Melanoma Growth Analysis in Blood Serum and Tissue Using Xenograft Model with Response to Cold Atmospheric Plasma Activated Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Animal
2.2. Device Characteristics and PAM Production
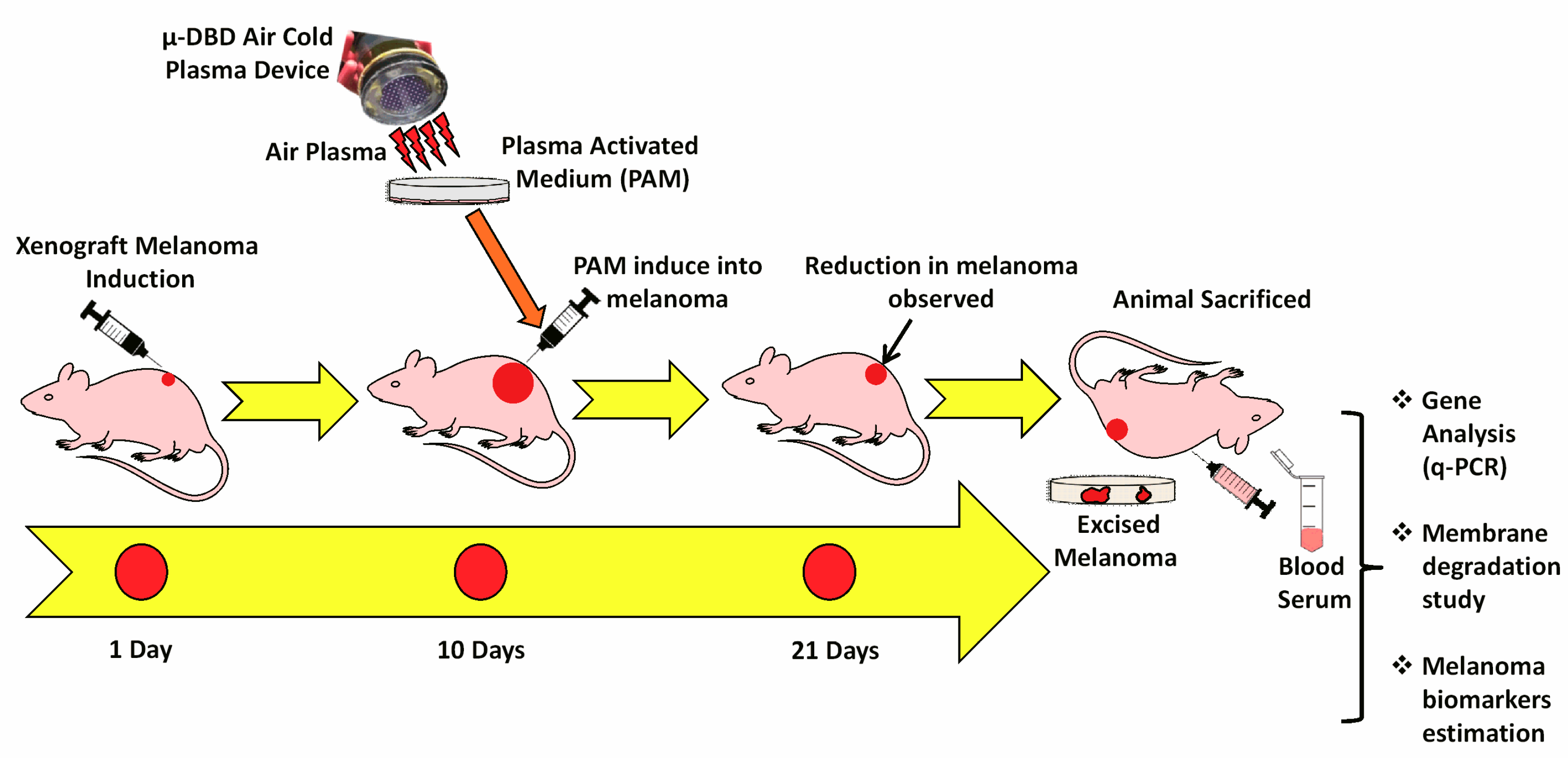
2.3. Melanoma Xenograft Induction
2.4. Protein Carbonylation Assay
2.5. Lipid Peroxidation Estimation (MDA Content)
2.6. LDH Assay
2.7. L-DOPA Estimation
2.8. Real-Time q-PCR Analysis
2.9. Immunohistochemistry and Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Cold Plasma Characteristics
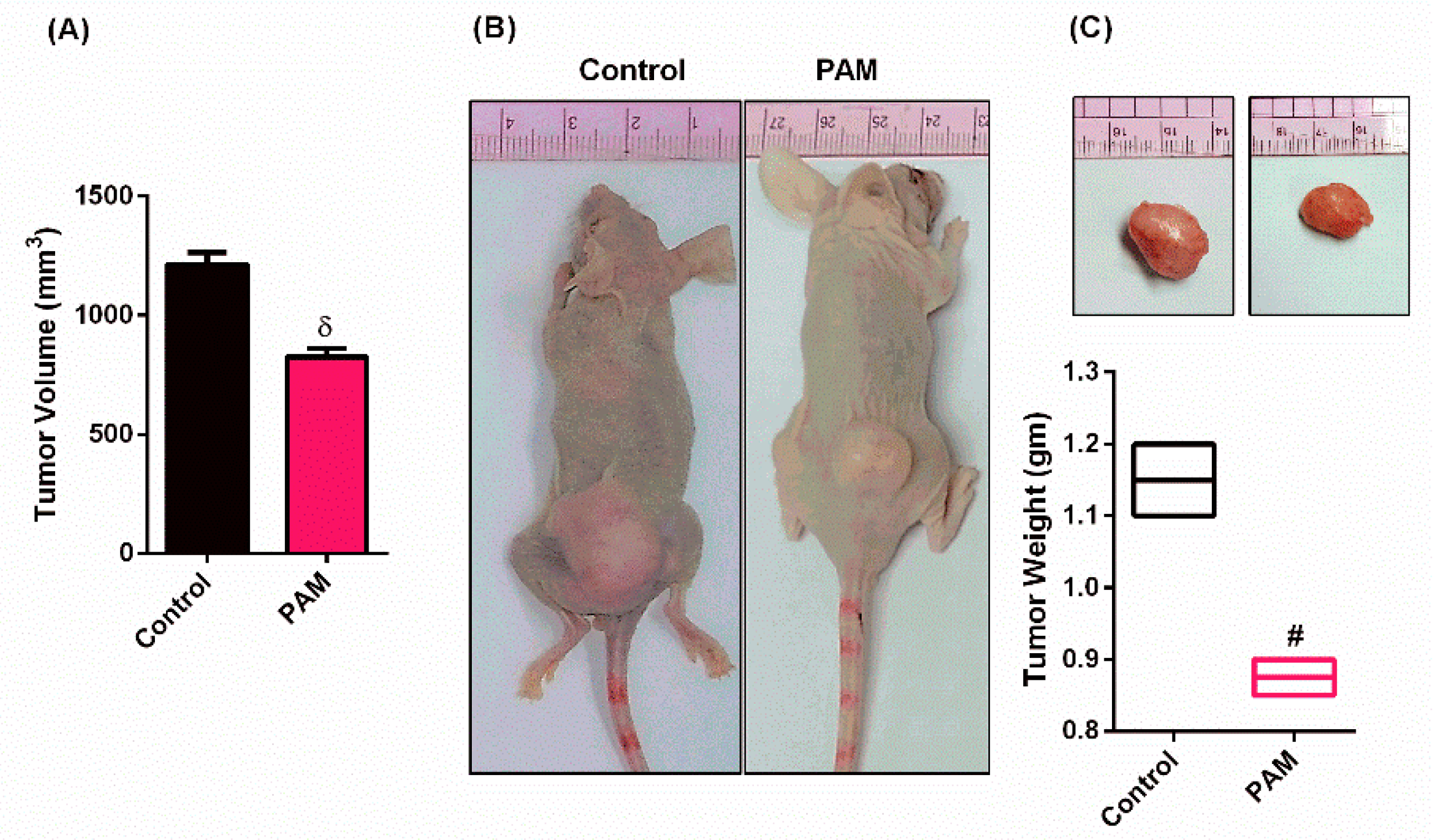
3.2. PAM Inhibited Melanoma Growth in Vivo
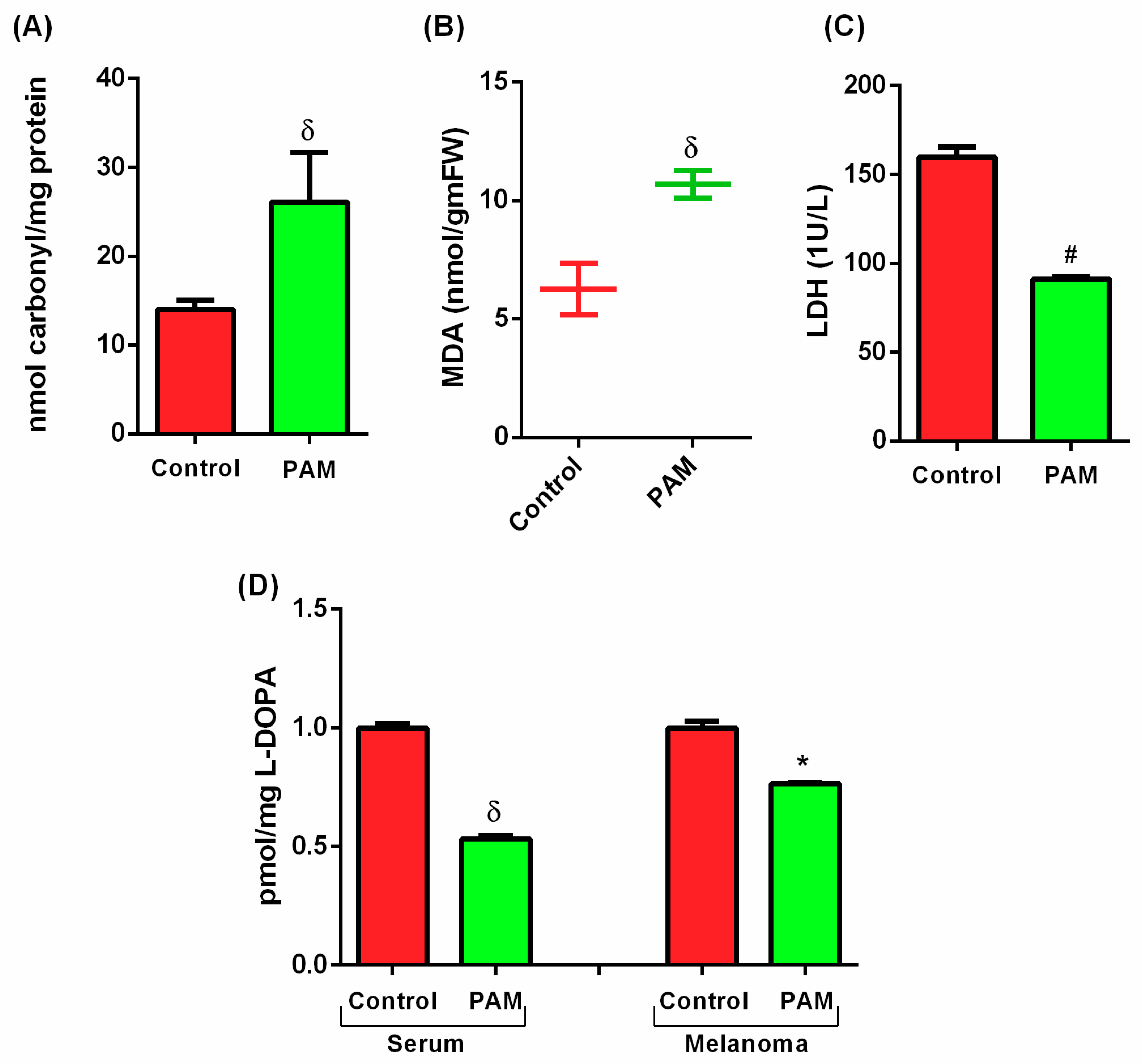
3.3. PAM Induced Oxidative Damage in Melanoma (Protein and Lipid Level)
3.4. Estimation of Melanoma Prognostic Markers Using PAM
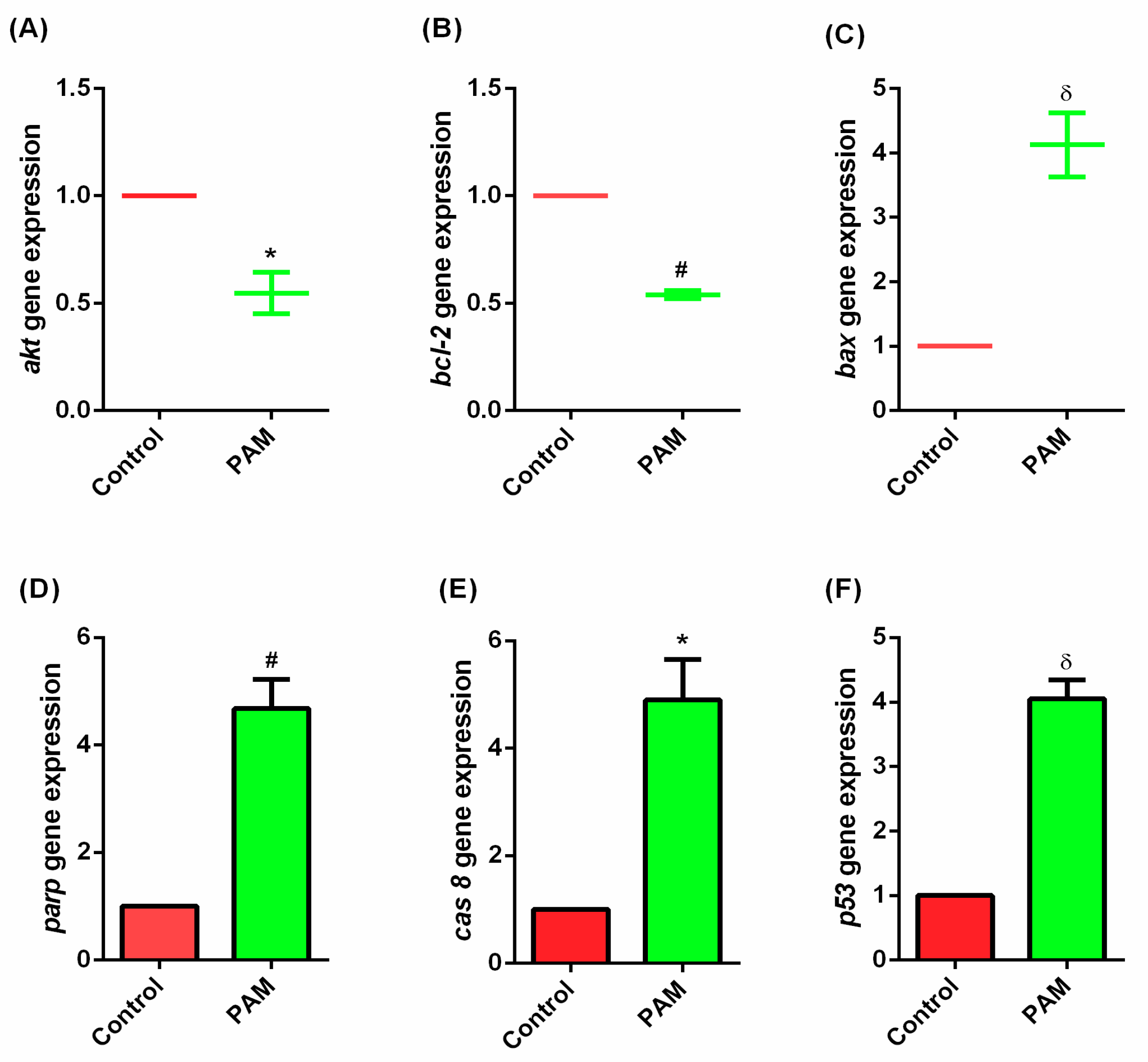
3.5. Gene Expression Study of Melanoma In-Vivo
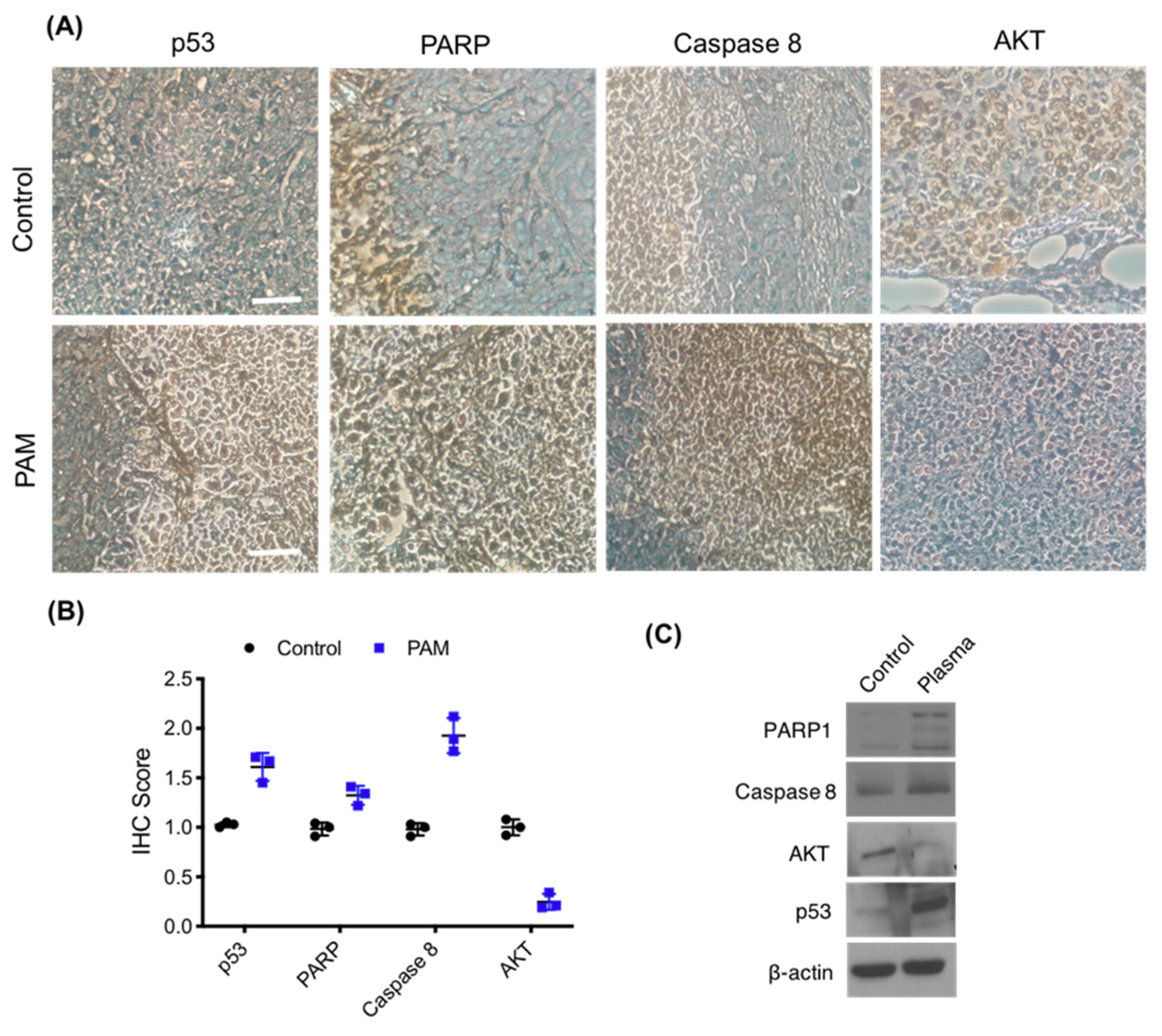
3.6. Immunohistochemistry of Melanoma Xenograft
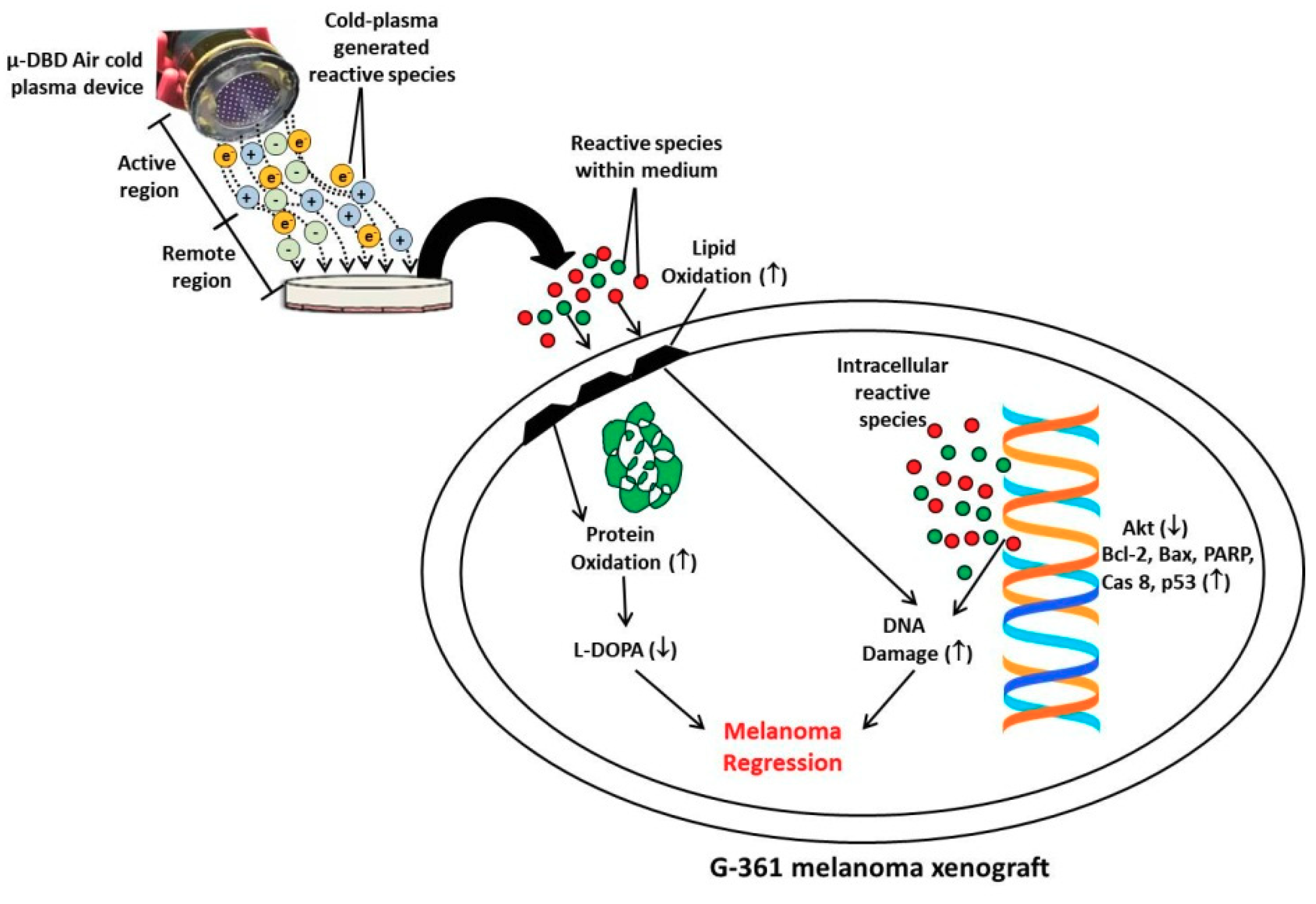
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giblin, A.V.; Thomas, J.M. Incidence, mortality and survival in cutaneous melanoma. J. Plast. Reconstr. Aes. 2007, 60, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Cho, H.; Won, Y.J.; Kong, H.J.; Roh, Y.H.; Jeong, K.H.; Jung, K.W. Nationwide Trends in the Incidence of Melanoma and Non-melanoma Skin Cancers from 1999 to 2014 in South Korea. Cancer Res. Treat. 2018, 50, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T. Narrative review: Braf opens the door for therapeutic advances in melanoma. Ann. Intern. Med. 2010, 153, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Oble, D.A.; Drappatz, J.; Velazquez, E.F.; Ramaiya, N.; Ramakrishna, N.; Day, A.L.; Kruse, A.; Mac-Rae, S.; Hoos, A.; et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat. Clin. Pract. Oncol. 2008, 5, 557–561. [Google Scholar] [CrossRef]
- Chapman, P.B.; Robert, C.; Larkin, J.; Haanen, J.B.; Ribas, A.; Hogg, D.; Hamid, O.; Ascierto, P.A.; Testori, A.; Lorigan, P.C.; et al. Vemurafenib in patients with BRAF V600 mutation-positive metastatic melanoma: Final overall survival results of the randomized BRIM-3 study. Ann. Oncol. 2017, 28, 2581–2587. [Google Scholar] [CrossRef]
- Brugnara, S.; Sicher, M.; Bonandini, E.M.; Donner, D.; Chierichetti, F.; Barbareschi, M.; Girardelli, C.R.; Caffo, O. Treatment with combined dabrafenib and trametinib in BRAFV600E-mutated metastatic malignant melanoma: A case of long-term complete response after treatment cessation. Drugs Context 2018, 7, 212515. [Google Scholar] [CrossRef][Green Version]
- Koelblinger, P.; Thuerigen, O.; Dummer, R. Development of encorafenib for BRAF-mutated advanced melanoma. Curr. Opin. Oncol. 2018, 30, 125–133. [Google Scholar] [CrossRef]
- Lo, J.A.; Fisher, D.E. The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science 2014, 346, 945–949. [Google Scholar] [CrossRef]
- Kufe, D.W.; Pollock, R.E.; Weichselbaum, R.R.; Bast, R.C., Jr.; Gansler, T.S.; Holland, J.F.; Frei, E., III. Holland-Frei Cancer Medicine, 6th ed.; BC Decker Publishing House: Toronto, ON, Canada, 2003; ISBN 1-55009-213-8. [Google Scholar]
- Liu, J.; Wang, Z. Increased Oxidative Stress as a Selective Anticancer Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 294303. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.; Shimizu, K.; Nagashima, T.; Tanaka, H.; Mizuno, M.; Kikkawa, F.; Hori, M.; Honda, H. Plasma-activated medium selectively eliminates undifferentiated human induced pluripotent stem cells. Regen. Ther. 2016, 5, 55–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, H.; Lu, R.; Xian, Y.; Gan, L.; Lu, X.; Yang, X. Effects of atmospheric pressure cold plasma on human hepatocarcinoma cell and its 5-fluorouracil resistant cell line. Phys. Plasmas 2015, 22, 122006. [Google Scholar] [CrossRef]
- Ermolaeva, S.A.; Sysolyatina, E.V.; Kolkova, N.I.; Bortsov, P.; Tuhvatulin, A.I.; Vasiliev, M.M.; Mukhachev, A.Y.; Petrov, O.F.; Tetsuji, S.; Naroditsky, B.S.; et al. Non-thermal argon plasma is bactericidal for the intracellular bacterial pathogen Chlamydia trachomatis. J. Med. Microbiol. 2012, 61, 793–799. [Google Scholar] [CrossRef]
- Volotskova, O.; Hawley, T.S.; Stepp, M.A.; Keidar, M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012, 2, 636. [Google Scholar] [CrossRef]
- Attri, P.; Park, J.; Ali, A.; Choi, E.H. How does plasma activated media treatment differ from direct cold plasma treatment? Anticancer Ag. Med. Chem. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Zhitong, C. Cold atmospheric plasma with self-organized patterns for cancer therapy. Front. Nanosci. Nanotech. 2019, 5, 1–4. [Google Scholar]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical application of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef]
- Ninomiya, K.; Ishijima, T.; Imamura, M.; Yamahara, T.; Enomoto, H.; Takahashi, K.; Tanaka, Y.; Uesugi, Y.; Shimuzu, N. Evaluation of extra- and intracellular OH radical generation, cancer cell injury, and apoptosis induced by a non-thermal atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 425401. [Google Scholar] [CrossRef]
- Xiang, J.; Wan, C.; Guo, R.; Guo, D. Is Hydrogen Peroxide a Suitable Apoptosis Inducer for All Cell Types? BioMed. Res. Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Troyano, A.; Sancho, P.; Fernandez, C.; Blas, E.; Bernardi, P.; Aller, P. The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathione-depleted human promonocytic cells. Cell Death Differ. 2003, 10, 889–898. [Google Scholar] [CrossRef]
- Adhikari, M.; Kaushik, N.; Ghimire, B.; Adhikari, B.; Baboota, S.; Al-Khedhairy, A.A.; Wahab, R.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Cold atmospheric plasma and silymarin nanoemulsion synergistically inhibits human melanoma tumorigenesis via targeting HGF/c-MET downstream pathway. Cell Commun. Signal. 2019, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Nguyen, F.D.; Noory, M.A.; Sharma, A. Current State of Animal (Mouse) Modeling in Melanoma Research. Cancer Growth Metast. 2015, 8, 81–94. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Attri, P.; Kaushik, N.; Choi, E.H. Preliminary Study of the Effect of DBD Plasma and Osmolytes on T98G Brain Cancer and HEK Non-Malignant Cells. Molecules 2013, 18, 4917–4928. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and Stiochiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Balch, C.M.; Soong, S.J.; Atkins, M.B.; Buzaid, A.C.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A., Jr.; Kirkwood, J.M.; et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J. Clin. 2004, 54, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Mena, M.A.; Pardo, B.; Casarejos, M.J.; Fahn, S.; Garcia de, Y.J. Neurotoxicity of levodopa on catecholamine-rich neurons. Mov. Disord. 1992, 7, 23–31. [Google Scholar] [CrossRef]
- Mytilineou, C.; Han, S.K.; Cohen, G. Toxic and protective effects of L-dopa on mesencephalic cell cultures. J. Neurochem. 1993, 61, 1470–1478. [Google Scholar] [CrossRef]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold Atmospheric Plasma for Selectively Ablating Metastatic Breast Cancer Cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of non-thermal plasma on mammalian cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef]
- Hara, H.; Taniguchi, M.; Kobayashi, M.; Kamiya, T.; Adachi, T. Plasma-activated medium-induced intracellular zinc liberation causes death of SH-SY5Y cells. Arch. Biochem. Biophys. 2015, 15, 51–60. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Boehm, D.; Curtin, J.; Cullen, P.J.; Bourke, P. Hydrogen peroxide and beyond-the potential of high-voltage plasma activated liquids against cancerous cells. Anticancer Ag. Med. Chem. 2017, 18, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Yoo, K.C.; Uddin, N.; Kim, J.S.; Lee, S.J.; Choi, E.H. Low doses of PEG-coated gold nanoparticles sensitize solid tumors to cold plasma by blocking the PI3K/AKT-driven signalling axis to suppress cellular transformation by inhibiting growth and EMT. Biomaterials 2016, 87, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Min, B.; Choi, K.H.; Hong, Y.J.; Miller, V.; Fridman, A.; Choi, E.H. Cytotoxic macrophage-release tumour necrosis factor-alpha (TNF-α) as a killing mechanism for cancer cell death after cold plasma activation. J. Phys. D. Appl. Phys. 2016, 49, 084001. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Adhikari, M.; Ghimire, B.; Linh, N.N.; Mishra, Y.K.; Lee, S.J.; Choi, E.H. Preventing the solid cancer progression via release of anticancer-cytokines in co-culture with cold plasma-stimulated macrophages. Cancers 2019, 11, 842. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed DBD plasma-generated reactive oxygen species triggers immunogenic cell death in A549 lung carcinoma cells through intracellular oxidative stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef]
- Lin, A.G.; Xiang, B.; Merlino, D.J.; Baybutt, T.R.; Sahu, J.; Fridman, A.; Snook, A.E.; Miller, V. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. Oncoimmunology 2018, 7, e1484978. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Seebauer, C.; Wende, C.; Schmidt, A. Physical plasma and leucocytes–immune or reactive? Biol. Chem. 2018, 400, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Rao, V.A. Specific protein carbonylation in human breast cancer tissue compared to adjacent healthy epithelial tissue. PLoS ONE 2018, 13, e0194164. [Google Scholar] [CrossRef] [PubMed]
- TAdachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.; Hori, M. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial nuclear network. Free Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, S.M.; Patil, V.W.; Ramraje, N.N.; Phadtare, J.M.; Gujarathi, S.U. Nitric Oxide, Carbonyl Protein, Lipid Peroxidation and Correlation Between Antioxidant Vitamins in Different Categories of Pulmonary and Extra Pulmonary Tuberculosis. Malays. J. Med. Sci. 2013, 20, 21–30. [Google Scholar] [PubMed]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.; Juan-García, A.; Font, G.; Ruiz, M.J. Reactive oxygen species induced by beauvericin, patulin and zearalenone in CHO-K1 cells. Toxicol. In Vitro 2009, 23, 1504–1509. [Google Scholar] [CrossRef]
- Palmer, S.R.; Erickson, L.A.; Ichetovkin, I.; Knauer, D.J.; Markovic, S.N. Circulating Serologic and Molecular Biomarkers in Malignant Melanoma. Mayo Clin. Proc. 2011, 86, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Kaplan, N.O.; Hall, T.C. Lactic dehydrogenase in human neoplastic tissues. Cancer Res. 1964, 24, 389–399. [Google Scholar]
- Buchanan, J.D.; Armstrong, D.A. Free radical inactivation of lactate dehydrogenase. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976, 30, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Siple, J.F.; Schneider, D.C.; Wanlass, W.A.; Rosenblatt, B.K. Levodopa therapy and the risk of malignant melanoma. Ann. Phamacother. 2000, 34, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.; Pawelek, J. L-tyrosine and L-DOPA as hormone-like regulators of melanocytes functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Nakamura, K.; Utsumi, F.; Kajiyama, H.; Kano, H.; Maruyama, S.; Kikkawa, F.; Hori, M. Cell survival and proliferation signaling pathways are downregulated by plasma-activated medium in glioblastoma brain tumor cells. Plasma Med. 2012, 2, 207–220. [Google Scholar] [CrossRef]
- Kumar, N.; Park, J.H.; Jeon, S.N.; Park, B.S.; Choi, E.H.; Attri, P. The action of microsecond-pulsed plasma-activating media on the inactivation of human lung cancer cells. J. Phys. D Appl. Phys. 2016, 49, 115401. [Google Scholar] [CrossRef]
- Hirst, D.; Robson, T. Targeting nitric oxide for cancer therapy. J. Pharm. Pharmacol. 2007, 59, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kominami, K.; Nakabayashi, J.; Nagai, T.; Tsujimura, Y.; Chiba, K.; Kimura, H.; Miyawaki, A.; Sawasaki, T.; Yokota, H.; Manabe, N. The molecular mechanism of apoptosis upon caspase-8 activation: Quantitative experimental validation of a mathematical model. Biochim. Biophys. Acta 2012, 1823, 1825–1840. [Google Scholar] [CrossRef] [PubMed]
- Wiman, K.G. p53 talks to PARP: The increasing complexity of p53-induced cell death. Cell Death Differ. 2013, 20, 1438–1439. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence (5’–3’) |
|---|---|
| 18s RNA F | CAGGTCTGTGATGCCCTTAGA |
| 18s RNA R | GCTTATGACCCGCACTTACTG |
| P53 F | GCCCCTCCTCAGCATCTTATC |
| P53 R | AAAGCTGTTCCGTCCCAGTAG |
| Bax F | AAGAAGCTGAGCGAGTGTCTC |
| Bax R | GCTGGCAAAGTAGAAAAGGGC |
| Casp8 F | CCCAAATCAACAAGAGCCTGC |
| Casp8 R | TCAGACAGTATCCCCGAGGTT |
| Akt F | CAAAGAAGTCAAAGGGGCTGC |
| Akt R | TGTAGCCAATGAAGGTGCCAT |
| Parp F | GGTAATTGGGAGAGGTAGCCG |
| Parp R | TCCCCACAGACACAACACAAA |
| Bcl-2 F | CCACCAAGAAAGCAGGAAAC |
| Bcl-2 R | GCAGGATAGCAGCACAGGAT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, M.; Adhikari, B.; Kaushik, N.; Lee, S.-J.; Kaushik, N.K.; Choi, E.H. Melanoma Growth Analysis in Blood Serum and Tissue Using Xenograft Model with Response to Cold Atmospheric Plasma Activated Medium. Appl. Sci. 2019, 9, 4227. https://doi.org/10.3390/app9204227
Adhikari M, Adhikari B, Kaushik N, Lee S-J, Kaushik NK, Choi EH. Melanoma Growth Analysis in Blood Serum and Tissue Using Xenograft Model with Response to Cold Atmospheric Plasma Activated Medium. Applied Sciences. 2019; 9(20):4227. https://doi.org/10.3390/app9204227
Chicago/Turabian StyleAdhikari, Manish, Bhawana Adhikari, Neha Kaushik, Su-Jae Lee, Nagendra Kumar Kaushik, and Eun Ha Choi. 2019. "Melanoma Growth Analysis in Blood Serum and Tissue Using Xenograft Model with Response to Cold Atmospheric Plasma Activated Medium" Applied Sciences 9, no. 20: 4227. https://doi.org/10.3390/app9204227
APA StyleAdhikari, M., Adhikari, B., Kaushik, N., Lee, S.-J., Kaushik, N. K., & Choi, E. H. (2019). Melanoma Growth Analysis in Blood Serum and Tissue Using Xenograft Model with Response to Cold Atmospheric Plasma Activated Medium. Applied Sciences, 9(20), 4227. https://doi.org/10.3390/app9204227







