Characteristics of Postural Muscle Activity in Response to A Motor-Motor Task in Elderly
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
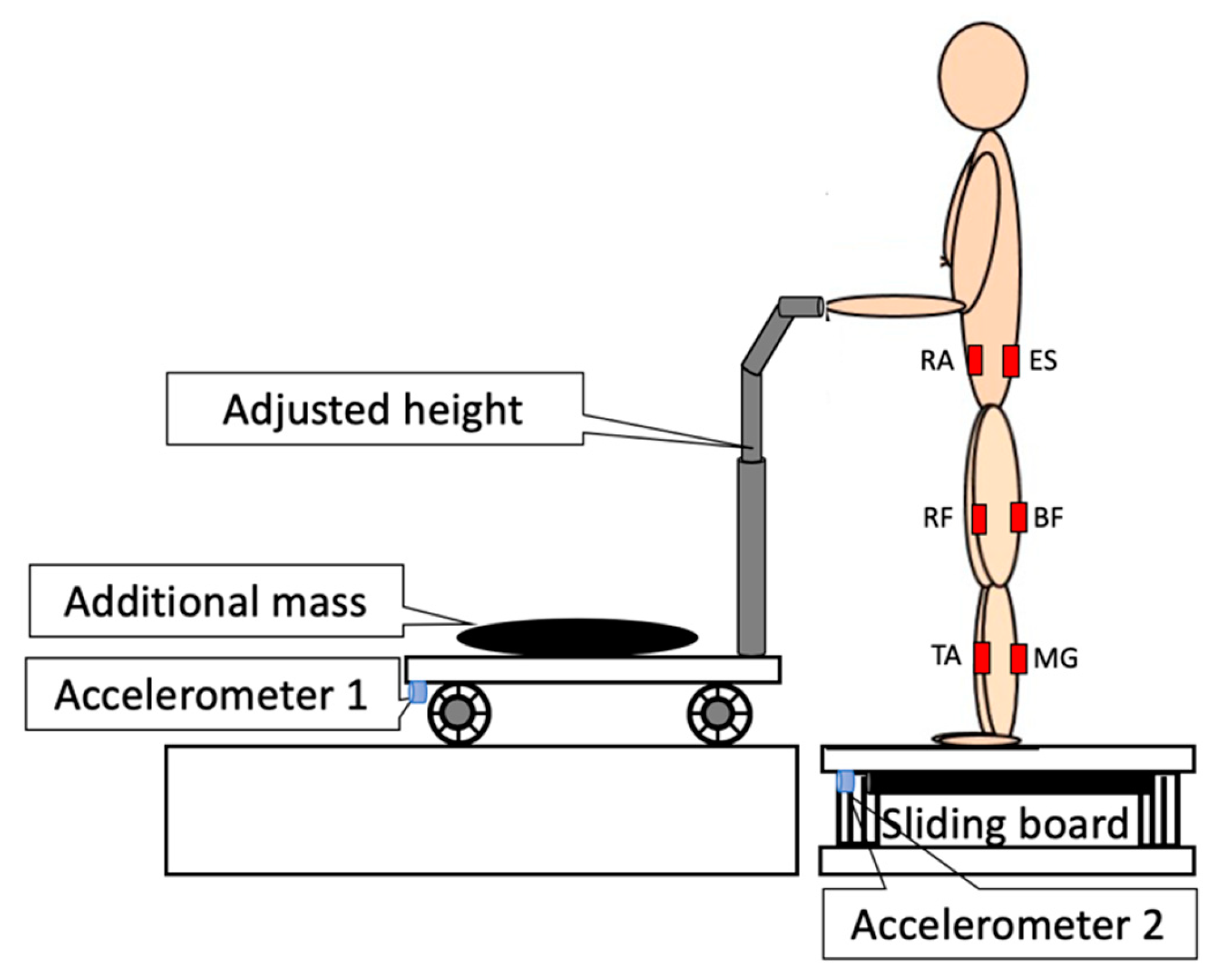
2.2. Procedure and Instrumentation
2.3. Data Processing
2.4. Statistics
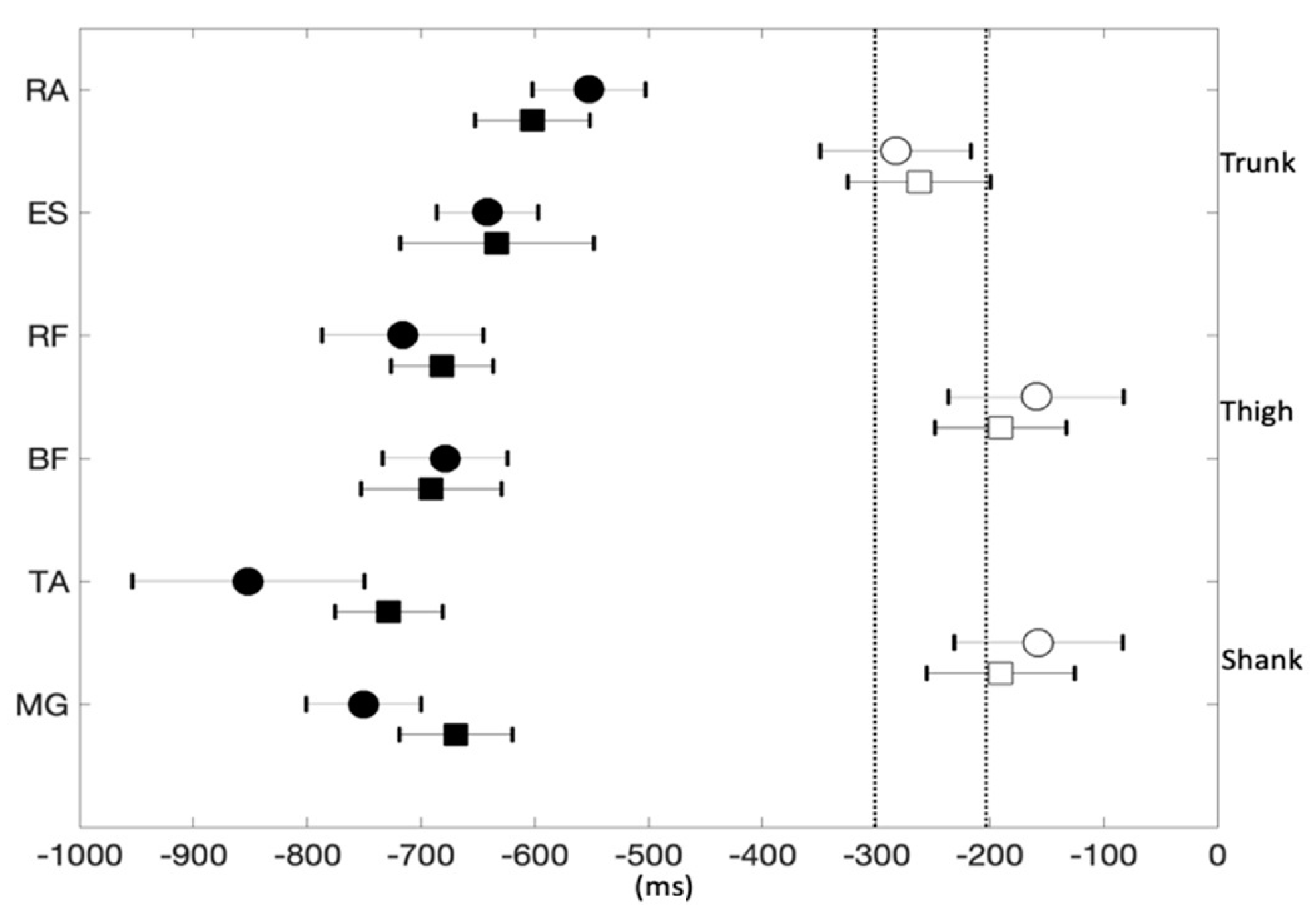
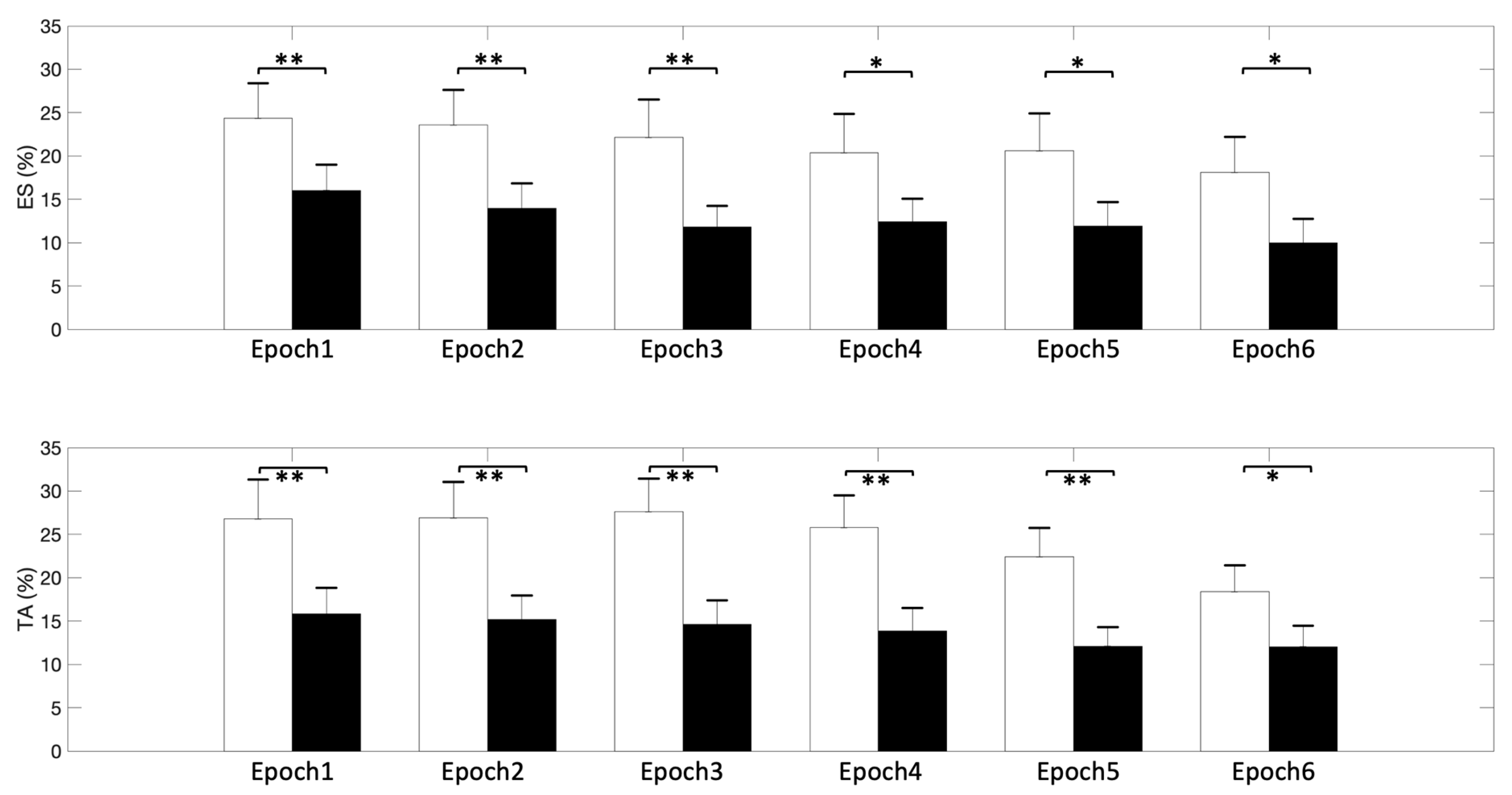
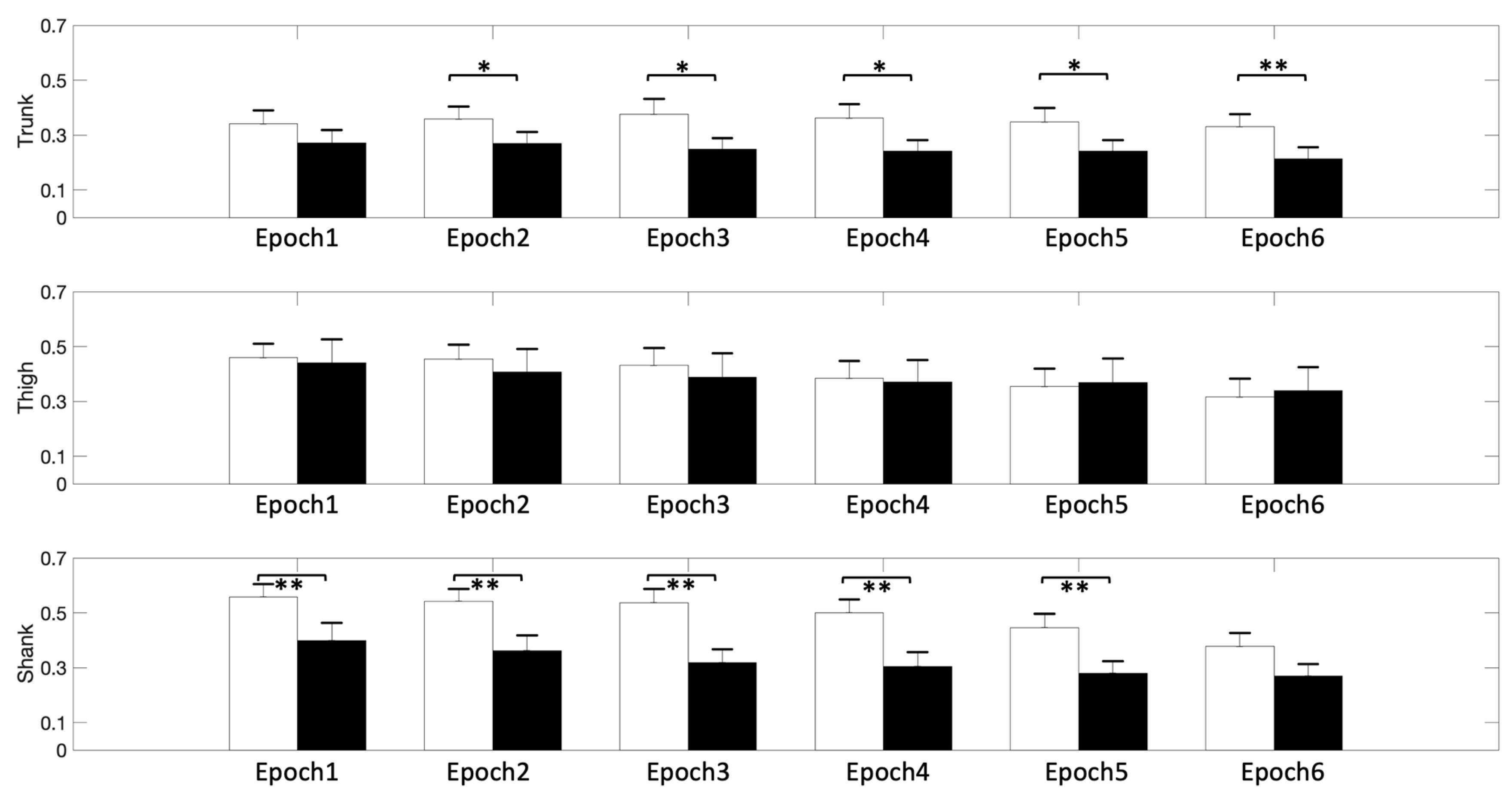
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aruin, A.S.; Latash, M.L. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp. Brain Res. 1995, 106, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.; Cresswell, A.; Thorstensson, A. Preparatory trunk motion accompanies rapid upper limb movement. Exp. Brain Res. 1999, 124, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.A.; Brown, R.H.; Stapley, P.J. Reaching to multiple targets when standing: The spatial organization of feedforward postural adjustments. J. Neurophysiol. 2009, 101, 2120–2133. [Google Scholar] [CrossRef]
- Lowrey, C.R.; Nashed, J.Y.; Scott, S.H. Rapid and flexible whole body postural responses are evoked from perturbations to the upper limb during goal-directed reaching. J. Neurophysiol. 2017, 117, 1070–1083. [Google Scholar] [CrossRef]
- Desmurget, M.; Grafton, S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn. Sci. 2000, 4, 423–431. [Google Scholar] [CrossRef]
- Carvalho, R.; Vasconcelos, O.; Goncalves, P.; Conceicao, F.; Vilas-Boas, J.P. The effects of physical activity in the anticipatory postural adjustments in elderly people. Motor Control 2010, 14, 371–379. [Google Scholar] [CrossRef][Green Version]
- Kubicki, A.; Bonnetblanc, F.; Petrement, G.; Ballay, Y.; Mourey, F. Delayed postural control during self-generated perturbations in the frail older adults. Clin. Interv. Aging 2012, 7, 65–75. [Google Scholar] [CrossRef]
- Bleuse, S.; Cassim, F.; Blatt, J.L.; Labyt, E.; Derambure, P.; Guieu, J.D.; Defebvre, L. Effect of age on anticipatory postural adjustments in unilateral arm movement. Gait Posture 2006, 24, 203–210. [Google Scholar] [CrossRef]
- Bugnariu, N.; Sveistrup, H. Age-related changes in postural responses to externally- and self-triggered continuous perturbations. Arch. Gerontol. Geriatr. 2006, 42, 73–89. [Google Scholar] [CrossRef]
- Kanekar, N.; Aruin, A.S. The effect of aging on anticipatory postural control. Exp. Brain Res. 2014, 232, 1127–1136. [Google Scholar] [CrossRef]
- Kanekar, N.; Aruin, A.S. Aging and balance control in response to external perturbations: Role of anticipatory and compensatory postural mechanisms. Age 2014, 36, 9621. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Chen, B.; Aruin, A.S. Older adults utilize less efficient postural control when performing pushing task. J. Electromyogr. Kinesiol. 2015, 25, 966–972. [Google Scholar] [CrossRef]
- Horak, F.B.; Nashner, L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef]
- Halicka, Z.; Lobotkova, J.; Bzduskova, D.; Hlavacka, F. Age-related changes in postural responses to backward platform translation. Physiol. Res. 2012, 61, 331–335. [Google Scholar]
- Bergamin, M.; Gobbo, S.; Zanotto, T.; Sieverdes, J.C.; Alberton, C.L.; Zaccaria, M.; Ermolao, A. Influence of age on postural sway during different dual-task conditions. Front. Aging Neurosci. 2014, 6, 271. [Google Scholar] [CrossRef]
- Boisgontier, M.P.; Beets, I.A.; Duysens, J.; Nieuwboer, A.; Krampe, R.T.; Swinnen, S.P. Age-related differences in attentional cost associated with postural dual tasks: Increased recruitment of generic cognitive resources in older adults. Neurosci. Biobehav. Rev. 2013, 37, 1824–1837. [Google Scholar] [CrossRef]
- Li, K.Z.H.; Bherer, L.; Mirelman, A.; Maidan, I.; Hausdorff, J.M. Cognitive involvement in balance, gait and dual-tasking in aging: A focused review from a neuroscience of aging perspective. Front. Neurol. 2018, 9, 913. [Google Scholar] [CrossRef]
- Mazaheri, M.; Hoogkamer, W.; Potocanac, Z.; Verschueren, S.; Roerdink, M.; Beek, P.J.; Peper, C.E.; Duysens, J. Effects of aging and dual tasking on step adjustments to perturbations in visually cued walking. Exp. Brain Res. 2015, 233, 3467–3474. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chen, B.; Liang, J.N.; Aruin, A.S. Control of vertical posture while standing on a sliding board and pushing an object. Exp. Brain Res. 2018, 236, 721–731. [Google Scholar] [CrossRef]
- Dietz, V.; Kowalewski, R.; Nakazawa, K.; Colombo, G. Effects of changing stance conditions on anticipatory postural adjustment and reaction time to voluntary arm movement in humans. J. Physiol. 2000, 524, 617–627. [Google Scholar] [CrossRef]
- Bateni, H.; Zecevic, A.; McIlroy, W.E.; Maki, B.E. Resolving conflicts in task demands during balance recovery: Does holding an object inhibit compensatory grasping? Exp. Brain Res. 2004, 157, 49–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, B.; Lee, Y.J.; Aruin, A.S. Control of grip force and vertical posture while holding an object and being perturbed. Exp. Brain Res. 2016, 234, 3193–3201. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S. Adaptive utilization of optical variables during postural and suprapostural dual-task performance: Comment on stoffregen, smart, bardy, and pagulayan (1999). J. Exp. Psychol. Hum. Percept. Perform. 2004, 30, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.L.; Redfern, M.S.; Jennings, J.R. Postural prioritization defines the interaction between a reaction time task and postural perturbations. Exp. Brain Res. 2007, 183, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Woollacott, M.; Kerns, K.A.; Baldwin, M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M232–M240. [Google Scholar] [CrossRef] [PubMed]
- Konrad, P. The ABC of EMG: A Practical Introduction to Kinesiological Electromyography; Noraxon Inc.: Scottsdale, AZ, USA, 2005. [Google Scholar]
- Li, X.; Zhou, P.; Aruin, A.S. Teager-kaiser energy operation of surface emg improves muscle activity onset detection. Ann. Biomed. Eng. 2007, 35, 1532–1538. [Google Scholar] [CrossRef]
- Solnik, S.; Rider, P.; Steinweg, K.; DeVita, P.; Hortobagyi, T. Teager-kaiser energy operator signal conditioning improves emg onset detection. Eur. J. Appl. Physiol. 2010, 110, 489–498. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hoozemans, M.J.; van Dieen, J.H. Oblique abdominal muscle activity in response to external perturbations when pushing a cart. J. Biomech. 2010, 43, 1364–1372. [Google Scholar] [CrossRef]
- Krishnan, V.; Latash, M.L.; Aruin, A.S. Early and late components of feed-forward postural adjustments to predictable perturbations. Clin. Neurophysiol. 2012, 123, 1016–1026. [Google Scholar] [CrossRef]
- Lee, Y.J.; Aruin, A.S. Three components of postural control associated with pushing in symmetrical and asymmetrical stance. Exp. Brain Res. 2013, 228, 341–351. [Google Scholar] [CrossRef]
- Slijper, H.; Latash, M.L. The effects of muscle vibration on anticipatory postural adjustments. Brain Res. 2004, 1015, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lee, Y.J.; Aruin, A.S. Anticipatory and compensatory postural adjustments in conditions of body asymmetry induced by holding an object. Exp. Brain Res. 2015, 233, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.M.; Farina, D.; Loram, I.D. Emg and posture in its narrowest sense. In Surface Electromyography: Physiology, Engineering, and Allications; Merletti, R., Farina, D., Eds.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Krishnan, V.; Aruin, A.S.; Latash, M.L. Two stages and three components of the postural preparation to action. Exp. Brain Res. 2011, 212, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Latash, M.L.; Levin, M.F.; Scholz, J.P.; Schoner, G. Motor control theories and their applications. Medicina 2010, 46, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lee, Y.J.; Aruin, A.S. Role of point of application of perturbation in control of vertical posture. Exp. Brain Res. 2017, 235, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hwang, J.M. The center of pressure and ankle muscle co-contraction in response to anterior-posterior perturbations. PLoS ONE 2018, 13, e0207667. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.M.; Dhaher, Y.Y.; Perreault, E.J. Contributions of feed-forward and feedback strategies at the human ankle during control of unstable loads. Exp. Brain Res. 2012, 217, 53–66. [Google Scholar] [CrossRef]
- Craig, C.E.; Goble, D.J.; Doumas, M. Proprioceptive acuity predicts muscle co-contraction of the tibialis anterior and gastrocnemius medialis in older adults’ dynamic postural control. Neuroscience 2016, 322, 251–261. [Google Scholar] [CrossRef]
- Piras, A.; Raffi, M.; Perazzolo, M.; Squatrito, S. Influence of heading perception in the control of posture. J. Electromyogr. Kinesiol. 2018, 39, 89–94. [Google Scholar] [CrossRef]
- Raffi, M.; Piras, A.; Persiani, M.; Perazzolo, M.; Squatrito, S. Angle of gaze and optic flow direction modulate body sway. J. Electromyogr. Kinesiol. 2017, 35, 61–68. [Google Scholar] [CrossRef]
- Ramkhalawansingh, R.; Butler, J.S.; Campos, J.L. Visual-vestibular integration during self-motion perception in younger and older adults. Psychol. Aging 2018, 33, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Argubi-Wollesen, A.; Wollesen, B.; Leitner, M.; Mattes, K. Human body mechanics of pushing and pulling: Analyzing the factors of task-related strain on the musculoskeletal system. Saf. Health Work 2017, 8, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Gerards, M.H.G.; McCrum, C.; Mansfield, A.; Meijer, K. Perturbation-based balance training for falls reduction among older adults: Current evidence and implications for clinical practice. Geriatr. Gerontol. Int. 2017, 17, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
| Muscle | Epoch 1 | Epoch 2 | Epoch 3 | Epoch 4 | Epoch 5 | Epoch 6 |
|---|---|---|---|---|---|---|
| RA | 10.51 ± 2.51 Ɨ | 12.68 ± 2.62 | 14.22 ± 2.62 | 13.77 ± 2.51 | 13.28 ± 3.14 | 13.15 ± 2.41 |
| ES | 20.18 ± 3.31 * | 18.77 ± 3.27 * | 16.97 ± 3.16 * | 16.39 ± 3.22 * | 16.23 ± 3.29 * | 14.05 ± 3.21 * |
| RF | 20.64 ± 4.07 | 21.10 ± 4.00 | 21.21 ± 4.17 | 19.02 ± 4.32 | 17.78 ± 4.50 | 16.82 ± 4.36 |
| BF | 24.45 ± 4.42 | 22.02 ± 4.06 | 19.82 ± 4.12 | 18.75 ± 4.10 | 18.44 ± 4.31 | 15.99 ± 4.30 |
| TA | 21.33 ± 3.52 * | 21.02 ± 3.34 * | 21.15 ± 3.11 * | 19.82 ± 2.88 * | 17.25 ± 2.56 * | 15.22 ± 2.53 * |
| MG | 26.55 ± 4.60 | 24.19 ± 4.34 | 21.60 ± 3.96 | 20.43 ± 3.68 | 19.00 ± 3.56 | 17.15 ± 3.52 |
| Epoch 1 | Epoch 2 | Epoch 3 | Epoch 4 | Epoch 5 | Epoch 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment | t (59) | p | t (59) | p | t (59) | p | t (59) | p | t (59) | p | t (59) | p |
| Trunk | −9.95 | <0.001 | −9.16 | <0.001 | −7.80 | <0.001 | −7.64 | <0.001 | −7.58 | <0.001 | −7.04 | <0.001 |
| Thigh | −8.66 | <0.001 | −8.74 | <0.001 | −7.80 | <0.001 | −7.78 | <0.001 | −7.46 | <0.001 | −6.71 | <0.001 |
| Shank | −9.76 | <0.001 | −9.15 | <0.001 | −8.22 | <0.001 | −8.12 | <0.001 | −8.19 | <0.001 | −7.94 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Liang, J.N.; Wen, Y.-T. Characteristics of Postural Muscle Activity in Response to A Motor-Motor Task in Elderly. Appl. Sci. 2019, 9, 4319. https://doi.org/10.3390/app9204319
Lee Y-J, Liang JN, Wen Y-T. Characteristics of Postural Muscle Activity in Response to A Motor-Motor Task in Elderly. Applied Sciences. 2019; 9(20):4319. https://doi.org/10.3390/app9204319
Chicago/Turabian StyleLee, Yun-Ju, Jing Nong Liang, and Yu-Tang Wen. 2019. "Characteristics of Postural Muscle Activity in Response to A Motor-Motor Task in Elderly" Applied Sciences 9, no. 20: 4319. https://doi.org/10.3390/app9204319
APA StyleLee, Y.-J., Liang, J. N., & Wen, Y.-T. (2019). Characteristics of Postural Muscle Activity in Response to A Motor-Motor Task in Elderly. Applied Sciences, 9(20), 4319. https://doi.org/10.3390/app9204319





