Abstract
The present review article attempts to summarize the use of deep eutectic solvents in the extraction of flavonoids, one of the most important classes of plant secondary metabolites. All of the applications reviewed have reported success in isolation and extraction of the target compounds; competitive, if not superior, extraction rates compared with conventional solvents; and satisfactory behavior of the extract in the latter applications (such as direct analysis, synthesis, or catalysis), wherever attempted.
1. Introduction
The global turn towards green chemistry is an integral approach towards conventional chemical practices. Following this trend, both raw materials and processes are being re-evaluated from the ground up, combining the research for naturally, sustainably sourced raw materials with eco-friendly, cost effective, and lean processes.
Research on multiple compound groups is being carried out globally, aiming at isolating substances with significant health, well-being, or other benefits from natural sources, through green methods.
The present review focuses on the extraction of flavonoids, an immense category of compounds found in plants and natural products. Furthermore, the review is dedicated to conventional and novel extraction methods using classic or natural deep eutectic solvents, a new category of green solvents with exceptional solvent properties, as well as generally green behavior.
An inclusive overview of the current research being carried out on the extraction of flavonoids using deep eutectic solvents is provided. The main conclusions, issues, and trends are analyzed in order to comprise a solid foundation for further research or more focused application.
1.1. Flavonoids
Flavonoids are a category of naturally occurring organic compounds found in fruits, vegetables, or grains. Flavonoids, as a group, include upwards of 8000 different identified compounds, which are responsible for a number of functions within plants, such as the coloration of the different parts of fruits or vegetables (leaves, flower, peel), as well as shielding against UV rays or external threats, such as pathogens. They can be located in other subsystems as well, such as the bark or the roots of the plant, where they might serve similar or different purposes [1].
The interest around extraction of flavonoids stems from the multiple health benefits they provide. Cardiovascular benefits, anticancer activity, and neurological system fortification are only some of the many actions this group. Flavonoid intake has been involuntarily pursued in medicine since ancient times. Herbal medicine, such as traditional Chinese or Mediterranean medicine, has helped breed plants that are considerably rich in flavonoids. This legacy has fueled much of the research listed in this project, directing green extraction towards traditional herbs, fruits, or common foods in the hopes of isolating useful substances, including flavonoids [2,3].
From a chemical structure standpoint, all flavonoids stem from a main skeleton that they share, and differentiate from each other based on the substituents attached to any part of the structure (Figure 1). They possess phenolic and pyrane rings in their structures and have many subclasses, such as flavanols, flavones, flavanones, chalcones, and anthocyanidines. The flavonoid skeleton is comprised of an aromatic ring linked on one side with a six-membered heterocyclic ring, which bears an oxygen atom instead of carbon next to the common side. The two rings connect to another aromatic ring to form the skeleton, as shown below:
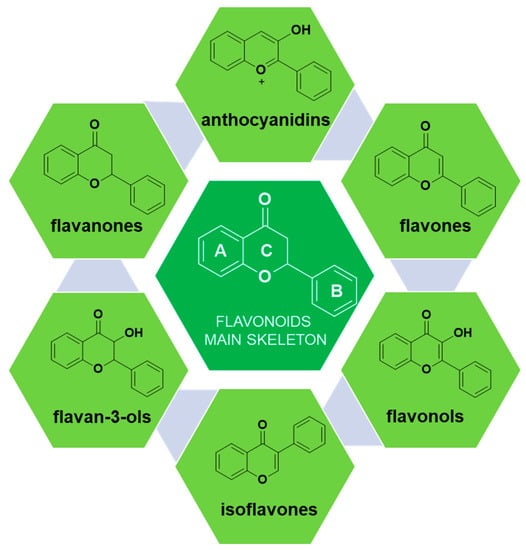
Figure 1.
Flavonoid skeleton and general structures of the main flavonoid categories.
1.2. Deep Eutectic Solvents
The extraction media of choice for green extractions could very well be Deep Eutectic Solvents (DES). DES are solvents that occur when a mixture of substances has a melting point that is much lower than that of the two constituents. In order to form a DES system, there needs to be a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), which when mixed at proper ratios create a new “mesh” of hydrogen-bond-interconnected molecules with interesting physicochemical properties. DES can be highly viscous, inhibiting their use in processes that require diffusion or flow, however, research into their chemical structure, as well as the use of additives (like water), largely alleviates this issue [4].
The potential to use multiple molecules as constituents and create a fluid mixture that can be used as a solvent is the first key interesting point regarding DES. Adding to this notion, the potential for many naturally occurring molecules to form DES, thus providing a natural solvent system with low vapor pressure; low cost, even at larger industrial scales; and the potential to remove the need for solvent retrieval (Natural DES–NaDES could remain in a consumable end product) highlights their importance. The evolution of DES, and more importantly, NaDES, means that the design of the newer solvents can focus on a solvent that is a capable and biocompatible storage media, a readily available active ingredient, a very efficient catalyst, or a molecular or compound carrier [5,6].
Competing with ionic liquids (ILs), another category of green solvents so far favors DES, since they are largely cheaper to produce, less toxic, and offer great variety. Seemingly, NaDES is the natural step forward from conventional ionic liquids, since they can be formed from green sources, can be polar or non-polar, hydrophilic or hydrophobic, and can be tailored for any situation. DES could become designer solvents, designed and synthesized for every extraction individually, with maximum efficiency in mind.
DES can be synthesized via a number of methods. Considering the research listed in this review, predominant methods are often simple, such as stirring at room to high temperatures (80 °C), freeze-dry mixing, or ultrasound-assisted mixtures [7,8]. The synthesis method can be selected with cost in mind (lower temperatures prevail), speed or efficiency (favoring higher temperatures and/or ultrasound assistance) or limited by the properties of the reagents (thermal-sensitive substances might require freeze-drying instead of heating due to thermal instability).
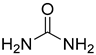
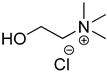
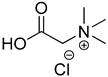
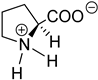
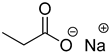
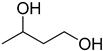
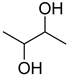
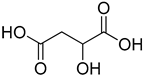
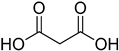
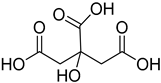
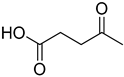
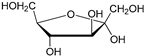
Compounds that are preferred for their natural origin and green character, and that have been heavily employed in NaDES synthesis are shown in Table 1. All the molecules in Table 1 have been a part of DES used in flavonoid extraction.

Table 1.
Common ingredients for deep eutectic solvents (DES) used in flavonoid extraction (based upon the literature cited in this review).
1.3. Application of DES on Flavonoid Extractions
This review examined multiple research topics using DES in various methods to extract flavonoids from natural sources. All of the extraction methods agree upon the potential of DES in the extraction of plant sources, given the success of the extractions as well as the promising results in relation to conventional solvents, particularly so wherever a comparison could be directly drawn [9,10].
The extractions might not have the collection of a compound as the end goal. Many aimed at providing better analytical methods through the use of DES [11,12,13,14], while others attempted the creation of a substrate for several reactions or subsequent extractions (through systems constructed with the aid of DES) [15,16,17].
Furthermore, all of the projects have been successful at applying DES to achieve the target extractability or otherwise consequent properties, boasting either high retrievability (for extraction), sufficiently low detection limits for analytical methods, or effective substrates and precursors for reactions. Whether utilizing DES in novel methods, attempting to increase efficiency with new approaches, such as negative pressure cavitation (NPC) [18] and these novel solvents, or applying them to older extraction systems, DES generally seem to adapt to the needs of each process.
All of the applications that incorporated the extraction of a substance through DES, at some point and to some degree, have provided useful information towards creating a clear picture as to what affects the process of extraction through DES, and more importantly, how this happens.
2. Factors Affecting the Extraction of Flavonoids Using DES Separation Techniques
The careful study of the current literature concerning the extraction of flavonoids using DES reveals a set of factors, namely, temperature, molecular structure and composition of the DES, extraction time, water content, the use of additives, solvent/sample ratio, and pH, which plays an important role in the efficiency and yield of the process.
2.1. Temperature
The temperature in which the extraction takes place is naturally expected to affect the time of the extraction, as well as its efficiency and performance. In general, higher temperatures increase molecular mobility and allow extracted molecules to diffuse to the solvent quicker. Extractions through DES are no exception. More so, extractions with DES rely on temperature to decrease the viscosities of DES, which are significantly high and render extractions cumbersome.
According to the literature, the desirable temperature range for extractions is from room temperature (25 °C) to about 60 °C. Higher temperatures, aside from demanding energy to sustain (moving away from the green character of extractions), might also endanger either the DES or the target substance, since many of the natural substances involved are thermally sensitive [19].
Very high temperatures also proved to decrease yield in some cases because of a decrease in the interactability between the target compound and the solvent of choice, regardless of the thermal endurance of either one (which is still a limiting factor nevertheless) [20].
2.2. Molecular Structure and Composition of the DES
The molecular structure of the DES refers to the ingredients used in its synthesis. Whether a binary or a ternary system, the molecules contained in the DES are responsible for its unique properties. In extractions, it is reported that the polarity of the DES is a very important factor affecting solubility. In screening multiple DES against many samples and target compounds, the conclusion implies that the polarity of the DES used needs to be close to the polarity of the target substance. Among similar DES, one with a polarity closer to the target will present the greatest extractability. Therefore, in selecting the proper DES for extraction, polarity similarity is a top priority as far as efficiency is concerned. This might be difficult given the many potential structures of DES, but potentially enables great efficiency through novel structures that approach the polarity of each given target [21,22].
Furthermore, the molecular interactions of both HBDs and HBAs with the target, as well as the background, need to be considered. Any competitive interactions between the ingredients of the DES and another presence in the system might interfere with the extraction efficiency to a great degree, potentially leading to a redesign of the extraction [23,24]. An example of this occurrence comes from Cui et. al, where a change in the ratio between the donor and acceptor led to decrease in yield. This decrease was not due to polarity change or some similar factor, but due to the chloride anion that the choline and betaine carried, and which reacted with the target. Reducing their presence in the DES reduced the yield simply because of the decrease in interaction between the fewer chloride anions and the target compound [20]. Within a more general scope, with regards to the ratio of HBA/HBD, it seems that an increase in the hydrogen bond donor content leads to a decrease in the viscosity of DES. In addition, an increase of hydroxyl groups in any ingredient of the DES would promote the formation of hydrogen bonds yielding a significantly more stable DES [22].
2.3. Toxicity
Generally, DES are reported as “safe” and “non-toxic” or of “low toxicity”, without any other justification than the safety and low toxicity of their components. However, this assumption can be true only in the case of NaDES, which are constituted by naturally occurring compounds, and therefore can be considered as inherently non-toxic. Thus, the literature concerning the toxicity of DES or NaDES is still scarce, and in the majority of the published works involving their use as extraction solvents, no toxicity tests are included.
Hayyan et al. [25] were among the first to study the toxicity and cytotoxicity of DES possessing choline chloride as the HBA and glycerine, ethylene glycol, triethylene glycol, and urea as the HBD. The tested DES and their individual components did not show toxicity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, or Pseudomonas aeruginosa. The interesting finding was that the DES under study showed significantly higher cytotoxicity than their individual components against Artemia salina leaches. This striking difference in cytotoxicity was attributed to the hydrogen bonding network present in DES and definitely merits further investigation.
The toxicity of a series of NaDES against L929 fibroblast-like cells was studied by Duarte et al. [26]. The results indicated that although no clear trend regarding cytotoxicity in relation with structure was observed, the presence of organic acids as HBDs results in increased cytotoxic activity. A series of 28 NaDES containing ChCl as the HBA and a variety of HBDs were tested for their cytotoxicity against the human embryonic kidney cell line (HEK-293) [27]. The results showed that ChCl, as well as the compounds used as HBDs, were less toxic than the corresponding NaDES and that the structure of the HBD and the HBA/HBD ratio play a role in cytotoxicity.
Radošević et al. [28,29] studied different NaDES regarding their antimicrobial activity against Salmonella typhimurium., Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Staphylococcus aureus, and Candida albicans as well as their cytotoxicity against human normal and cancer cell lines (HEK293T, HeLa, MCF-7). NADES containing an organic acid were found to possess good antimicrobial activity, whereas their individual components were not active. Moreover, the majority of the tested NaDES showed low cytotoxicity, with the exception of ChCl-oxalic acid, which exhibited moderate cytotoxicity selectively against cancer cells. This observation is very important and the authors claim that this can be attributed to the fact that cancer cells have higher energy demands than normal cells.
In the work of Macario et al. [30], a series of DES comprised of ChCl, tetramethylammonium chloride ([N1111]Cl), and tetrabutylammonium chloride ([N4444]Cl) as HBAs, in combination various HBDs, were extensively studied for their cytotoxicity against two human skin cell lines, HaCaT32–35 (chosen as model for cosmetic applications) and MNT-136–38 (selected as a model to understand the potential of the DES under study for the treatment of skin disorders). The ChCl- and [N1111]Cl-containing DES were not cytotoxic, and some of them even increased cell viability. Thus, these DES can be safely characterized as “benign”, at least for these cell lines, and for skin-related applications. The [N4444]Cl containing DES was cytotoxic against these cell lines, and as no clear trend regarding the relation of cytotoxicity with the HBD used was deduced, this HBA should not be considered safe for further applications.
The in vivo safety of DES and NaDES is much less studied. The first published research in which both in vitro and in vivo toxicity of DES was conducted is the work of Hayyan et al. in 2015 [31]. Four DES possessing ChCl as the HBA along with glycerine, ethylene glycol, triethylene glycol, and urea were tested against five human cancer cell lines and one normal cell line, and the individual components were tested as well. The cytotoxicity of DES in the various cell lines was found to be not negligible, and the ChCl/HBD ratio as well as the HBD structure seem to play important roles in toxicity. The in vitro acute toxicity studies indicated that the examined DES were more toxic than their individual components.
The cytotoxicity of two NaDES having ChCl as the HBA and glucose and fructose as HBDs, as well as the DES N,N-diethylethanol ammonium chloride-triethylene glycol, was studied by Mbous et al. [32] against 6 cancer cell lines. NaDES were found to be less toxic than the DES in the in vitro tests. In the in vivo tests, however, the NaDES showed higher toxicity than the DES, a result that was attributed to the higher viscosity of the NaDES.
Toxicological studies of DES and NaDES should be conducted before they are used in any application involving administration to living organisms, animals, or humans. In this context, Chen et al. [33] performed an acute toxicity study to test the safety of ChCl-glycerine DES, which was to be used as a drug carrier for salvianolic acid B. They were grateful to find that the LD50 value (Median Lethal Dose) of the tested DES was 7733 mg/kg, with a 95% confidence interval of 7130–8387 mg/kg for oral administration; thus, it can be safely administered orally, as it did not promote acute toxicity.
Belebna et al. [34] recently published the toxicity evaluation of an extract from green coffee beans rich in polyphenolic compounds. The extraction medium was NaDES (betaine-glycerol) and the studies were conducted in vivo on rats in order to investigate the potential of administering the extract as a dietary supplement. The NaDES extract induced several adverse effects after a high dose was administered orally, and the authors correctly highlight that in the case of developing food supplements based on NaDES extracts, the dose should be carefully defined after a detailed in vivo study.
2.4. Viscosity
The usually high viscosity of DES or NaDES is the major drawback that can restrict their use as extraction solvents, as it hampers penetration of the solvent in the extraction matrix. Increasing the temperature of the extraction process can lead to a decrease in viscosity, however, this option is not always the ideal choice, as it is energy consuming and some heat-sensitive phytochemicals may not tolerate the elevated temperature.
A simple way to overcome this problem is the addition of a co-solvent in the extraction medium. In most cases, this co-solvent is water, which maintains the green character of the process; however, organic solvents such as methanol have also been used. In this way, the viscosity is lowered and the extraction is facilitated [35,36]. In the recent work of Koutsoukos et al., water was used as a co-solvent for the extraction of phenolic compounds from brown propolis using ChCl/tartaric acid NaDES, with methanol used as the co-solvent for the extraction of carotenoids from apricot pulp and shrimp head by-products using the same NaDES [37].
The concentration of water in the DES–water or NaDES–water mixture affects the efficiency of the extraction, as has been shown by Bi et al. [38], who showed that a mixture of the ChCl/1,4-butanediol NaDES with 35% water is the optimum medium for the extraction of myricetin and amentoflavone from Chamaecyparis obtusa. As another indicative example, the work of Zhao et al. [39] indicates that very efficient extraction of rutin from the flower buds of Sophora japonica can be successful using the DES ChCl/triethyleneglycol containing 20% water. The researchers studied the viscosities of 20 DES and concluded that the viscosity increases significantly when more hydrogen bonds are possible among the DES constituents.
The amount of added water in a DES is a factor that should be carefully monitored when DES or NaDES are applied as extraction solvents. Dai et al. [40] showed that the viscosity of DES is affected by the water content, and that if more than 50% water is present the hydrogen bond framework of the DES components is destroyed.
Another approach to overcome the problem of high viscosity is to take advantage of the enormous number of possible combinations of natural compounds that can produce NaDES, in order to design solvents of low viscosity. The latest research from Marrucho et al. [41,42,43] introduced a new concept—the design of less-polar NaDES with lower viscosity, formed by mixing fatty acids of different alkyl chain lengths or by combining menthol with various organic acids.
2.5. Extraction Time
Extraction times show little variance among extractions. Greater extraction times increase costs, while shorter extractions run the risk of leaving considerable quantities of target substances in the sample, rendering the process ineffective. Most of the processes reviewed have very high retrieval percentages, with extraction times ranging from 20 minutes to 2 hours. Naturally, the type of extraction also defines the extraction time necessary, with energy-assisted methods such heating, ultrasound, or microwave requiring less extraction time, but, in turn, more energy to conduct. Overall, the use of DES has enabled undeniably short extraction times for all extraction methods employed.
2.6. Water Content
Water content is another crucial factor to the efficiency of NaDES in flavonoid extractions. Water might be found in a DES system unintentionally (during the synthesis, or from remaining in a container) or intentionally through co-solution to create an aqueous system. While some NaDES could be applied as extraction media on their own, their increased viscosity would hamper the speed of extractions. Furthermore, using pure DES as extraction media could increase the costs of extractions, rendering the process cost-inefficient at the laboratory or industrial scale. To tackle this issue, aqueous solutions of NaDES have been experimentally used instead, attempting the extraction of multiple substances with aqueous NaDES solutions of various concentrations, ranging from 20% all the way up to 80%. According to the literature, particularly in studies in which the water content was a part of optimization, percentages close to 20% water content [20,44] are the balance between creating a fluid extraction system and maintaining the hydrogen bond mesh of the DES [40]. Higher water contents tend to break the hydrogen bond structure of the DES, decreasing its effectiveness. This, however, does not imply that higher water contents would be ineffective or undesirable, as every extraction, having a great number of variables, could take advantage of higher or lower percentages of water.
2.7. DES as Additives, or Additives to DES
DES have been tested both as additives to traditional extraction or analysis systems, or have had additives combined with them in extractions, with ionic liquids being the prevalent example [45]. Sharing similar properties in structure and behavior as solvents meant that the combination of DES and ILs was inevitable.
Additives that co-exist with DES in extractions include, of course, other DES ingredients, which form ternary systems that aim at isolating multiple compounds, enhancing the efficiency of a single extraction or otherwise supplementing the processes which the system will traverse. DES can be made of multiple ingredients, as attempted on multiple occasions, however, there is no guarantee that even a carefully planned and synthesized ternary DES system will be more efficient than a binary, simpler one. Depending on the target, a ternary system could be a better or a worse option [46].
Other additives can be added to assist the extraction of a substance or any other action, however, similarly to the ternary systems, any addition may promote or hamper an extraction, depending on the target. An example by Georgantzi et al. [47] shows that the addition of β-cyclodextrin alters the extractability of select flavonoids depending on the selected DES, benefiting one but worsening the other, with varying levels of significance based on the miscellaneous parameters of the extraction.
2.8. Solvent/Sample Ratio
The ratio of sample (solid or otherwise) to solvent used can also affect the extraction. Immersing a miniscule amount of sample into the solvent means the extraction could be inefficient at a larger scale, since only a small amount of sample is being processed at a given time. On the other hand, smothering the solvent with a copious amount of solid sample might mean the dispersion of solvent around the sample would be slower, the contact surface of the sample with the solvent could eventually decrease (compared to a lesser amount of sample), and the system would end up underperforming. Most of the literature examples, after statistical analysis or reference to previous successful work, have converged on a ratio of solid sample to solvent of 1:10, balancing the amount of sample processed with the efficiency of the method [48,49].
2.9. pH
The pH level of the system may dictate the form of the target compound in some cases, eventually affecting its solubility in the DES. Some DES ingredients might also be affected by the pH themselves, changing their polarity or general behavior, with beneficial or undesirable results. The form of the target (a result of the pH) may completely change the design of the extraction, given that a solvent with a completely different polarity would be extracted by a different DES than originally planned [50,51].
2.10. Separation Techniques
The majority of the research with regards to the use of DES in flavonoid extractions still revolves around the optimization of conditions and evaluating the performance of the extraction at a level deemed satisfactory after having modified certain parameters. Most cited attempts employed HPLC (High Performance Liquid Chromatography), UV-Vis (Ultra-Violet – Visible Spectroscopy) [12], or other instrumental analysis, aiming to analyze the extract in order to evaluate the performance of the DES mixtures to capture the target compounds. Therefore, some reviews do not capitalize upon the separation of the flavonoids from each other or from a group of organic compounds extracted via the DES as a research point in itself, rather than as a means to examine the performance of the initial extraction. For example, research aiming at simply verifying the flavonoid content of the extract to confirm a successful NaDES extraction employed the Folin–Ciocalteu reagent in a properly prepared extract sample, which, after reaction and incubation, could be studied through UV-Vis or another method to verify the total phenolic content, and subsequently, the flavonoid content [52].
Whenever deemed necessary, often due to the extraction methodology, some researchers opted for filtration or centrifugation prior to the commonplace HPLC analysis that would confirm the extraction of the target flavonoids [53].
A popular and efficient solution for the enrichment and the separation of the flavonoids from the DES extract involves the use of column chromatography through a packed column with a macroporous resin (such as ME-2 [44], NKA-9, or AB-8 [54]), which is cleaned with deionized water, and then after exposure to the extract is eluted with aqueous ethanol. This method, however, requires further processing of the solution to isolate a particular flavonoid from a potential group of flavonoids extracted from a source. The packed column method may provide exceptionally high yields of up to 98.92% [55].
A back extraction using an antisolvent is a simpler method of isolating the flavonoids from the DES. After centrifugation of the sample, the supernatant is diluted with an organic antisolvent such as methanol [46], and subsequently centrifuged again to create a biphasic system. The newly occurring supernatant is the target system, leaving only the solvent to be evaporated (i.e., by vacuum centrifugal evaporation [8]).
Finally, a novel method for extracting the flavonoids from the DES involves the mimicking of DNA denaturation in the DES, as described by Tian et al. [56]. According to their research, the main goal in removing the flavonoids from the DES is the breakdown of the hydrogen mesh that holds the DES together, which is a process similar to denaturation. The group designed an effective method of extracting the flavonoids on a chrome metal organic framework (Materials of Institut Lavoisier: MIL-100 (Cr)), from which the isolation of the flavonoids becomes easier and more selective. Initially, the DES is diluted in water (10% DES Solution), then NaCl is added, causing the HB mesh to breakdown. The subsequent addition of the MIL allows for the readily collectible flavonoids to attach to it and be easily removed from the diluted DES.
An overview of all the applications of NaDES on flavonoid extractions examined in this review is presented in Table 2, including the bioactivity of the target flavonoids as mentioned in each study.

Table 2.
Overview of extraction examples from the literature, presenting the plant source, DES employed, target compound, and the bioactive role the writers mention (literature presented in chronological order).
3. Conclusions
The use of DES in the extractions of flavonoids has yielded overwhelmingly promising results. All of the applications reviewed have reported success in isolation and extraction of the target compounds, as well as competitive, if not superior, extraction rates compared with conventional solvents, in addition to the satisfactory behavior of the extract in the latter applications (such as direct analysis, synthesis or catalysis), wherever attempted.
The issue of selectivity of the DES or NaDES used for the extraction of flavonoids has not been extensively researched yet. In fact, the majority of the published works focus on the extraction efficiency evaluated in terms of total flavonoid or total phenolic content, or anti-oxidant activity, and do not usually analyze the selectivity of the solvents on the extraction of certain molecules. Obviously, this is the next step that should be investigated. As a very good indicative example, Vieira et al. [65] have screened a series of DES comprised of ChCl and carboxylic acids as solvents for the extraction of phytochemicals from the leaves of walnut trees (Juglans. regia L.). They found differences in selectivity in the extraction of 3-O-caffeoylquinic acid, quercetin 3-O-glucose, and quercetin O-pentoside among the various DES, and these results enabled them to choose the optimum system for their process.
DES are extremely versatile, with variables such as the number of ingredients, their ratios, and the ingredients themselves. This versatility limits their number to the foresight and the resilience of the researcher, who can design and apply any DES to any system, through any method, with the promise that the resulting extraction will be efficient while being green, given the general properties of DES. The relatively low cost of DES ingredients coupled with an increased selectivity after careful planning means that a lean-process future would definitely involve DES in the extraction compendium.
The fact that the DES extract system has a satisfactory performance in analytical methods [12] or chemical processes means that there is the potential for circumventing the last stage of extraction (separating the extract from the DES), using the system in its entirety instead. DES, being perfectly capable solvents, albeit viscous, can act as the solvent or carrier for the extract in its following stages, in some cases aiding in the reactions it might partake in or in the overall environment the extract will inhabit. The natural origin of NaDES would mean that such systems can be used in their entirety, even in products to be used or consumed by people (such as cosmetics or pharmaceuticals), without the need for extract isolation and further processing.
A pioneer example of NaDES being utilized as both an extraction medium and a biocompatible, consumer-grade carrier is the patented process by Lavaud et.al. [74], which encompasses all of the advantages and the potential they provide. This patent officially links the laboratory research results regarding the use of betaine-based NaDES, mixed with glycerol or water, for the extraction and storage of natural extracts from plants or microorganisms, and the subsequent use of the extract directly as a uniform, natural-origin product. Evidently, the patent of such a method means that the industry is ready and willing to employ NaDES as an immediate extraction-carrier system, even on a consumer level.
This summary of flavonoid extractions via DES incorporates the essence of green chemistry, and the future of chemistry in general. Tailor-made solvents are applied to carefully designed conditions to collect a very valuable substance from a natural source with as little waste as possible. The simplicity of this sentence is deceptive, since careful design and research is required to replace conventional methods, however, these promising results can only act as fuel for future research.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.A.; Liu, D. Flavonoids and Cardiovascular Health. In Complementary and Alternative Therapies and the Aging Population; Watson, R.R., Ed.; Academic Press: Elsevier: Amsterdam, The Netherlands, 2009; pp. 371–392. [Google Scholar]
- Watson, R.; Preedy, V.; Zibadi, S. Polyphenols in Human Health and Disease; Academic Press: Elsevier: Amsterdam, The Netherlands, 2014; Volume 1–2. [Google Scholar]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Weiz, G.; Braun, L.; Lopez, R.; de Maria, P.D.; Breccia, J.D. Enzymatic deglycosylation of flavonoids in deep eutectic solvents aqueous mixtures Paving the way for sustainable flavonoid chemistry. J. Mol. Catal. B: Enzym. 2016, 130, 70–73. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multifunctioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents:application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Liu, Y.; Garzon, J.; Friesen, J.B.; Zhang, Y.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Countercurrent assisted quantitative recovery of metabolites from plant-associated natural deep eutectic solvents. Fitoterapia 2016, 112, 30–37. [Google Scholar] [CrossRef]
- Fu, N.; Lv, R.; Guo, Z.; Guo, Y.; You, X.; Tang, B.; Han, D.; Yan, H.; Row, K.H. Environmentally friendly and non-polluting solvent pretreatment of palm samples for polyphenol analysis using choline chloride deep eutectic solvents. J. Chromatogr. A 2017, 1492, 1–11. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.-Z.; Wang, L.-T.; Kang, Y.-F.; Meng, Y.; Jiao, J.; Fu, Y.-J. Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from sea buckthorn leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Espino, M.; de los Angeles Fernandez, M.; Raba, J.; Silva, M.F. Enhanced electrochemical detection of quercetin by Natural Deep Eutectic Solvents. Anal. Chim. Acta 2016, 936, 91–96. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Towards green analysis of virgin olive oil phenolic compounds: Extraction by a natural deep eutectic solvent and direct spectrophotometric detection. Food Chem. 2016, 212, 43–47. [Google Scholar] [CrossRef]
- Li, X.; Dai, Y.; Row, K.H. Preparation of two-dimensional magnetic molecularly imprinted polymers based on boron nitride and a deep eutectic solvent for the selective recognition of flavonoids. Analyst 2019, 144, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, L.; Tang, W.; Row, K.H.; Zhu, T. Optimization of the chromatographic behaviors of quercetin using choline chloride-based deep eutectic solvents as HPLC mobile-phase additives. Sep. Sci. Technol. 2018, 53, 397–403. [Google Scholar] [CrossRef]
- Taylor, K.M.; Taylor, Z.E.; Handy, S.T. Rapid synthesis of aurones under mild conditions using a combination of microwaves and deep eutectic solvents. Tetrahedron Lett. 2017, 58, 240–241. [Google Scholar] [CrossRef]
- Fu, N.; Li, L.; Liu, X.; Fu, N.; Zhang, C.; Hu, L.; Li, D.; Tang, B.; Zhu, T. Specific recognition of polyphenols by molecularly imprinted polymers based on a ternary deep eutectic solvent. J. Chromatogr. A 2017, 1530, 23–34. [Google Scholar] [CrossRef]
- Unlu, A.E.; Prasad, B.; Anavekar, K.; Bubenheim, P.; Liese, A. Investigation of a green process for the polymerization of catechin. Prep. Biochem. Biotechnol. 2017, 47, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Greiner, R.; Orlien, V.; Lebovka, N.I. Negative pressure cavitation extraction: A novel method for extraction of food bioactive compounds from plant materials. Trends Food Sci. Technol. 2016, 52, 98–108. [Google Scholar] [CrossRef]
- Mulia, K.; Muhammad, F.; Krisanti, E. Extraction of vitexin from binahong (Anredera cordifolia (Ten.) Steenis) leaves using betaine -1,4 butanediol natural deep eutectic solvent (NADES). AIP Conf. Proc. 2017, 1823, 020018-1–020018-4. [Google Scholar]
- Cui, Q.; Peng, X.; Yao, X.-H.; Wei, Z.-F.; Luo, M.; Wang, W.; Zhao, C.-J.; Fu, Y.-J.; Zu, Y.-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Yao, X.-H.; Zhang, D.-Y.; Duan, M.-H.; Cui, Q.; Xu, W.-J.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Fu, Y.-J. Preparation and determination of phenolic coumpounds from Pyrola Incarnata Fisch. with a green polyols based-deep eutectic solvent. Sep. Purif. Technol. 2015, 149, 116–123. [Google Scholar] [CrossRef]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, H.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef]
- Shang, X.; Tan, J.-N.; Du, Y.; Liu, X.; Zhang, Z. Environmentally-Friendly Extraction of Flavonoids from Cyclocarya paliurus (Batal.) Iljinskaya Leaves with Deep Eutectic Solvents and Evaluation of Their Antioxidant Activities. Molecules 2018, 23, 2110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, Y.; Wu, W.; Liu, D.; Ji, Y.; Ren, S. Roles of a hydrogen bond donor and a hydrogen bond acceptor in the extraction of toluene from n-heptane using deep eutectic solvents. Green Chem. 2016, 18, 3089–3097. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.; Hayyan, A.; Al-Saadi, M.A.; Al Nashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents-solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Ahmadi, R.; Hemmateenejad, B.; Safavi, A.; Shojaeifard, Z.; Mohabbati, M.; Firuzi, O. Assessment of cytotoxicity of choline chloride-based natural deep eutectic solvents against human HEK-293 cells: A QSAR analysis. Chemosphere 2018, 209, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Bubalo, M.C.; Srček, V.G.; Grgas, D.; Dragičević, T.L.; Redovniković, I.R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Cvjetko Bubalo, M.; Frece, J.; Gaurina Srček, V.; Radojčić Redovniković, I. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Macário, I.P.E.; Oliveira, H.; Menezes, A.C.; Ventura, S.P.M.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef]
- Hayyan, M.; Looi, C.Y.; Hayyan, A.; Wong, W.F.; Hashim, M.A. In vitro and in vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS ONE 2015, 10, 2. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the cytotoxicity and metabolic pathways of binary natural deep eutectic solvent systems. Sci.Rep. 2017, 7, 41257. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, M.; Zhang, L. The effect of deep eutectic solvent on the pharmacokinetics of salvianolic acid B in rats and its acute toxicity test. J. Chromatogr. B 2017, 1063, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Belebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of natural deep eutectic solvent betaine: Glycerol in rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, R.; Atilhan, M.; Aparicio, S. On the properties of (choline chloride+ lactic acid) deep eutectic solvent with methanol mixtures. J. Mol. Liq. 2018, 272, 815–820. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline Chloride and Tartaric Acid, a Natural Deep Eutectic Solvent for the Efficient Extraction of Phenolic and Carotenoid Compounds. J. Clean. Prod. 2019, in press. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Kou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.; Marrucho, I.M. Menthol-based eutectic mixtures: Hydrophobic low viscosity solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.; Marrucho, I. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilib. 2017, 448, 135–142. [Google Scholar] [CrossRef]
- Florindo, C.; Romero, L.; Rintoul, I.; Branco, L.C.; Marrucho, I.M. From phase change materials to green solvents: Hydrophobic low viscous fatty acid–based deep eutectic solvents. ACS Sustain. Chem. Eng. 2018, 6, 3888–3895. [Google Scholar] [CrossRef]
- Wei, Z.-F.; Wang, X.-Q.; Peng, X.; Wang, W.; Zhao, C.-J.; Gang Zu, Y.; Fu, Y.-J. Fast and Green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind. Crop. Prod. 2015, 63, 175–181. [Google Scholar] [CrossRef]
- Ma, W.; Row, K.H. Optimized extraction of bioactive compounds from Herba Artemisiae Scopariae with ionic liquids and deep eutectic solvents. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 459–466. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef]
- Georgantzi, C.; Lioliou, A.-E.; Paterakis, N.; Makris, D.P. Combination of Lactic Acid-based Deep Eutectic Solvents (DES) with β-cyclodextrin: Performance screening using ultrasound-assisted extraction of Polyphenols from selected native Greek medicinal plants. Agronomy 2017, 7, 54. [Google Scholar] [CrossRef]
- Tang, B.; Park, H.E.; Row, K.H. Simultaneous Extraction of Flavonoids from Chamaecyparis obtusa Using Deep Eutectic solvents as Additives of Conventional Extractions Solvents. J. Chromatogr. Sci. 2015, 53, 836–840. [Google Scholar] [CrossRef]
- Tang, W.; Li, G.; Chen, B.; Zhu, T.; Row, K.H. Evaluating ternary deep eutectic solvents as novel media for extraction of flavonoids from Ginkgo biloba. Sep. Sci. Technol. 2017, 52, 91–99. [Google Scholar] [CrossRef]
- Kanberoglu, G.S.; Yilmaz, E.; Soylak, M. Application of Deep Eutectic Solvent in ultrasound-assisted emulsification microextraction of quercetin from some fruits and vegetables. J. Mol. Liq. 2019, 279, 571–577. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Stefou, I.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Development of sodium propionate-based deep eutectic solvents for polyphenol extraction from onion solid wastes. Clean Technol. Environ. Policy 2019. [Google Scholar] [CrossRef]
- Jeong, K.M.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Kim, E.M.; Lee, J. One-step sample preparation for convenient examination of volatile monoterpenes and phenolic compounds in peppermint leaves using deep eutectic solvents. Food Chem. 2018, 251, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-L.; Peng, X.; Huang, Y.-Y.; Li, L.; Wei, Z.-F.; Zu, Y.-G.; Fu, Y.-J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crop. Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.-L.; Li, P.; Liu, E.-H. Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platycladi Cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, J.; Li, Y.; Bi, W.; Chen, D.D.Y. Recovery of Natural Products from Deep Eutectic Solvents by Mimicking Denaturation. ACS Sustain. Chem. Eng. 2019, 7, 9976–9983. [Google Scholar] [CrossRef]
- Li, J.; Han, Z.; Zou, Y.; Yu, B. Efficient extraction of major catechins in Camellia sinensis leaves using green choline chloride-based deep eutectic solvents. RSC Adv. 2015, 5, 93937–93944. [Google Scholar] [CrossRef]
- Garcia, A.; Rodriguez-Juan, E.; Rodriguez-Gutierrez, G.; Rios, J.J.; Fernandez-Bolanos, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.-Z.; Luo, M.; Wag, W.; Huang, Y.-Y.; Efferth, T.; Wang, H.-M.; Fu, Y.-J. Efficient extraction and preparative separation of four main isoflavonoids from Dalbergia odorifera T. Chen leaves by deep eutectic solvents-based negative pressure cavitation extraction followed by macroporous resin column chromatography. J. Chromatogr. B 2016, 1033–1034, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Two-phase systems developed with hydrophilic and hydrophobic deep eutectic solvents for simultaneously extracting various bioactive compounds with different polarities. Green Chem. 2018, 20, 1879–1886. [Google Scholar] [CrossRef]
- Wang, H.; Ma, X.; Cheng, Q.; Wang, L.; Zhang, L. Deep Eutectic Solvent—Based ultra-high pressure extraction of Baicalin from Scutellaria baicalensis Georgi. Molecules 2018, 23, 3233. [Google Scholar] [CrossRef]
- Yang, M.; Cao, J.; Cao, F.; Lu, C.; Su, E. Efficient Extraction of Bioactive Flavonoids from Ginkgo biloba Leaves Using Deep Eutectic Solvent/Water Mixture as Green Media. Chem. Biochem. Q. 2018, 32, 315–324. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinsons, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Hamany Djande, C.Y.; Piater, L.A.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Differential extraction of phytochemicals from the multipurpose tree, Moringa oleifera, using green extraction solvents. South. Afr. J. Bot. 2018, 115, 81–89. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, I.C.F.R.; Ferreira, O. Enhanced extraction of phenolic compounds using choline chloride based deep eutectic solvents from Juglans regia L. Ind. Crop. Prod. 2018, 115, 261–271. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, M.; Guo, H.; Xu, J.; Ye, J.; Zhao, J.; Zhao, L. Ultrasound-assisted deep eutectic solvent as green and efficient media for the extraction of flavonoids from Radix scutellariae. NJC 2019, 43, 644–650. [Google Scholar] [CrossRef]
- Pavic, V.; Flacer, D.; Jakovljevic, M.; Maja, M.; Jokic, S. Assessment of total phenolic content in vitro antioxidant and antibacterial activity of Ruta graveolens L. Extracts obtained by choline chloride based natural deep eutectic solvents. Plants 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, W.M.A.; Lorwirachsutee, A.; Theodoropoulos, C.; Gonzalez-Miquel, M. Polyol-Based Deep Eutectic Solvents for Extraction of Natural Polyphenolic Antioxidants from Chlorella vulgaris. ACS Sustain. Chem. Eng. 2019, 7, 5018–5026. [Google Scholar] [CrossRef]
- Mocan, A.; Diuzheva, A.; Badarau, S.; Cadmiel, M.; Andruch, V.; Carradori, S.; Campestre, C.; Tartaglia, A.; De Simone, M.; Vodnar, D.; et al. Liquid Phase and Microwave-Assisted Extractions for Multicomponent Phenolic Pattern Determination of Five Romanian Galium Species Coupled with Bioassays. Molecules 2019, 24, 1226. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Voroblev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges deep eutectic solvents and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Q.; Yin, L.-J.; Zheng, X.; Gao, M.-Z.; Meng, Y.; Wang, W. Efficient extraction of flavonoids from Flos Sophorae Immaturus by tailored and sustainable deep eutectic solvent as green extraction media. J. Pharm. Biomed. Anal. 2019, 170, 285–294. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.-E.; Jang, H.W.; Lim, T.-G.; Yoo, M.; Nam, T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, A.; Laguerre, M.; Birtic, S.; Fabiano Tixier, A.S.; Roller, M.; Chemat, F.; Bily, A.C. Eutectic Extraction Solvents, Extraction Methods by eutecticgenesis using said solvents, and extracts derived from said extraction methods. U.S. Patent WO 2016/162703, 13 October 2016. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).