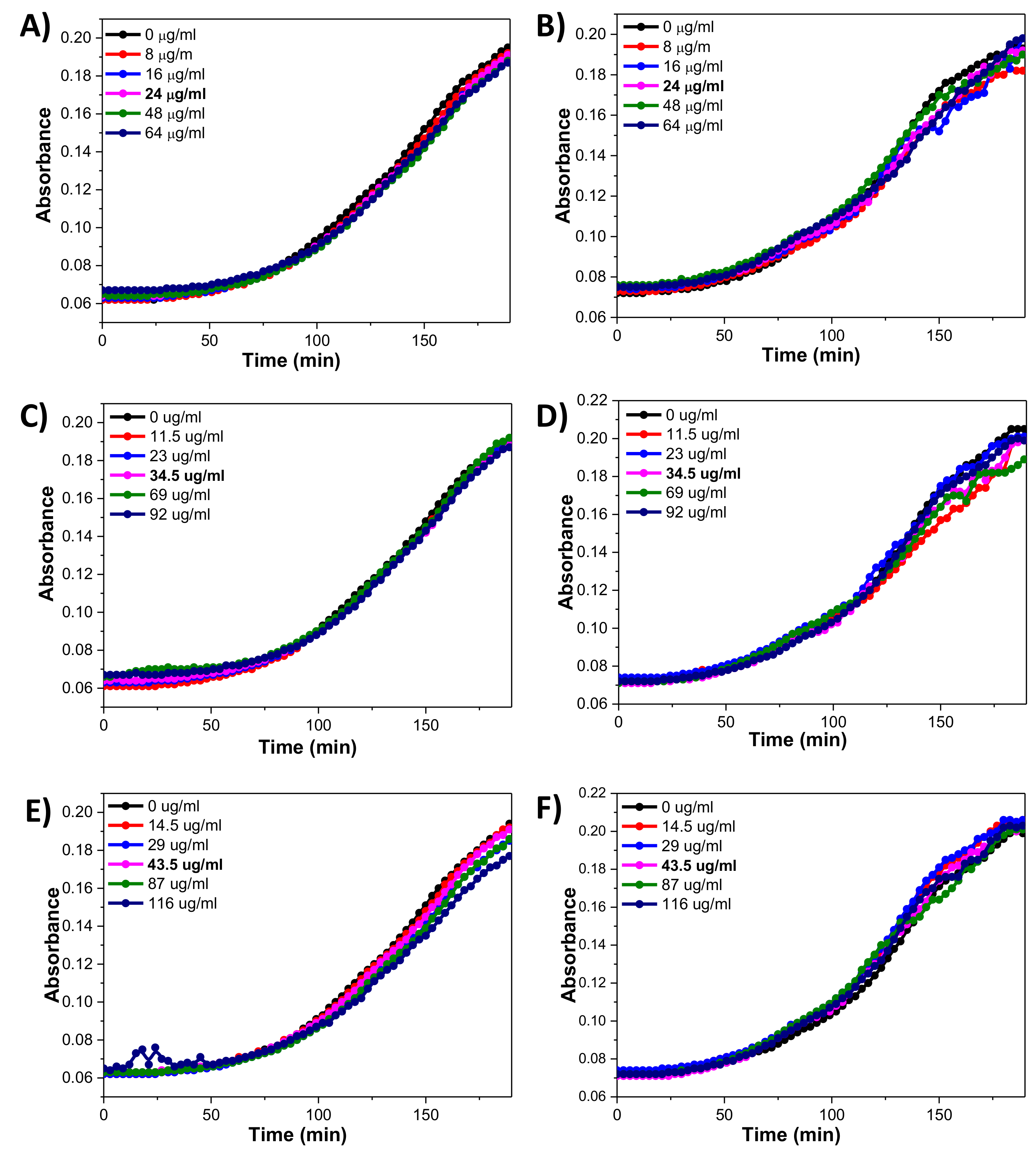
3.9. Bacterial Drug Resistance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bunn, H.F.; Poyton, R.O. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996, 76, 839–885. [Google Scholar]
- Decker, H.; van Holde, K.E. Oxygen and the Evolution of Life; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Wolfbeis, O.S. Sensor paints. Adv. Mater. 2008, 20, 3759–3763. [Google Scholar] [CrossRef]
- Semenza, G.L. Life with oxygen. Science 2007, 318, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.E.V.D.; Silva, R.J.N.B.D.; Camões, M.F.G.F.C. Optimization of the determination of chemical oxygen demand in wastewaters. Anal. Chim. Acta 2011, 699, 161–169. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, P.; Huang, X.; Ren, Z.J. A novel microbial fuel cell sensor with a gas diffusion biocathode sensing element for water and air quality monitoring. Chemosphere 2018, 203, 21–25. [Google Scholar] [CrossRef]
- Kelly, C.A.; Cruz-Romero, M.; Kerry, J.P.; Papkovsky, D.P. Assessment of Performance of the Industrial Process of Bulk Vacuum Packaging of Raw Meat with Nondestructive Optical Oxygen Sensing Systems. Sensors 2018, 18, 1395. [Google Scholar] [CrossRef]
- Parra, L.; Lloret, G.; Lloret, J.; Rodilla, M. Physical sensors for precision aquaculture: A Review. IEEE Sens. J. 2018, 18, 3915–3923. [Google Scholar] [CrossRef]
- Thomas, P.C.; Halter, M.; Tona, A.; Raghavan, S.R.; Plant, A.L.; Forry, S.P. A noninvasive thin film sensor for monitoring oxygen tension during in vitro cell culture. Anal. Chem. 2009, 81, 9239–9246. [Google Scholar] [CrossRef]
- Chang, G.; Morigaki, K.; Tatsu, Y.; Hikawa, T.; Goto, T.; Imaishi, H. Vertically integrated human P450 and oxygen sensing film for the assays of P450 metabolic activities. Anal. Chem. 2011, 83, 2956–2963. [Google Scholar] [CrossRef]
- Zou, X.S.; Pan, T.T.; Chen, L.; Tian, Y.Q.; Zhang, W.W. Luminescence materials for pH and oxygen sensing in microbial cells–structures, optical properties, and biological applications. Crit. Rev. Biotechnol. 2017, 37, 723–738. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Chem, A. Fiber-optic chemical sensors and biosensors. Anal. Chem. 2015, 74, 2663–2677. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Hirakawa, T.; Watanabe, I.; Kikuchi, Y. Determination of blood pO2 using a micromachined Clark-type oxygen electrode. Anal. Chim. Acta 2001, 431, 249–259. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the clark electrode. Bioessays 2015, 37, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.; Franklin, C. Hypoxemia and hypoxia. In Common Surgical Diseases: An Algorithmic Approach to Problem Solving; Millikan, J.A.M.W., Saclarides, T.J., Eds.; Springer: New York, NY, USA, 2008; pp. 391–394. [Google Scholar]
- Gnaiger, E.; Steinlechner-Maran, R.; Méndez, G.; Eberl, T.; Margreiter, R. Control of mitochondrial respiration by oxygen. J. Bioenergy 1995, 27, 583–596. [Google Scholar]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430. [Google Scholar] [CrossRef]
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef]
- Nadanaciva, S.; Rana, P.; Beeson, G.C.; Chen, D.; Ferrick, D.A.; Beeson, C.C.; Will, Y. Assessment of drug-induced mitochondrial dysfunction via altered cellular respiration and acidification measured in a 96-well platform. J. Bioenergy Biomembr. 2012, 44, 421–437. [Google Scholar] [CrossRef]
- Pang, H.L.; Kwok, N.Y.; Chow, M.C.; Yeung, C.H.; Wong, K.Y.; Chen, X.; Wang, X. ORMOSIL oxygen sensors on polystyrene microplate for dissolved oxygen measurement. Sens. Actuators B Chem. 2007, 123, 120–126. [Google Scholar] [CrossRef]
- Carraway, E.R.; Demas, J.N.; Degraff, B.A.; Bacon, J.R. Photophysics and photochemistry of oxygen sensors based on luminescent transition-metal complexes. Anal. Chem. 1991, 63, 337–342. [Google Scholar] [CrossRef]
- And, A.M.; Lepre, A. Controlling the response characteristics of luminescent porphyrin plastic film sensors for oxygen. Anal. Chem. 1997, 69, 4653–4659. [Google Scholar]
- O’Donovan, C.; Hynes, J.; Yashunski, D.; Papkovsky, D.B. Phosphorescent oxygen-sensitive materials for biological applications. J. Mater. Chem. 2005, 15, 2946–2951. [Google Scholar] [CrossRef]
- O’Riordan, T.C.; Fitzgerald, K.; Ponomarev, G.V.; Mackrill, J.; Hynes, J.; Taylor, C.; Papkovsky, D.B. Sensing intracellular oxygen using near-infrared phosphorescent probes and live-cell fluorescence imaging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1613–R1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Maslov, K.; Wang, L.V. Single-cell label-free photoacoustic flowoxigraphy in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 5759–5764. [Google Scholar] [CrossRef] [PubMed]
- Esipova, T.V.; Karagodov, A.; Miller, J.; Wilson, D.F.; Busch, T.M.; Vinogradov, S.A. Two new “protected” oxyphors for biological oximetry: Properties and application in tumor imaging. Anal. Chem. 2011, 83, 8756–8765. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.S.; Pan, T.T.; Jiang, J.P.; Li, G.; Song, C.; Sun, R.F.; Yang, Z.Y.; Sun, D.Z.; Hou, C.H.; Chen, M.W.; et al. Poly(epsilon-caprolactone)-containing graft copolymers for ratiometric extracellular oxygen sensing. Sens. Actuators B Chem. 2017, 248, 108–118. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, T.; Xiang, G.; Mei, Z.; Jiang, J.; Li, G.; Zou, X.; Chen, M.; Sun, D.; Jiang, S. Highly efficient ratiometric extracellular oxygen sensors through physical incorporation of a conjugated polymer and PtTFPP in graft copolymers. Sens. Actuators B Chem. 2018, 273, 242–252. [Google Scholar] [CrossRef]
- Su, F.; Alam, R.; Mei, Q.; Tian, Y.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. Nanostructured oxygen sensor-using micelles to incorporate a hydrophobic platinum porphyrin. PLoS ONE 2012, 7, e33390. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Li, G.; Mei, Z.P.; Shi, J.Y.; Mao, Y.Y.; Pan, T.T.; Liao, C.Z.; Zhang, J.B.; Tian, Y.Q. Preparation and application of ratiometric polystyrene-based microspheres as oxygen sensors. Anal. Chim. Acta 2018, 1030, 194–201. [Google Scholar] [CrossRef]
- Wang, X.H.; Peng, H.S.; Cheng, K.; Liu, X.M.; Liu, Y.A.; Yang, W. Two-photon oxygen nanosensors based on a conjugated fluorescent polymer doped with platinum porphyrins. Methods Appl. Floures. 2018, 6, 035008. [Google Scholar] [CrossRef]
- Mao, Y.Y.; Mei, Z.P.; Liang, L.F.; Zhou, B.P.; Tian, Y.Q. Robust and magnetically recoverable dual-sensor particles: Real-time monitoring of glucose and dissolved oxygen. Sens. Actuators B Chem. 2018, 262, 371–379. [Google Scholar] [CrossRef]
- Khanna, K.; Varshney, S.; Kakkar, A. Miktoarm star polymers: Advances in synthesis, self-assembly, and applications. Polym. Chem. 2010, 1, 1171–1185. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, S. Macromol Supramolecular Self-Assembly of Nonlinear Amphiphilic and Double Hydrophilic Block Copolymers in Aqueous Solutions, Macromol. Rapid Commun. 2009, 30, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.J.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kim, T.H.; Wu, W.C.; Huang, C.M.; Wei, H.; Mount, C.W.; Tian, Y.; Jang, S.H.; Pun, S.H.; Jen, A.K. pH-dependent, thermosensitive polymeric nanocarriers for drug delivery to solid tumors. Biomaterials 2013, 34, 4501–4509. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.S.; Jamshidi, R.; Tor, Y. 10-phenanthrolines as tunable fluorophores. Angew. Chem. Int. Ed. 1999, 38, 2721–2725. [Google Scholar] [CrossRef]
- Lai, S.-W.; Hou, Y.-J.; Che, C.-M.; Pang, H.-L.; Wong, K.-Y.; Chang, C.K.; Zhu, N. Electronic spectroscopy, photophysical properties, and emission quenching studies of an oxidatively robust perfluorinated platinum porphyrin. Inorg. Chem. 2004, 43, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Yang, C.; Shi, J.; Hao, C.; Qiao, Y.; Li, J.; Deng, M.; Tian, Y.; Chen, M. Dual ph and oxygen luminescent nanoprobes based on graft polymers for extracellular metabolism monitoring and intracellular imaging. Sens. Actuators B Chem. 2019, 291, 306–318. [Google Scholar] [CrossRef]
- Yang, F.; Gao, H.; Li, S.-S.; An, R.-B.; Sun, X.-Y.; Kang, B.; Xu, J.-J.; Chen, H.-Y. A fluorescent τ-probe: Quantitative imaging of ultra-trace endogenous hydrogen polysulfide in cells and in vivo. Chem. Sci. 2018, 9, 5556–5563. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Li, Y.; Zhuang, Q.; Gu, J. Real-time monitoring of dissolved oxygen with inherent oxygen-sensitive centers in metal–organic frameworks. Chem. Mater. 2016, 28, 2652–2658. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Tian, Y.; Shumway, B.R.; Gao, W.; Youngbull, C.; Holl, M.R.; Johnson, R.H.; Meldrum, D.R. Influence of matrices on oxygen sensing of three sensing films with chemically conjugated platinum porphyrin probes and preliminary application for monitoring of oxygen consumption of escherichia coli (E. Coli). Sens. Actuators B Chem. 2010, 150, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Buck, S.M.; Kopelman, R.; Philbert, M.A.; Brasuel, M.; Monson, E.; Behrend, C.; Ross, B.; Rehemtulla, A.; Koo, Y.E.L. Fluorescent Pebble Nano-Sensors and Nanoexplorers for Real-Time Intracellular and Biomedical Applications. Top. Fluoresc. Spectrosc. 2005, 10, 69–126. [Google Scholar]
- Gao, X.; Yang, L.; Petros, J.A.; Marshall, F.F.; Simons, J.W.; Nie, S. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005, 16, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, W.C.; Chen, C.Y.; Jang, S.H.; Zhang, M.; Strovas, T.; Anderson, J.; Cookson, B.; Li, Y.; Meldrum, D. Utilization of micelles formed from poly (ethylene glycol)-block-poly (ϵ-caprolactone) block copolymers as nanocarriers to enable hydrophobic red two-photon absorbing emitters for cells imaging. J. Biomed. Mater. Res. 2010, 93, 1068–1079. [Google Scholar]
- Ashok, B.; Arleth, L.; Hjelm, R.P.; Rubinstein, I.; Önyüksel, H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: Effects of PEG chain length and PC incorporation. J. Pharm. Sci. 2004, 93, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Agarwal, S.; Pan, T.; Qiao, Y.; Zhang, L.; Shi, Z.; Kong, X.; Day, K.; Chen, M.; Meldrum, D. Multifunctional phpma-derived polymer for ratiometric ph sensing, fluorescence imaging, and magnetic resonance imaging. ACS Appl. Mater. Interfaces 2018, 10, 1556–1565. [Google Scholar] [CrossRef]
- Hütter, E.; Renner, K.; Jansen-Dürr, P.; Gnaiger, E. Biphasic oxygen kinetics of cellular respiration and linear oxygen dependence of antimycin A inhibited oxygen consumption. Mol. Biol. Rep. 2002, 29, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Arsianti, A.; Hanafi, M.; Saepudin, E.; Morimoto, T.; Kakiuchi, K. Synthesis and biological activity of 2-hydroxynicotinoyl-serine-butyl esters related to antibiotic UK-3A. Bioorg. Med. Chem. Lett. 2010, 20, 4018–4020. [Google Scholar] [CrossRef] [PubMed]

| Product- | Molar Ratioa | Yield | GPC | 1H-NMR | Mass ratiob | CMC (μg/mL) | ||
|---|---|---|---|---|---|---|---|---|
| Mn (g/mol) | Mw (g/mol) | PDI | Mn (g/mol) | |||||
| P1 | 1:224 | 72.4% | 13610 | 17800 | 1.31 | 33300 | 1:2.33 | 3.10 |
| P2 | 1:112 | 45.6% | 11940 | 12680 | 1.27 | 18700 | 1:0.87 | 2.49 |
| P3 | 1:56 | 44.0% | 8970 | 9960 | 1.11 | 13600 | 1:0.36 | 3.31 |
| Micelles- | Concentration of Polymers (mg/mL) a | Concentrations of PtTFPP (mg/mL) b | Quantum Yield (%) | Encapsulation efficiency for PtTFPP (%) c | PtTFPP loading ratio (wt%) d | Size (nm) e | PDI e |
|---|---|---|---|---|---|---|---|
| M1 | 1.4 | 0.219 | 15.9 | 65.7 | 15.4 | 203.8 | 0.221 |
| M2 | 2.1 | 0.226 | 11.4 | 79.1 | 9.7 | 89.3 | 0.176 |
| M3 | 2.7 | 0.195 | 6.0 | 82.9 | 6.7 | 40.0 | 0.213 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Pan, T.; Li, J.; Yang, C.; Wen, J.; Zhong, K.; Wu, S.; Su, F.; Tian, Y. Extracellular Oxygen Sensors Based on PtTFPP and Four-Arm Block Copolymers. Appl. Sci. 2019, 9, 4404. https://doi.org/10.3390/app9204404
Qiao Y, Pan T, Li J, Yang C, Wen J, Zhong K, Wu S, Su F, Tian Y. Extracellular Oxygen Sensors Based on PtTFPP and Four-Arm Block Copolymers. Applied Sciences. 2019; 9(20):4404. https://doi.org/10.3390/app9204404
Chicago/Turabian StyleQiao, Yuan, Tingting Pan, Jiaze Li, Cheng Yang, Jiaxing Wen, Ke Zhong, Shanshan Wu, Fengyu Su, and Yanqing Tian. 2019. "Extracellular Oxygen Sensors Based on PtTFPP and Four-Arm Block Copolymers" Applied Sciences 9, no. 20: 4404. https://doi.org/10.3390/app9204404
APA StyleQiao, Y., Pan, T., Li, J., Yang, C., Wen, J., Zhong, K., Wu, S., Su, F., & Tian, Y. (2019). Extracellular Oxygen Sensors Based on PtTFPP and Four-Arm Block Copolymers. Applied Sciences, 9(20), 4404. https://doi.org/10.3390/app9204404





