Effect of the Pillar Size on the Electrochemical Performance of Laser-Induced Silicon Micropillars as Anodes for Lithium-Ion Batteries
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
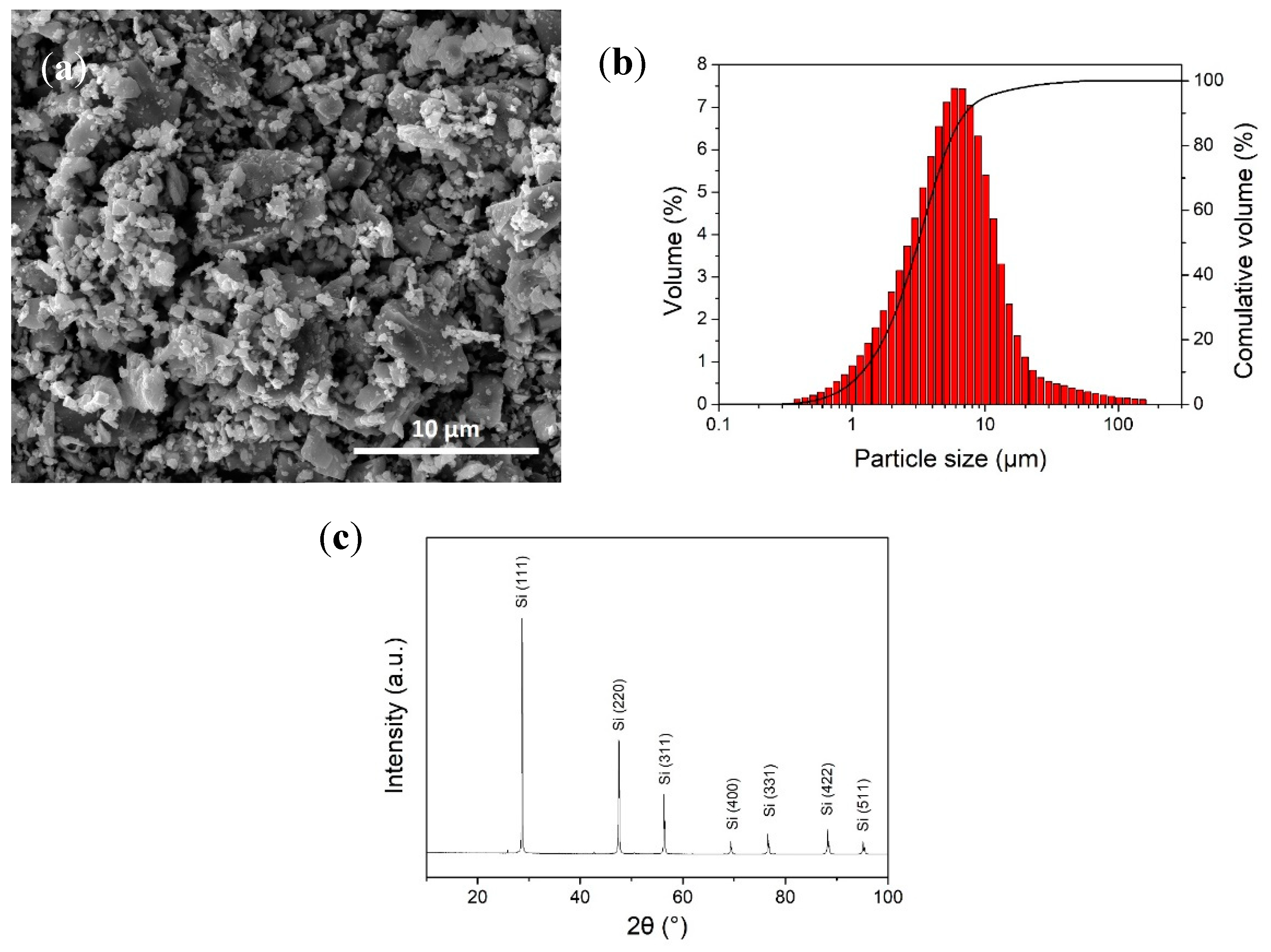
2.1. Sample Preparation
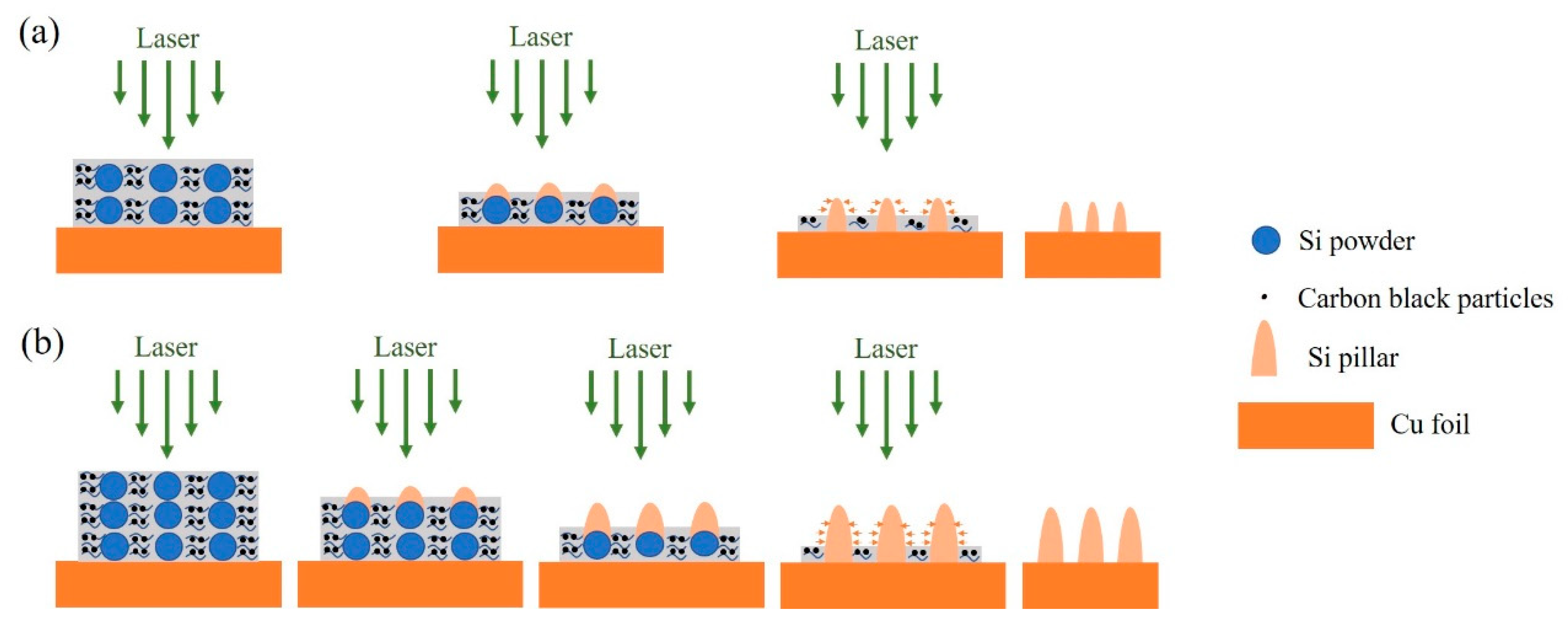
2.2. Si Micropillar Growth
2.3. Material Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
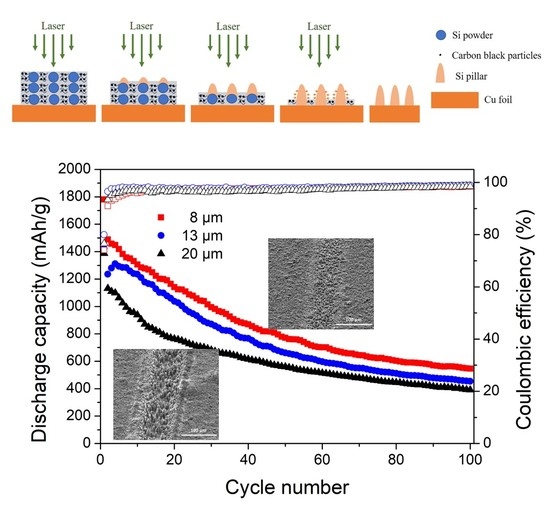
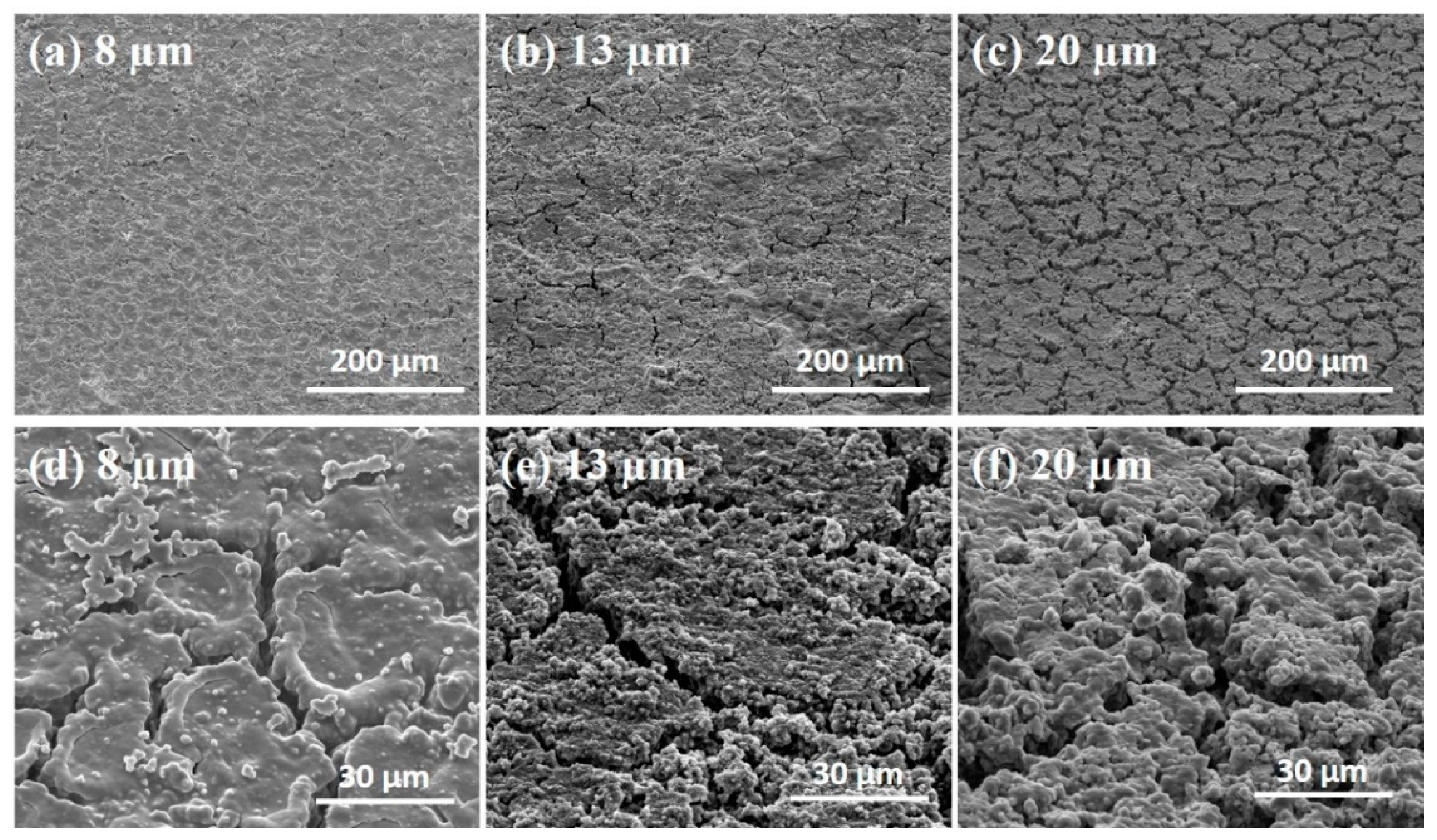
3.1. Si Micropillar Growth
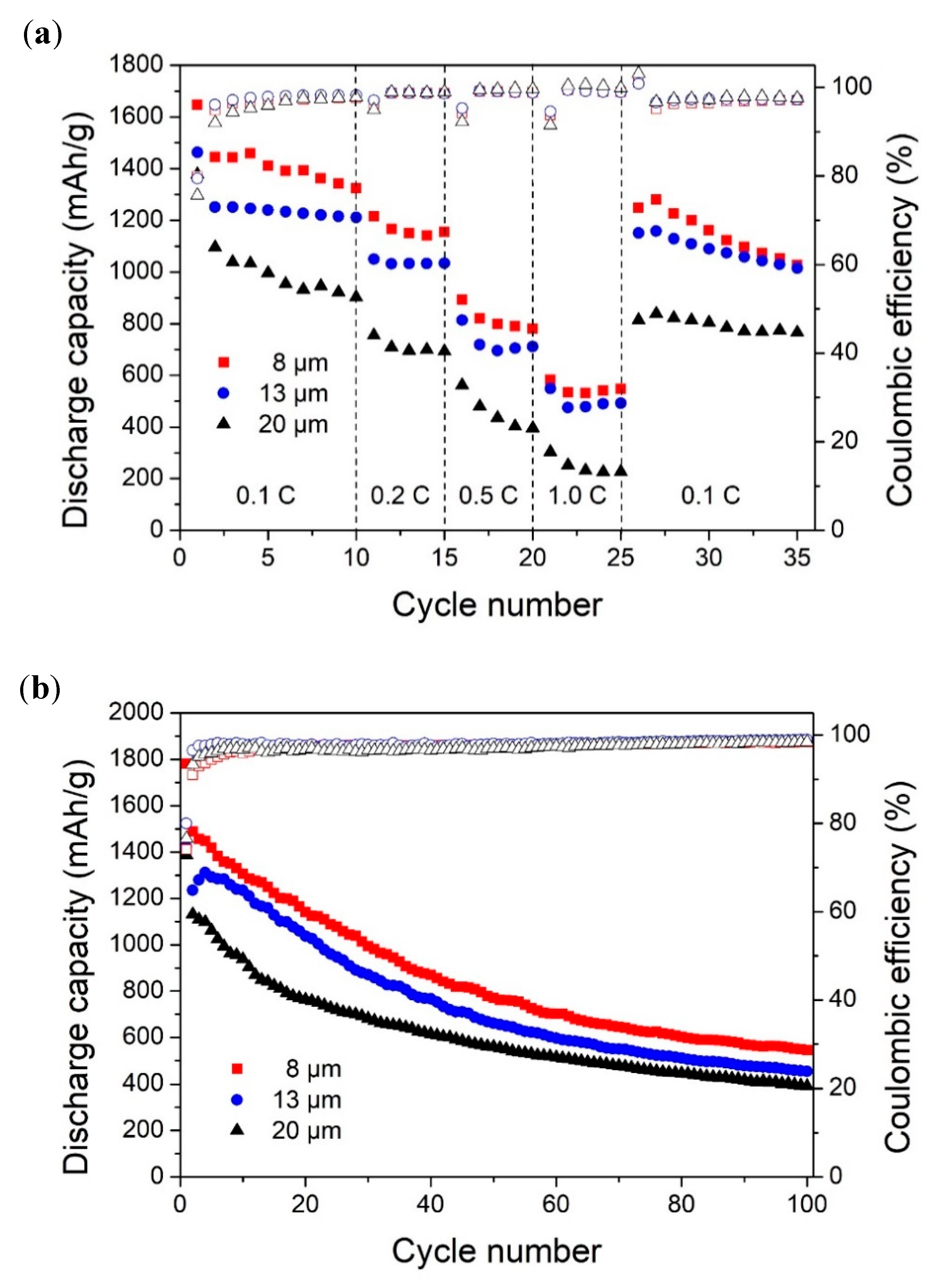
3.2. Electrochemical Performance
- (1)
- Smaller micropillars had a higher surface-area-to-volume ratio, which could reduce the occurrence of cracks on the surface and maintain the mechanical integrity.
- (2)
- Increased surface area and denser distribution of smaller micropillars provided more contact area between electrolyte and Si for large Li+ flux.
- (3)
- Shorter Li+ diffusion pathway in micropillars with a smaller diameter, which ensured a fast and homogeneous lithiation [35].
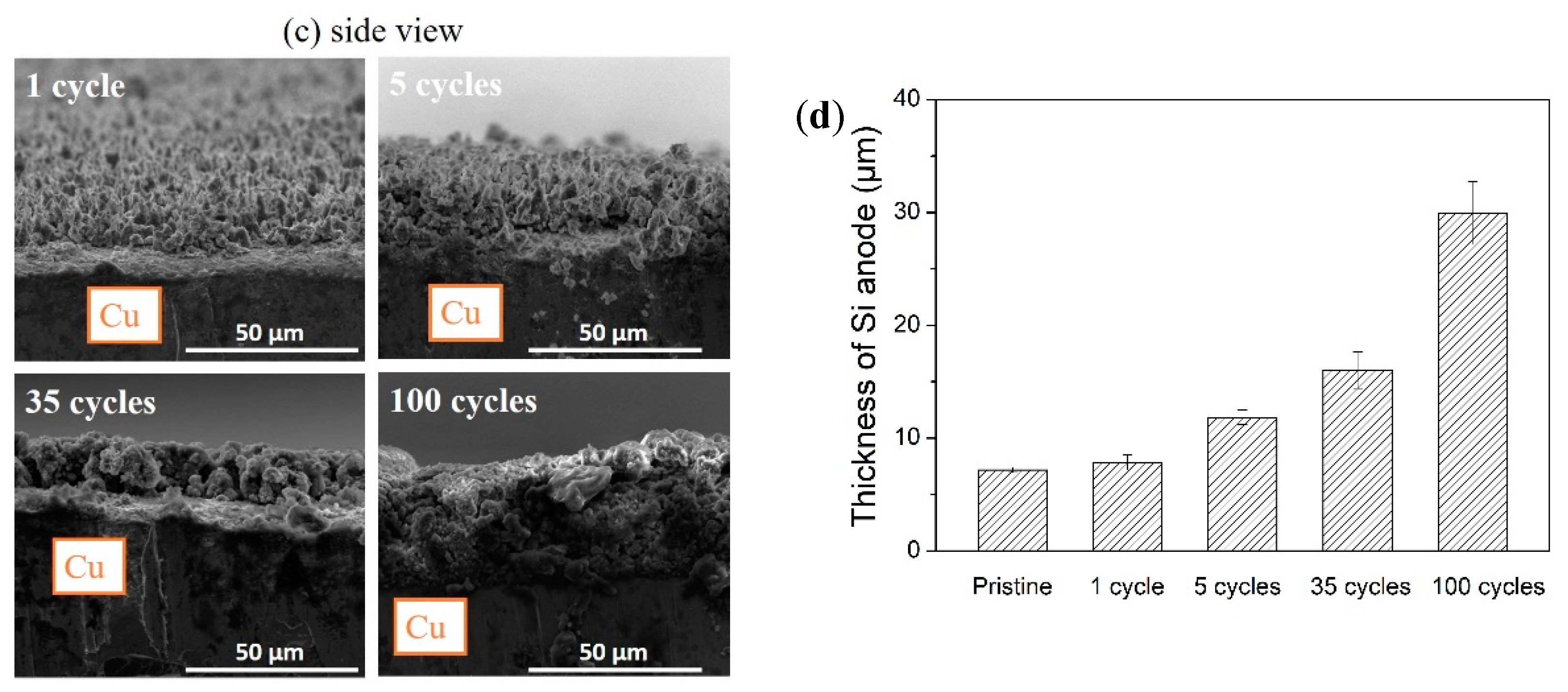
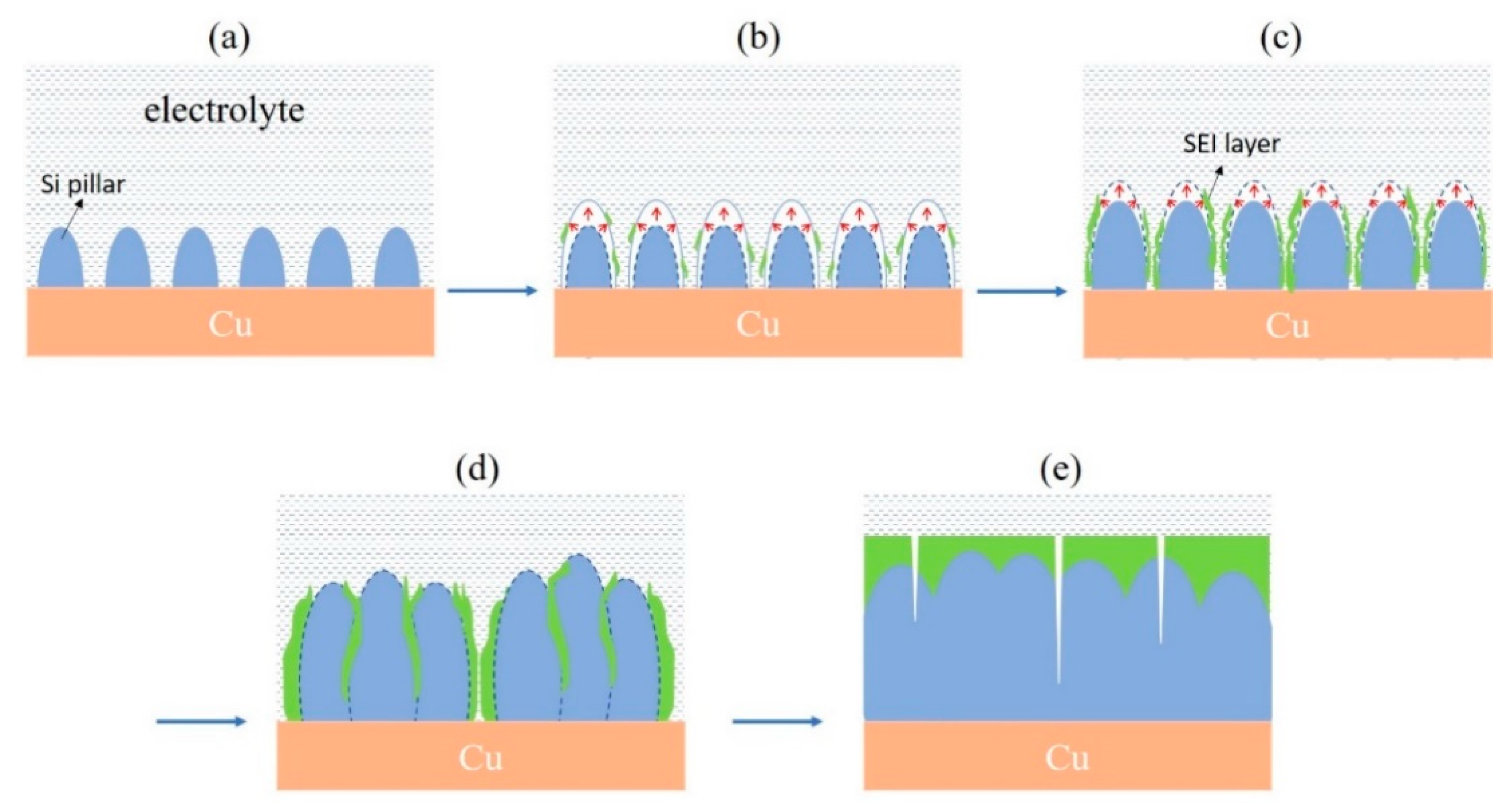
3.3. Morphological Change of Si Micropillars during Electrochemical Cycling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kinoshita, K.; Zaghib, K. Negative electrodes for Li-ion batteries. J. Power Source 2002, 110, 416–423. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652. [Google Scholar] [CrossRef] [PubMed]
- Maddipatla, R.; Loka, C.; Choi, W.; Lee, K.-S. Nanocomposite of Si/C Anode Material Prepared by Hybrid Process of High-Energy Mechanical Milling and Carbonization for Li-Ion Secondary Batteries. Appl. Sci. 2018, 8, 2140. [Google Scholar] [CrossRef]
- Lu, G.; Qiu, S.; Lv, H.; Fu, Y.; Liu, J.; Li, X.; Bai, Y.-J. Li-ion storage performance of MnO nanoparticles coated with nitrogen-doped carbon derived from different carbon sources. Electrochim. Acta 2014, 146, 249–256. [Google Scholar] [CrossRef]
- Lv, H.; Qiu, S.; Lu, G.; Fu, Y.; Li, X.; Hu, C.; Liu, J. Nanostructured antimony/carbon composite fibers as anode material for lithium-ion battery. Electrochim. Acta 2015, 151, 214–221. [Google Scholar] [CrossRef]
- De Sutter, L.; Berckmans, G.; Marinaro, M.; Smekens, J.; Firouz, Y.; Wohlfahrt-Mehrens, M.; van Mierlo, J.; Omar, N. Comprehensive Aging Analysis of Volumetric Constrained Lithium-Ion Pouch Cells with High Concentration Silicon-Alloy Anodes. Energies 2018, 11, 2948. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Li, X.; Gu, H.; Liu, J.; Wei, H.; Qiu, S.; Fu, Y.; Lv, H.; Lu, G.; Wang, Y.; Guo, Z. Multi-walled carbon nanotubes composited with nanomagnetite for anodes in lithium ion batteries. RSC Adv. 2015, 5, 7237–7244. [Google Scholar] [CrossRef]
- Fu, Y.; Gu, H.; Yan, X.; Liu, J.; Wang, Y.; Huang, J.; Li, X.; Lv, H.; Wang, X.; Guo, J. Chromium (III) oxide carbon nanocomposites lithium-ion battery anodes with enhanced energy conversion performance. Chem. Eng. J. 2015, 277, 186–193. [Google Scholar] [CrossRef]
- Qiu, S.; Lu, G.; Liu, J.; Lyu, H.; Hu, C.; Li, B.; Yan, X.; Guo, J.; Guo, Z. Enhanced electrochemical performances of MoO 2 nanoparticles composited with carbon nanotubes for lithium-ion battery anodes. RSC Adv. 2015, 5, 87286–87294. [Google Scholar] [CrossRef]
- Lyu, H.; Liu, J.; Qiu, S.; Cao, Y.; Hu, C.; Guo, S.; Guo, Z. Carbon composite spun fibers with in situ formed multicomponent nanoparticles for a lithium-ion battery anode with enhanced performance. J. Mater. Chem. A 2016, 4, 9881–9889. [Google Scholar] [CrossRef]
- Guo, S.; Lu, G.; Qiu, S.; Liu, J.; Wang, X.; He, C.; Wei, H.; Yan, X.; Guo, Z. Carbon-coated MnO microparticulate porous nanocomposites serving as anode materials with enhanced electrochemical performances. Nano Energy 2014, 9, 41–49. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, A.-M.; Wan, L.-J.; Guo, Y.-G. Spin-coated silicon nanoparticle/graphene electrode as a binder-free anode for high-performance lithium-ion batteries. Nano Res. 2012, 5, 845–853. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Bai, Z.; Tu, W.; Zhu, J.; Li, J.; Deng, Z.; Li, D.; Tang, H. POSS-Derived Synthesis and Full Life Structural Analysis of Si@ C as Anode Material in Lithium Ion Battery. Polymers 2019, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Key, B.; Bhattacharyya, R.; Morcrette, M.; Seznec, V.; Tarascon, J.-M.; Grey, C.P. Real-time NMR investigations of structural changes in silicon electrodes for lithium-ion batteries. J. Am. Chem. Soc. 2009, 131, 9239–9249. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th anniversary article: Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, B.; Lesh, G.; Huggins, R. All-solid lithium electrodes with mixed-conductor matrix. J. Electrochem. Soc. 1981, 128, 725–729. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. Engl. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Jin, Y.; Tan, Y.; Zong, L.; Hu, Y.; Chen, L.; Chen, Y.; Zhang, Q.; Zhu, J. Scalable Production of Si Nanoparticles Directly from Low Grade Sources for Lithium-Ion Battery Anode. Nano. Lett. 2015, 15, 5750–5754. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Y, J. Huang, Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.-S.; Yao, Y.; Cui, Y.; Cho, J. One dimensional Si/Sn-based nanowires and nanotubes for lithium-ion energy storage materials. J. Mater. Chem. 2011, 21, 9825–9840. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, J.W.; Sung, Y.-E.; M, S. Oh, Failure modes of silicon powder negative electrode in lithium secondary batteries. Electrochem. Solid State Lett. 2004, 7, A306–A309. [Google Scholar] [CrossRef]
- Chan, C.K.; Ruffo, R.; Hong, S.S.; Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Source 2009, 189, 1132–1140. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- Chockla, A.M.; Harris, J.T.; Akhavan, V.A.; Bogart, T.D.; Holmberg, V.C.; Steinhagen, C.; Mullins, C.B.; Stevenson, K.J.; A, B. Korgel, Silicon nanowire fabric as a lithium ion battery electrode material. J. Am. Chem. Soc. 2011, 133, 20914–20921. [Google Scholar] [CrossRef]
- Chan, C.K.; Patel, R.N.; O’Connell, M.J.; Korgel, B.A.; Cui, Y. Solution-grown silicon nanowires for lithium-ion battery anodes. ACS Nano 2010, 4, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Ruffo, R.; Hong, S.S.; Chan, C.K.; Huggins, R.A.; Cui, Y. Impedance analysis of silicon nanowire lithium ion battery anodes. J. Phys. Chem. C 2009, 113, 11390–11398. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon nanotube battery anodes. Nano Lett. 2009, 9, 3844–3847. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Hu, L.; Paik, U. One-Dimensional Silicon Nanostructures for Li Ion Batteries. J. Phys. Chem. Lett. 2014, 5, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Gohier, A.; Laïk, B.; Pereira-Ramos, J.-P.; Cojocaru, C.S.; Tran-Van, P. Influence of the diameter distribution on the rate capability of silicon nanowires for lithium-ion batteries. J. Power Source 2012, 203, 135–139. [Google Scholar] [CrossRef]
- Choi, M.; Kim, J.C.; Kim, D.W. Waste Windshield-Derived Silicon/Carbon Nanocomposites as High-Performance Lithium-Ion Battery Anodes. Sci. Rep. 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, Y.; Yan, J. Laser sintering of silicon powder and carbon nanofibers for porous composite thick films. Appl. Phys. Expr. 2015, 8, 026501. [Google Scholar] [CrossRef]
- Yan, J.; Okada, K. Fabrication of silicon-based porous nanocomposite films by focused infrared light sintering. CIRP Ann. 2016, 65, 217–220. [Google Scholar] [CrossRef]
- Yan, J.; Noguchi, J.; Terashi, Y. Fabrication of single-crystal silicon micro pillars on copper foils by nanosecond pulsed laser irradiation. CIRP Ann. 2017, 66, 253–256. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kimura, K.; Nishihara, H.; Kasukabe, T.; Kyotani, T.; Kobayashi, H. Fabrication of Si nanopowder from Si swarf and application to high-capacity and low cost Li-ion batteries. J. Alloys Compd. 2017, 720, 529–540. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, H.; Chang, H.; Kim, J.; Roh, K.M.; Choi, J.H.; Cho, B.G.; Park, E.; Kim, H.; Luo, J.; et al. Aerosol-assisted extraction of silicon nanoparticles from wafer slicing waste for lithium ion batteries. Sci. Rep. 2015, 5, 9431. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Huang, Y.-H.; Lan, C.-K.; Chen, B.-H.; Duh, J.-G. Scalable Upcycling Silicon from Waste Slicing Sludge for High-performance Lithium-ion Battery Anodes. Electrochim. Acta 2015, 173, 82–90. [Google Scholar] [CrossRef]
- Gauthier, M.; Mazouzi, D.; Reyter, D.; Lestriez, B.; Moreau, P.; Guyomard, D.; Roué, L. A low-cost and high performance ball-milled Si-based negative electrode for high-energy Li-ion batteries. Energy Environ. Sci. 2013, 6, 2145–2155. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Deng, J.; Qi, D.; Leow, W.R.; Wei, J.; Yin, S.; Dong, Z.; Yazami, R.; Chen, Z. Unravelling the Correlation between the Aspect Ratio of Nanotubular Structures and Their Electrochemical Performance To Achieve High-Rate and Long-Life Lithium-Ion Batteries. Angew. Chem. 2014, 126, 13706–13710. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured Materials for Advanced Energy Conversion and Storage Devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar]
- Luo, F.; Chu, G.; Xia, X.; Liu, B.; Zheng, J.; Li, J.; Li, H.; Gu, C.; Chen, L. Thick solid electrolyte interphases grown on silicon nanocone anodes during slow cycling and their negative effects on the performance of Li-ion batteries. Nanoscale 2015, 7, 7651–7658. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; McDowell, M.T.; Choi, J.W.; Cui, Y. Anomalous shape changes of silicon nanopillars by electrochemical lithiation. Nano Lett. 2011, 11, 3034–3039. [Google Scholar] [CrossRef]
- Leveau, L.; Laïk, B.; Pereira-Ramos, J.-P.; Gohier, A.; Tran-Van, P.; Cojocaru, C.-S. Silicon nano-trees as high areal capacity anodes for lithium-ion batteries. J. Power Source 2016, 316, 1–7. [Google Scholar] [CrossRef]
- Corn, S.H.; Falconer, J.L.; Czanderna, A. The copper–silicon interface: Composition and interdiffusion. J. Vac. Sci. Technol. A Vac. Surf. Films 1988, 6, 1012–1016. [Google Scholar] [CrossRef]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of fluoroethylene carbonate (FEC) on the performance and surface chemistry of Si-nanowire Li-ion battery anodes. Langmuir 2011, 28, 965–976. [Google Scholar] [CrossRef] [PubMed]



| Laser Type | Nd:YAG Laser |
|---|---|
| Wavelength (nm) | 532 |
| Environment | In air |
| Beam profile | Gaussian |
| Beam diameter (μm) | 85 |
| Pulse width (ns) | 48.4 |
| Repetition frequency (kHz) | 10 |
| Average power (mW) | 300, 600 |
| Laser fluence (mJ/cm2) | 529, 1057 |
| Scanning speed (mm/s) | 1 |
| Line spacing for area irradiation (μm) | 57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Tachikawa, N.; Katayama, Y.; Li, L.; Yan, J. Effect of the Pillar Size on the Electrochemical Performance of Laser-Induced Silicon Micropillars as Anodes for Lithium-Ion Batteries. Appl. Sci. 2019, 9, 3623. https://doi.org/10.3390/app9173623
Yang X, Tachikawa N, Katayama Y, Li L, Yan J. Effect of the Pillar Size on the Electrochemical Performance of Laser-Induced Silicon Micropillars as Anodes for Lithium-Ion Batteries. Applied Sciences. 2019; 9(17):3623. https://doi.org/10.3390/app9173623
Chicago/Turabian StyleYang, Xueyuan, Naoki Tachikawa, Yasushi Katayama, Lin Li, and Jiwang Yan. 2019. "Effect of the Pillar Size on the Electrochemical Performance of Laser-Induced Silicon Micropillars as Anodes for Lithium-Ion Batteries" Applied Sciences 9, no. 17: 3623. https://doi.org/10.3390/app9173623
APA StyleYang, X., Tachikawa, N., Katayama, Y., Li, L., & Yan, J. (2019). Effect of the Pillar Size on the Electrochemical Performance of Laser-Induced Silicon Micropillars as Anodes for Lithium-Ion Batteries. Applied Sciences, 9(17), 3623. https://doi.org/10.3390/app9173623