Low Level of Allergens in the Argentinean Plant Zuccagnia punctata Cav.: Screening and Quality Control of North-Western Propolis Using an LC-DAD-QTOF System
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Extract Preparation
2.2. Instrumental Analysis
3. Results
3.1. Caffeic Acid Derivatives with Known Sensitizing Properties
3.1.1. First Group: Strong Sensitizing Agents
3.1.2. Second Group: Moderate Sensitizing Agents
3.1.3. Third Group: Weak to Negative Sensitizing Agents
3.2. Potential Allergenic/Antiallergenic Compounds in NAPs
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.G. Chemical composition of European propolis: Expected and unexpected results. Zeitschrift fur Naturforschung C J. Biosci. 2002, 57, 530–533. [Google Scholar] [CrossRef]
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology, Ethnobotany; Timber Press: Cambridge, UK, 2003; ISBN 0881925748. [Google Scholar]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Walgrave, S.E.; Warshaw, E.M.; Glesne, L.A. Allergic contact dermatitis from propolis. Dermatitis 2005, 16, 209–215. [Google Scholar] [PubMed]
- Murtaza, G.; Karim, S.; Akram, M. Caffeic Acid Phenethyl Ester and Therapeutic Potentials. Biomed Res. Int. 2014, 2014, 145342. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Longo, R.; Vanella, A. Antioxidant activity of propolis: Role of caffeic acid phenethyl ester and galangin. Fitoterapia 2002, 73, S21–S29. [Google Scholar] [CrossRef]
- BasistaSołtys, K. Allergy to Propolis in Beekeepers-A Literature Review. Occup. Med. Health Aff. 2013. [Google Scholar] [CrossRef]
- Hausen, B.M.; Evers, P.; Stüwe, H.-T.; König, W.A.; Wollenweber, E. Propolis allergy (IV) Studies with further sensitizers from propolis and constituents common to propolis, poplar buds and balsam of Peru. Contact Dermat. 1992, 26, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Hausen, B.M. Evaluation of the main contact allergens in propolis (1995 to 2005). Dermatitis 2005, 16, 127–129. [Google Scholar] [PubMed]
- Hausen, B.M.; Wollenweber, E. Propolis allergy: (III). Sensitization studies with minor constituents. Contact Dermat. 1988, 19, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Oliwiecki, S.; Beck, M.H.; Hausen, B.M. Occupational allergic contact dermatitis from caffeates in poplar bud resin in a tree surgeon. Contact Dermat. 1992, 27, 127–128. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Gambichler, T.; Boms, S.; Freitag, M. Contact dermatitis and other skin conditions in instrumental musicians. BMC Dermatol. 2004, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Solórzano, E.; Vera, N.; Cuello, S.; Ordoñez, R.; Zampini, C.; Maldonado, L.; Bedascarrasbure, E.; Isla, M.I. Chalcones in bioactive Argentine propolis collected in arid environments. Nat. Prod. Commun. 2012, 7, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Villagra, P.E.; Giordano, C.; Alvarez, J.A.; Cavagnaro, J.B.; Guevara, A.; Carmen, S.; Passera, C.B.; Greco, S.E. Ser planta en el desierto: Estrategias de uso de agua y resistencia al estrés hídrico en el Monte Central de Argentina. Ecol. Austral 2011, 21, 29–42. [Google Scholar]
- Vera, N.; Solorzano, E.; Ordoñez, R.; Maldonado, L.; Bedascarrasbure, E.; Isla, M.I. Chemical composition of Argentinean propolis collected in extreme regions and its relation with antimicrobial and antioxidant activities. Nat. Prod. Commun. 2011, 6, 823–827. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Agüero, M.B.; Gonzalez, M.; Lima, B.; Svetaz, L.; Sánchez, M.; Zacchino, S.; Feresin, G.E.; Schmeda-Hirschmann, G.; Palermo, J.; Daniel Wunderlin, D.; et al. Argentinean propolis from Zuccagnia punctata cav. (Caesalpinieae) exudates: Phytochemical characterization and antifungal activity. J. Agric. Food Chem. 2010, 58, 194–201. [Google Scholar]
- Solorzano, E.R.; Bortolini, C.; Bogialli, S.; Di Gangi, I.M.; Favaro, G.; Maldonado, L.; Pastore, P. Use of a LC-DAD-QTOF system for the characterization of the phenolic profile of the argentinean plant Zuccagnia punctata and of the related propolis: New biomarkers. J. Funct. Foods 2017, 33, 425–435. [Google Scholar] [CrossRef]
- De Rijke, E.; Out, P.; Niessen, W.M.; Ariese, F.; Gooijer, C.; Brinkman, U.A. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, J.; Chen, S.; Liu, X.; Zhou, Y.; Zhang, X.; Xu, X. Recent developments in qualitative and quantitative analysis of phytochemical constituents and their metabolites using liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2013, 72, 267–291. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, D.; Ganzera, M. Recent advances on HPLC/MS in medicinal plant analysis. J. Pharm. Biomed. Anal. 2011, 55, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Saftić, L.; Peršurić, Ž.; Kraljević Pavelić, S. LC–QQQ and LC–QTOF MS methods for comprehensive detection of potential allergens in various propolis extracts. Eur. Food Res. Technol. 2019, 1–15. [Google Scholar] [CrossRef]
- Medana, C.; Carbone, F.; Aigotti, R.; Appendino, G.; Baiocchi, C. Selective analysis of phenolic compounds in propolis by HPLC-MS/MS. Phytochem. Anal. 2008, 19, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Matei, M.F.; Ullrich, F.; Kuhnert, N. How to distinguish between cinnamoylshikimate esters and chlorogenic acid lactones by liquid chromatography-tandem mass spectrometry. J. Mass Spectrom. 2011, 46, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Simonetti, P. Evaluation of allergens in propolis by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 1675–1682. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharm. Sci. 2013, 308249. [Google Scholar] [CrossRef]
- Agüero, M.B.; Svetaz, L.; Sánchez, M.; Luna, L.; Lima, B.; López, M.L.; Zacchino, S.; Palermo, J.; Wunderlin, D.; Feresin, G.E.; et al. Argentinean Andean propolis associated with the medicinal plant Larrea nitida Cav. (Zygophyllaceae). HPLC-MS and GC-MS characterization and antifungal activity. Food Chem. Toxicol. 2011, 49, 1970–1978. [Google Scholar]
- Chae, H.; Kang, O.; Oh, Y.; Choi, J.; Keum, J.; Kim, S.; Kim, Y.-S.; Mun, S.-H.; Shin, D.-W.; Han, S.-H.; et al. Gomisin N has anti-allergic effect and inhibits inflammatory cytokine expression in mouse bone marrow-derived mast cells. Immunopharmacol. Immunotoxicol. 2011, 33, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Chung, H.; Kim, D.; Hwang, J.; Lee, S. Macelignan attenuated allergic lung in fl ammation and airway hyper-responsiveness in murine experimental asthma. Life Sci. 2013, 92, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Dorr, R.T.; Timmermann, B.N. Nordihydroguaiaretic acid: A review of its numerous and varied biological activities. Pharm. Biol. 2004, 42, 149–158. [Google Scholar] [CrossRef]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.E.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. Antifungal chalcones and new caffeic acids esters from Zuccagnia punctata acting against soybean infecting fungi. J. Agric. Food Chem. 2004, 52, 3297–3300. [Google Scholar] [CrossRef]
- Ramachandra, M.S.; Subbaraju, G. V Synthesis and bioactivity of novel caffeic acid esters from Zuccagnia punctata. J. Asian Nat. Prod. Res. 2006, 8, 683–688. [Google Scholar] [CrossRef]
- Tanaka, T. Flavonoids for allergic diseases: Present evidence and future perspective. Curr. Pharm. Des. 2014, 20, 879–885. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshimura, M.; Yamaguchi, F.; Kouchi, T.; Tsuji, R.; Saito, M.; Obata, A.; Kikuchi, M. Anti-allergic activity of naringenin chalcone from a tomato skin extract. Biosci. Biotechnol. Biochem. 2004, 68, 1706–1711. [Google Scholar] [CrossRef]
- Itoh, T.; Ninomiya, M.; Nozawa, Y.; Koketsu, M. Chalcone glycosides isolated from aerial parts of Brassica rapa L. “hidabeni” suppress antigen-stimulated degranulation in rat basophilic leukemia RBL-2H3 cells. Bioorg. Med. Chem. 2010, 18, 7052–7057. [Google Scholar] [CrossRef]
- Udompataikul, M.; Srisatwaja, W. Comparative trial of moisturizer containing licochalcone A vs. hydrocortisone lotion in the treatment of childhood atopic dermatitis: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 660–665. [Google Scholar] [CrossRef]
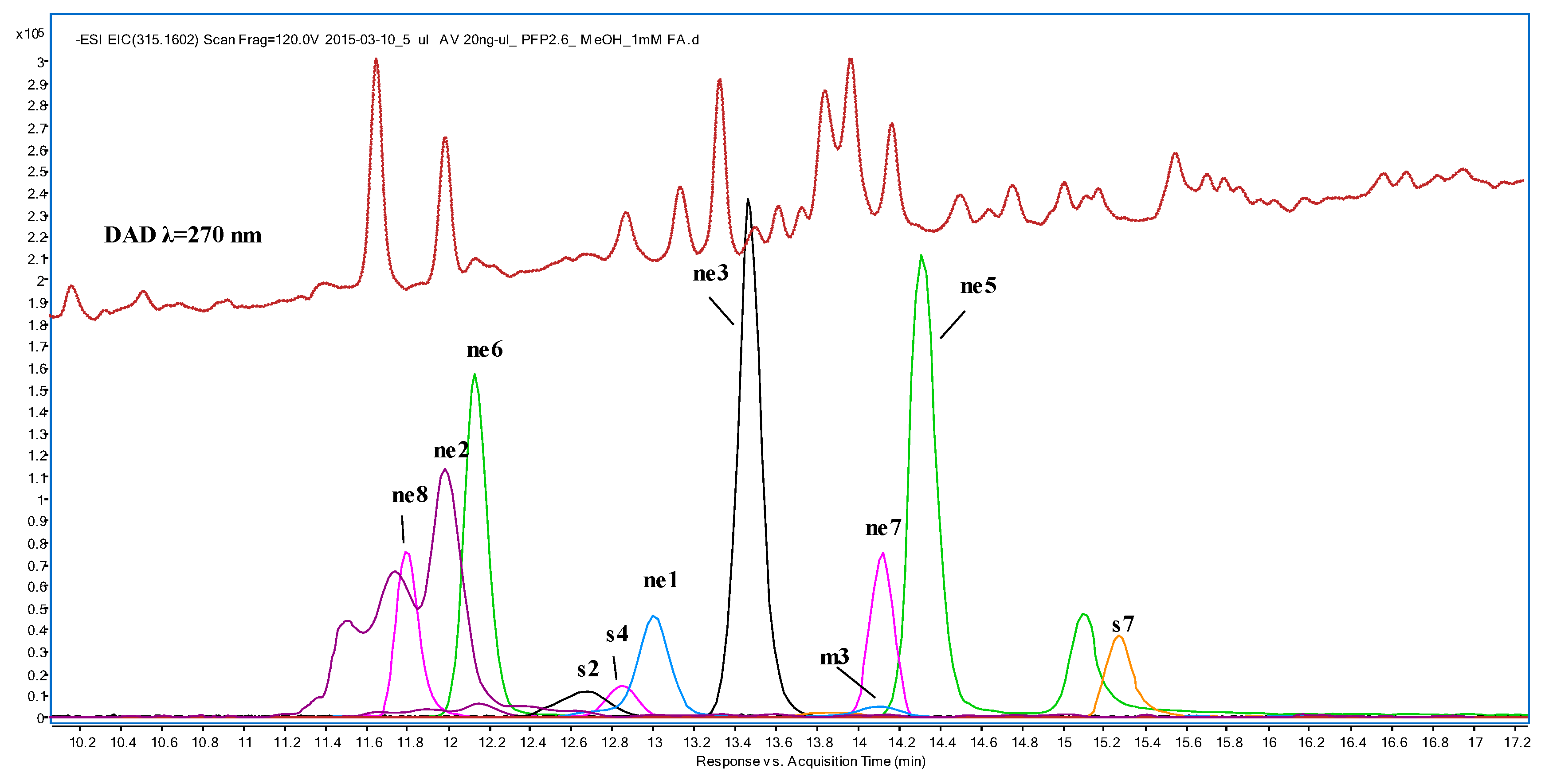
| N | tr | [M-H]− | Compounds | Fragments and Relative Abundances (%) | Samples | ||
|---|---|---|---|---|---|---|---|
| ZP | SM | AV | |||||
| Strong sensitizing agents (s) | |||||||
| s1 | 283.0976 | CAPE | nd | ||||
| s2 | 12.62 | 247.0976 | prenyl caffeate a | 247.0976 (14); 179.0350 (21); 178.0272 (10); 161.0244 (7); 135.0452 (27); 134.0373 (100) | nd | 8.8 a | 8.8 a |
| s3 | 383.2228 | farnesyl caffeate | nd | ||||
| s4 | 12.92 | 269.0819 | benzyl caffeate | 269.0819 (5); 178.0272 (5); 161.0244 (13); 135.0452 (11); 134.0373 (100); 80.9982 (21) | nd | 2.3 | 8.0 |
| s5 | 283.0976 | benzyl isoferulate | nd | ||||
| s6 | 227.0714 | benzyl salicylate | nd | ||||
| s7 | 15.33 | 315.1602 | geranyl caffeate | 315.1602 (8); 179.035 (12); 178.0272 (10); 134.0373 (100); 133.0289 (8); 135.0452 (8) | 9.4 | 17.8 | 18.9 |
| Moderate sensitizing agents(m) | |||||||
| m1 | 283.0976 | coniferyl benzoate | nd | ||||
| m2 | 213.0557 | resorcinol monobenzoate | nd | ||||
| m3 | 14.10 | 295.0976 | cinnamyl caffeate | 295.0976 (77); 178.0272 (42); 135.0452 (13); 134.0373 (100) | nd | 1.4 | 3.6 |
| m4 | 6.78 | 193.0506 | methyl caffeate | 193.0510 (24); 178.0272 (48); 149.0608 (24); 134.0373 (17) | nd | 0.5 | nd |
| m5 | 179.0713 | coniferyl alcohol | nd | ||||
| m6 | 297.1132 | phenylethyl isoferulate | nd | ||||
| m7 | 15.99 | 369.1344 | acetate of phenylethyl caffeate | - | nd | b | b |
| Weak to Negative sensitizing agents(w) | |||||||
| w1 | 283.0976 | benzyl ferulate | nd | ||||
| w2 | 237.0931 | benzyl cinnamate | nd | ||||
| w3 | 263.1077 | cinnamyl cinnamate | nd | ||||
| w4 | 261.1132 | 3-methyl-2-butenyl isoferulate | nd | ||||
| w5 | 161.0608 | methyl cinnamate | nd | ||||
| w6 | 211.0764 | benzyl benzoate | nd | ||||
| w7 | 179.0350 | caffeic acid | nd | ||||
| w8 | 122.1213 | benzoic acid | nd | ||||
| w9 | 181.0506 | dihydrocaffeic acid | nd | ||||
| w10 | 14.24 | 151.0401 | vanillin | - | b | b | b |
| w11 | 221.1911 | farnesol | nd | ||||
| w12 | 309.1132 | cynnamyl isoferulate | nd | ||||
| w13 | 133.0659 | cynnamic alcohol | nd | ||||
| w14 | 107.0502 | benzyl alcohol | nd | ||||
| w15 | 439.1551 | coniferyl dicinnamate | nd | ||||
| w16 | 267.0663 | tectochrysin | - | nd | b | b | |
| w17 | 193.0506 | ferulic acid | - | nd | b | b | |
| Major components, not established allergenic potential (ne) | |||||||
| ne1 | 13.02 | 327.1238 | 1-methyl-3-(4′-hydroxyphenyl)-propyl caffeic acid ester | 327.1238 (100); 179.035 (16); 163.0401 (20); 135.0452 (10); 134.03731 (7); 119.0502 (7) | 21.8 | 22.8 | 25.9 |
| ne2 | 11.91 | 343.1187 | 1-methyl-3-(3′,4′-dihydroxyphenyl)-propyl caffeic acid ester | 343.1187 (100); 179.035 (30); 181.0506 (3); 163.0765 (3); 161.0244 (4); 135.0452 (22) | 29.0 | 34.8 | 54.7 |
| ne3 | 13.53 | 255.1027 | 4′-hydroxy-2′-methoxydihydrochalcone | 255.1027 (28); 239.0713 (2); 149.0244 (9); 136.0165 (100); 121.0290 (2); 108.0216 (31); 80.0267 (5) | 100 | 61.2 | 85.6 |
| ne4 | 13.01 | 241.0875 | 2′,4′-dihydroxydihydrochalcone | 241.0870 (100); 223.0764 (6); 197.0971 (15); 150.0322 (98); 135.0087 (59); 122.0373 (69); 109.0295 (67); 91.0189 (36) | 23.6 | 19.0 | 21.5 |
| ne5 | 14.38 | 239.0714 | 2′,4′-dihydroxychalcone | 239.0714 (100); 197.0608 (20); 135.0088 (37); 91.0189 (15) | 96.3 | 68.0 | 95.0 |
| ne6 | 12.19 | 239.0714 | 7-hydroxyflavanone | 239.0714 (100); 197.0608 (60); 135.0088 (37); 91.0189 (16) | 63.9 | 34.9 | 46.1 |
| ne7 | 14.13 | 269.0819 | 2′,4′-dihydroxy-3′-methoxychalcone | 269.0819 (18); 254.0585 (71); 149.9959 (100); 122.0009 (9); 106.0060 (21); 94.0060 (27) | 34.7 | 22.9 | 31.2 |
| ne8 | 11.80 | 269.0819 | 7-hydroxy-8-methoxyflavanone | 269.0819 (18); 254.0585 (71); 149.9959 (100); 122.0009 (9); 106.0060 (21); 94.0060 (27) | 22.4 | 13.9 | 27.1 |
| ne9 | 13.82 | 301.1445 | NDGA | 301.1445 (100); 273.1132 (4); 122.0373 (78) | nd | 1.5 | nd |
| ne10 | 315.1602 | MNDGA | nd | ||||
| ne11 | 13.41 | 285.1496 | 4-[4-(4-hydroxy-phenyl)-2,3-dimethyl butyl] benzene-1,2-diol | 285.1496 (100); 122.0973 (32) | nd | 0.2 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solorzano, E.R.; Di Gangi, I.M.; Roverso, M.; Favaro, G.; Bogialli, S.; Pastore, P. Low Level of Allergens in the Argentinean Plant Zuccagnia punctata Cav.: Screening and Quality Control of North-Western Propolis Using an LC-DAD-QTOF System. Appl. Sci. 2019, 9, 3546. https://doi.org/10.3390/app9173546
Solorzano ER, Di Gangi IM, Roverso M, Favaro G, Bogialli S, Pastore P. Low Level of Allergens in the Argentinean Plant Zuccagnia punctata Cav.: Screening and Quality Control of North-Western Propolis Using an LC-DAD-QTOF System. Applied Sciences. 2019; 9(17):3546. https://doi.org/10.3390/app9173546
Chicago/Turabian StyleSolorzano, Eliana Rita, Iole Maria Di Gangi, Marco Roverso, Gabriella Favaro, Sara Bogialli, and Paolo Pastore. 2019. "Low Level of Allergens in the Argentinean Plant Zuccagnia punctata Cav.: Screening and Quality Control of North-Western Propolis Using an LC-DAD-QTOF System" Applied Sciences 9, no. 17: 3546. https://doi.org/10.3390/app9173546
APA StyleSolorzano, E. R., Di Gangi, I. M., Roverso, M., Favaro, G., Bogialli, S., & Pastore, P. (2019). Low Level of Allergens in the Argentinean Plant Zuccagnia punctata Cav.: Screening and Quality Control of North-Western Propolis Using an LC-DAD-QTOF System. Applied Sciences, 9(17), 3546. https://doi.org/10.3390/app9173546






