Effects of a Non-Thermal Atmospheric Pressure Plasma Jet with Different Gas Sources and Modes of Treatment on the Fate of Human Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Mesenchymal Stem Cells
2.2. Non-Thermal Atmospheric Pressure Plasma Jet
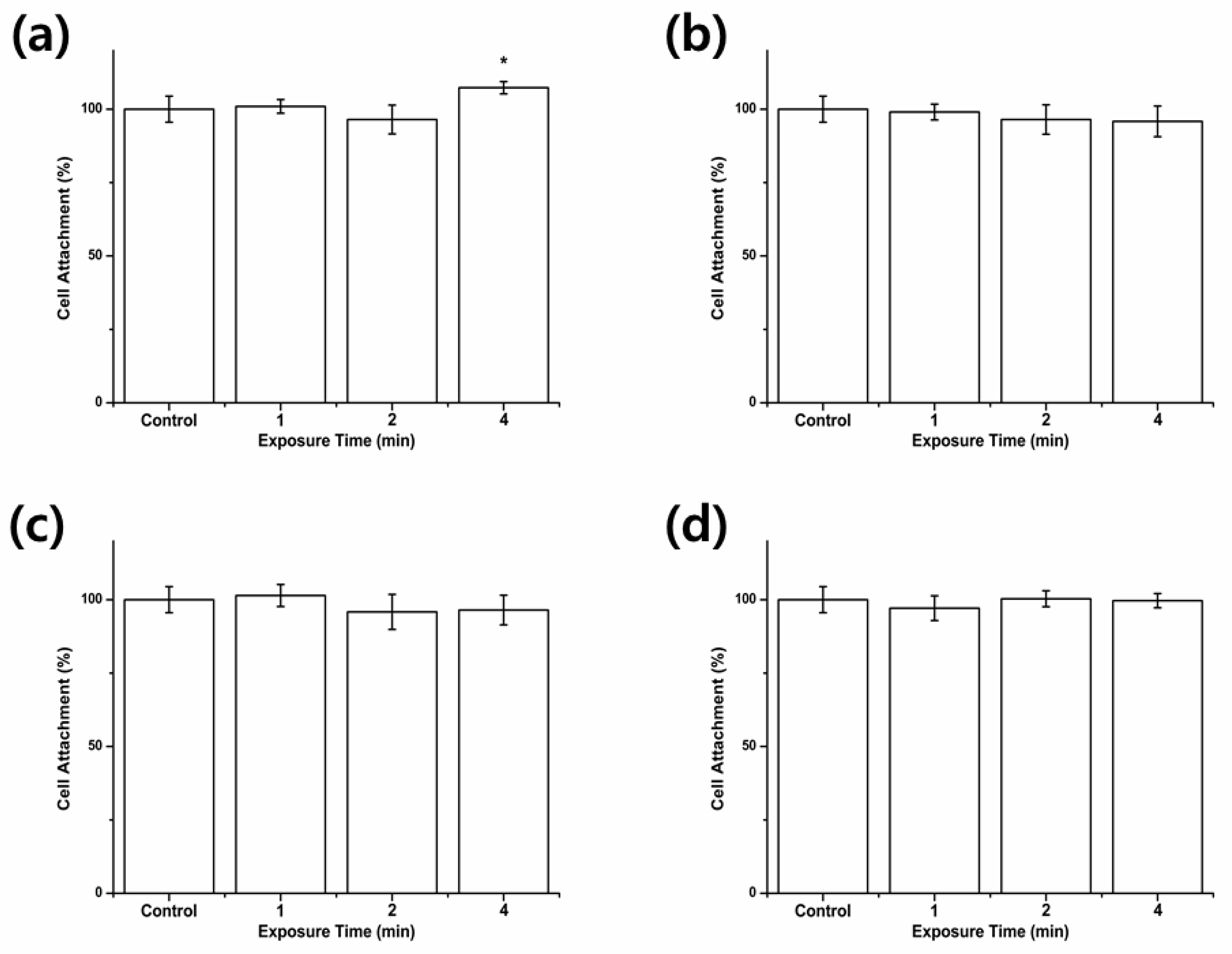
2.3. Cell Attachment
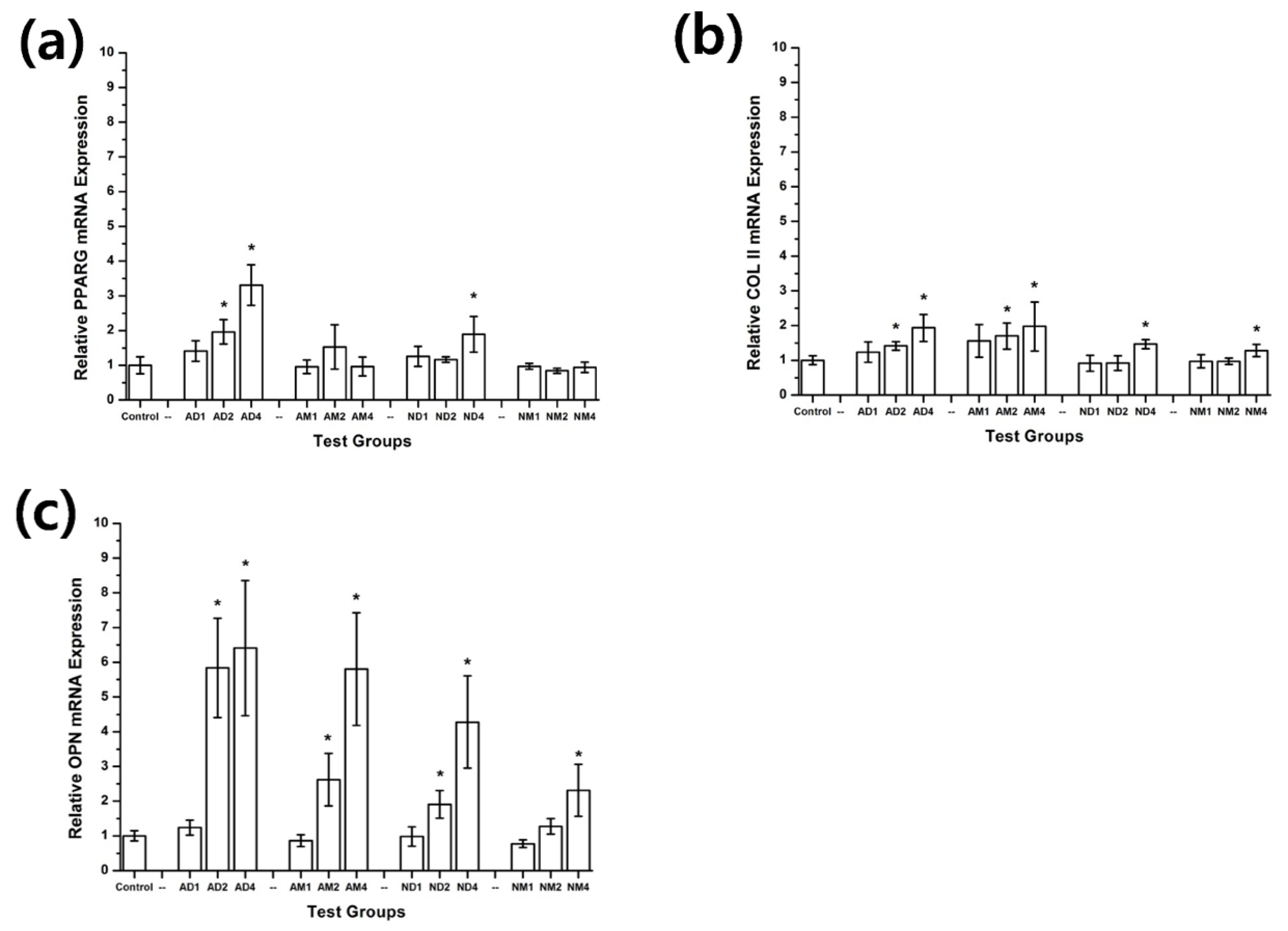
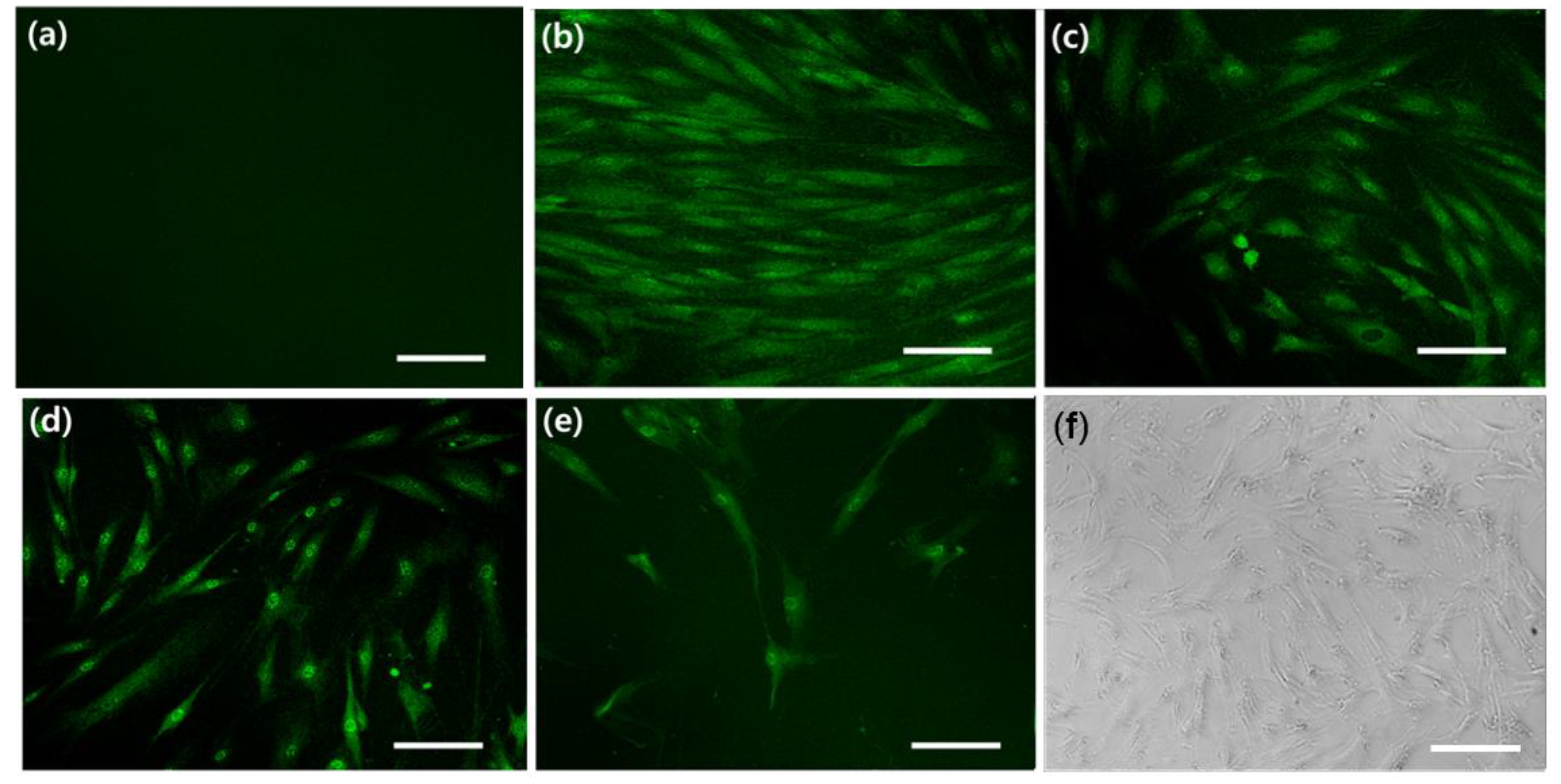
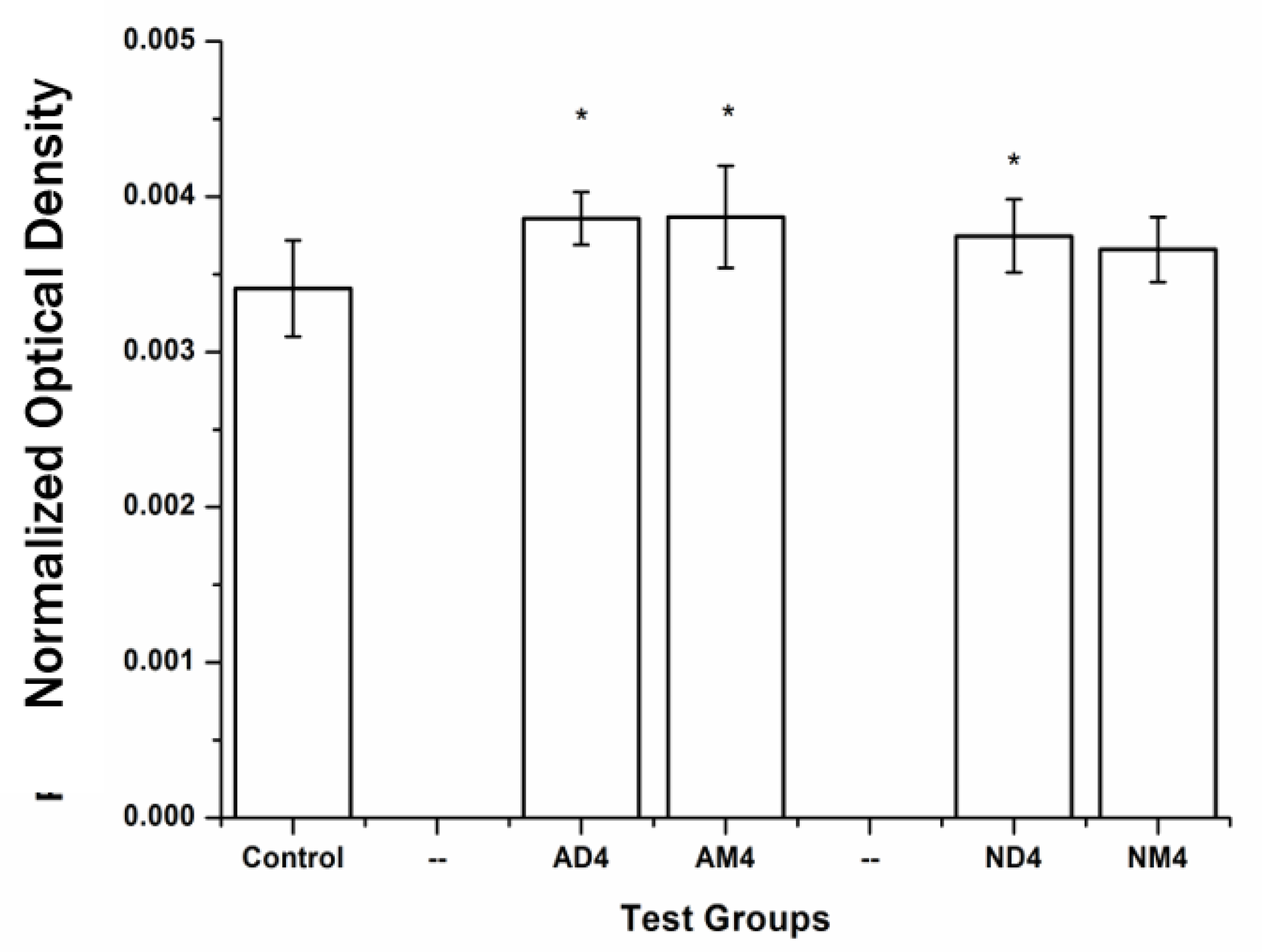
2.4. Cell Differentiation
2.5. Statistical Analysis
3. Results
3.1. Cell Attachment
3.2. Cell Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.; Boynton, R.E.; Liu, B.S.; Murphy, J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell Biochem. 1997, 64, 295–312. [Google Scholar] [CrossRef]
- Matsushita, K.; Wu, Y.; Okamoto, Y.; Pratt, R.E.; Dzau, V.J. Local renin angiotensin expression regulates human mesenchymal stem cell differentiation to adipocytes. Hypertension 2006, 48, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Kim, Y.H.; Choi, E.H.; Kim, K.N. Development of ultra-hydrophilic and non-cytotoxic dental vinyl polysiloxane impression materials using a non-thermal atmospheric-pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 195201. [Google Scholar] [CrossRef]
- Lee, E.J.; Kwon, J.S.; Uhm, S.H.; Song, D.H.; Kim, Y.H.; Choi, E.H.; Kim, K.N. The effects of non-thermal atmospheric pressure plasma jet on cellular activity at SLA-treated titanium surfaces. Curr. Appl. Phys. 2013, 13, S36–S41. [Google Scholar] [CrossRef]
- Laroussi, M.; Tendero, C.; Lu, X.; Alla, S.; Hynes, W.L. Inactivation of bacteria by the plasma pencil. Plasma Process Polym. 2006, 3, 470–473. [Google Scholar] [CrossRef]
- Sladek, R.E.J.; Stoffels, E. Deactivation of Escherichia coli by the plasma needle. J. Phys. D Appl. Phys. 2005, 38, 1716–1721. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Ayan, H.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Brooks, A.; Friedman, G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Kieft, I.E.; Kurdi, M.; Stoffels, E. Reattachment and apoptosis after plasma-needle treatment of cultured cells. IEEE Trans. Plasma Sci. 2006, 34, 1331–1336. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, Y.H.; Choi, E.H.; Kim, K.N. The effects of non-thermal atmospheric pressure plasma jet on attachment of osteoblast. Curr. Appl. Phys. 2013, 13, S42–S47. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial Cell Proliferation is Enhanced by Low Dose Non-Thermal Plasma Through Fibroblast Growth Factor-2 Release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, H.; Lee, H.J.; Kim, G.C.; Kim, D.Y.; Han, S.; Song, K. Non-Thermal Atmospheric Pressure Plasma Efficiently Promotes the Proliferation of Adipose Tissue-Derived Stem Cells by Activating NO-Response Pathways. Sci. Rep. 2016, 6, 39298. [Google Scholar] [CrossRef] [PubMed]
- Miletic, M.; Mojsilovic, S.; Dordevic, I.O.; Maletic, D.; Puac, N.; Lazovic, S.; Malovic, G.; Milenkovic, P.; Petrovic, Z.L.; Bugarski, D. Effects of non-thermal atmospheric plasma on human periodontal ligament mesenchymal stem cells. J. Phys. D Appl. Phys. 2013, 46, 345401. [Google Scholar] [CrossRef]
- Lazovic, S.; Puac, N.; Miletic, M.; Pavlica, D.; Jovanovic, M.; Bugarski, D.; Mojsilovic, S.; Maletic, D.; Malovic, G.; Milenkovic, P.; et al. The effect of a plasma needle on bacteria in planktonic samples and on peripheral blood mesenchymal stem cells. New J. Phys. 2010, 12, 083037. [Google Scholar] [CrossRef]
- Kwon, J.S.; Illeperuma, R.P.; Kim, J.; Kim, K.M.; Kim, K.N. Cytotoxicity evaluation of zinc oxide-eugenol and non-eugenol cements using different fibroblast cell lines. Acta Odontol. Scand. 2014, 72, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Lee, S.B.; Kim, C.K.; Kim, K.N. Modified cytotoxicity evaluation of elastomeric impression materials while polymerizing with reduced exposure time. Acta Odontol. Scand. 2011, 70, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Guo, X.J. Osteopontin—A Protein with Diverse Functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Gene Dev. 2000, 14, 1293–1307. [Google Scholar] [PubMed]
- Lai, H.C.; Zhuang, L.F.; Liu, X.; Wieland, M.; Zhang, Z.Y.; Zhang, Z.Y. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J. Biomed. Mater. Res. A 2010, 93, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.S.W.; Schwartz, M.P.; Durney, A.R.; Anseth, K.S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 2008, 7, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kawazoe, N.; Fan, Y.; Ito, Y.; Tanaka, J.; Tateishi, T.; Zhang, X.; Chen, G. Chondrogenic differentiation of human mesenchymal stem cells on photoreactive polymer-modified surfaces. Biomaterials 2008, 29, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.E.; Petrie, T.A.; Creighton, F.P.; Garcia, A.J. Human mesenchymal stem cell differentiation on self-assembled monolayers presenting different surface chemistries. Acta Biomater. 2010, 6, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006, 12, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Rahimi, C.; Hescheler, J.; Wartenberg, M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000, 476, 218–223. [Google Scholar] [CrossRef]
- Sundaresan, M.; Yu, Z.X.; Ferrans, V.J.; Irani, K.; Finkel, T. Requirement for Generation of H2o2 for Platelet-Derived Growth-Factor Signal-Transduction. Science 1995, 270, 296–299. [Google Scholar] [CrossRef] [PubMed]

| Genes | Forward Sequences | Backward Sequences |
|---|---|---|
| PPARG | 5′-GCA GGA GCA GAG CAA AGA G-3′ | 5′-TGG TCG TTC AAG TCA AGA TTT AC-3′ |
| COL II | 5′-GGA GCA GCA AGA GCA AGG AGA AG-3′ | 5′-TGG ACA GCA GGC GTA GGA AGG-3′ |
| OPN | 5′-AGA CCA TGC AGA GAG CGA G-3′ | 5′-ACG TCT GCT TGT GTG CTG G-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.-Y.; Kwon, J.-S.; Kumar, N.; Choi, E.H.; Kim, K.-M. Effects of a Non-Thermal Atmospheric Pressure Plasma Jet with Different Gas Sources and Modes of Treatment on the Fate of Human Mesenchymal Stem Cells. Appl. Sci. 2019, 9, 4819. https://doi.org/10.3390/app9224819
Kang T-Y, Kwon J-S, Kumar N, Choi EH, Kim K-M. Effects of a Non-Thermal Atmospheric Pressure Plasma Jet with Different Gas Sources and Modes of Treatment on the Fate of Human Mesenchymal Stem Cells. Applied Sciences. 2019; 9(22):4819. https://doi.org/10.3390/app9224819
Chicago/Turabian StyleKang, Tae-Yun, Jae-Sung Kwon, Naresh Kumar, Eun Ha Choi, and Kwang-Mahn Kim. 2019. "Effects of a Non-Thermal Atmospheric Pressure Plasma Jet with Different Gas Sources and Modes of Treatment on the Fate of Human Mesenchymal Stem Cells" Applied Sciences 9, no. 22: 4819. https://doi.org/10.3390/app9224819
APA StyleKang, T.-Y., Kwon, J.-S., Kumar, N., Choi, E. H., & Kim, K.-M. (2019). Effects of a Non-Thermal Atmospheric Pressure Plasma Jet with Different Gas Sources and Modes of Treatment on the Fate of Human Mesenchymal Stem Cells. Applied Sciences, 9(22), 4819. https://doi.org/10.3390/app9224819







