Investigations into the Potential Abrasive Release of Nanomaterials due to Material Stress Conditions-Part A: Carbon Black Nano-Particulates in Plastic and Rubber Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
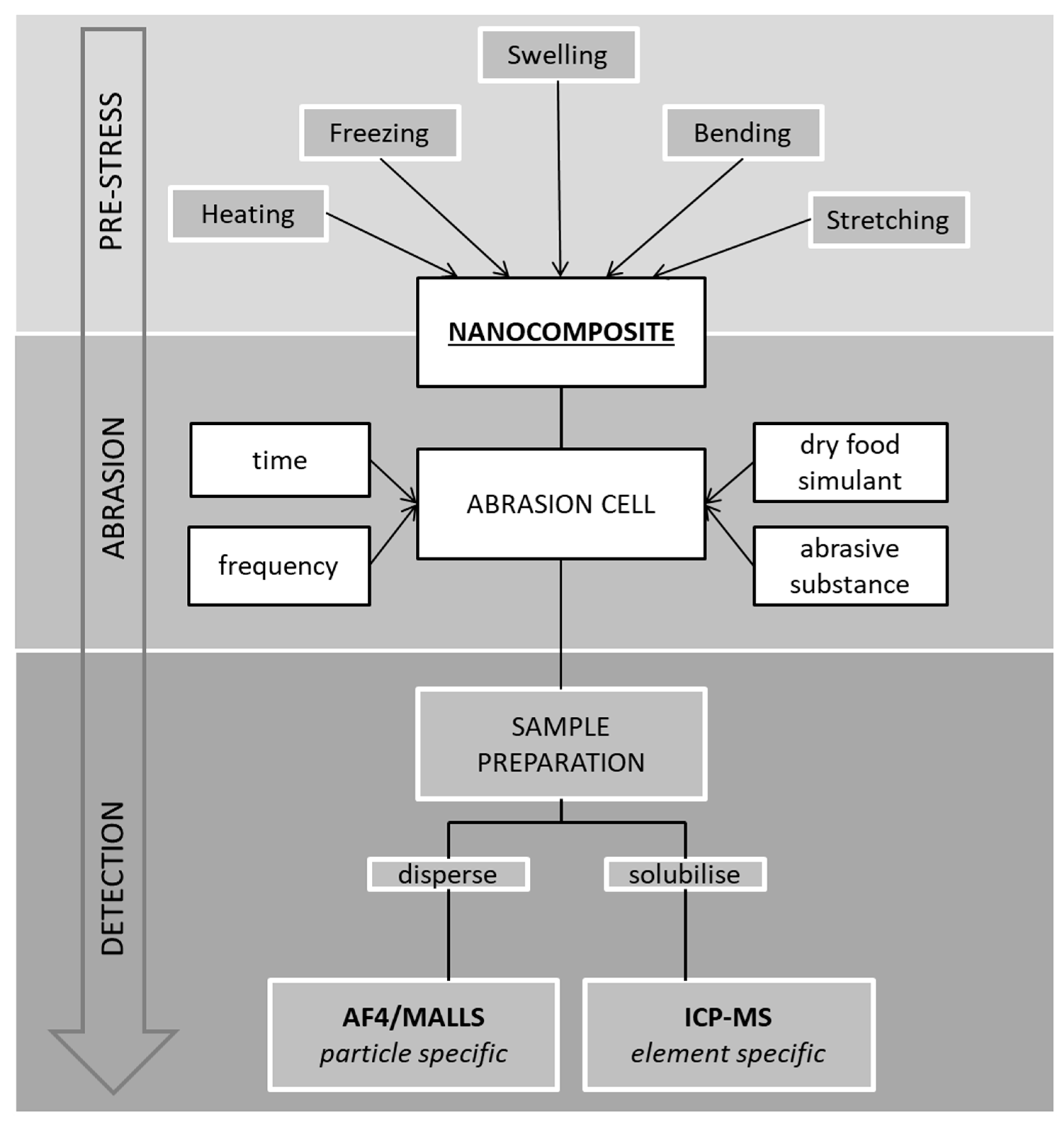
2.2. Nano-Release Study-General Experimental Design
- Pre-stress:relevant mechanical, thermal, and chemical stress conditions that are realistic in practice
- Abrasion test:basic stress test to see whether NMs can be abraded from the nanocomposite’s surface using a dry simulant with an abrasive character simulating intensive use and handling of the nanocomposite.
- Analytical detection of released NMs:depending on the nature of NM, particle- and/or element-specific detection techniques are used to demonstrate whether or not NMs were released from the nanocomposite
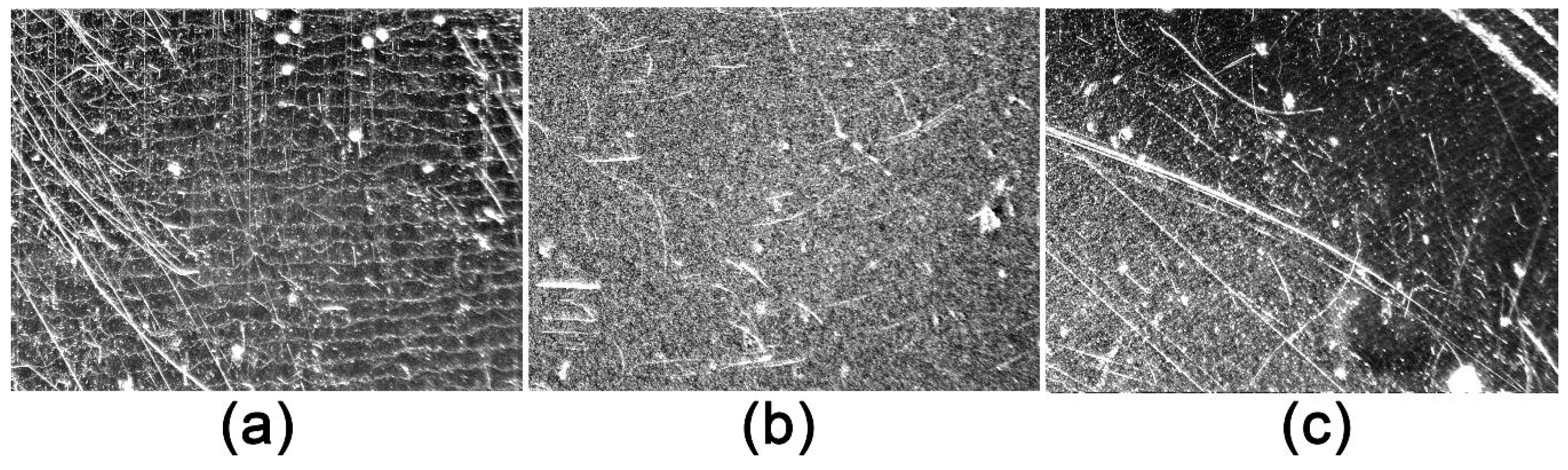
2.2.1. Basic Material Stressing-Abrasion Test
2.2.2. Additional Material Stress Conditions that Might Induce the Release of CB
- Thermal stress by heat:The LDPE and EPDM compounds were placed in closed foil pans and stored at 100 °C for 24 h in a temperature controlled oven.
- Thermal stress by freezing:The LDPE and EPDM compounds were placed in closed foil pans and stored at −50 °C for 24 h in a temperature controlled freezer.
- Chemical stress by swelling with solvents:The LDPE and EPDM compounds were placed in stainless steel cells, immersed in 100 mL of isooctane or toluene for LDPE and EPDM, respectively, and then stored for 24 h at 40 °C in a temperature controlled oven. All samples were stored for two days under a fume-hood to evaporate any remaining solvent prior to being subjected to the abrasion test.
- Mechanical stress test by stretching:Rectangular strips of 88 × 59 × 3 mm and of 88 × 59 × 2 mm were prepared from the LDPE and EPDM plaques, respectively. These specimens were clamped in a universal tensile strength testing apparatus equipped with a traverse path sensor. The LDPE strips were vertically stretched by 5 mm and the EPDM specimens by 50 mm. Cut-outs for the subsequent abrasion test were taken from the center of the strips.
2.3. Analytical Set-Up for the Detection of CB Nano-Particulates in the Abrasion
2.3.1. Preparation of CB Reference Dispersions
2.3.2. AF4 and MALLS Measurements
3. Results
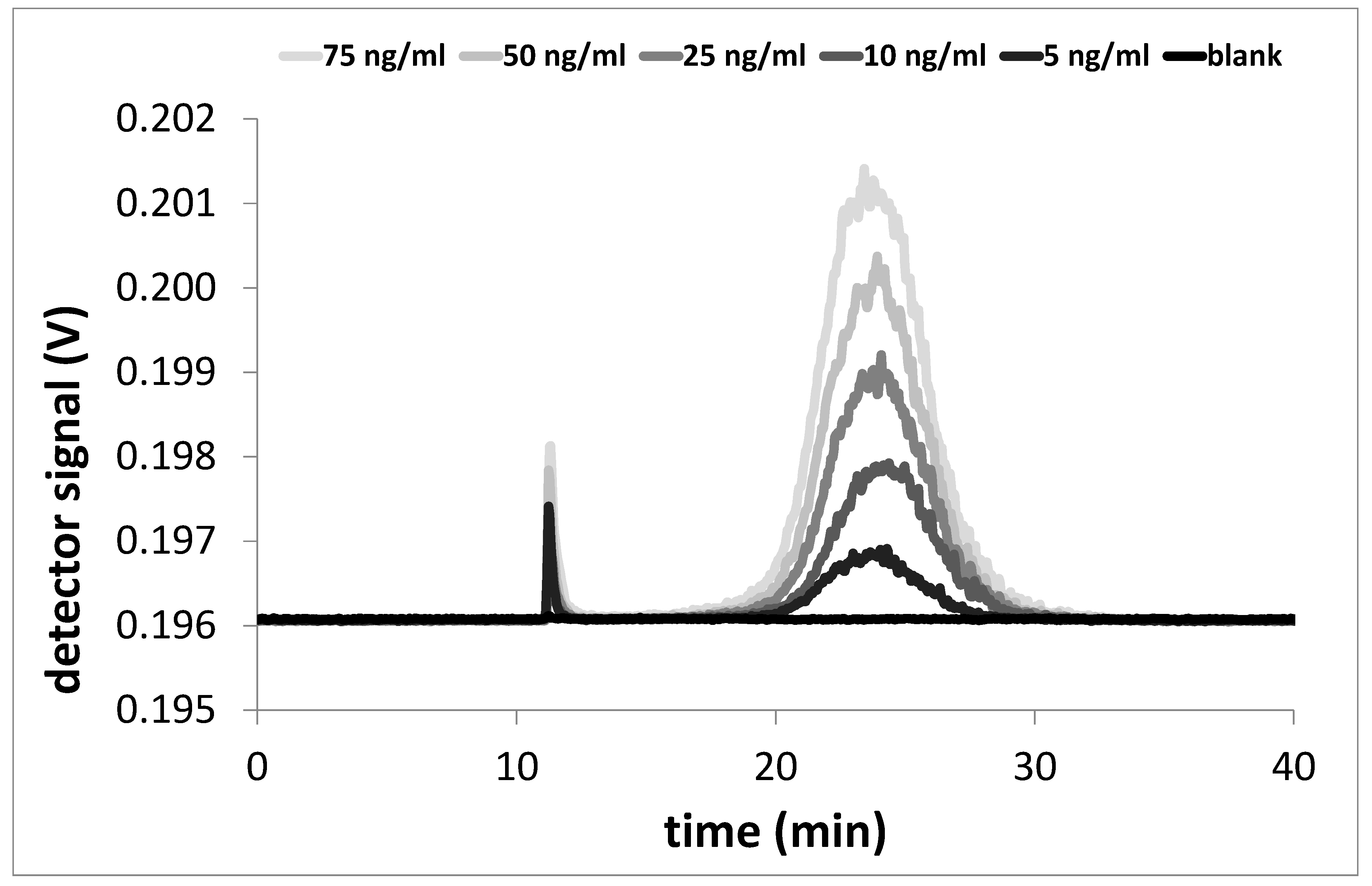
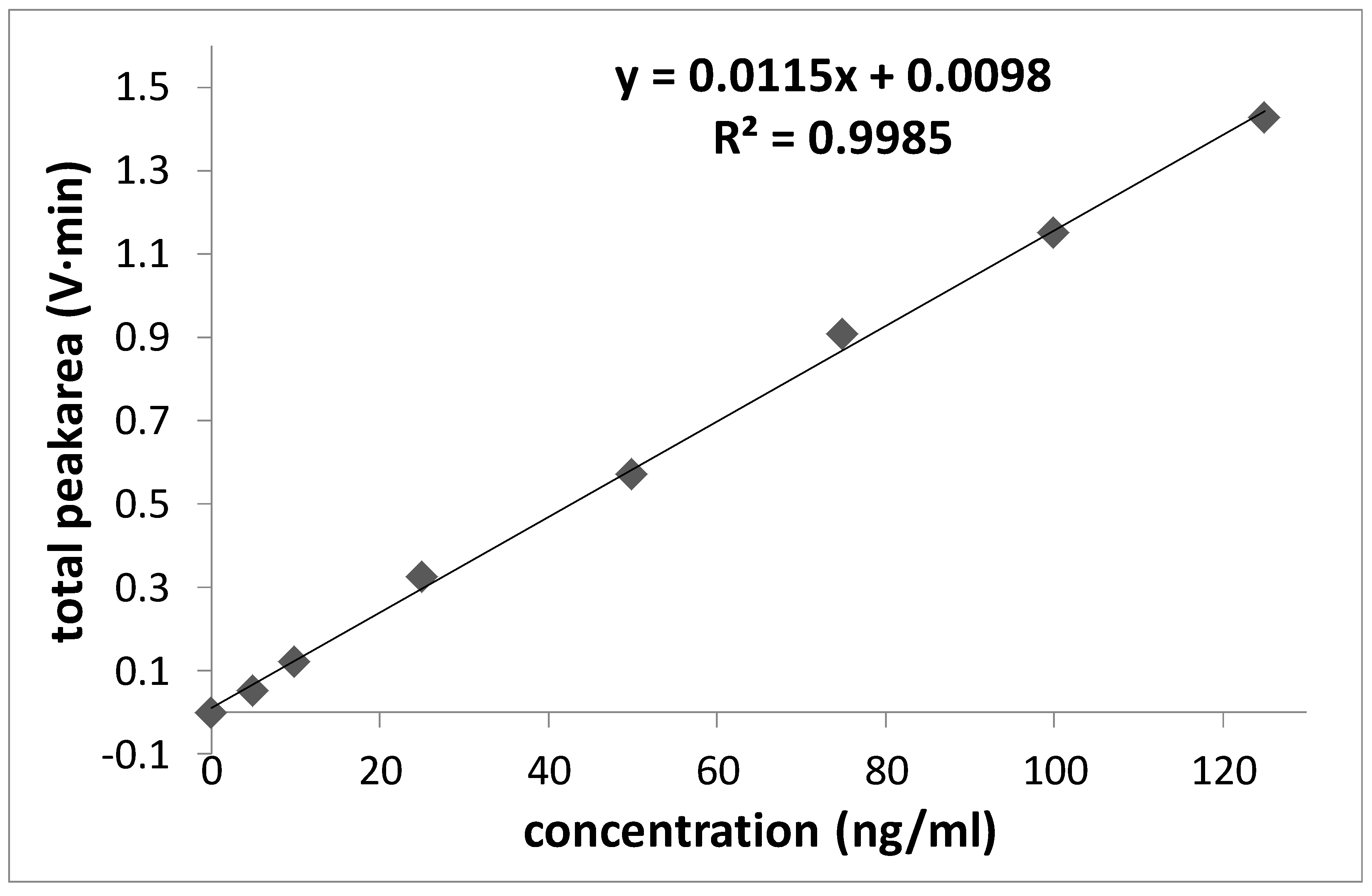
3.1. Characterisation and Quantification of CB in Dispersion
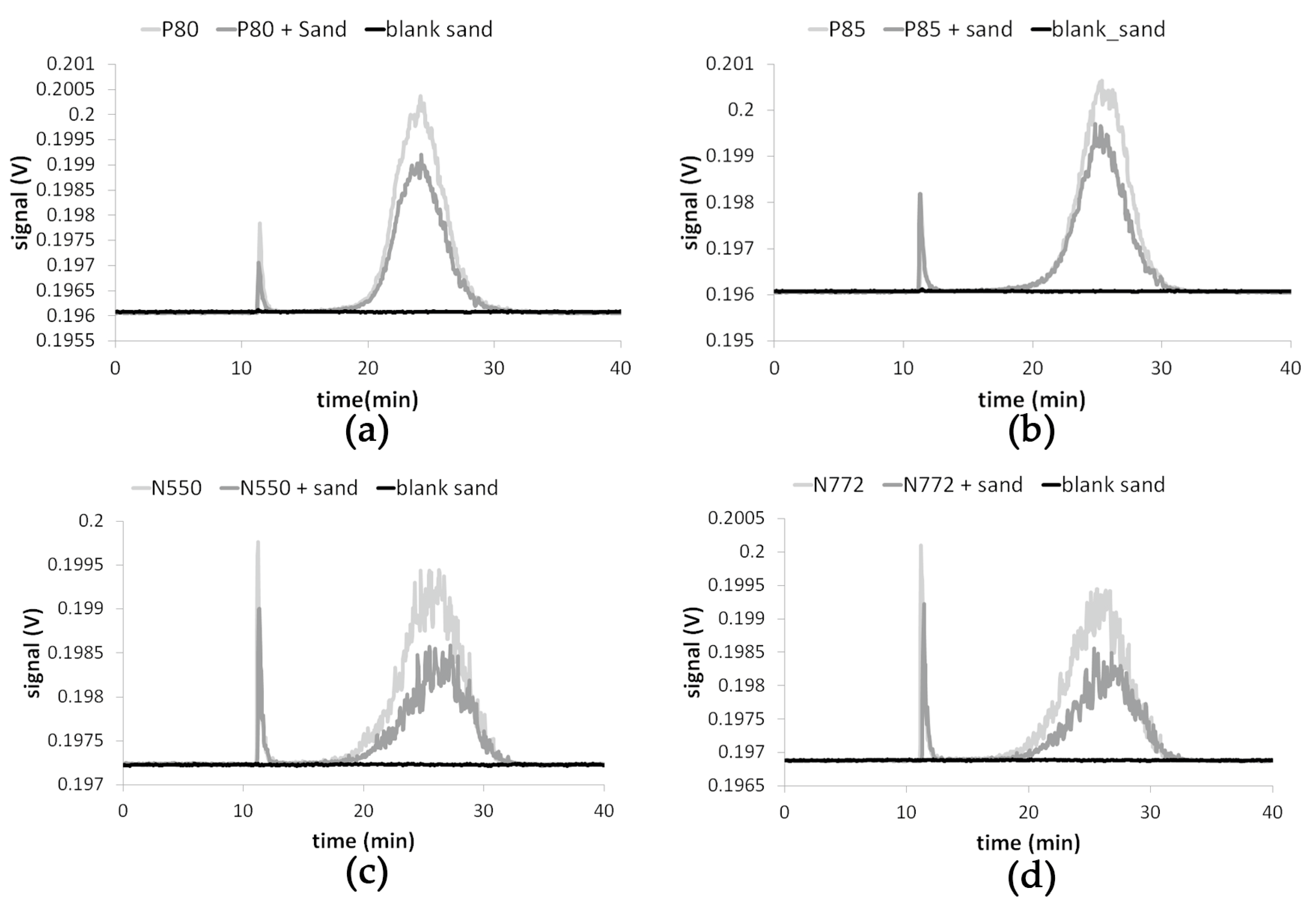
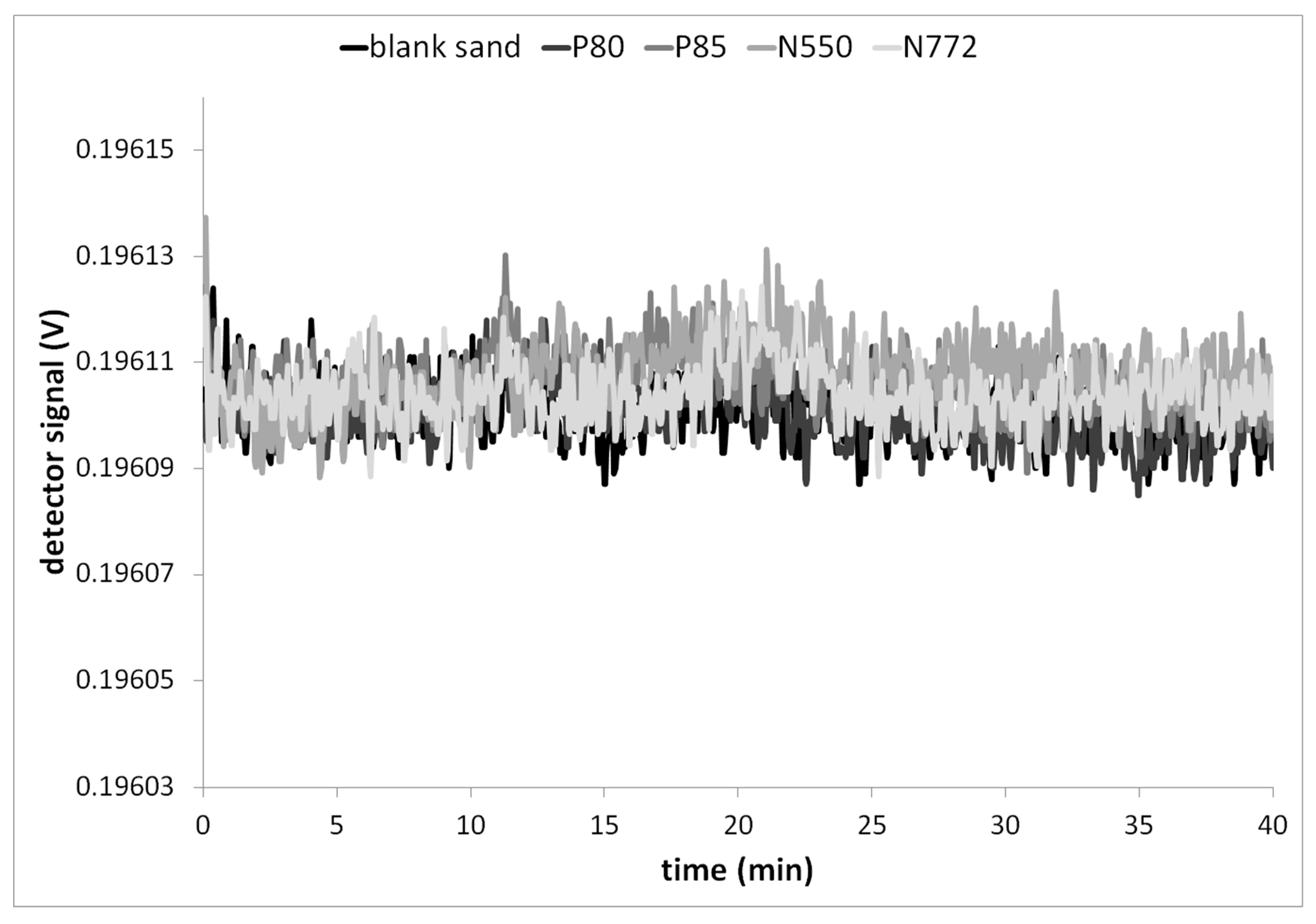
3.2. Recovery of CB Redispersed from Quartz
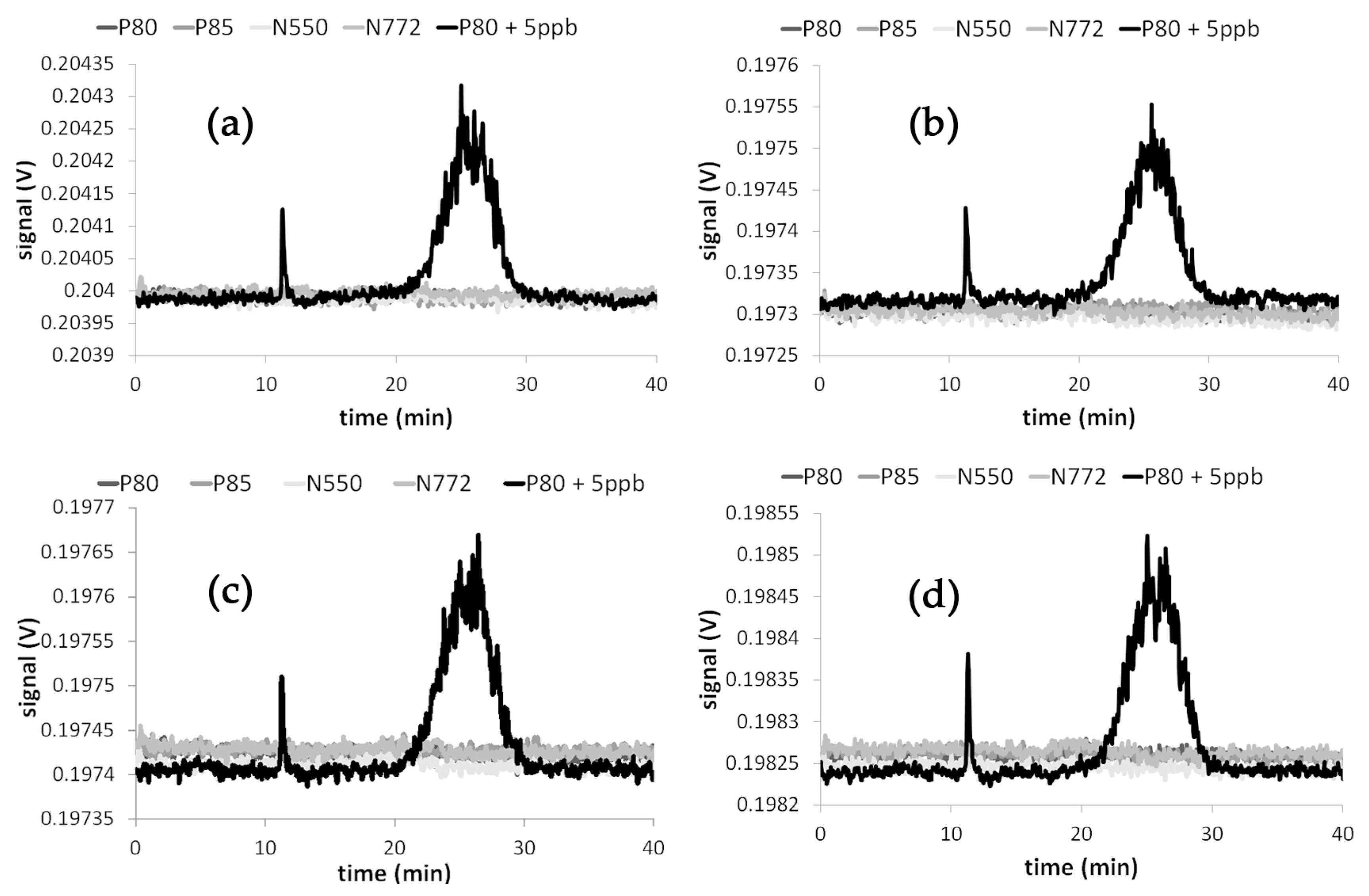
3.3. Results of the Nano-Release Study
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Ko, S. Nano-food packaging: An overview of market, migration research, and safety regulations. J. Food Sci 2015, 80, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Pocas, F.; Franz, R. Overview on European Regulatory Issues, Legislation, and EFSA Evaluations of Nanomaterials. In Nanomaterials for Food Packaging-Materials, Processing Technologies, and Safety Issues; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Peters, R.J.B.; Bouwmeester, H.; Gottardo, S.; Amenta, V.; Arena, M.; Brandhoff, P.; Marvin, H.J.P.; Mech, A.; Moniz, F.B.; Pesudo, L.Q.; et al. Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci. Technol. 2016, 54, 155–164. [Google Scholar]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, 5327. [Google Scholar]
- Duncan, T.V.; Pillai, K. Release of Engineered Nanomaterials from Polymer Nanocomposites: Diffusion, Dissolution, and Desorption. ACS Appl. Mater. Interfaces 2015, 7, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of mechanical properties, migration, and potential Aapplications in active food packaging systems containing nanoclays and nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Commission Regulation (EU). On plastic materials and articles intended to come into contact with food; Official Journal of the European Union: Brussels, Belgium, 14 January 2011. [Google Scholar]
- Bott, J.; Störmer, A.; Franz, R. A model study into the migration potential of nanoparticles from plastics nanocomposites for food contact. Food Packag. Shelf Life 2014, 2, 73–80. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Mathematic modelling of migration of nanoparticles from food contact polymers. In The Use of Nanomaterials in Food Contact Materials-Design, Application, Safety; Veraart, R., Ed.; DEStech Publications Inc.: Lancaster, PA, USA, 2017. [Google Scholar]
- Bott, J.; Störmer, A.; Franz, R. Migration of nanoparticles from plastic packaging materials containing carbon black into foodstuffs. Food Addit. Contam. 2014, 31, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V. Release of engineered nanomaterials from polymer nanocomposites: The effect of matrix degradation. ACS Appl. Mater. Interfaces 2015, 7, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rosas, E.; Vilar, G.; Janer, G.; González-Gálvez, D.; Puntes, V.; Jamier, V.; Aubouy, L.; Vázquez-Campos, S. Influence of nanomaterial compatibilization strategies on polyamide nanocomposites properties and nanomaterial release during the use phase. Environ. Sci. Technol. 2016, 50, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Bott, J.; Franz, R. Investigations into the Potential Abrasive Release of Nanomaterials due to Material Stress Conditions—Part B: Silver, Titanium Nitride, and Laponite Nanoparticles in Plastic Composites. Appl. Sci. 2019, 9. in press. [Google Scholar]
- Andersson, M.; Wittgren, B.; Wahlund, K.-G. Accuracy in Multiangle Light Scattering Measurements for Molar Mass and Radius Estimations. Model Calculations and Experiments. Anal. Chem. 2003, 75, 4279–4291. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC monographs on the evaluation of carcinogenic risks to humans. In Carbon Black, Titanium Dioxide and Talc; International Agency For Research On Cancer: Lyon, France, 2010. [Google Scholar]
- Ntim, S.A.; Norris, S.; Scott, K.; Thomas, T.A.; Noonan, G.O. Consumer use effects on nanoparticle release from commercially available ceramic cookware. Food Control 2018, 87, 31–39. [Google Scholar] [CrossRef]
- Laux, P.; Tentschert, J.; Riebeling, C.; Braeuning, A.; Creutzenberg, O.; Epp, A.; Fessard, V.; Haas, K.-H.; Haase, A.; Hund-Rinke, K.; et al. Nanomaterials: Certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018, 92, 121–141. [Google Scholar] [CrossRef]
- Noonan, G.O.; Whelton, A.J.; Carlander, D.; Duncan, T.V. Measurement methods to evaluate engineered nanomaterial release from food contact materials. Compre. Rev. Food Sci. Food Saf. 2014, 13, 679–692. [Google Scholar] [CrossRef]
- Singh, G.; Stephan, C.; Westerhoff, P.; Carlander, D.; Duncan, T.V. Measurement eethods to detect, characterize, and quantify engineered nanomaterials in foods. Compre. Rev. Food Sci. Food Saf. 2014, 13, 693–704. [Google Scholar] [CrossRef]
- Gray, C.A.; Muranko, H. Studies of robustness of industrial aciniform aggregates and agglomerates--carbon black and amorphous silicas: A review amplified by new data. J. Occup. Environ. Med. 2006, 48, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Voll, M.; Kleinschmit, P. Carbon, 6. Carbon Black, in Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2010. [Google Scholar]
- Wang, M.-J.; Gray, C.A.; Reznek, S.R.; Mahmud, K.; Kutsovsky, Y.; Black, C. Encyclopedia Of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 52–91. [Google Scholar]
- Watson, A.Y.; Valberg, P.A. Carbon Black and Soot: Two Different Substances. AIHAJ 2001, 62, 218–228. [Google Scholar] [CrossRef] [PubMed]


| CB type | Polymer Matrix, Thickness | CB Loading(%) |
|---|---|---|
| Printex® 80 “PX 80” | LDPE plaque, 3 mm | 2.5 |
| Printex® 85 “PX 85” | LDPE plaque, 3 mm | 2.5 |
| N550 | EPDM plaque, 2 mm | 37.2 |
| N772 | EPDM plaque, 2 mm | 45.6 |
| Property | PX 80 | PX 85 | N550 | N772 |
|---|---|---|---|---|
| OAN a (mL/100g) | 105 | 54 | 122.8 | 65.9 |
| BET surface area (m2/g) | 220 | 200 | 39.0 | 30.0 |
| Mean primary particle size (nm) | 16 | 16 | 55 | 75 |
| CB Type | rg,min (nm) | rg,max (nm) | rg,main (nm) | dsphere (nm) | Icoil (nm) |
|---|---|---|---|---|---|
| PX 80 | 15 | 250 | 55 | 140 | 134 |
| PX 85 | 30 | 250 | 100 | 260 | 244 |
| N550 | 30 | 400 | 170 | 440 | 416 |
| N772 | 50 | 400 | 210 | 540 | 514 |
| Concentration of Standard (ng/mL) | Total Area by MALLS (mV·min) | |||
|---|---|---|---|---|
| PX 80 | PX 85 | N550 | N772 | |
| 0 | −0.003 | 0.003 | 0.000 | 0.000 |
| 5 | 0.053 | 0.063 | 0.001 | 0.001 |
| 10 | 0.123 | 0.168 | 0.003 | 0.004 |
| 25 | 0.323 | 0.288 | 0.070 | 0.043 |
| 50 | 0.572 | 0.671 | 0.135 | 0.099 |
| 75 | 0.907 | 1.023 | 0.192 | 0.174 |
| 100 | 1.149 | 1.405 | 0.328 | 0.238 |
| 125 | 1.426 | 1.832 | 0.433 | 0.352 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bott, J.; Franz, R. Investigations into the Potential Abrasive Release of Nanomaterials due to Material Stress Conditions-Part A: Carbon Black Nano-Particulates in Plastic and Rubber Composites. Appl. Sci. 2019, 9, 214. https://doi.org/10.3390/app9020214
Bott J, Franz R. Investigations into the Potential Abrasive Release of Nanomaterials due to Material Stress Conditions-Part A: Carbon Black Nano-Particulates in Plastic and Rubber Composites. Applied Sciences. 2019; 9(2):214. https://doi.org/10.3390/app9020214
Chicago/Turabian StyleBott, Johannes, and Roland Franz. 2019. "Investigations into the Potential Abrasive Release of Nanomaterials due to Material Stress Conditions-Part A: Carbon Black Nano-Particulates in Plastic and Rubber Composites" Applied Sciences 9, no. 2: 214. https://doi.org/10.3390/app9020214
APA StyleBott, J., & Franz, R. (2019). Investigations into the Potential Abrasive Release of Nanomaterials due to Material Stress Conditions-Part A: Carbon Black Nano-Particulates in Plastic and Rubber Composites. Applied Sciences, 9(2), 214. https://doi.org/10.3390/app9020214






