A Novel Way for Whey: Cheese Whey Fermentation Produces an Effective and Environmentally-Safe Alternative to Chlorine
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Whey Fermentation
2.2. Biochemical Analysis
2.3. Determination of Antibacterial Activities
2.3.1. Minimum Inhibitory Concentration (MIC) Throughout the 120 h Fermentation
2.3.2. Well-Diffusion Assay
2.4. Impact of Fermented Whey as a MP Lettuce Sanitizer
2.4.1. pH Measurement
2.4.2. Texture Analysis
2.4.3. Color Measurement
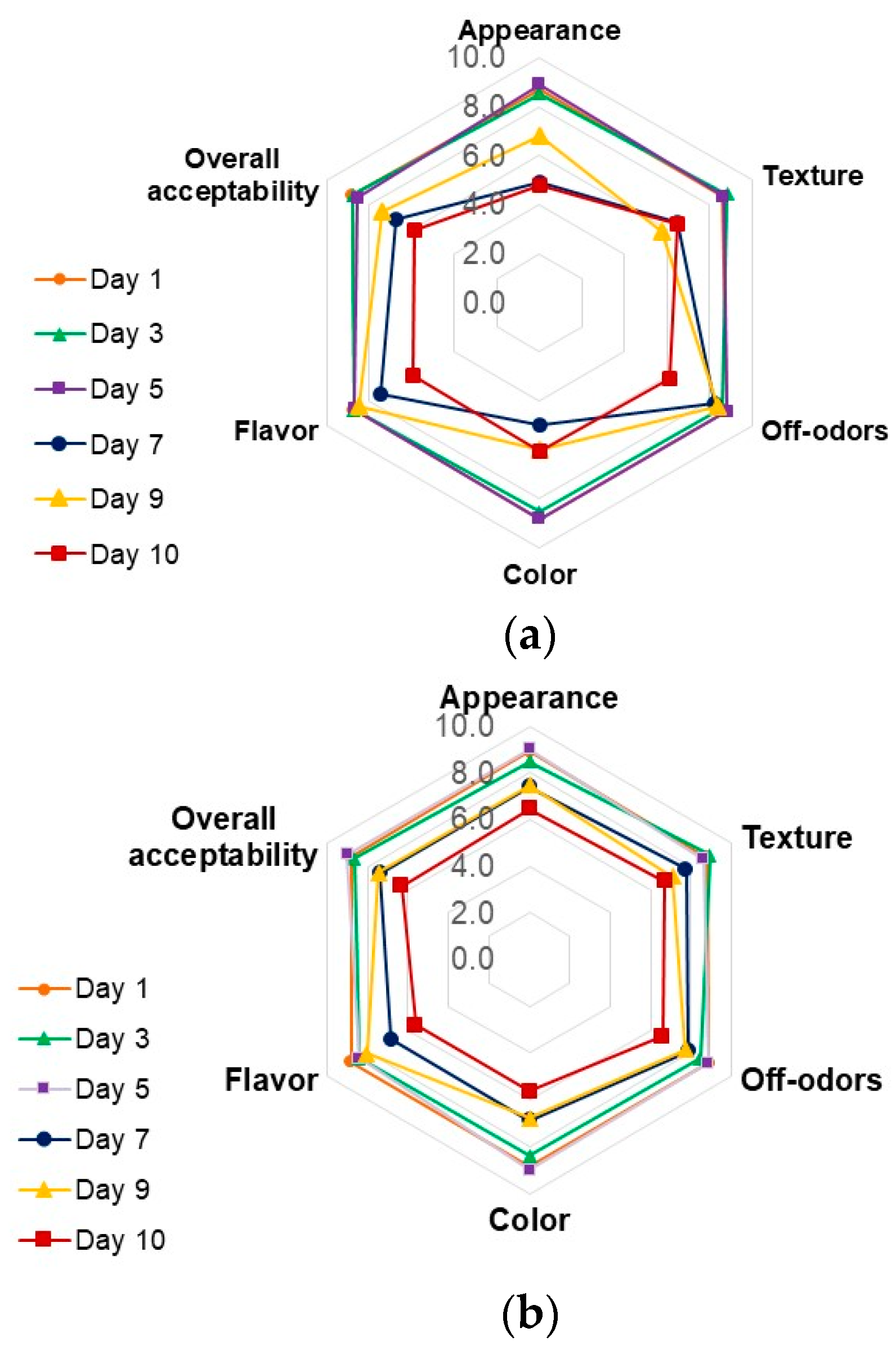
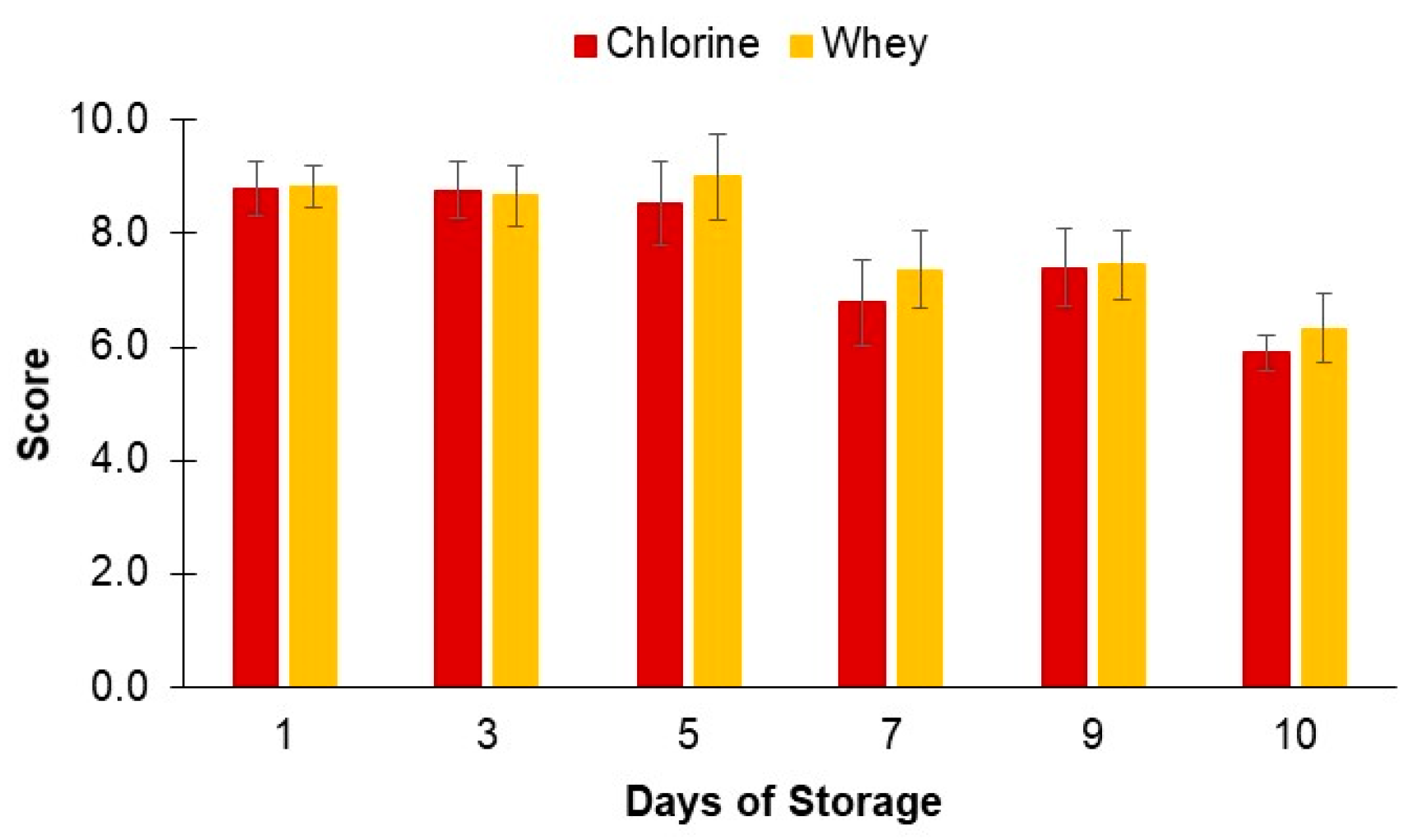
2.4.4. Sensory Evaluation
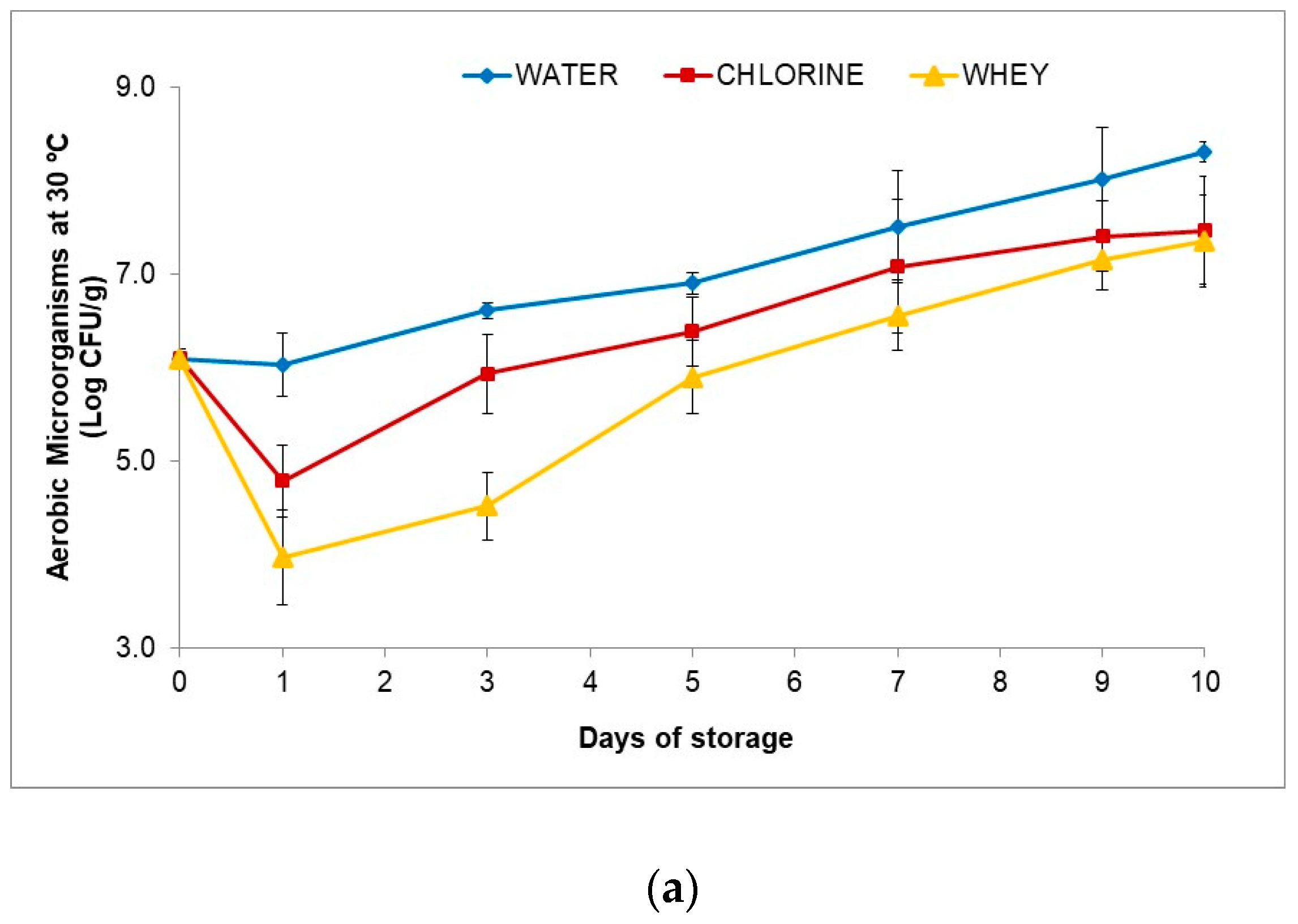
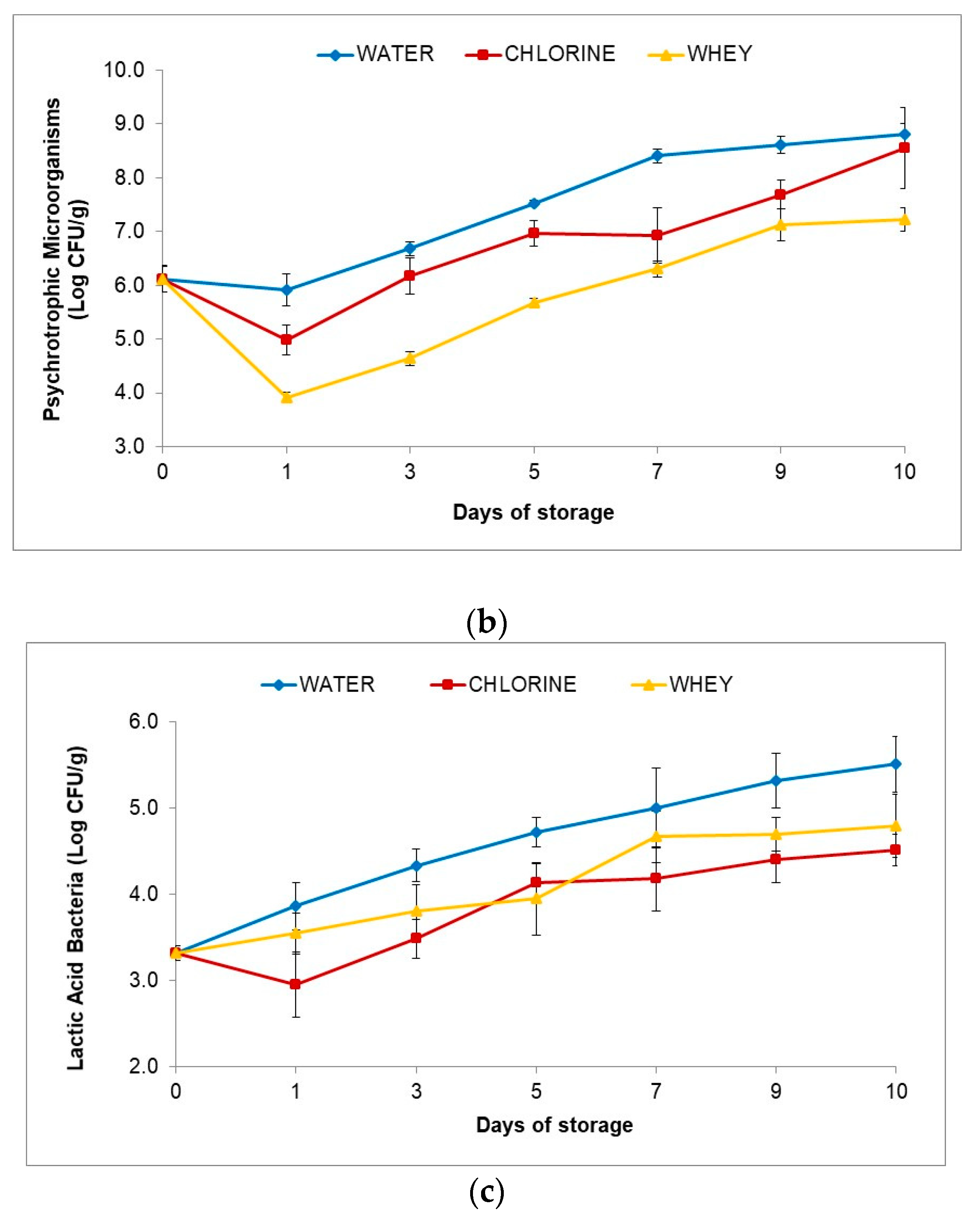
2.4.5. Microbial Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Longer Fermentation Periods Are Important for the Highest Lactic Acid Production and the Lowest Sugar Content
3.2. Antibacterial Activity Is Also Dependent on the Length of Fermentation
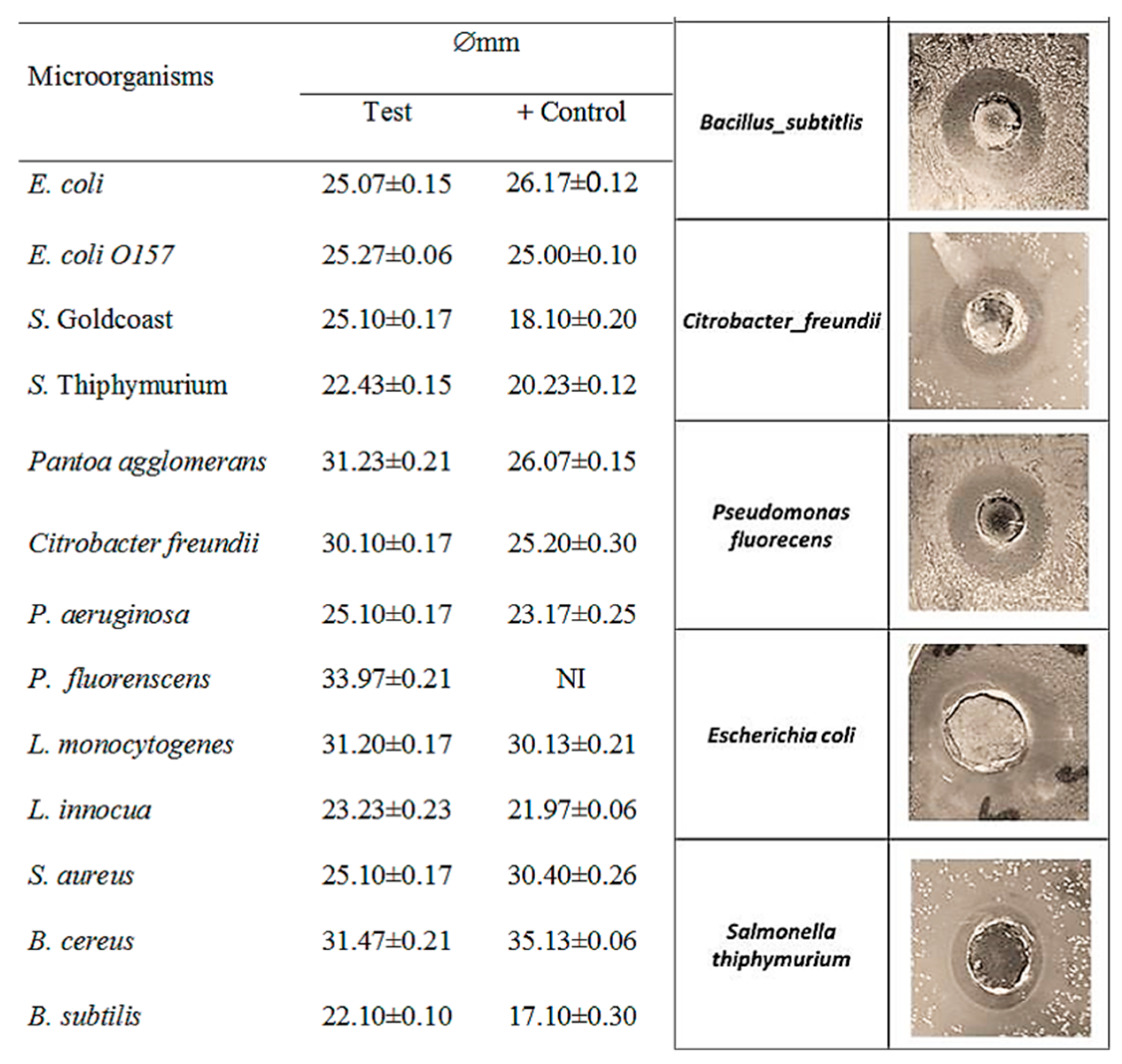
3.3. Five Days-Fermented Whey Presents Dose-Dependent Antibacterial Activities and Is Effective Against Several Bacterial Species
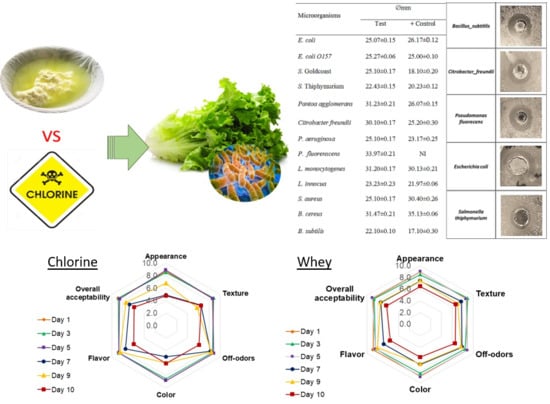
3.4. Industrial Whey Is an Effective Disinfecting Agent When Applied to Lettuce, with Better or Similar Results When Compared Chlorine
3.4.1. O2 and CO2
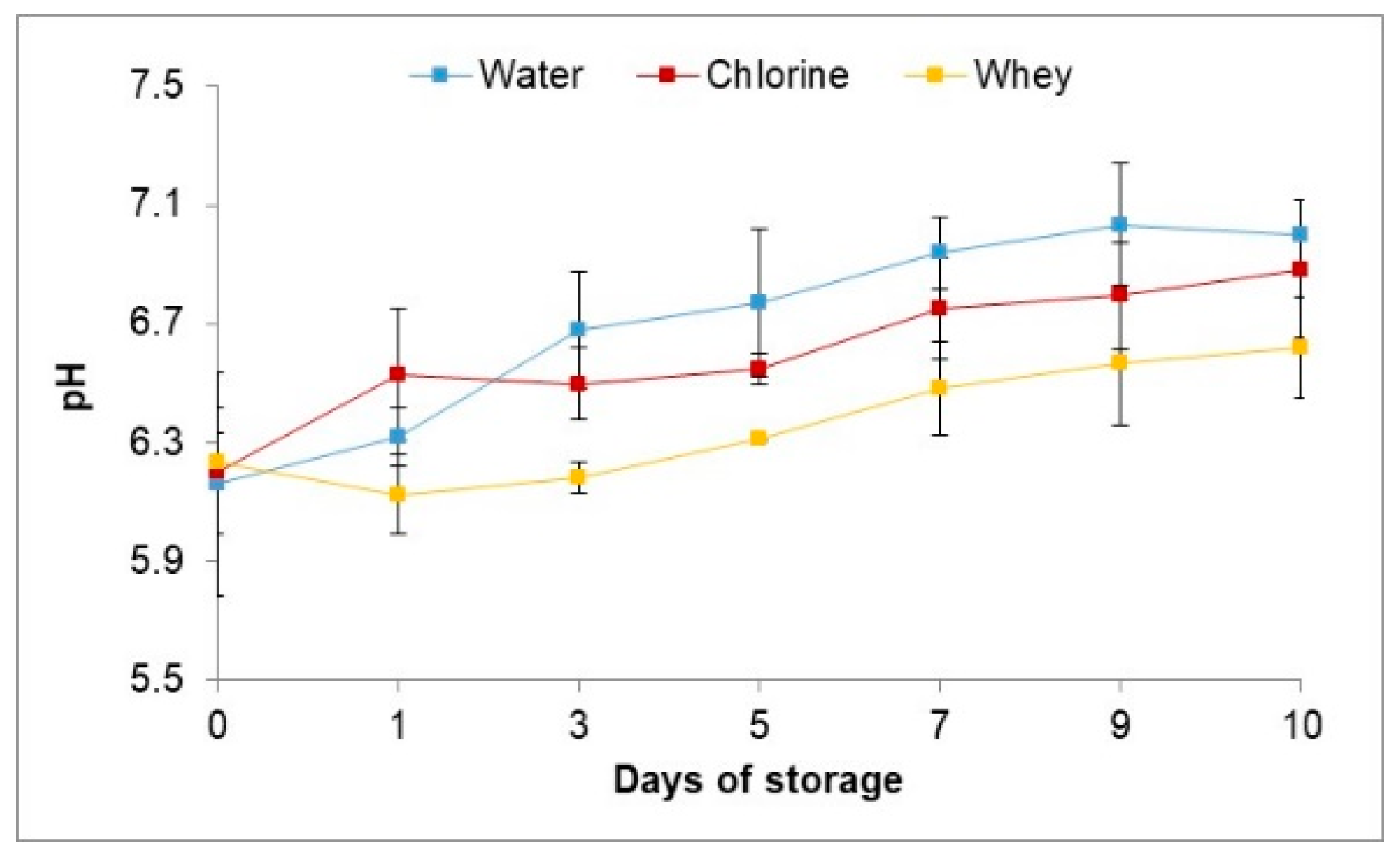
3.4.2. pH
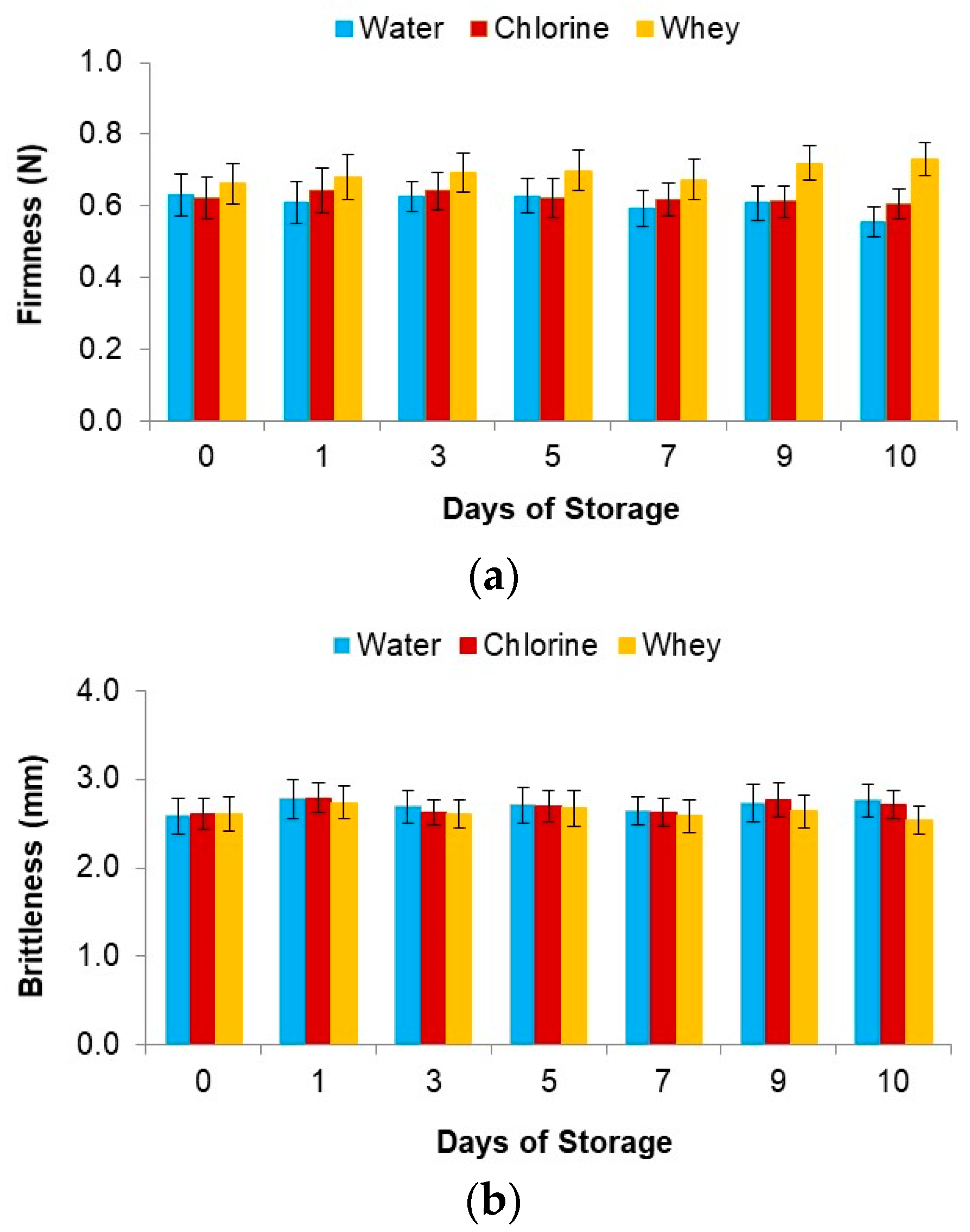
3.4.3. Texture
3.4.4. Color Measurement
3.4.5. Sensory Evaluation
3.4.6. Microbial Markers
- 1.
- Aerobic Microorganisms at 30 °C
- 2.
- Psychrotrophic Microorganisms
- 3.
- Lactic Acid Bacteria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryan, M.P. The biotechnological potential of whey. Rev. Environ. Sci. Biotechnol. 2016, 15, 479–498. [Google Scholar] [CrossRef]
- Prazeres, A.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445, 385–396. [Google Scholar] [CrossRef]
- Murari, C.S.; Moraes, D.C.; Bueno, G.F.; Del Bianchi, V.L. Avaliação da redução na poluição dos laticínios, a partir da fermentação do soro de leite em etanol pela levedura Kluyveromyces Marxianus 229. Revista do Instituto de Laticínios Cândido Tostes, Juiz de Fora 2013, 68, 42–50. [Google Scholar] [CrossRef]
- Lievore, P.; Simões, D.R.S.; Silva, K.M.; Drunkler, N.L.; Barana, A.C.; Nogueira, A.; Demiate, I.M. Chemical characterisation and application of acid whey in fermented milk. J. Food Sci. Technol. 2015, 52, 2083–2092. [Google Scholar] [CrossRef]
- Smithers, G. Whey and whey proteins—From “gutter-to-gold”. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Martin-Diana, A.; Rico, D.; Frias, J.; Mulcahy, J. Whey permeate as a bio-preservative for shelf life maintenance. Innov. Food Sci. Emerg. 2006, 7, 112–123. [Google Scholar] [CrossRef]
- Panesar, P.; Kennedy, J.; Gandhi, D.; Bunko, K. Bioutilisation of whey for lactic acid production. Food Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.; Barat, J.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Rizello, C.G.; Losito, I.; Gobbetti, M.; Carbonara, T.; Bari, M.D.; Zambonin, P.G. Antibacterial Activities of Peptides from the Water-Soluble Extracts of Italian Cheese Varieties. J. Dairy Sci. 2005, 88, 2348–2360. [Google Scholar] [CrossRef]
- Almaas, H.; Eriksen, E.; Sekse, C.; Comi, I.; Flengsrud, R.; Holm, H.; Jensen, E.; Jacobsen, M.; Laangsrud, T.; Vegarud, G.E. Antibacterial peptides derived from caprine whey proteins, by digestion with human gastrointestinal juice. Br. J. Nutr. 2011, 106, 896–905. [Google Scholar] [CrossRef]
- Medeiros, G.V.; Queiroga, R.E.; Costa, W.A.; Gadelha, C.A.; Lacerda, R.R.; Lacerda, J.G.; Pinto, L.S.; Braganhol, F.; Teixeira, F.C.; Barbosa, P.S.; et al. Proteomic of goat milk whey and its bacteriostatic and antitumour potential. Int. J. Biol. Macromol. 2018, 113, 116–123. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Roux, E.; Perrin, C.; Miclo, L.; Dary-Mourot, A. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Res. Int. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Théolier, J.; Hammami, R.; Labelle, P.; Fliss, I.; Jean, J. Isolation and identification of antimicrobial peptides derived by peptic cleavage of whey protein isolate. J. Funct. Foods 2013, 5, 706–714. [Google Scholar] [CrossRef]
- Dullius, A.; Goettertb, M.I.; Souza, C.V. Whey protein hydrolysates as a source of bioactive peptides for functional foods—Biotechnological facilitation of industrial scale-up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Murray, K.; Fan Wu, F.; John Shi, J.; Sophia Jun Xue, S.J.; Warriner, K. Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Qual. Saf. 2017, 1, 289–301. [Google Scholar] [CrossRef]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Charlier, C.; Goffinet, F.; Azria, A.; Leclercq, A.; Lecuit, M. Inadequate management of pregnancy-associated listeriosis: Lessons from four case reports. Clin. Microbiol. Infect. 2014, 20, 246–249. [Google Scholar] [CrossRef]
- Francis, G.A.; Thomas, C.; O’Breirne, D. Isolation and pulsed-field gel electrophoresis typing of Listeria monocytogenes from modified atmosphere packaged fresh-cut vegetables collected in Ireland. J. Food Protect. 2006, 69, 2524–2558. [Google Scholar] [CrossRef]
- Gombas, D.; Chen, Y.; Clavero, R.; Scott, V. Survey of Listeria monocytogenes in Ready-to-Eat Foods. J. Food Protect. 2003, 66, 559–569. [Google Scholar] [CrossRef]
- Lianou, A.; Sofos, J.N. A review of the incidence and transmission of Listeria monocytogenes in ready-to-eat products in retail and food service environments. J. Food Protect. 2007, 70, 2172–2198. [Google Scholar] [CrossRef]
- World Health Organization & Food and Agriculture Organization of the United Nations. Microbiological Hazards in Fresh Leafy Vegetables and Herbs: Meeting Report. World Health Organization. 2008. Available online: https://apps.who.int/iris/handle/10665/44031 (accessed on 22 November 2018).
- Scientific Committee on Food. Risk Profile on the Microbiology Contamination of Fruits and Vegetables Eaten Raw. Report of the Scientific Commission on Food 2002, European Commission on Health and Consumer Protection Directorate-General. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out125_en.pdf (accessed on 22 November 2018).
- Meireles, A.; Giaouris, E.; Simões, M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016, 82, 71–85. [Google Scholar] [CrossRef]
- Francis, G.A.; Gallone, A.; Nychas, G.J.; Sofos, J.N.; Colelli, G.; Amodio, M.L.; Spano, G. Factors affecting quality and safety of fresh-cut produce. Crit. Rev. Food Sci. Nutr. 2012, 52, 595–610. [Google Scholar] [CrossRef]
- Warriner, K.; Namvar, A. Recent advances in fresh produce post-harvest decontamination technologies to enhance microbiological safety. Stewart Postharvest Rev. 2013, 9, 1–8. [Google Scholar] [CrossRef]
- Santos, M.I.; Cavaco, A.; Gouveia, J.; Novais, M.R.; Nogueira, P.J.; Pedroso, L.; Ferreira, M.A.S.S. Evaluation of minimally processed salads commercialized in Portugal. Food Control 2012, 23, 275–281. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Ölmez, H. Inactivation of Escherichia coli and Listeria monocytogenes on iceberg lettuce by dip wash treatments with organic acids. Lett. Appl. Microbiol. 2007, 44, 619–624. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Adler, B.B.; Lang, M.M. Efficacy of chlorine and a peroxyacetic acid sanitizer in killing Listeria monocytogenes on iceberg and romaine lettuce using simulated commercial processing conditions. J. Food Protect. 2004, 67, 1238–1242. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; Huang, B.; He, J.; Wang, Y. In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control 2011, 22, 1992–1999. [Google Scholar] [CrossRef]
- Guha, A.; Banerjee, S.; Bera, D. Production of lactic acid from sweet meat industry waste by Lactobacillus delbruki. Int. J. Res. Eng. Technol. 2013, 2, 630–634. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Diana, I.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of bio-control agents to improve the safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 302–310. [Google Scholar] [CrossRef]
- Trias, R.; Bañeras, L.; Badosa, E.; Montesinos, E. Bioprotection of Golden Delicious apples and Iceberg lettuce against foodborne bacterial pathogens by lactic acid bacteria. Int. J. Food Microbiol. 2008, 123, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Bjorkroth, J.; Koort, J. Lactic acid bacteria: Taxonomy and Biodiversity. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: London, UK, 2011; Volume 3, pp. 45–48. [Google Scholar]
- Madureira, A.R.; Soares, J.C.; Amorim, M.; Tavares, T.; Gomes, A.M.; Pintado, M.M.; Malcata, F.X. Bioactivity of probiotic whey cheese: Characterization of the content of peptides and organic acids. J. Sci. Food Agric. 2013, 93, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.; Lovitt, R. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Proc. 2012, 2, 50–56. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kumari, S.; Panesar, R. Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Res. 2010, 2010, 473137. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.I.S.; Lima, A.I.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B.; Pedroso, L.; Sousa, I.; Ferreira, M.A.S.S. Preliminary study on the effect of fermented cheese whey on Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella Goldcoast populations inoculated onto fresh organic lettuce. Foodborne Pathog. Dis. 2016, 13, 423–427. [Google Scholar] [CrossRef]
- Santos, M.I.S.; Martins, S.R.; Pedroso, L.; Sousa, I.; Ferreira, M.A.S.S. Potential bio-activity of whey fermented extract as sanitizer of organic grown lettuce. Food Control 2015, 50, 477–481. [Google Scholar] [CrossRef]
- Gottlieb, C.T.; Thomsen, L.E.; Ingmer, H. Antimicrobial peptides effectively kill a broad spectrum of Listeria monocytogenes and Staphylococcus aureus strains independently of origin, sub-type, or virulence factor expression. BMC Microbiol. 2008, 8, 205. [Google Scholar] [CrossRef]
- Bouhdid, S.; Abrini, J.; Amensour, M.; Zhiri, A.; Espuny, M.J.; Manresa, A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. J. Appl. Microbiol. 2010, 109, 1139–1149. [Google Scholar] [CrossRef]
- Pintado, C.M.B.S.; Ferreira, M.A.S.S.; Sousa, I. Properties of whey-protein based films containing organic acids and nisin to control Listeria monocytogenes. J. Food Prot. 2009, 72, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Bosnea, L.; Psarianos, C.; Koutinas, A.; Marchant, R.; Banat, I.M. Lactic acid production by mixed cultures of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and Lactobacillus helveticus. Bioresour. Technol. 2008, 99, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on lactose thresholds in lactose intolerance and galactosaemia. EFSA J. 2010, 8, 1777. [Google Scholar] [CrossRef]
- Beuchat, L.R. Surface disinfection of raw produce. Dairy Food Environ. Sanit. 1992, 12, 6–9. [Google Scholar]
- Health Protection Agency. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods; Health Protection Agency: London, UK, 2009.
- Barth, M.; Hankinson, T.; Zhuang, H.; Breidt, F. Microbiological Spoilage of Fruit and Vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009; pp. 135–183. [Google Scholar]
- Tauxe, R.; Kruse, H.; Hedberg, C.; Potter, M.; Madden, J.; Wachsmuth, K. Microbiological Hazards and Emerging Issues Associated with Produce: A Preliminary Report to the National Advisory Committee on Microbiological Criteria for Foods. J. Food Prot. 1997, 60, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.G.; Roura, S.I.; Del Valle, C.E.; Fritz, R. Characterization of native microbial population of Swiss Chard (Beta vulgaris, type cicla). LWT Food Sci. Technol. 2002, 35, 331–337. [Google Scholar] [CrossRef]
- Beuchat, L.R. Use of Sanitizers in Raw Fruit and Vegetable Processing. In Minimally Processed Fruits and Vegetables; Alzamora, S.M., Tapia, M.S., Lopez-Malo, A., Eds.; An Aspen Publication: Gaithersburg, MD, USA, 2000; pp. 63–78. [Google Scholar]
- Nykänen, A.; Lapveteläinen, A.; Kallio, H.; Salminen, S. Effects of whey, whey-derived lactic acid and sodium lactate on the surface microbial counts of rainbow trout packed in vacuum pouches. LWT Food Sci. Technol. 1998, 31, 361–365. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Jiang, Y.; Doucette, C.; Fillmore, S. Antimicrobial activity of bacteriocin-producing lactic acid bacteria isolated from cheeses and yogurts. AMB Express 2012, 2, 48. [Google Scholar] [CrossRef]

| Days of Fermentation | Strains | |
|---|---|---|
| Listeria monocytogenes 4b (MIC) | Escherichia coli O157:H7 (MIC) | |
| 1 | NI | 25.0 |
| 2 | NI | 25.0 |
| 3 | NI | 25.0 |
| 4 | 25.0 | 25.0 |
| 5 | 1.56 | 3.13 |
| Inhibition Halo (mm) | |||
|---|---|---|---|
| Dilutions in water (v/v) | Milk | Non-Fermented Whey | Fermented Whey ** |
| 25% | 0 | 0 | 20.70 ± 0.05 + |
| 50% | 0 | 0 | 24.70 ± 0.05 * |
| 100% | 0 | 0 | 31.20 ± 0.17 # |
| Days of Storage | O2 (%) | CO2 (%) | ||||
|---|---|---|---|---|---|---|
| Water | Chlorine | Whey | Water | Chlorine | Whey | |
| Day 0 | 19.79 ± 0.41 | 19.9 ± 0.61 | 19.4 ± 0.50 | 0.01 ± 0.04 | 0.00 ± 0.00 | 0.02 ± 0.07 |
| Day 1 | 19.31 ± 0.34 | 18.5 ± 0.86 | 18.0 ± 0.71 | 0.06 ± 0.09 | 0.43 ± 0.25 | 0.60 ± 0.32 |
| Day 3 | 19.44 ± 0.55 | 16.2 ± 2.31 | 17.5 ± 1.69 | 0.23 ± 0.32 | 0.95 ± 0.78 | 0.46 ± 0.49 |
| Day 5 | 19.22 ± 0.71 | 17.1 ± 1.68 | 17.7 ± 1.90 | 0.57 ± 0.46 | 0.96 ± 0.80 | 0.56 ± 0.61 |
| Day 7 | 19.02 ± 0.81 | 17.5 ± 1.80 | 17.8 ± 1.42 | 0.56 ± 0.47 | 0.93 ± 0.83 | 0.63 ± 0.58 |
| Day 9 | 18.97 ± 0.91 | 16.5 ± 1.86 | 17.8 ± 2.00 | 0.64 ± 0.54 | 1.33 ± 0.70 | 0.66 ± 1.01 |
| Day 10 | 19.20 ± 0.94 | 16.7 ± 1.18 | 17.3 ± 2.17 | 0.61 ± 0.57 | 1.33 ± 0.52 | 0.78 ± 0.78 |
| Days Storage | Water | Chlorine Solution | Fermented Whey Solution | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| 0 | 72.0 ± 4.4 | −20.9 ± 1.4 | 39.0 ± 1.1 | 97.6 ± 2.4 | −19.8 ± 1.2 | 37.2 ± 0.9 | 72.1 ± 1.7 | −21.3 ± 0.9 | 38.3 ± 1.7 |
| 1 | 70.2 ± 3.3 | −20.8 ± 2.4 | 33.1 ± 1.2 | 72.7 ± 3.2 | −20.4 ± 0.9 | 36.7 ± 1.0 | 103.3 ± 2.8 | −21.3 ± 0.6 | 40.9 ± 2.5 |
| 3 | 71.6 ± 3.7 | −17.3 ± 1.4 | 33.1 ± 1.9 | 69.2 ± 3.4 | −20.7 ± 0.9 | 39.3 ± 2.5 | 107.1 ± 3.1 | −20.9 ± 0.9 | 37.8 ± 2.2 |
| 5 | 101.8 ± 2.3 | 3.8 ± 1.3 | 2.4 ± 1.8 | 100.5 ± 1.5 | −19.2 ± 0.8 | 34.3 ± 1.3 | 61.5 ± 3.3 | −19.5 ± 1.3 | 36.3 ± 2.3 |
| 7 | 74.5 ± 2.9 | −19.1 ± 1.4 | 36.1 ± 1.9 | 95.2 ± 1.9 | −10.8 ± 1.0 | 17.8 ± 2.3 | 64.9 ± 2.2 | −20.2 ± 0.8 | 37.4 ± 2.2 |
| 9 | 94.2 ± 3.8 | −19.4 ± 1.7 | 39.7 ± 2.7 | 98.5 ± 1.8 | −20.1 ± 1.5 | 35.2 ± 2.7 | 95.0 ± 2.5 | −19.6 ± 1.5 | 37.9 ± 2.3 |
| 10 | 97.1 ± 4.0 | −19.0 ± 1.6 | 36.5 ± 1.7 | 105.6 ± 3.3 | −16.2 ± 1.6 | 35.8 ± 1.9 | 93.8 ± 3.3 | −19.8 ± 1.6 | 39.9 ± 2.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S. Santos, M.I.; Fradinho, P.; Martins, S.; G. Lima, A.I.; M. S. Boavida Ferreira, R.; Pedroso, L.; S. S. Ferreira, M.A.; Sousa, I. A Novel Way for Whey: Cheese Whey Fermentation Produces an Effective and Environmentally-Safe Alternative to Chlorine. Appl. Sci. 2019, 9, 2800. https://doi.org/10.3390/app9142800
S. Santos MI, Fradinho P, Martins S, G. Lima AI, M. S. Boavida Ferreira R, Pedroso L, S. S. Ferreira MA, Sousa I. A Novel Way for Whey: Cheese Whey Fermentation Produces an Effective and Environmentally-Safe Alternative to Chlorine. Applied Sciences. 2019; 9(14):2800. https://doi.org/10.3390/app9142800
Chicago/Turabian StyleS. Santos, Maria Isabel, Patrícia Fradinho, Sandro Martins, Ana Isabel G. Lima, Ricardo M. S. Boavida Ferreira, Laurentina Pedroso, Maria Adélia S. S. Ferreira, and Isabel Sousa. 2019. "A Novel Way for Whey: Cheese Whey Fermentation Produces an Effective and Environmentally-Safe Alternative to Chlorine" Applied Sciences 9, no. 14: 2800. https://doi.org/10.3390/app9142800
APA StyleS. Santos, M. I., Fradinho, P., Martins, S., G. Lima, A. I., M. S. Boavida Ferreira, R., Pedroso, L., S. S. Ferreira, M. A., & Sousa, I. (2019). A Novel Way for Whey: Cheese Whey Fermentation Produces an Effective and Environmentally-Safe Alternative to Chlorine. Applied Sciences, 9(14), 2800. https://doi.org/10.3390/app9142800