Research on Measurement Technology of Thermophysical Properties for Full-Scale Phase Change Material Product in a Container
Abstract
Featured Application
Abstract
1. Introduction
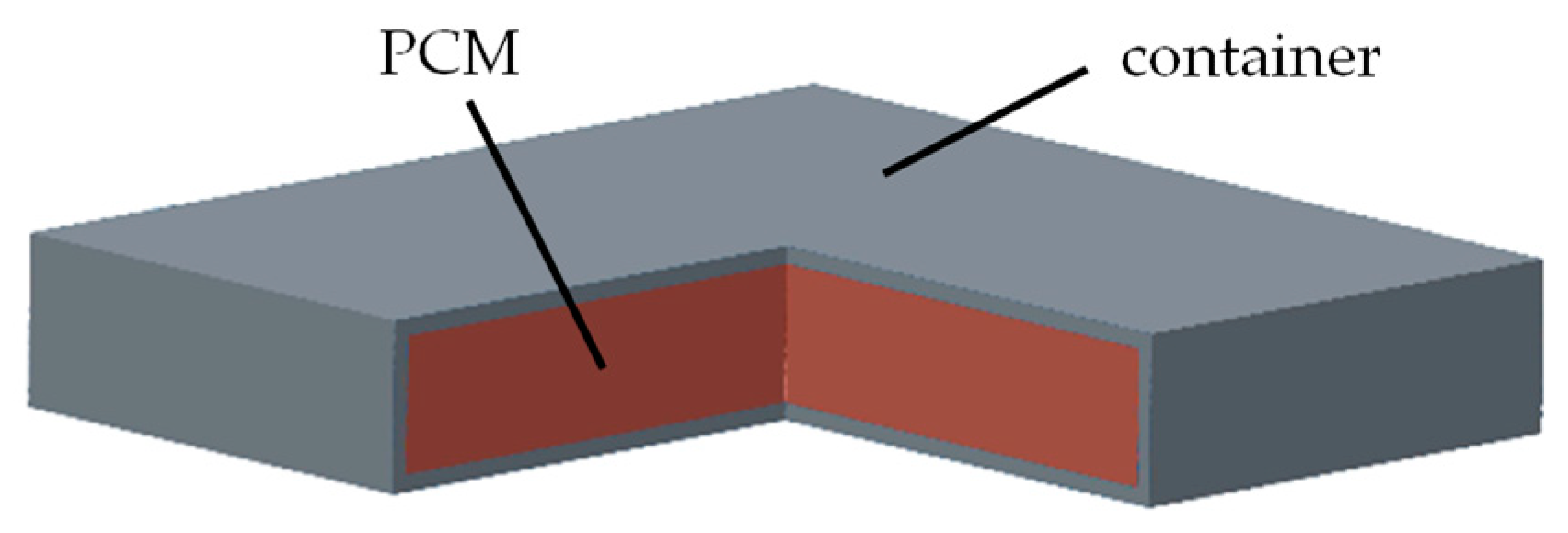
2. Measurement Method of Thermophysical Parameters for PCM in the Container
2.1. Measurement Apparatus
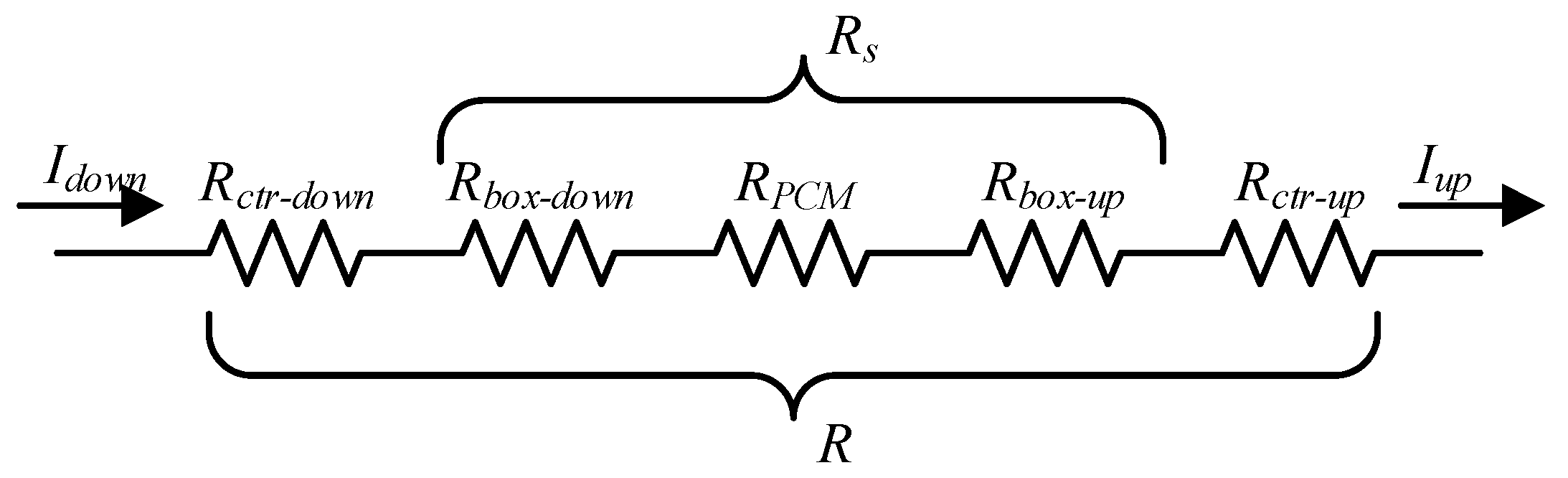
2.2. HFMA Method of PCM in the Container Considering Contact Thermal Resistance
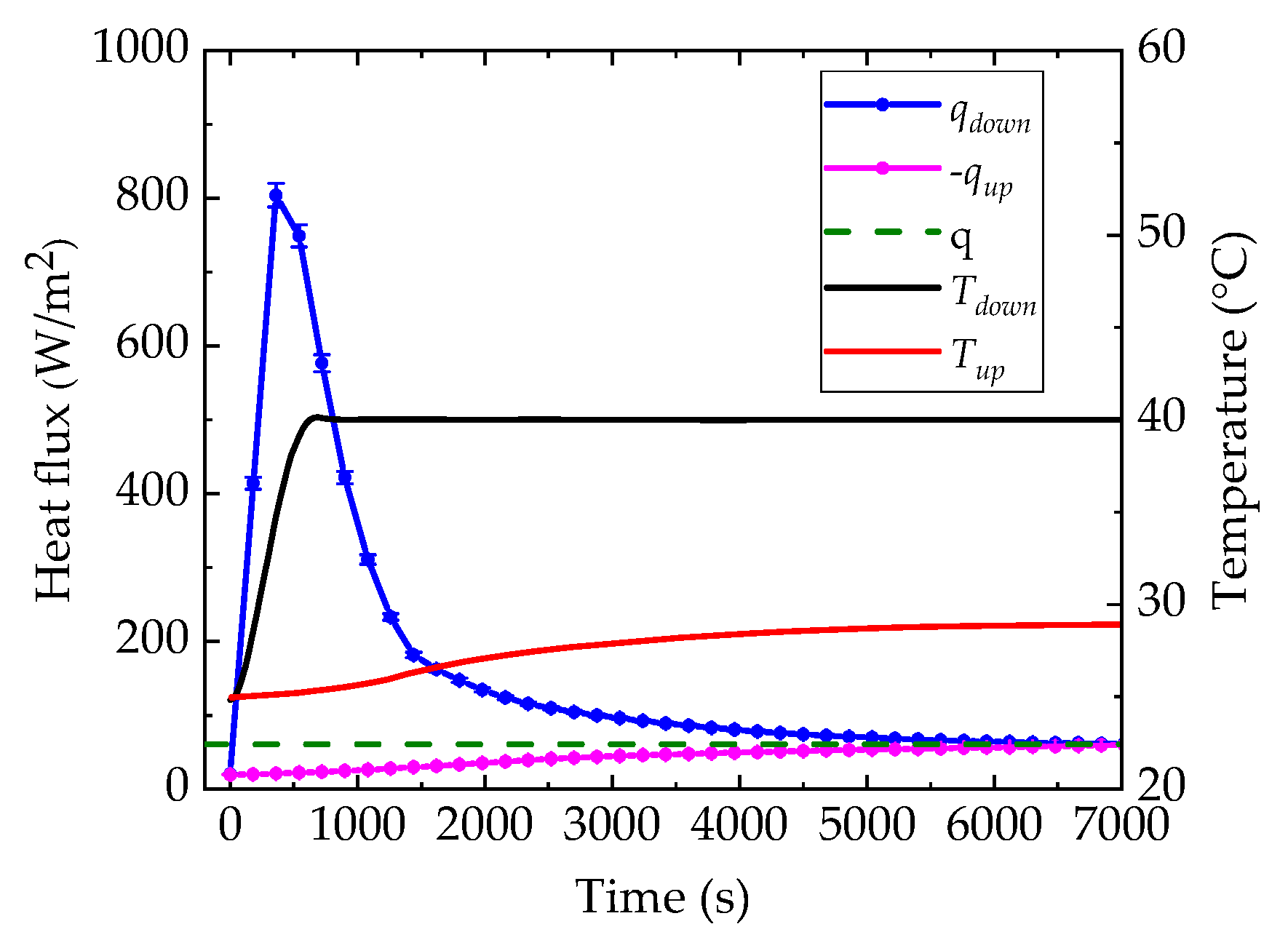
2.3. DHFMA Method of PCM in the Container
3. Calibration
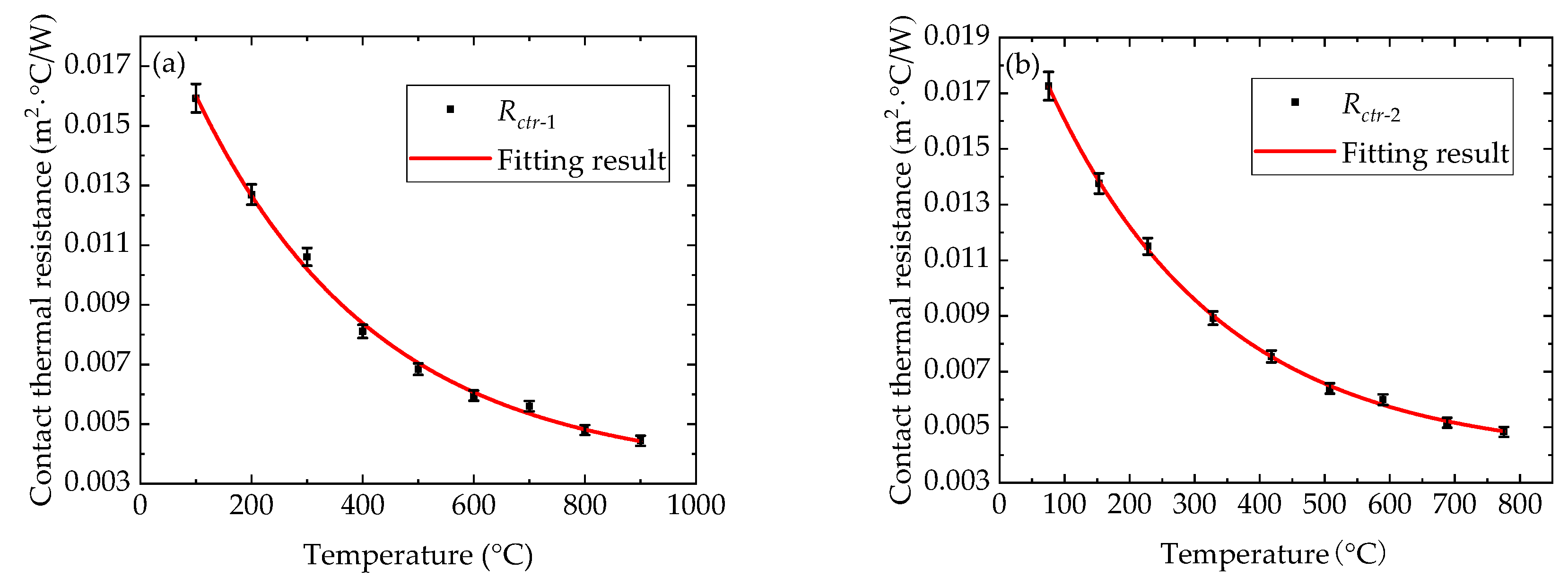
3.1. Calibration Method of Contact Thermal Resistance
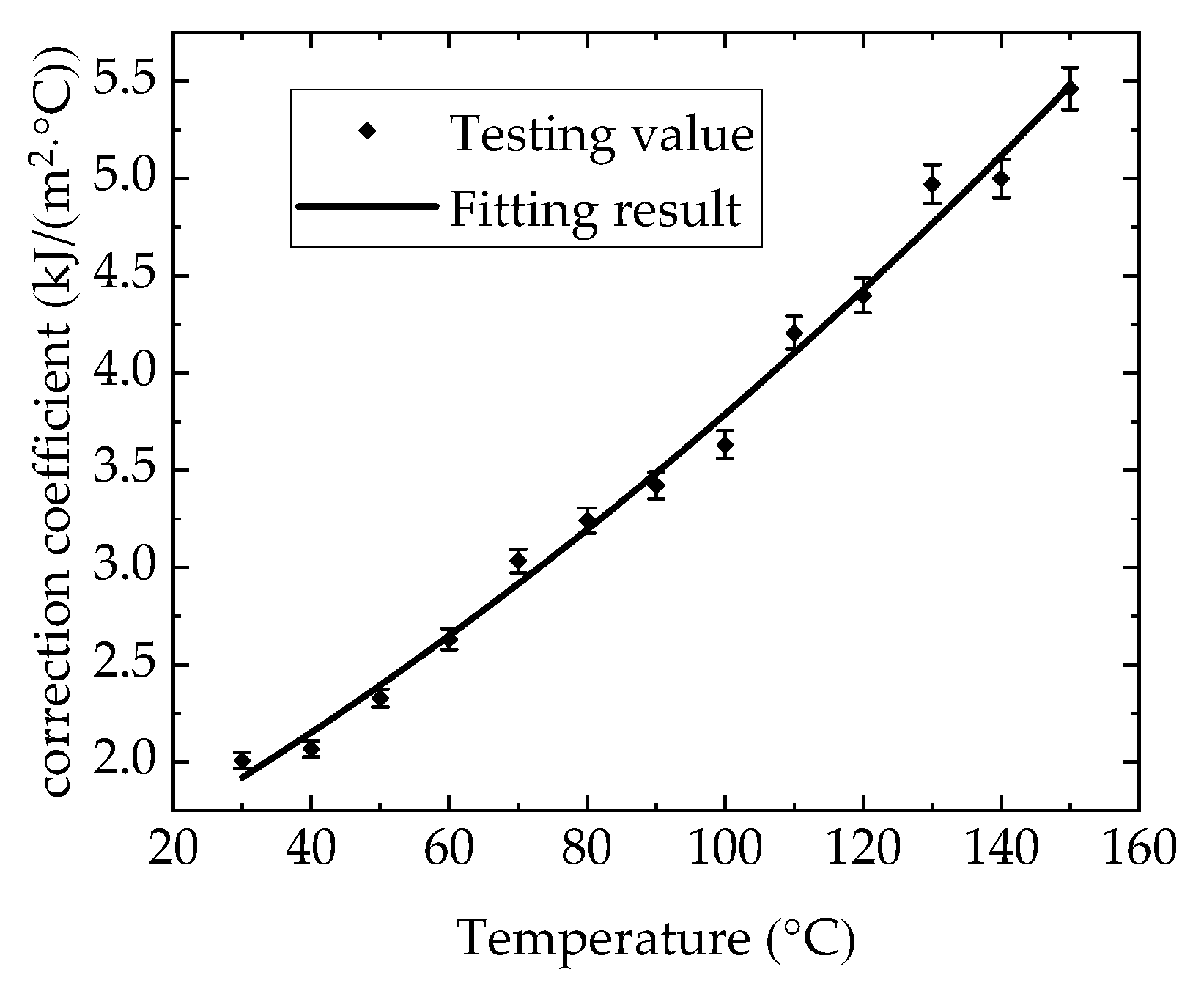
3.2. Calibration Method of Calorific Absorption Correction Coefficient of Heat Flux Sensor
4. Experimental Results
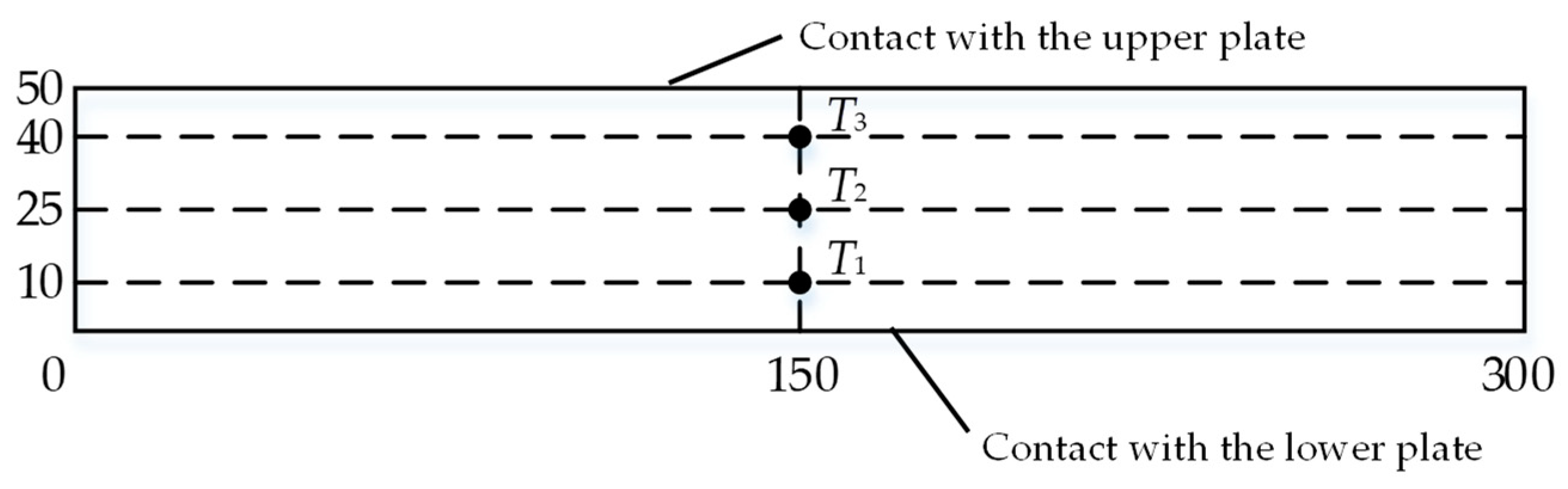
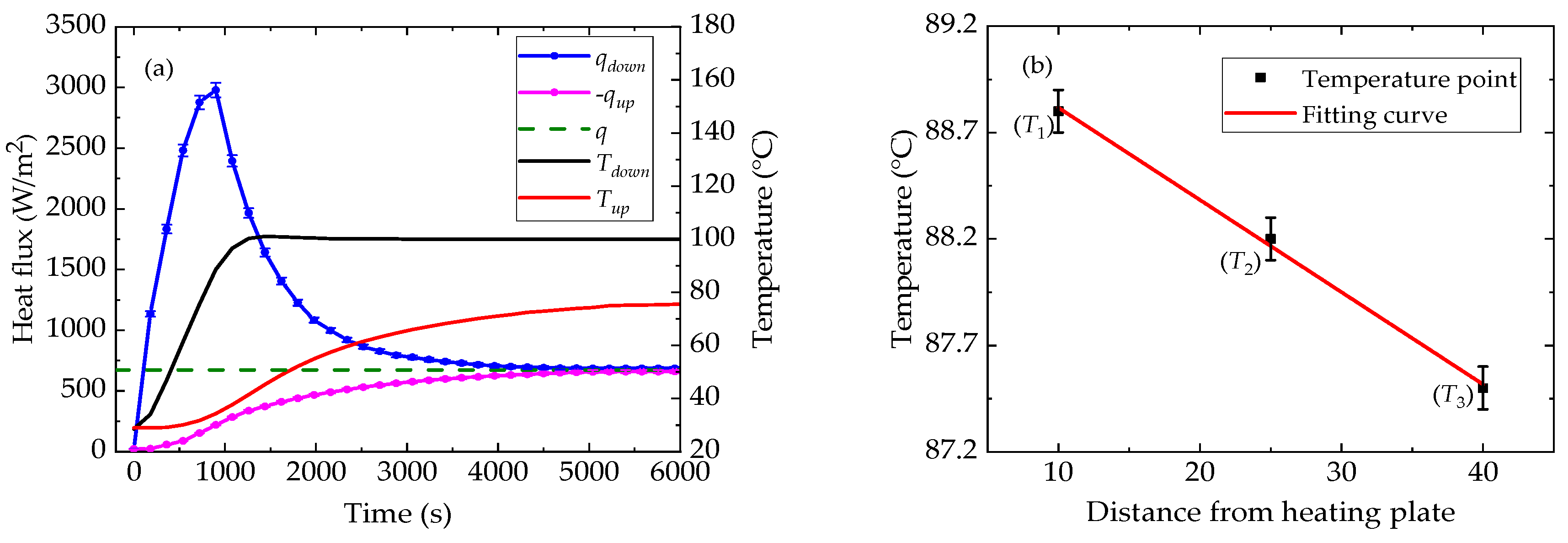
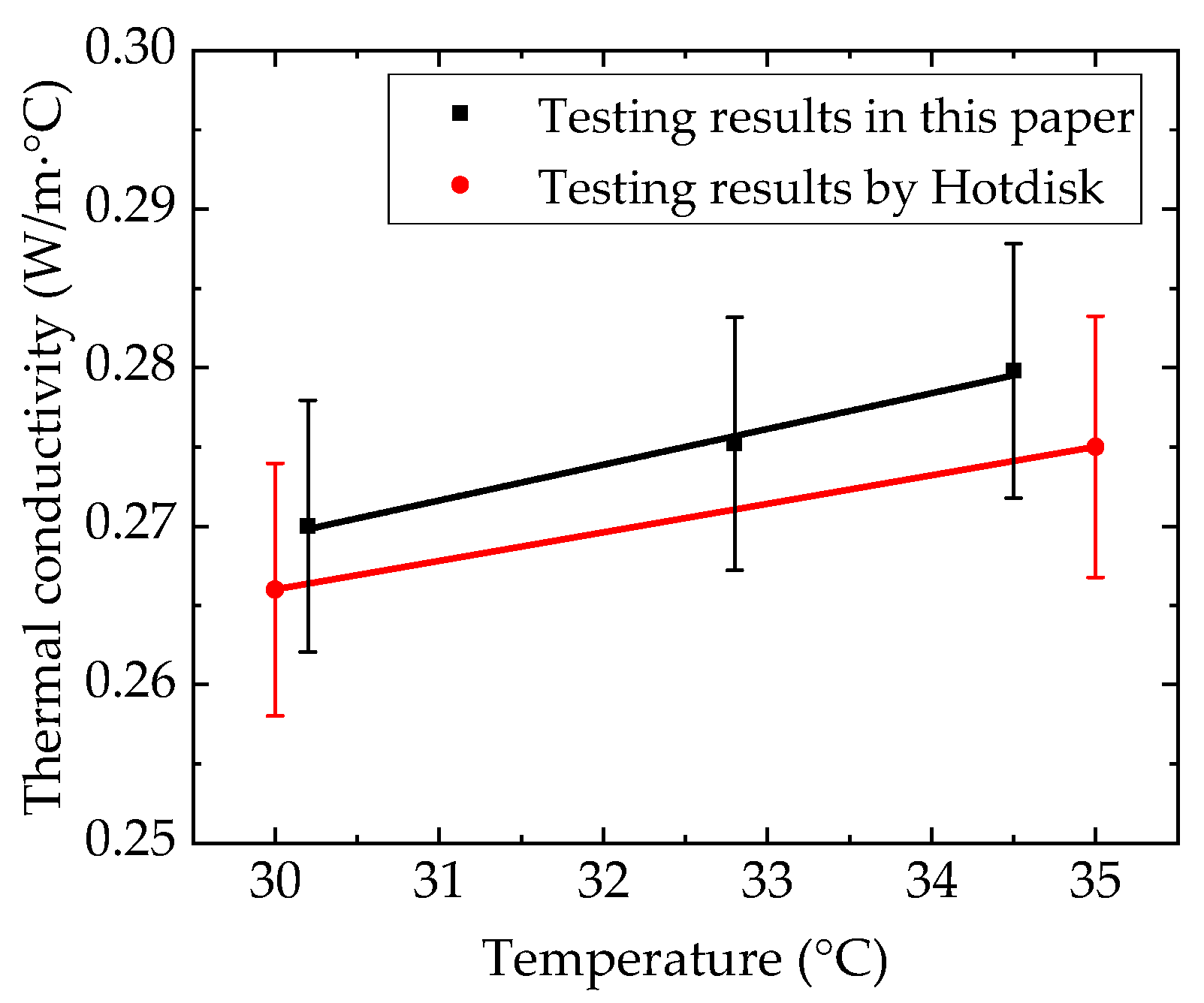
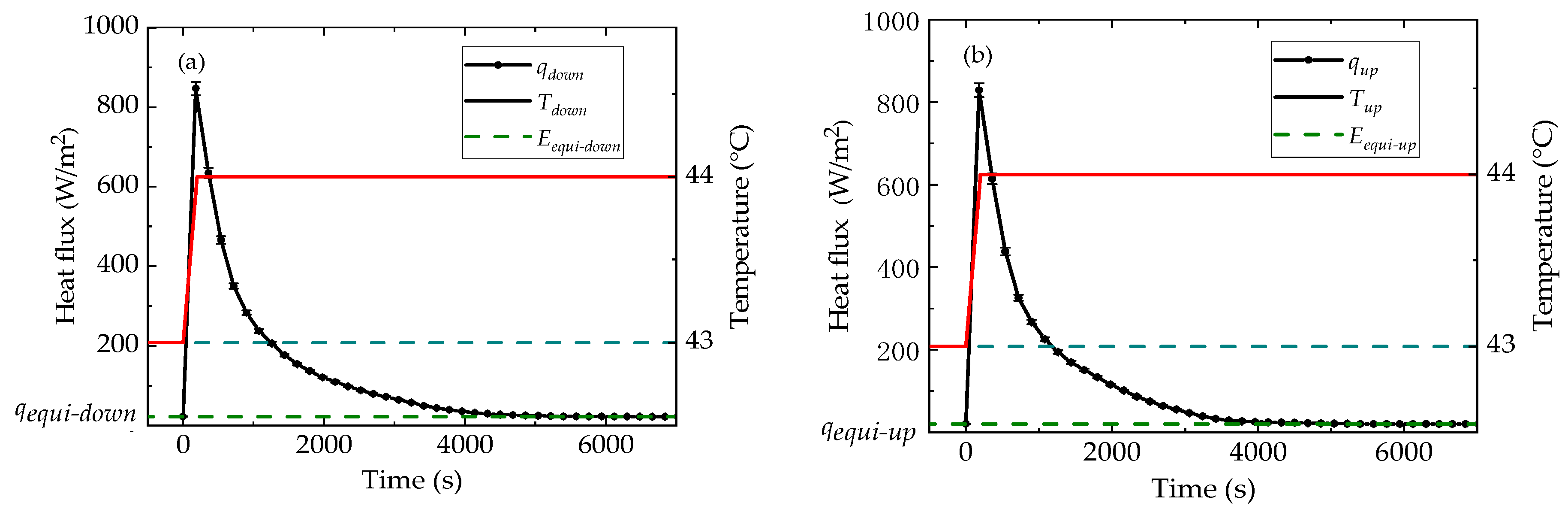
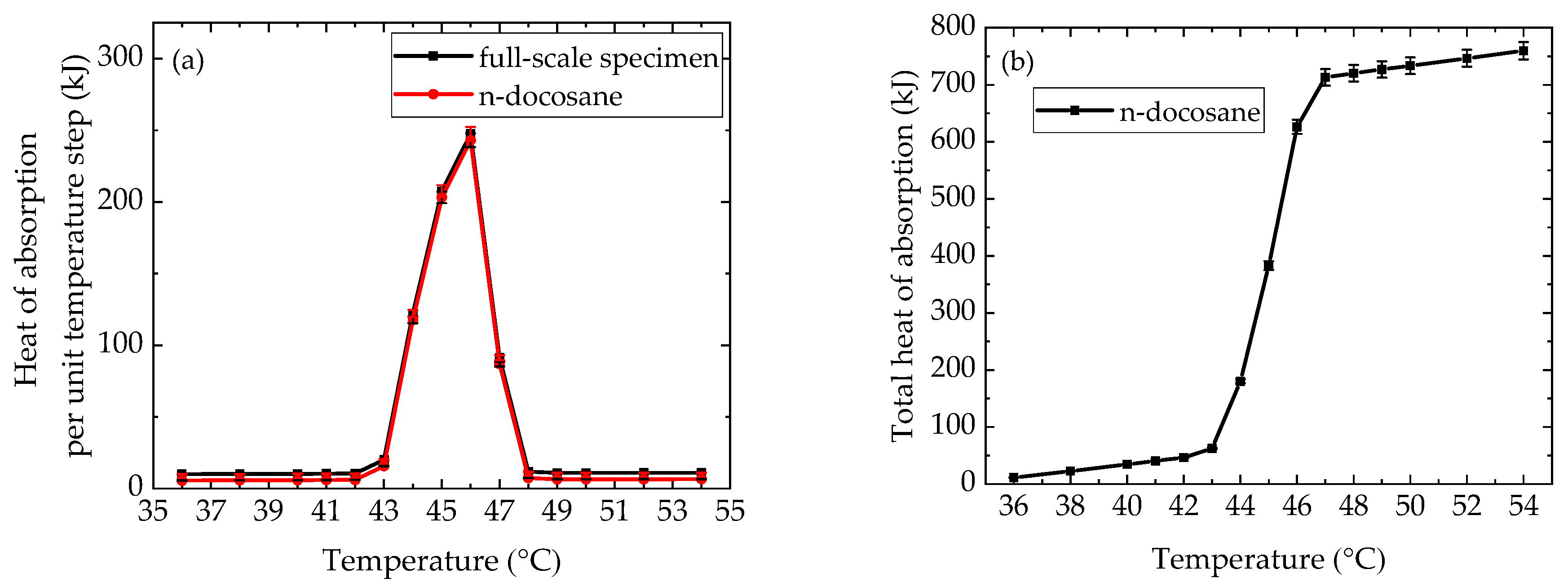
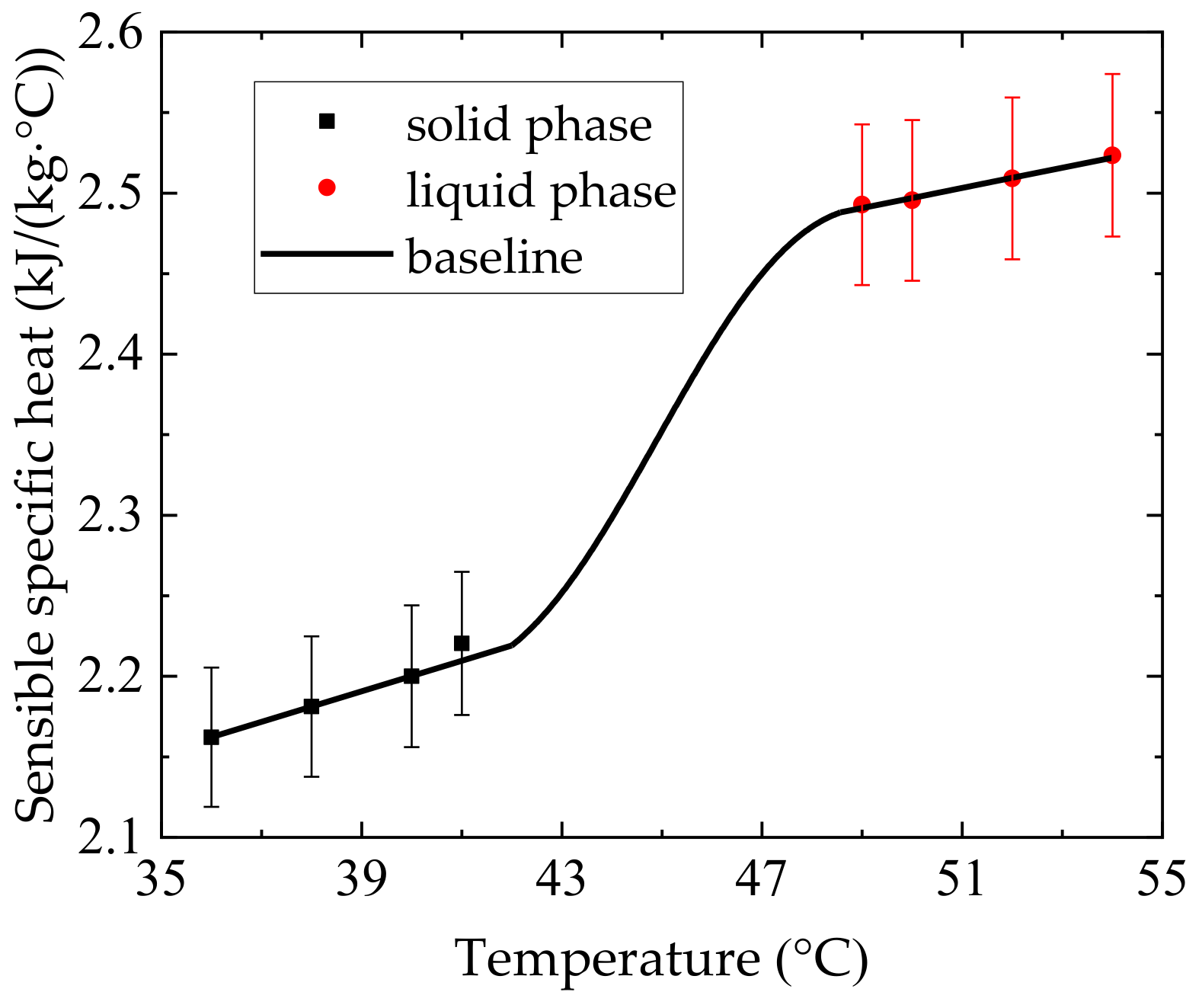
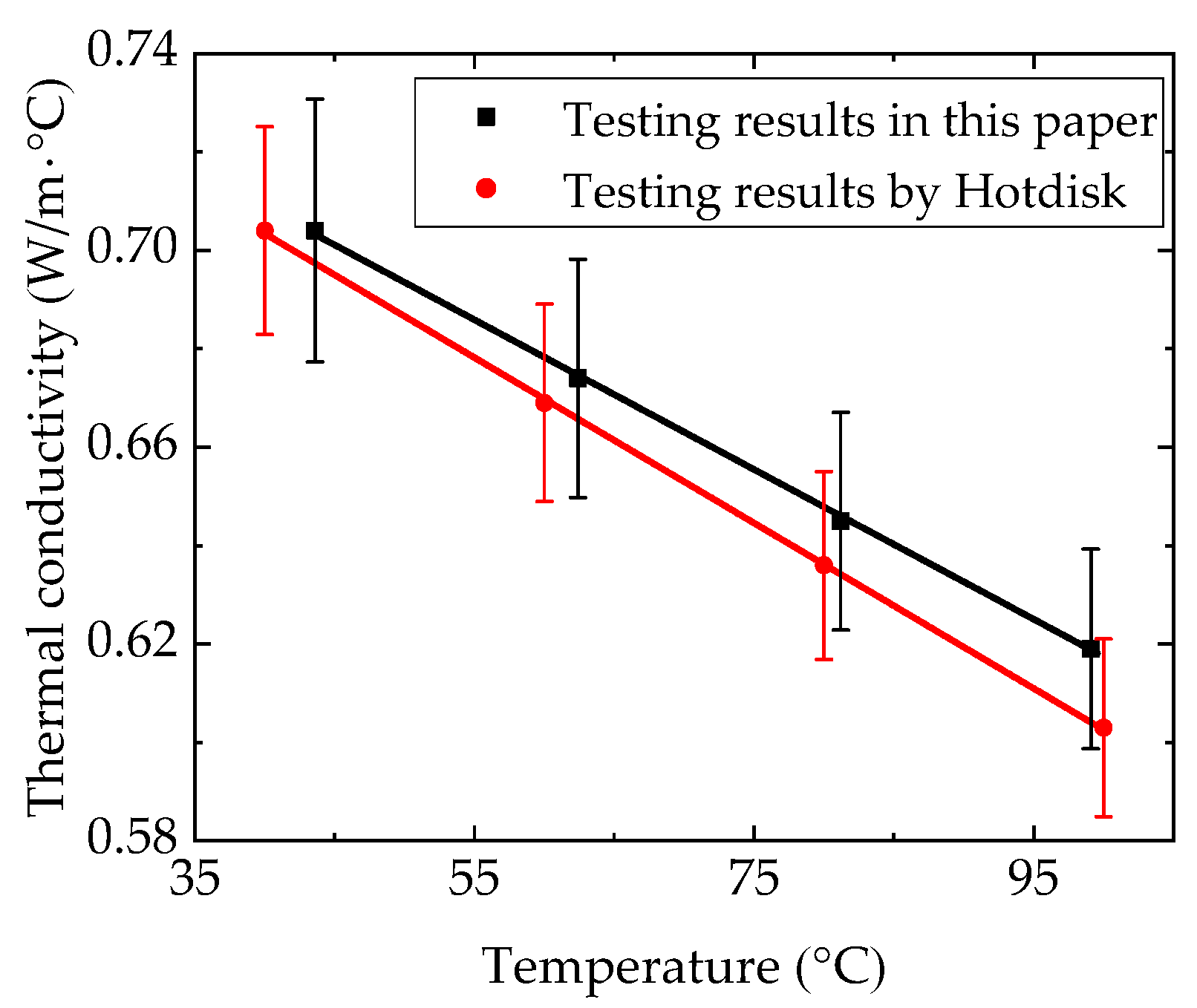
4.1. Thermophysical Properties of n-docosane in the Container
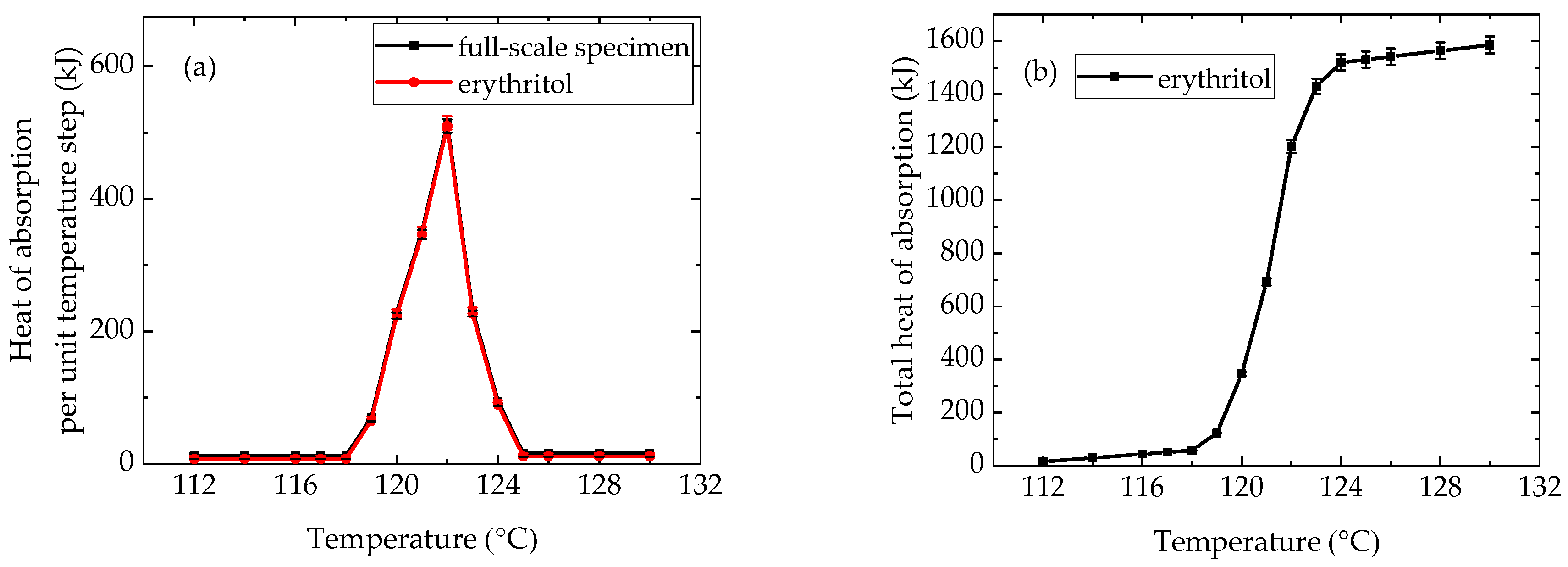
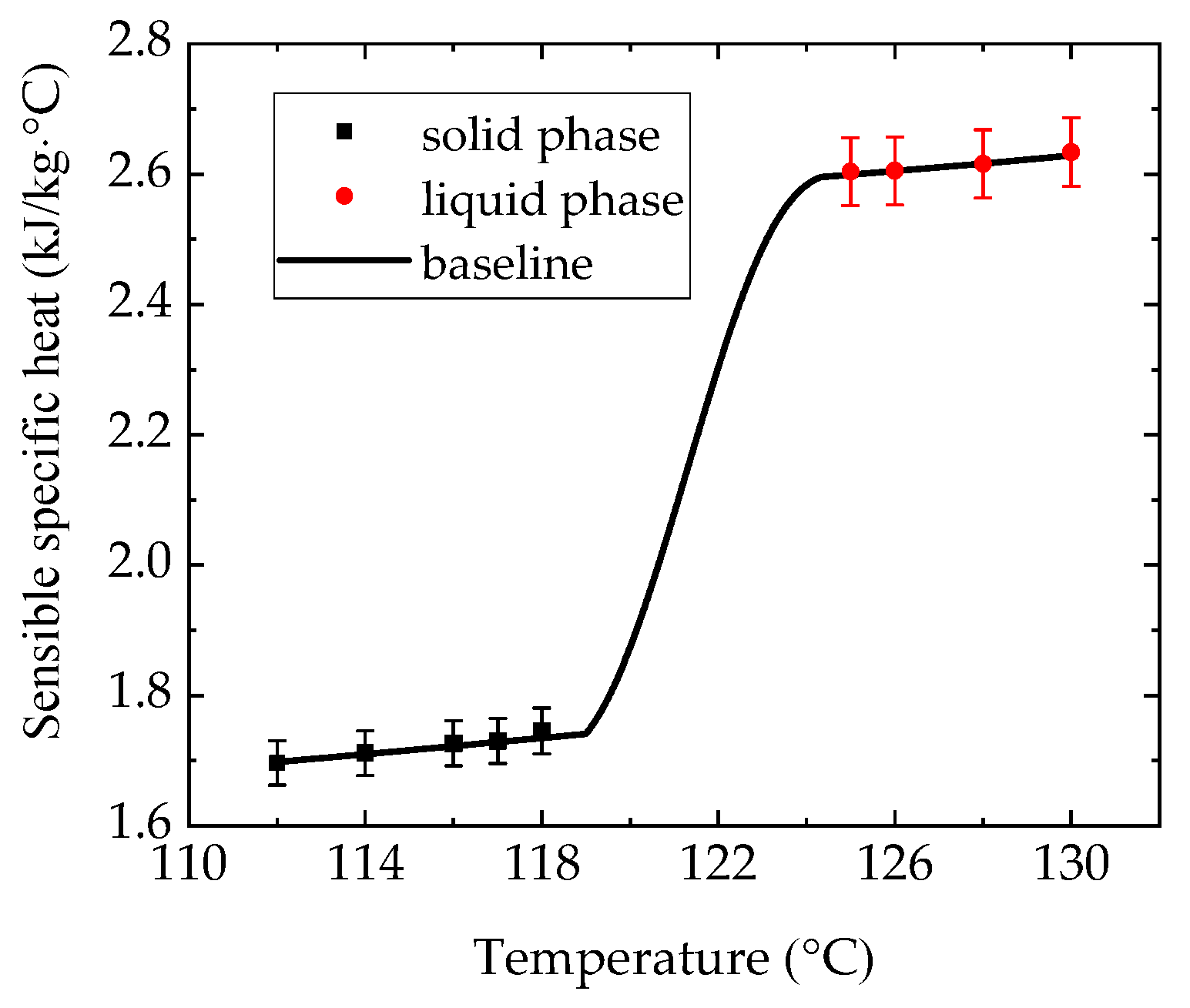
4.2. Thermophysical Properties of Erythritol in the Container
4.3. Uncertainty Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| CV | volume specific heat capacity (kJ/(m3·°C)) |
| CV-304L | volume specific heat capacity of 304L stainless steel (kJ/(m3·°C)) |
| CV-box | volume specific heat capacity of the container (kJ/(m3·°C)) |
| Cm-PCM | mass specific heat capacity of the PCM (kJ/(kg·°C)) |
| E | voltage value read by heat flux meter (μV) |
| Eequi-down | voltage value read by heat flux meter on the lower plate returning to the steady state (μV) |
| Eequi-up | voltage value read by heat flux meter on the upper plate returning to the steady state (μV) |
| Ei-down | voltage value read by heat flux meter on the lower plate at the ith temperature step (μV) |
| Ei-up | voltage value read by heat flux meter on the upper plate at the ith temperature step (μV) |
| Eequi | voltage value returning to the steady state (μV) |
| h | enthalpy (kJ/kg) |
| hA | areal enthalpy (J/m2) |
| hsensible | sensible enthalpy (kJ/kg) |
| L | latent enthalpy (kJ/kg) |
| m | mass (kg) |
| Q | heat of absorption (kJ) |
| q | heat flow (W/m2) |
| R | thermal resistance (m2·°C/W) |
| Rbox-down | thermal resistance the lower wall of the container (m2·°C/W) |
| Rbox-up | thermal resistance the upper wall of the container (m2·°C/W) |
| Rctr | total contact thermal resistance (m2·°C/W) |
| Rctr-up | contact thermal resistance between specimen and upper plate (m2·°C/W) |
| Rctr-down | contact thermal resistance between specimen and lower plate (m2·°C/W) |
| S | sensitivity coefficient of heat flux sensor (W/m2·μV) |
| SHFM | correction coefficient of heat flux sensor (kJ/m2·°C) |
| T | temperature (°C) |
| Ta | initial temperature of temperature step (°C) |
| Tb | termination temperature of temperature step (°C) |
| TC | the temperature of the cold surface of the PCM product after fitting (°C) |
| Tmean | mean temperature of temperature step (°C) |
| TH | the temperature of the hot surface of the PCM product after fitting (°C) |
| ΔT | temperature difference (°C) |
| u | standard uncertainty |
| u’ | relative standard uncertainty |
| V | volume |
| Greek symbols | |
| δ | thickness (m) |
| δbox-down | thickness of the lower wall of the container (m) |
| δbox-up | thickness of the upper wall of the container (m) |
| λ | thermal conductivity (W/m·°C) |
| Δτ | time interval (s) |
| Subcripts | |
| box | container |
| s | PCM with a container |
| C22 | n-docosane |
| down | lower plate |
| ery | erythritol |
| PCM | phase change material without container |
| up | upper plate |
| 304L | 304L stainless steel |
References
- Xie, N.; Huang, Z.W.; Luo, Z.G.; Gao, X.N.; Fang, Y.T.; Zhang, Z.G. Inorganic salt hydrate for thermal energy storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef]
- Mahavar, S.; Sengar, N.; Rajawat, P.; Verma, M.; Dashora, P. Design development and performance studies of a novel single family solar cooker. Renew. Energy 2012, 47, 67–76. [Google Scholar] [CrossRef]
- Hasan, A.; Hejase, H.; Abdelbaqi, S.; Assi, A.; Hamdan, M.O. Comparative effectiveness of different phase change materials to improve cooling performance of heat sinks for electronic devices. Appl. Sci. 2016, 6, 226. [Google Scholar] [CrossRef]
- Sqanson, T.D.; Birur, G.C. NASA thermal control technologies for robotic spacecraft. Appl. Therm. Energy 2003, 23, 1055–1065. [Google Scholar] [CrossRef]
- Renteria, J.D.; Nika, D.L.; Balandin, A.A. Graphene thermal properties: Applications in thermal management and energy storage. Appl. Sci. 2014, 4, 525–547. [Google Scholar] [CrossRef]
- Frusteri, F.; Leonardi, V.; Vasta, S.; Restrccia, G. Thermal conductivity measurement of a PCM based storage system containing carbon fibers. Appl. Therm. Energ. 2005, 25, 1623–1633. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Appl. Therm. Energy 2007, 27, 1271–1277. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Thermal characterization of nitrates and nitrates/expanded graphite mixture phase change materials for solar energy storage. Energ. Convers. Manag. 2013, 73, 86–94. [Google Scholar] [CrossRef]
- Wang, W.L.; Yang, X.X.; Fang, Y.T.; Ding, J.; Yan, J.Y. Enhanced thermal conductivity and thermal performance of form-stable composite phase change materials by using beta-Aluminum nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Chen, M.Z.; Wan, L.; Lin, J.T. Effect of phase-change materials on thermal and mechanical properties of asphalt mixtures. J. Test Eval. 2012, 40, 746–753. [Google Scholar] [CrossRef]
- Motahar, S.; Nikkam, N.; Alemrajabi, A.A.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M. Experimental investigation on thermal and rheological properties of n-octadecane with dispersed TiO2 nanoparticles. Int. Commun. Heat. Mass. 2014, 56, 114–120. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Effective thermal conductivity of open-cell metal foams impregnated with pure paraffin for latent heat storage. Int. J. Therm. Sci. 2014, 81, 94–105. [Google Scholar] [CrossRef]
- Aktay, K.S.D.C.; Tamme, R.; Muller-Steinhagen, H. Thermal conductivity of high-temperature multicomponent materials with phase change. Int. J. Thermophys. 2008, 29, 678–692. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Li, L.; Zhao, M. Research on temperature dependent effective thermal conductivity of composite-phase change materials (PCMs) wall based on steady-state method in a thermal chamber. Energy Build. 2016, 126, 408–414. [Google Scholar] [CrossRef]
- Abyzov, A.M.; Shakhov, F.M. Measurement of the effective thermal conductivity of particulate materials by the steady-state heat flow method in a cuvette. Meas. Sci. Technol. 2014, 25, 125009. [Google Scholar] [CrossRef]
- Stacey, C.; Simplin, A.J.; Jarrett, R.N. Techniques for reducing thermal contact resistance in steady-state thermal conductivity measurements on polymer composites. Int. J. Thermophys. 2016, 37, 107. [Google Scholar] [CrossRef]
- Sun, X.Q.; Lee, K.O.; Medina, M.A.; Chu, Y.H.; Li, C.C. Melting temperature and enthalpy variations of phase change materials (PCMs): A differential scanning calorimetry (DSC) analysis. Phase Transit. 2018, 91, 667–680. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Sun, Y.P.; Liu, M. Eutectic Na2CO3-NaCl salt: A new phase change material for high temperature thermal storage. Sol. Energy Mat. Sol. C 2016, 152, 155–160. [Google Scholar] [CrossRef]
- Banu, D.; Feldman, D.; Hawes, D. Evaluation of thermal storage as latent heat in phase change material wallboard by differential scanning calorimetry and large scale thermal testing. Thermochim. Acta 1998, 317, 39–45. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Jiang, Y.; Jiang, Y. A simple method, the T-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201–205. [Google Scholar]
- Peck, J.H.; Kim, J.J.; Kang, C.D.; Hong, H.K. A study of accurate latent heat measurement for a PCM with a low melting temperature using T-history method. Int. J. Refrig. 2006, 29, 1225–1232. [Google Scholar] [CrossRef]
- Lázaro, A.; Günther, E.; Mehling, H.; Hiebler, S.; Marin, J.M.; Zalba, B. Verification of a T-history installation to measure enthalpy versus temperature curves of phase change materials. Meas. Sci. Technol. 2006, 17, 2168–2174. [Google Scholar] [CrossRef]
- Kravvaritis, E.D.; Antonopoulos, K.A.; Tzivanidis, C. Improvements to the measurement of the thermal properties of phase change materials. Meas. Sci. Technol. 2010, 21, 045103. [Google Scholar] [CrossRef]
- Shukla, N.; Fallahi, A.; Kosny, J. Performance characterization of PCM impregnated gypsum board for building applications. Energy Procedia 2012, 30, 370–379. [Google Scholar] [CrossRef]
- Kosny, J.; Kossecka, E.; Brzezinski, A.; Tleoubaev, A.; Yarbrough, D. Dynamic thermal performance analysis of fiber insulations containing bio-based phase change materials (PCMs). Energy Build. 2012, 52, 122–131. [Google Scholar] [CrossRef]
- Biswas, K.; Shukla, Y.; Desjarlais, A.; Rawal, R. Thermal characterization of full-scale PCM products and numerical simulations, including hysteresis, to evaluate energy impacts in an envelope application. Appl. Therm. Energy 2018, 138, 501–512. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, P.; Dai, J.M. Design and construction of a new steady-state apparatus for medium thermal conductivity measurement at high temperature. Rev. Sci. Instrum. 2017, 88, 104903. [Google Scholar] [CrossRef]
- Chang, S.J.; Wi, S.; Lee, J.; Kim, S. Thermal performance analysis of phase change materials composed of double layers considering heating and cooling period. J. Ind. Eng. Chem. 2019, 72, 255–264. [Google Scholar] [CrossRef]
- Li, J.F.; Lu, W.; Zeng, Y.B.; Luo, Z.P. Simultaneous enhancement of latent heat and thermal conductivity of docosane-based phase change material in the presence of spongy grapheme. Sol. Energy Mat. Sol. C 2014, 128, 48–51. [Google Scholar] [CrossRef]
- Rao, Z.H.; Wang, S.F.; Zhang, Y.L.; Peng, F.F.; Cai, S.H. Molecular dynamics simulation of the thermophysical properties of phase change material. Acta. Phys. Sin. 2013, 62, 056601. [Google Scholar]
- Hearn, J.D.; Smith, G.D. Ozonolysis of mixed oleic acid/n-docosane particles: The roles of phase, morphology, and metastable states. J. Phys. Chem. A 2007, 111, 11059–11065. [Google Scholar] [CrossRef] [PubMed]
- Oya, T.; Nomura, T.; Tsubotga, M.; Okinaka, N. Thermal conductivity enhancement of erythritol as PCM by using graphite and nickel particles. Appl. Therm. Energy 2013, 61, 825–828. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shin, H.K.; Park, M.; Rhee, K.Y.; Park, S.J. Thermal characterization of erythritol/expanded graphite composites for high thermal storage capacity. Carbon 2014, 68, 67–72. [Google Scholar] [CrossRef]
- Karthik, M.; Faik, A.; Blanco-Rodríguez, P.; Rodríguez-Aseguinolaza, J.; D’Aguanno, B. Preparation of erythritol–graphite foam phase change composite with enhanced thermal conductivity for thermal energy storage applications. Carbon 2015, 94, 266–276. [Google Scholar] [CrossRef]
- Talja, R.A.; Roos, Y.H. Phase and state transition effects on dielectric, mechanical, and thermal properties of polyols. Thermochim. Acta 2001, 380, 109–121. [Google Scholar] [CrossRef]
- Kholmanov, I.; Kim, J.; Ou, E.; Ruoff, R.S.; Shi, L. Continuous carbon nonatube-ultrathin graphite hybrid foams for increased thermal conductivity and suppressed subcooling in composite phase change materials. ACS Nano 2015, 9, 11699–11707. [Google Scholar] [CrossRef] [PubMed]
- Diarce, G.; Gandarias, I.; Campos-Celador, Á.; Garcia-Romero, A.; Griesser, U.J. Eutectic mixtures of sugar alcohols for thermal energy storage in the 50–90 °C temperature range. Sol. Energy Mat. Sol. C 2015, 134, 215–226. [Google Scholar] [CrossRef]
- Jensen, C.; Xing, C.; Folsom, C.; Ban, H.; Phillips, J. Design and validation of a high-temperature comparative thermal-conductivity measurement system. Int. J. Thermophys. 2012, 33, 311–329. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dai, J.; Xiao, P. Research on Measurement Technology of Thermophysical Properties for Full-Scale Phase Change Material Product in a Container. Appl. Sci. 2019, 9, 2422. https://doi.org/10.3390/app9122422
Wang Y, Dai J, Xiao P. Research on Measurement Technology of Thermophysical Properties for Full-Scale Phase Change Material Product in a Container. Applied Sciences. 2019; 9(12):2422. https://doi.org/10.3390/app9122422
Chicago/Turabian StyleWang, Yong, Jingmin Dai, and Peng Xiao. 2019. "Research on Measurement Technology of Thermophysical Properties for Full-Scale Phase Change Material Product in a Container" Applied Sciences 9, no. 12: 2422. https://doi.org/10.3390/app9122422
APA StyleWang, Y., Dai, J., & Xiao, P. (2019). Research on Measurement Technology of Thermophysical Properties for Full-Scale Phase Change Material Product in a Container. Applied Sciences, 9(12), 2422. https://doi.org/10.3390/app9122422




