Abstract
Lithium-sulfur (Li-S) batteries have shown a high theoretical specific capacity of 1675 mAh g−1. However, amongst the issues they have, the low electron conductivity of sulfur and its dissolution represent the biggest challenge limiting its practical applications. This contributes to the low utilization of the active sulfur at the cathode—a phenomenon known as the “shuttling effect.” To overcome these limitations, some strategies such as physical confinement (sulfur–carbon composite), chemical adsorption (N and/or S doping) electrolyte design, and separator design have already been proposed. Calcium citrate is the most attractive carbon source because no activation process is necessary and the fabrication process is very simple. In this experiment, we synthesized calcium citrate and sulfur (S) to conduct a charging–discharging test and compared them by adding thiourea (TU) as well as S in the carbonized calcium citrate (CaC). This effective and simple technique for material production can accommodate the charge/discharge reactions and preserve the structure over long cycles. A CaC/TU-S composite is acceptable for an initial discharge capacity of 1051.6 mAh g−1 over 100 cycles at 1 C. The results show that the CaC-S and CaC/TU-S composites have a good, stable specific capacity.
1. Introduction
The demand for sustainable energy storage is becoming more important owing to the emergence of applications such as electric vehicles and portable electronic devices [1]. Among the various types of rechargeable batteries, lithium-sulfur (Li-S) batteries have been considered one of most promising next-generation storage systems. Sulfur (S) has a theoretical specific capacity that is 3–5 times higher (1672 mAh g−1) than that of commercialized Li battery cathode materials such as transition metal oxides and iron phosphates [2], and the resultant Li-S battery yields very high energy density (2567 Wh kg−1). However, there are still several major issues facing Li-S batteries that impede their practical use, namely: (a) The low electronic conductivity of S (5 × 10−30 S cm−1 at 25 °C) significantly increases the overpotential and the insolubility of the discharge products (e.g., Li2S2/Li2S), leading to low utilization of S. This results in a reduction of the practical specific capacity to far less than the theoretical capacity [3]. This necessitates the use of carbon as a conducting additive in the sulfur cathode of Li-S batteries. (b) Soluble lithium polysulfide (PS, S4–82−) intermediates formed during the cycling process can diffuse in the organic liquid electrolytes, which causes the PS shuttle phenomenon [3,4]. This phenomenon results in active mass loss from the cathode, the reduction of coulombic efficiency, and capacity decay upon cycling. To overcome these limitations, some strategies such as physical confinement (sulfur–carbon composites), chemical adsorption (N and/or S doping) [5], electrolyte design [6,7], and separator design [4] have already been proposed. Among the physical confinement methods, one approach is to incorporate sulfur into nano-sized hosts such as mesoporous carbon. Recently, extensive research has been carried out on composites of sulfur and carbon matrix technologies such as carbon nano-tubes [8], graphene [9], and activated carbon [10] due to their excellent accessibility, producibility, electrochemical inertness, and wide temperature range. However, the production of these carbon matrices is usually very complicated and expensive [11]. Therefore, we attempted to reduce the time and cost by shortening the carbonization process. Calcium citrate is considered one of the most attractive carbon sources since a simple and effective carbonization method was reported for preparing porous carbon without any activation process, using calcium citrate as carbon source [11].
To make low-cost, high-efficiency Li-S batteries, carbon materials were made and tested using calcium citrate [12]. However, since calcium citrate alone cannot help with electrical conductivity, we improved the electrical conductivity through N-doping [13]. Thiourea (TU) is a very attractive substance used for N doping. It has the chemical formula CH4N2S and is a planar molecule [14]. It has nitrogen elements and sulfur elements that ensure high electrical conductivity when combined with sulfur. Several studies have shown that doping a carbon material with nitrogen, sulfur, and phosphorous can enhance both electrochemical activity and electrical conductivity [15]. N-doped carbons support the interaction of sulfur atoms with the carbon matrix [16]. Sulfur doping can also develop the affinity for polysulfides in the carbon matrix for better cycling performance [17]. The combined force of the sulfur is very high, producing a good adsorption effect to control the polysulfide during charge and discharge processes [18]. In this experiment, we synthesized calcium citrate and sulfur to conduct a charging–discharging test and compared them by adding thiourea and sulfur in the calcium citrate.
2. Materials and Methods
2.1. Synthesis of the Mesoporous Carbon–Sulfur Composites
Calcium citrate (Ca3(C6H5O7) 4H2O, Sigma Aldrich, St. Louis, Missouri MO, USA) was used as the carbon precursor and thiourea (CH4N2S) was added for co-doping nitrogen and sulfur (Figure 1). Porous carbon with high specific surface area could be synthesized by a simple one-step CaO template method (involving simultaneous templating and carbonization), without using any metal catalyst and activation processes [19,20]. Thiourea has a dual combination of C- and S-, and therefore it has good binding power and a single combination of N-, which helps it combine with sulfur. A certain amount of calcium citrate powder and thiourea (CH4N2S) (10:1 w/w) were loaded in an alumina boat after mixing in a mortar. Thiourea was used as both the N and S precursor to synthesize the N- and S-doped carbon samples. The mixed samples were heated at 900 °C under a nitrogen gas flow at a heating rate of 2 °C min−1 and maintained for 2 h. The resulting product was subsequently immersed with dilute 30 vol % HCl solution to remove insoluble substances such as CaO, which was further washed with deionized water until pH = 7. Then, the samples were dried under vacuum at 110 °C for 12 h to obtain the final carbon samples. Mesoporous carbon samples prepared in this manner were designated the CaC (without thiourea) and CaC/TU (with thiourea).
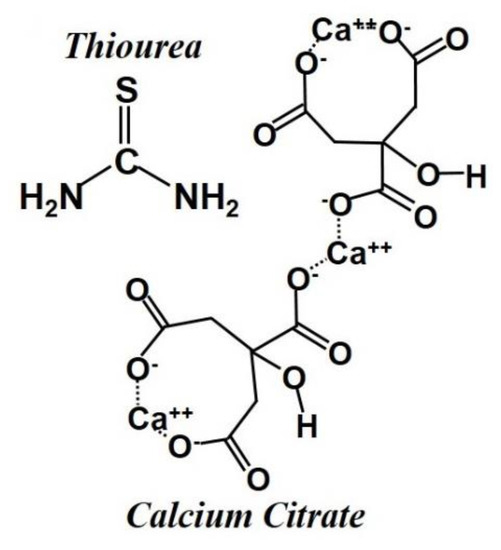
Figure 1.
Chemical structures of calcium citrate and thiourea.
Sulfur-loaded samples were prepared via wet impregnation of a sulfur solution and melt-diffusion methods on the CaC and CaC/TU carbon samples. Sulfur powder (S, ACS reagent, Aldrich) was dissolved in carbon disulfide (CS2, ACS reagent, Aldrich). The obtained yellowish solution was impregnated into the CaC and CaC/TU samples, and dried at 60 °C for 6 h in a fume hood and transferred to a Teflon-lined autoclave vessel for melt-diffusion. The sample vessels were heated at 150 °C for 12 h in atmosphere. These porous carbon–sulfur composites are denoted as CaC-S and CaC/TU-S, respectively.
2.2. Characterization
The pore size and surface area distribution of the mesoporous carbon were measured using the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods. The surface morphologies and element dispersive mapping of the samples were determined using scanning electron micrographs (SEM; Su 70, Hitachi, Japan) and field-emission transmission electron micrographs (FE-TEM; JEM 2100F, JEOL, Japan). X-ray diffraction (XRD) patterns were measured using an Empyrean (Panalytical) instrument. X-ray diffraction (XRD, Panalytical Malvern, 40 kV, 15 mA) patterns were recorded using Cu Kα radiation at λ = 1.5406 Å. Thermogravimetric analysis (TGA) data was used to collect the sulfur content of the samples using a Perkin-Elmer TGA 7 run to 600 °C at 10 °C min−1 under a N2 atmosphere. The valence states of the elements and surface compositions for the samples were evaluated by X-ray photoelectron spectrometry (XPS, Thermo Electron Corporation, K-Alpha+) using an Al Kα X-ray source (hν = 1486.6 eV) at a spot diameter of 400 mm with charge compensation. The emitted photoelectrons were detected using a multichannel detector at a take-off angle of 90° with respect to the sample surface. During the measurement, the base pressure in the turbo-pumped analysis chamber was maintained at 1.2 × 10-9 mbar. The survey spectra were acquired at a pass energy of 200 eV and resolution of 1 eV, while the high-resolution spectra were acquired at a pass energy of 50 eV and resolution of 0.1 eV. All the binding energies were referenced to the adventitious carbon (C 1s) core-level peak at 284.6 eV.
2.3. Fabrication of Electrode and Electrochemical Measurements
For the working electrodes, a mixture of 80 wt % active materials (CaC-S and CaC/TU-S) and 10 wt % carbon black (Denka black 50% compressed; Li-250, Singapore), and 10 wt % polyvinylidene fluoride binder was coated on the aluminum-current collector using a doctor’s blade. The coated electrodes were dried in a vacuum drying oven at 80 °C for 2 h. The loading density of sulfur and thickness of the electrodes were 0.7 mg cm−2 and 20 μm, respectively.
To assemble 2032 coin-type half cells, the CaC-S and CaC/TU-S electrodes served as the working electrode and Li metal served as the counter electrode. The commercial Celgard 2400 polypropylene (PP)-membrane separator with 25 μm thickness was sandwiched between the working and counter electrodes as a separator. For an electrolyte, 1.0 M lithium bis-(trifluoromethyl-sulfonyl) imide (LiTFSI) and 0.4 M LiNO3 in a solvent mixture of 1:1 (volume) dioxolane (DOL):dimethoxyethane (DME) was used. Electrochemical impedance spectroscopy (EIS) measurements were conducted on the WBCS 3000 and ZIVE sp1 devices (WonAtech Co., Ltd., ROK). The EIS was tested between 10 kHz and 0.1 Hz with amplitude of 10 mV.
3. Results and Discussion
As shown in Figure 2a, the nitrogen adsorption–desorption isotherm of the CaC and CaC/TU is typical type IV behavior with hysteresis loops according to the IUPAC classification [21], with a sharp capillary condensation step at relative pressures (P/P0) in the range of 0.45~0.99 evincing a mesoporous structure as well as a narrow mesopores size distribution. In addition, a steep increase of nitrogen uptake above 0.99 and a slow increase of nitrogen uptake below 0.45 can also be observed, indicating that both samples also have macropores and micropores in their structure The pore size distribution (Figure 2b) implies that the CaC/TU has smaller pores than CaC, but it is difficult to find any significant difference in their average pore sizes, as shown in Table 1. The pore size is estimated to be about 7.20 nm and 7.30 nm for the CaC and CaC/TU, and BET-specific surface area of the CaC and CaC/TU were 816 m2 g−1 and 929 m2 g−1. The TEM image was measured to support BET data in Figure 2c. The images also show the formation of the pores, with both the CaC and CaC/TU samples showing porosity.
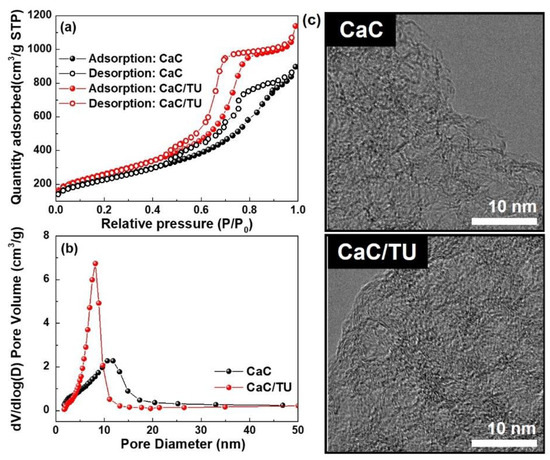
Figure 2.
(a) Nitrogen adsorption-desorption isotherms and (b) BJH pore size distributions of the mesoporous carbon CaC (carbonized calcium citrate) and CaC/TU (with thiourea). (c) Transmission electron micrographs of the CaC and CaC/TU powders at high magnification.

Table 1.
Textural properties of the mesoporous carbon.
In order to confirm the existence of the sulfur and nitrogen elements in the CaC and CaC/TU samples, scanning transmission electron microscopy was carried out as shown in Figure 3. The dispersion of carbon, sulfur, and nitrogen in the carbon matrix was confirmed by the corresponding elemental mapping images. While the sulfur, nitrogen, and carbon elements were found in the CaC/TU sample, only carbon elements could be found in the CaC sample; there were no nitrogen or sulfur elements. In addition, the distribution of carbon elements looks like porous carbon, even though the dark-field image is not clear. This is good evidence to support that thiourea could make N/S-doping states in a porous carbon matrix.
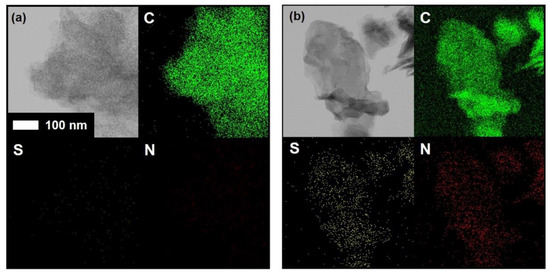
Figure 3.
Scanning transmission electron micrographs and elements mapping images of the (a) CaC and (b) CaC/TU powders.
XPS was used to analyze the chemical constituents and functional groups of CaC/TU as shown in Figure 4a. As calculated from the XPS survey result (data not shown in this paper), the atomic percentages of C, O, N, and S in CaC/TU were 85.9, 5.12, 4.91, and 4.07 wt %, respectively. However, the XPS spectrum of CaC reveals the existence of C and O elements, and the absence of N and S elements. The high-resolution N 1s spectrum of the CaC/TU depicts three individual configurations attributed to pyridinic-N (398.1 eV), pyrrolic-N (400.1 eV), and graphitic-N (401.3 eV), which is very common in N-doped carbon materials [22,23]. The pyridinic-N atom is located at the edges of the graphitic carbon layer by substituting a carbon atom on the C6 ring (Figure 4b) [24], which contributes one pair of lone electrons and imparts basic properties to the carbon surface. Thus, the adsorption ability (with lithium polysulfides, Li2Sx-N bonding) of the carbons should be improved due to the strong interatomic attractions between the electrophilic Li+ ion and the above-mentioned electronegative N atoms [25], thus enhancing the cycling performance of Li-S batteries. As shown in Figure 4b, the pyrrolic-N is a nitrogen atom on a five-membered ring and contributes two p-electrons to the π system, which may also favor surface adsorption, but its interaction is not as strong as that of pyridinic-N. The S 2p spectrum of CaC/TU can be deconvoluted into three peaks, corresponding to the S 2p3/2 (at 163.9 eV), S 2p1/2 (at 165.1 eV), and C-SOx (oxidized S) bonds (at 168.8 eV). G. Zhou et al. reported that the hetero-doped nitrogen/sulfur sites were demonstrated to show strong binding energy (Eb,N/S = 1.82 eV; this value is much higher than that of single doping of nitrogen or sulfur—Eb,N = 1.43 eV and Eb,S = 1.02 eV) and be capable of anchoring polysulfides based on first-principles calculations and experimental evidence through electrochemical performance [17]. Therefore, the N and S co-doping guarantee the strong affinity of polysulfides to the carbon surface.
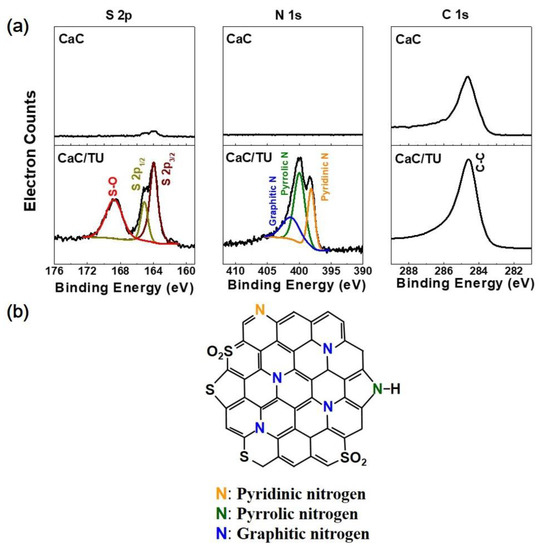
Figure 4.
(a) XPS spectra of the CaC and CaC/TU powders. (b) Schematic for the formation of N- and S-doped mesoporous carbon via reaction between calcium citrate and thiourea.
Figure 5a shows the XRD patterns of samples like elemental sulfur, CaC and CaC/TU mesoporous carbon powders, and the CaC-S and CaC/TU-S composites. The XRD patterns of both CaC and CaC/TU powders show two broad peaks at ~23 and 44°, attributed to the (002) and (100) reflections, respectively. The broad low-intensity diffraction peaks reflect the disordered carbon structures. After sulfur loading in the mesoporous carbon templates, both the CaC-S and CaC/TU-S composites show overlapped sharp sulfur peaks on mesoporous carbon diffraction, indicating the presence of well-crystallized sulfur (orthorhombic sulfur phase, JCPDS card no. 08-0247).
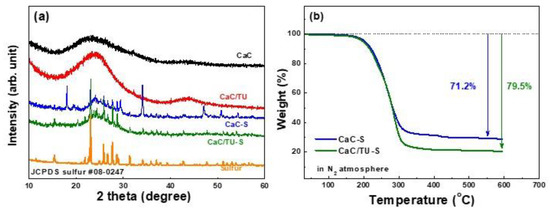
Figure 5.
(a) XRD patterns of elemental sulfur, the CaC, CaC/TU, CaC-S, and CaC/TU-S powders. (b) TGA profiles of the CaC-S and CaC/TU-S powders.
The thermal decomposition characteristics of the CaC-S and CaC/TU-S composites in a nitrogen atmosphere were investigated using TGA. The TGA curves (Figure 5b) show that the weight losses of the CaC-S and CaC/TU-S composites ranged up to 71.2 wt % and 79.5 wt %, respectively, consistent with the proportions of the added amounts. Consequently, the sulfur content of the CaC/TU-S composite was higher than that of CaC-S, owing to the increased BET surface area and pore volume.
Figure 6a shows the first charge–discharge profiles of the CaC-S and CaC/TU-S cells at 0.1 C (1 C = 1672 mA g−1). The specific capacities were measured and calculated by compensating loaded sulfur weight ratio. In the first cycles, both CaC-S and CaC/TU-S cells showed three plateaus in the discharge curve. The first plateau occurred nearly at 2.4 V, and then sloped down to a second plateau at 2.1 V and a third plateau was seen in a rapid drop to 1.8 V. These phenomena are represented by the formation of long-chain soluble polysulfides (Li2Sx: 6 < x < 8) in the first region and short-chain solid sulfides (Li2S2 and Li2S) in the second, and loss of recursive ability in the third region [26,27,28]. This shows the greater coulombic efficiency in the CaC/TU-S cell. The coulombic efficiencies of CaC-S and CaC/TU-S cells were 92% and 99% for the first cycle, respectively. While the initial charge capacity was exactly the same in both cells, the initial discharge capacity of the CaC-S cell (1120 mAh g−1) was slightly higher than that of the CaC/TU-S cell (1052 mAh g−1). It is difficult to find significant differences between two first charge-discharge profiles. The CaC-S cell not only had a lower discharge capacity value than the CaC/TU-S cell, but a lower retention as well. When increased from the 10th to the 100th cycle, the ratios of reduction in discharge capacity of CaC-S and CaC/TU-S cells were 71% and 86%, respectively.
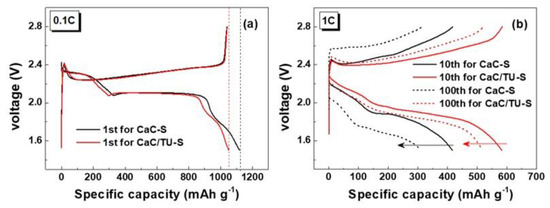
Figure 6.
Charge/discharge profiles of the CaC-S and CaC/TU-S cells: (a) 1st cycle at 0.1 C rate; (b) 10th and 100th cycles at 1 C rate.
However, the CaC/TU-S cell exhibited higher discharge capacities than the CaC-S cell, as compared in the cycling response in Figure 7a. The cells were cycled at 0.1 C for the first five cycles, followed by an additional 100 cycles at 1 C. Both samples showed fast capacity fading during the initial five cycles at 0.1 C, possibly due to the dissolution of lithium polysulfides mainly present on the surface of mesoporous carbon [4]. During the 1 C cycling, the CaC/TU-S cell delivered an initial capacity of 667 mAh g−1 and a capacity of 520 mAh g−1 after 100 cycles, giving a capacity retention of 78.0%. In contrast, the CaC-S cell showed an initial capacity of 513 mAh g−1 and a capacity of 310 mAh g−1 after 100 cycles, giving a capacity retention of only 60.5%. The CaC/TU-S also showed a stable coulombic efficiency as high as 98.7% after 100 cycles at 1 C, while the coulombic efficiency of the CaC-S cell fluctuated at lower levels than that of the CaC/TU-S cell, indicating electrochemical inefficiencies in the CaC-S cell over repeated cycles.
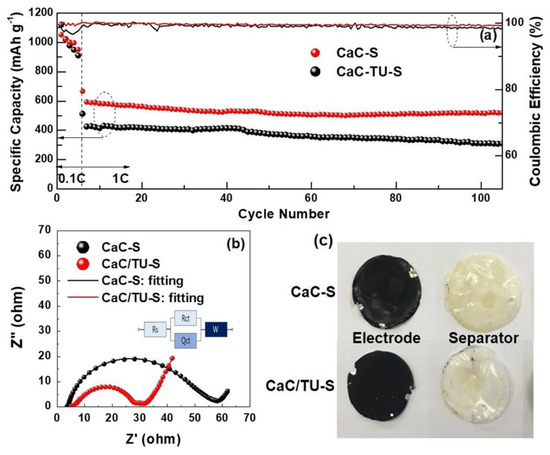
Figure 7.
(a) Cycling performance of the CaC-S and CaC/TU-S cells; (b) Nyquist plots of cycled cells; and (c) Photographs of electrode, polypropylene (PP) separator, and Li metal of cycled cells disassembled in fully charged state after the initial 100 cycles.
To provide further insights into the Li-S storage properties, electrochemical impedance spectroscopy was conducted to compare the electrical conductivities of the CaC-S and CaC/TU-S cells after 105 cycles. Impedance is a powerful diagnostic tool to investigate physical and chemical processes that occur on electrode/electrolyte interfaces during cycling and to identify the causes of degradation. The equivalent circuit is shown in the inset of Figure 7b, where Rs and Rct represent the electrolyte resistance and charge transfer resistance between the solid electrolyte interphase layer and electrode interface, respectively, and W is the Warburg impedance for Li+ diffusion in the electrode. The Rct of the CaC/TU-S cell was much smaller than that of the CaC-S cell, supporting the hypothesis that nitrogen and sulfur co-doped mesoporous carbon leads to a stronger bonding strength and better encapsulation of insulating sulfur in the mesoporous carbon pores than that of the sample without any doping state.
As can be clearly seen in Figure 7c, the black surface of the CaC-S electrode faded partially to grey, and the original white color of the PP separator changed to yellow after 105 cycles. It is evident that the amount of sulfur residues dissolved out of the CaC-S electrode was much larger than that out of the CaC/TU-S, which showed much smaller capacity drop as compared in Figure 7a.
We compared the data reported in several preceding papers (Table 2) and found that the data for CaC/TU was either slightly better than or similar to those for reported materials. Sulfur remains on the surface of porous carbon, leading to a reduced reactivity of the sulfur as well as facile dissolution of polysulfides into the electrolyte. Calcium citrate is prepared by adding only the thiourea without activation—a simpler process than for other materials, which is a great advantage. Performance was also observed to remain stable. Later, we will use the advantages of the CaC/TU-S composite to increase the cycle life and conduct experiments, and investigate what will happen with high loading mass.

Table 2.
Cathode materials and their properties for Li-S battery.
4. Conclusions
We synthesized porous carbon by carbonizing calcium citrate and thiourea. This is an effective and simple technique to produce a material that can accommodate charge/discharge reactions and preserve the structure during long cycles. CaC/TU-S composites had an acceptable initial discharge capacity of 1051.6 mAh g−1 over 100 cycles at 1 C. The results indicate that CaC-S and CaC/TU-S composites have a good, stable specific capacity.
Author Contributions
Experiments and paper writing, Y.C.; review and editing, S.-Y.L.; methodology, J.-S.B. and S.-J.L.; data curation, H.K.K.; conceptualization and editing, E.D.J.; editing, H.-C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by Busan center at Korea Basic Science Institute (KBSI-C030321).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ji, X.; Nazar, L.F. Advances in Li–S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.-M.; Gentle, I.R.; Lu, G.Q.M. Carbon–sulfur composites for Li–S batteries: Status and prospects. J. Mater. Chem. A 2013, 1, 9382–9394. [Google Scholar] [CrossRef]
- Oh, C.; Yoon, N.; Choi, J.; Choi, Y.; Ahn, S.; Lee, J.K. Enhanced Li–S battery performance based on solution-impregnation-assisted sulfur/mesoporous carbon cathodes and a carbon-coated separator. J. Mater. Chem. A 2017, 5, 5750–5760. [Google Scholar] [CrossRef]
- Ferrero, G.; Sevilla, M.; Fuertes, A. Mesoporous carbons synthesized by direct carbonization of citrate salts for use as high-performance capacitors. Carbon 2015, 88, 239–251. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, K.; Dokko, K.; Watanabe, M. Recent advances in electrolytes for lithium–sulfur batteries. Adv. Energy Mater. 2015, 5, 1500117. [Google Scholar] [CrossRef]
- Apul, O.G.; Karanfil, T. Adsorption of synthetic organic contaminants by carbon nanotubes: A critical review. Water Res. 2015, 68, 34–55. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Ionothermal synthesis of sulfur-doped porous carbons hybridized with graphene as superior anode materials for lithium-ion batteries. Chem. Commun. 2012, 48, 10663–10665. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef]
- Gao, Y.-P.; Zhai, Z.-B.; Huang, K.-J.; Zhang, Y.-Y. Energy storage applications of biomass-derived carbon materials: Batteries and supercapacitors. New J. Chem. 2017, 41, 11456–11470. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Chen, X.Y.; Wang, B. An activation-free protocol for preparing porous carbon from calcium citrate and the capacitive performance. Microporous Mesoporous Mater. 2012, 158, 155–161. [Google Scholar] [CrossRef]
- Fuertes, A.; Ferrero, G.; Sevilla, M. One-pot synthesis of microporous carbons highly enriched in nitrogen and their electrochemical performance. J. Mater. Chem. A 2014, 2, 14439–14448. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- See, K.A.; Jun, Y.-S.; Gerbec, J.A.; Sprafke, J.K.; Wudl, F.; Stucky, G.D.; Seshadri, R. Sulfur-functionalized mesoporous carbons as sulfur hosts in Li–S batteries: Increasing the affinity of polysulfide intermediates to enhance performance. ACS Appl. Mater. Interfaces 2014, 6, 10908–10916. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 2015, 6, 7760. [Google Scholar] [CrossRef]
- Niu, S.; Lv, W.; Zhou, G.; He, Y.; Li, B.; Yang, Q.-H.; Kang, F. N and S co-doped porous carbon spheres prepared using l-cysteine as a dual functional agent for high-performance lithium–sulfur batteries. Chem. Commun. 2015, 51, 17720–17723. [Google Scholar] [CrossRef]
- Morishita, T.; Ishihara, K.; Kato, M.; Inagaki, M. Preparation of a carbon with a 2 nm pore size and of a carbon with a bi-modal pore size distribution. Carbon 2007, 45, 209–211. [Google Scholar] [CrossRef]
- Morishita, T.; Ishihara, K.; Kato, M.; Tsumura, T.; Inagaki, M. Mesoporous carbons prepared from mixtures of magnesium citrate with poly (vinyl alcohol). Tanso 2007, 2007, 19–24. [Google Scholar] [CrossRef]
- Branton, P.J.; Hall, P.G.; Sing, K.S.; Reichert, H.; Schüth, F.; Unger, K.K. Physisorption of argon, nitrogen and oxygen by MCM-41, a model mesoporous adsorbent. J. Chem. Soc. Faraday Trans. 1994, 90, 2965–2967. [Google Scholar] [CrossRef]
- Yuan, S.; Bao, J.L.; Wang, L.; Xia, Y.; Truhlar, D.G.; Wang, Y. Graphene-Supported Nitrogen and Boron Rich Carbon Layer for Improved Performance of Lithium–Sulfur Batteries Due to Enhanced Chemisorption of Lithium Polysulfides. Adv. Energy Mater. 2016, 6, 1501733. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Tian, Y.; Su, Y.; Wang, J.; Yang, W.; Li, N.; Chen, S.; Bao, L. 3D coral-like nitrogen-sulfur co-doped carbon-sulfur composite for high performance lithium-sulfur batteries. Sci. Rep. 2015, 5, 13340. [Google Scholar] [CrossRef] [PubMed]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined effect of nitrogen-and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Hou, T.Z.; Chen, X.; Peng, H.J.; Huang, J.Q.; Li, B.Q.; Zhang, Q.; Li, B. Design principles for heteroatom-doped nanocarbon to achieve strong anchoring of polysulfides for lithium–sulfur batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Tian, T.; Cao, S.; Coxon, P.R.; Xi, K.; Fairen-Jimenez, D.; Vasant Kumar, R.; Cheetham, A.K. Graphene-wrapped sulfur/metal organic framework-derived microporous carbon composite for lithium sulfur batteries. APL Mater. 2014, 2, 124109. [Google Scholar] [CrossRef]
- Xi, K.; Kidambi, P.R.; Chen, R.; Gao, C.; Peng, X.; Ducati, C.; Hofmann, S.; Kumar, R.V. Binder free three-dimensional sulphur/few-layer graphene foam cathode with enhanced high-rate capability for rechargeable lithium sulphur batteries. Nanoscale 2014, 6, 5746–5753. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, J.; Dong, Z.; Liu, Y.; Wu, Y.; Xu, C.; Du, G. Biomass derived activated carbon with 3D connected architecture for rechargeable lithium—Sulfur batteries. Electrochim. Acta 2014, 116, 146–151. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Q.; Tan, Y.; Tian, W.; Zhu, L.; Yang, C. Microporous carbon derived from Apricot shell as cathode material for lithium–sulfur battery. Microporous Mesoporous Mater. 2015, 204, 235–241. [Google Scholar] [CrossRef]
- Xue, M.; Chen, C.; Tan, Y.; Ren, Z.; Li, B.; Zhang, C. Mangosteen peel-derived porous carbon: Synthesis and its application in the sulfur cathode for lithium sulfur battery. J. Mater. Sci. 2018, 53, 11062–11077. [Google Scholar] [CrossRef]
- Vu, D.-L.; Seo, J.-S.; Lee, H.-Y.; Lee, J.-W. Activated carbon with hierarchical micro–mesoporous structure obtained from rice husk and its application for lithium–sulfur batteries. RSC Adv. 2017, 7, 4144–4151. [Google Scholar] [CrossRef]
- Wang, D.; Fu, A.; Li, H.; Wang, Y.; Guo, P.; Liu, J.; Zhao, X.S. Mesoporous carbon spheres with controlled porosity for high-performance lithium-sulfur batteries. J. Power Sources 2015, 285, 469–477. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).