Application of Deep Eutectic Solvents to Prepare Mixture Extracts of Three Long-Lived Trees with Maximized Skin-Related Bioactivities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Synthesis of DESs
2.4. Determination of ISO as a Common Marker Compound for Extraction Efficiency Using LC-UV
2.5. Antioxidant Activity Assay
2.6. Anti-Tyrosinase Activity Assay
2.7. Anti-Elastase Activity Assay
2.8. Experimental Design and Statistical Analyses
3. Results and Discussion
3.1. Preparation of DESs
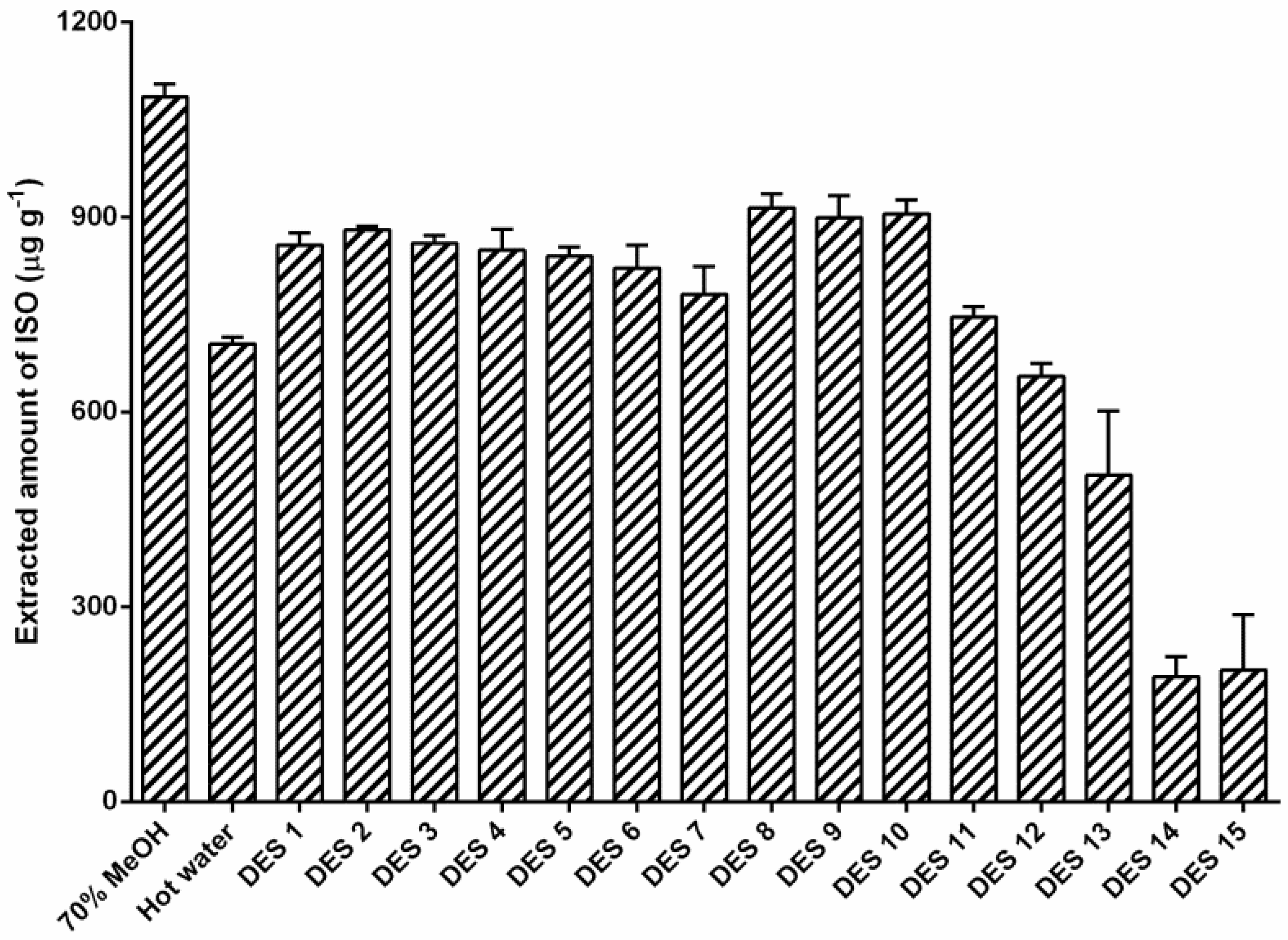
3.2. Selection of DESs for Mixture Design Studies Based on the ISO Extraction Yields
3.3. Simplex-Centroid Mixture Design
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging—Contributors and inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Shivanand, P.; Nilam, M.; Viral, D. Herbs play an important role in the field of cosmetics. Int. J. PharmTech Res. 2010, 2, 632–639. [Google Scholar]
- Juhász, M.L.; Levin, M.K.; Marmur, E.S. The use of natural ingredients in innovative Korean cosmeceuticals. J. Cosmet. Dermatol. 2018, 17, 305–312. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Dudley, E.; Plummer, S.; Tang, J.; Newton, R.P.; Brenton, A.G. Quantitative determination of major active components in Ginkgo biloba dietary supplements by liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Shar, A.H.; Shahen, M.; Wang, H.; Alagawany, M.; El-Hack, M.E.A.; Kalhoro, S.A.; Rashid, M.; Shar, P.A. Pharmacological Uses of Ginkgo Biloba Extracts for Cardiovascular Disease and Coronary Heart Diseases. Int. J. Pharmacol. 2019, 15, 1–9. [Google Scholar]
- van Beek, T.A.; Montoro, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef] [PubMed]
- van Beek, T.A. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–55. [Google Scholar] [CrossRef]
- Zuo, W.; Yan, F.; Zhang, B.; Li, J.; Mei, D. Advances in the studies of Ginkgo biloba leaves extract on aging-related diseases. Aging Dis. 2017, 8, 812. [Google Scholar] [CrossRef]
- Chan, P.-C.; Xia, Q.; Fu, P.P. Ginkgo biloba leave extract: Biological, medicinal, and toxicological effects. J. Environ. Sci. Health C 2007, 25, 211–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the black death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Fu, C.-S.; Yang, W.-J.; Wang, X.-L.; Feng, D.; Wang, X.-N.; Ren, D.-M.; Lou, H.-X.; Shen, T. Investigation of constituents from Cinnamomum camphora (L.) J. Presl and evaluation of their anti-inflammatory properties in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2018, 221, 37–47. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G. Antifungal activity of plant extracts against Colletotrichum lagenarium, the causal agent of anthracnose in cucumber. J. Sci. Food Agric. 2012, 92, 1937–1943. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Jeong, S.I.; Moon, S.E.; Kil, B.S.; Yun, S.I.; Lee, K.Y.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oil of Cryptomeria japonica. Phytother. Res. 2007, 21, 295–299. [Google Scholar] [CrossRef]
- Shyur, L.-F.; Huang, C.-C.; Lo, C.-P.; Chiu, C.-Y.; Chen, Y.-P.; Wang, S.-Y.; Chang, S.-T. Hepatoprotective phytocompounds from Cryptomeria japonica are potent modulators of inflammatory mediators. Phytochemistry 2008, 69, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-S.; Lin, H.-Y.; Chang, S.-T. Chemical composition and antifungal activity of essential oils from different tissues of Japanese cedar (Cryptomeria japonica). J. Agric. Food Chem. 2005, 53, 614–619. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Hong, C.; Gwak, K.; Park, M.; Smith, D.; Choi, I. Whitening and antioxidant activities of bornyl acetate and nezukol fractionated from Cryptomeria japonica essential oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Domokos, E.; Cosarca, S.; Miklos, A.; Imre, S.; Domokos, J.; Dehelean, C.A. Study of the ultrasound-assisted extraction of polyphenols from beech (Fagus sylvatica L.) bark. Bioresources 2018, 13, 2247–2267. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef] [PubMed]
- The Commission of the European Communities. Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. Off. J. Eur. Union 2006, 257, 1–528. [Google Scholar]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep eutectic solvent-based valorization of spent coffee grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yuan, C.; Dong, C.; Shuang, S.; Choi, M.M. In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 437–445. [Google Scholar] [CrossRef]
- Kim, Y.; Narayanan, S.; Chang, K.-O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antivir. Res. 2010, 88, 227–235. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, Y.; Zhang, X.; Chen, X.; Liu, Z.; Tian, X. Isoquercetin ameliorates myocardial infarction through anti-inflammation and anti-apoptosis factor and regulating TLR4-NF-κB signal pathway. Mol. Med. Rep. 2018, 17, 6675–6680. [Google Scholar] [CrossRef]
- Chatatikun, M.; Chiabchalard, A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement. Altern. Med. 2017, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, I.K.; Sarangi, D.D.; Sundararajan, V.; George, S.; Mohideen, S.S. Poly herbal formulation with anti-elastase and anti-oxidant properties for skin anti-aging. BMC Complement. Altern. Med. 2018, 18, 33. [Google Scholar]
- Handa, C.L.; de Lima, F.S.; Guelfi, M.F.G.; Georgetti, S.R.; Ida, E.I. Multi-response optimisation of the extraction solvent system for phenolics and antioxidant activities from fermented soy flour using a simplex-centroid design. Food Chem. 2016, 197, 175–184. [Google Scholar] [CrossRef]
- Bau, T.R.; Garcia, S.; Ida, E.I. Optimization of a fermented soy product formulation with a kefir culture and fiber using a simplex-centroid mixture design. Int. J. Food Sci. Nutr. 2013, 64, 929–935. [Google Scholar] [CrossRef] [PubMed]

| DES No. | Component 1 | Component 2 | Component 3 | Molar Ratio |
|---|---|---|---|---|
| 1a | Glycerol | Xylitol | 2:1 | |
| 2 a | Maltose | 3:1 | ||
| 3 a | Sorbitol | 2:1 | ||
| 4 a | Fructose | 3:1 | ||
| 5 b | Sucrose | 3:1 | ||
| 6 b | Glucose | 3:1 | ||
| 7 b | Maltitol | 3:1 | ||
| 8 b | Malic acid | 1:1 | ||
| 9 b | Malic acid | 1:2 | ||
| 10 b | Lactic acid | Glucose | 1:2 | |
| 11 b | Fructose | Sucrose | 1:1 | |
| 12 b | Sucrose | Glucose | 1:1:1 | |
| 13 b | Betaine | Sucrose | 1:1 | |
| 14 b | Sucrose | 1:2 | ||
| 15 b | Glucose | 1:1 |
| No. | Independent Variables a | Dependent Variables b | ||||
|---|---|---|---|---|---|---|
| X1 (mg) | X2 (mg) | X3 (mg) | Y1 | Y2 | Y3 | |
| 1 | 1 (60) | 0 (0) | 0 (0) | 3.259 | 60.44 | 72.11 |
| 2 | 0 (0) | 1 (60) | 0 (0) | 3.175 | 68.35 | 78.86 |
| 3 | 0 (0) | 0 (0) | 1 (60) | 3.175 | 39.61 | 50.67 |
| 4 | 0.5 (30) | 0.5 (30) | 0 (0) | 3.246 | 50.55 | 76.96 |
| 5 | 0.5 (30) | 0 (0) | 0.5 (30) | 3.252 | 62.48 | 82.92 |
| 6 | 0 (0) | 0.5 (30) | 0.5 (30) | 3.131 | 30.50 | 9.83 |
| 7 | 0.33 (20) | 0.33 (20) | 0.33 (20) | 3.271 | 54.99 | 62.64 |
| 8 | 0.33 (20) | 0.33 (20) | 0.33 (20) | 3.252 | 50.98 | 72.07 |
| 9 | 0.33 (20) | 0.33 (20) | 0.33 (20) | 3.258 | 42.50 | 73.04 |
| Response a | Model | R2 | R2adj |
|---|---|---|---|
| 3.26X1 + 3.17X2 + 3.17X3 + 0.12X1X2 + 0.14X1X3 − 0.18X2X3 + 1.31X1X2X3 | 0.9905 | 0.9619 | |
| b | 219231.94X1 + 317719.32X2 + 60577.95X3 − 531992.97X1X2 + 441173.67X1X3 − 617876.6X2X3 | 0.9400 | 0.8399 |
| 8.49X1 + 8.88X2 + 7.12X3 + 0.35X1X2 + 5.2X1X3 − 19.46X2X3 + 45.87X1X2X3 | 0.9911 | 0.9645 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Jung, D.; Li, K.; Park, K.; Ko, J.; Yang, M.; Lee, J. Application of Deep Eutectic Solvents to Prepare Mixture Extracts of Three Long-Lived Trees with Maximized Skin-Related Bioactivities. Appl. Sci. 2019, 9, 2581. https://doi.org/10.3390/app9132581
Jin Y, Jung D, Li K, Park K, Ko J, Yang M, Lee J. Application of Deep Eutectic Solvents to Prepare Mixture Extracts of Three Long-Lived Trees with Maximized Skin-Related Bioactivities. Applied Sciences. 2019; 9(13):2581. https://doi.org/10.3390/app9132581
Chicago/Turabian StyleJin, Yan, Dasom Jung, Ke Li, Keunbae Park, Jaeyoung Ko, Misuk Yang, and Jeongmi Lee. 2019. "Application of Deep Eutectic Solvents to Prepare Mixture Extracts of Three Long-Lived Trees with Maximized Skin-Related Bioactivities" Applied Sciences 9, no. 13: 2581. https://doi.org/10.3390/app9132581
APA StyleJin, Y., Jung, D., Li, K., Park, K., Ko, J., Yang, M., & Lee, J. (2019). Application of Deep Eutectic Solvents to Prepare Mixture Extracts of Three Long-Lived Trees with Maximized Skin-Related Bioactivities. Applied Sciences, 9(13), 2581. https://doi.org/10.3390/app9132581






