Nondestructive Evaluation of Apple Fruit Quality by Frequency-Domain Diffuse Reflectance Spectroscopy: Variations in Apple Skin and Flesh
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Samples
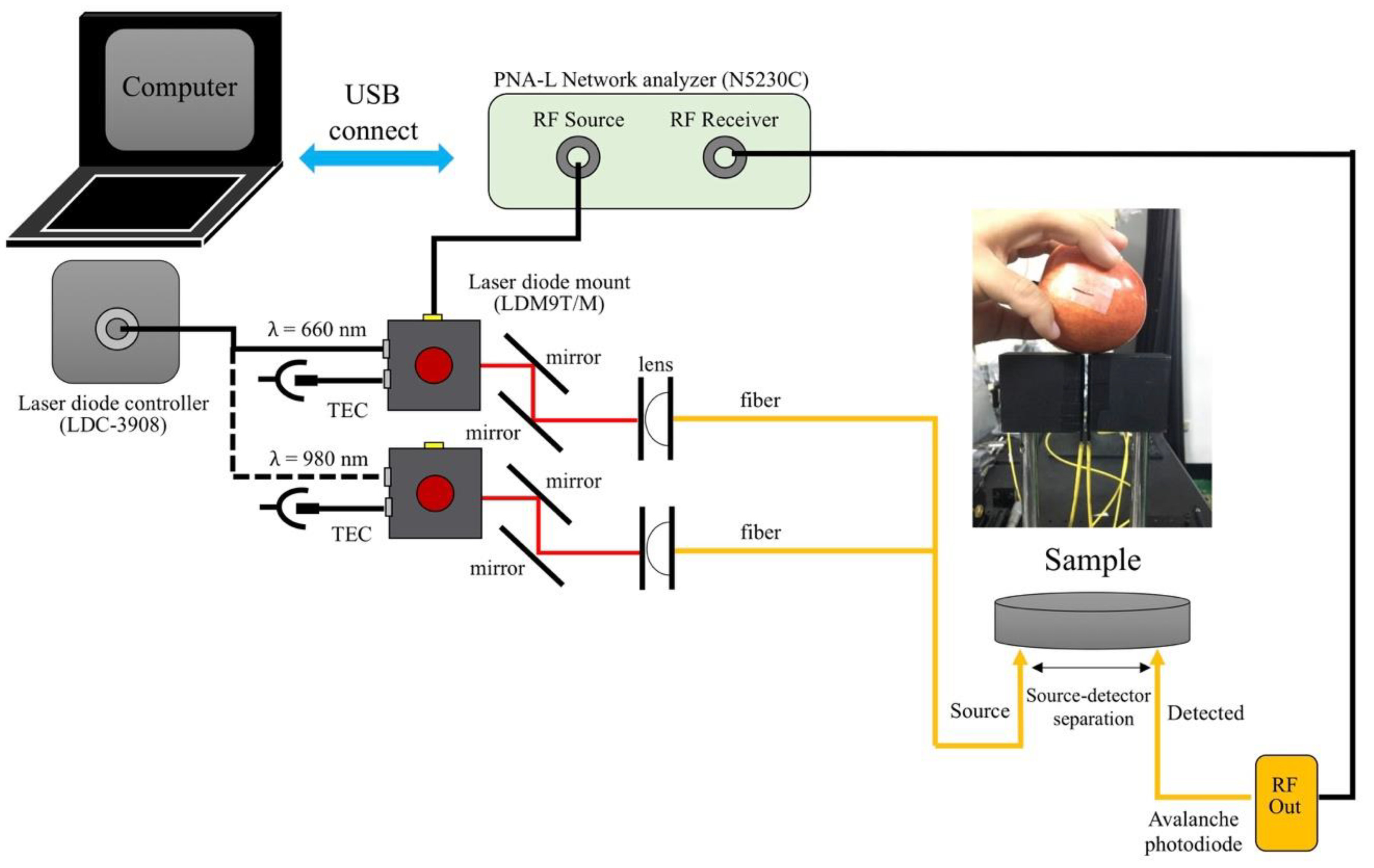
2.2. Frequency-Domain Photon Migration (FDPM) Diffuse Reflectance Spectroscopy (DRS) System and Artificial Neural Network (ANN)
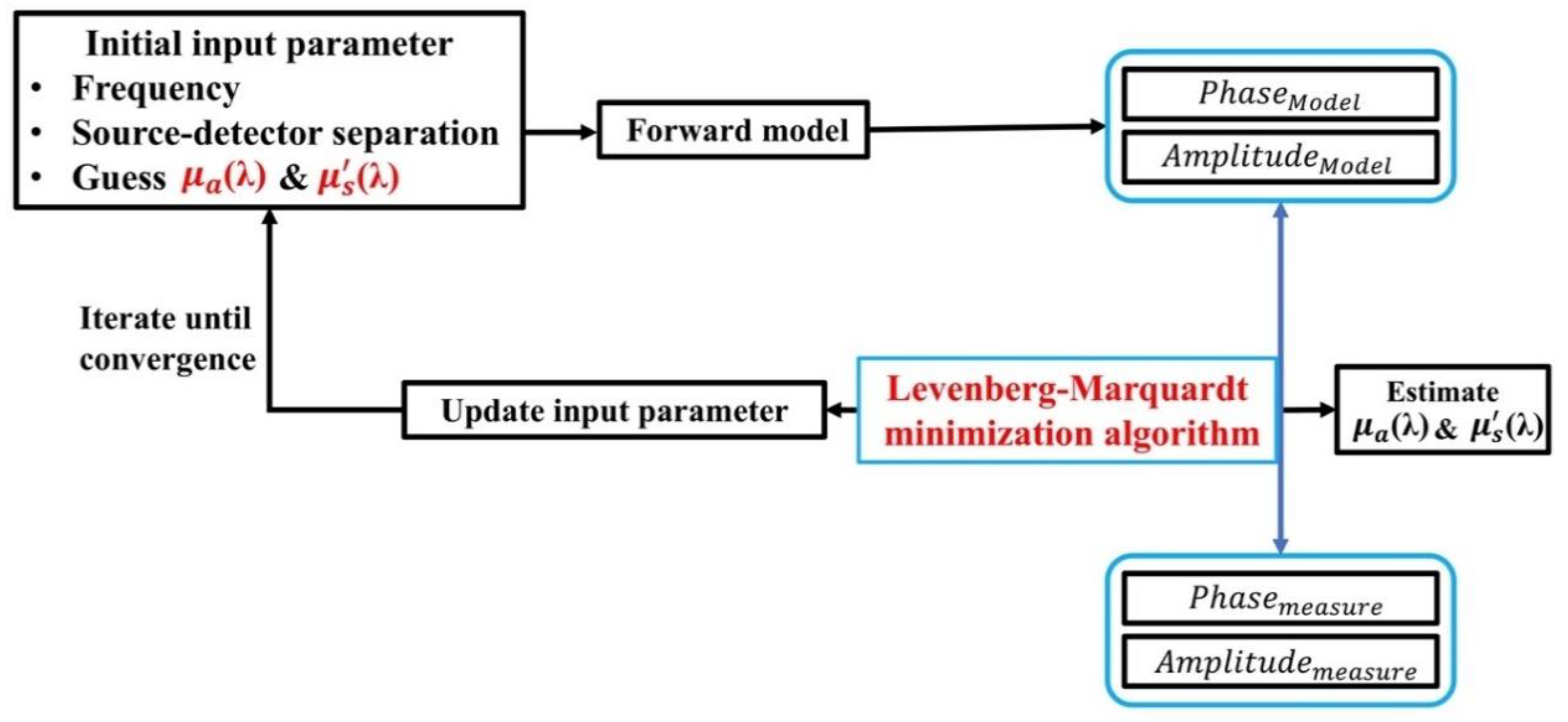
2.3. Optical Properties Measurement
3. Results and Discussion
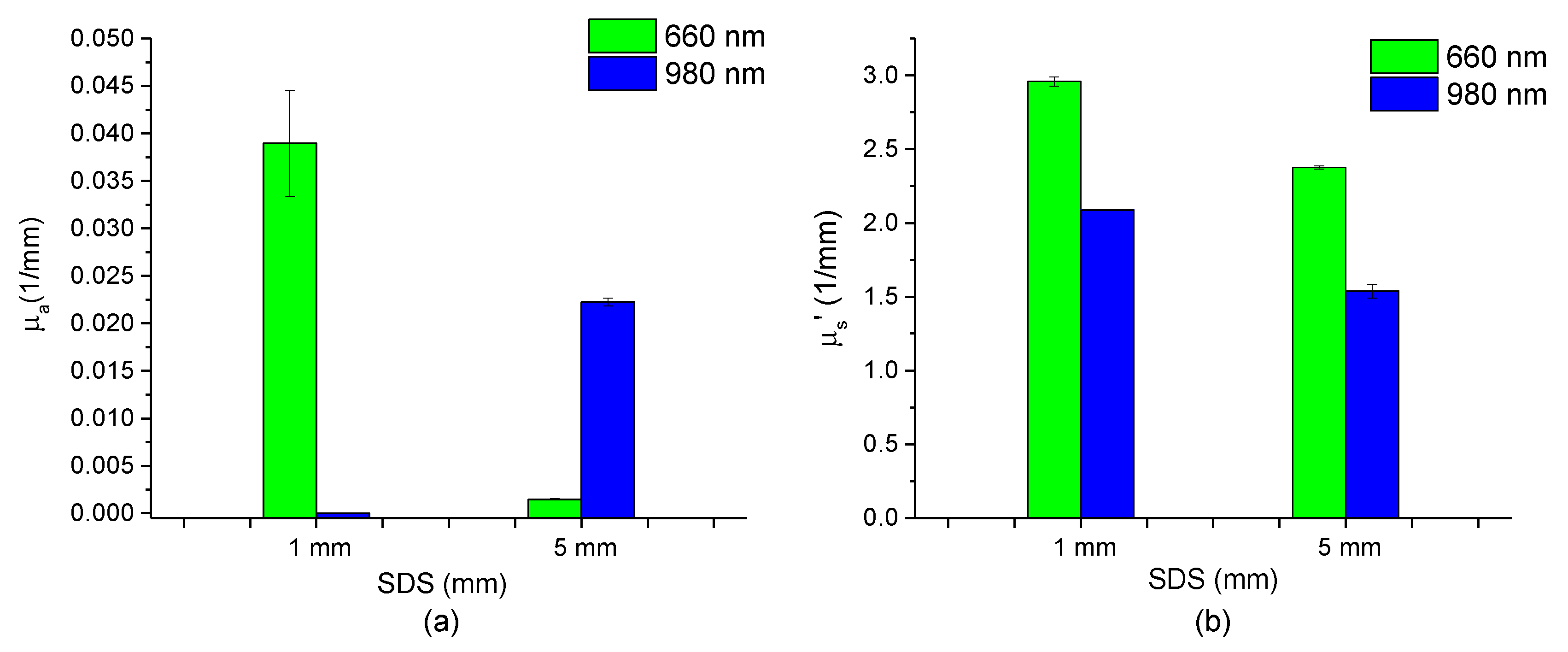
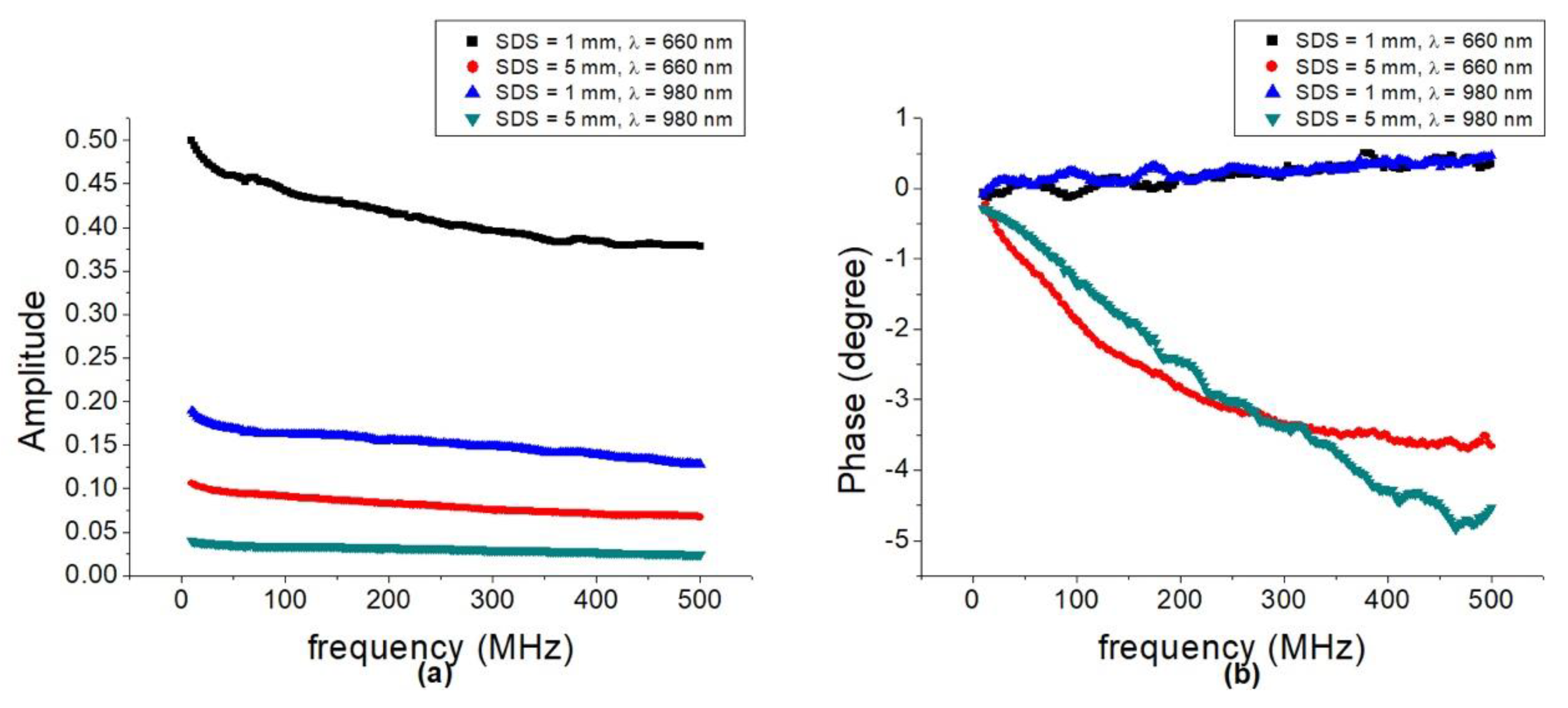
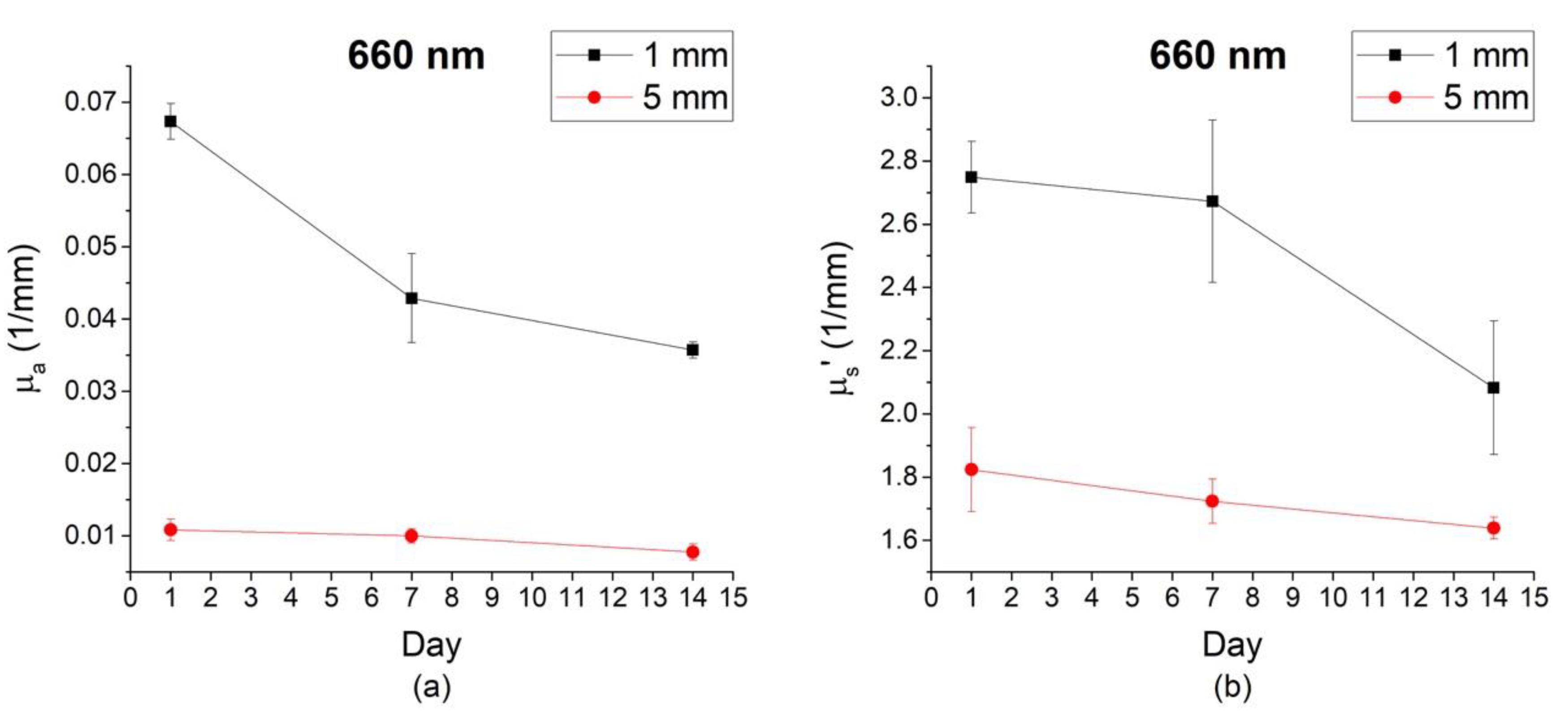
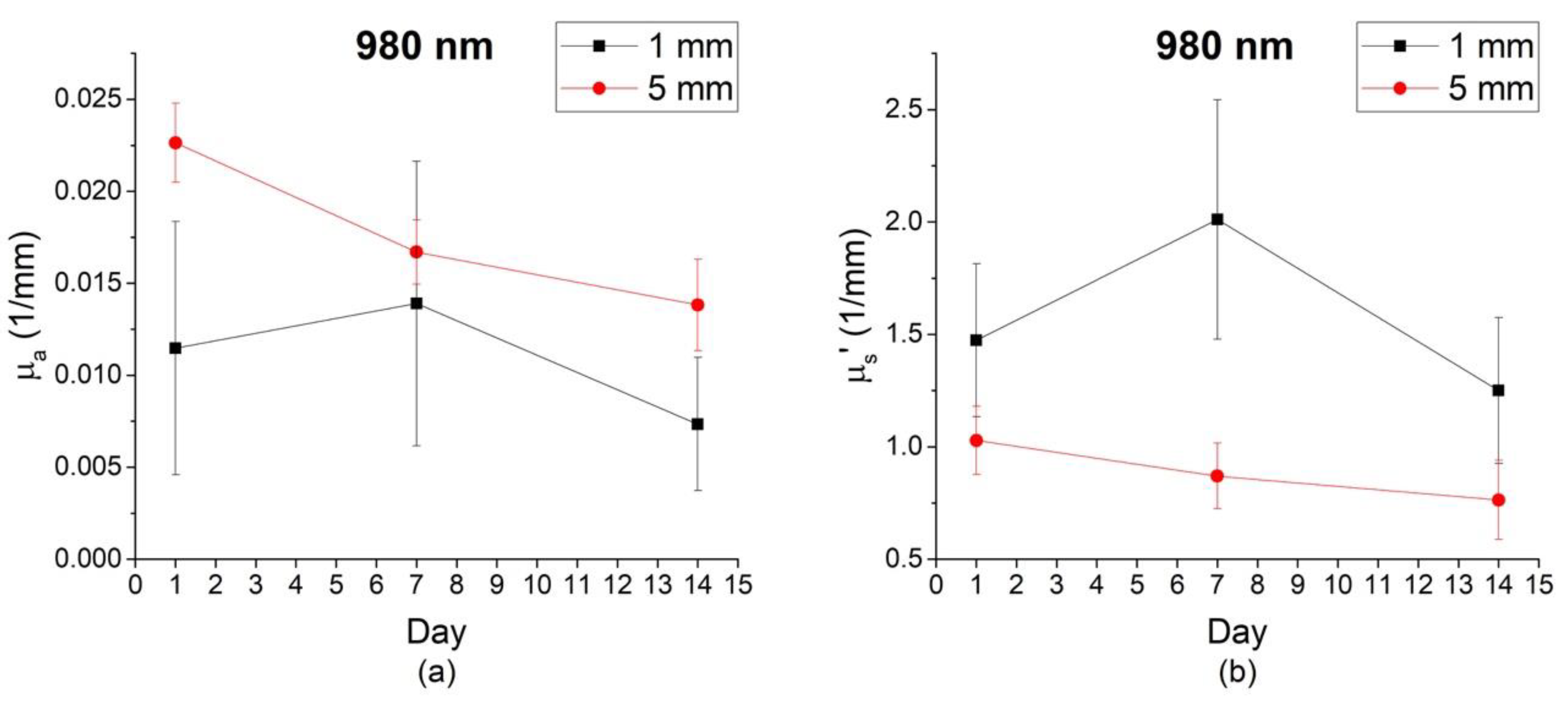
3.1. Optical Properties of Gala Apples at Different Depths
3.2. Optical Properties of Gala Apples at Different Depths and Time During Storage
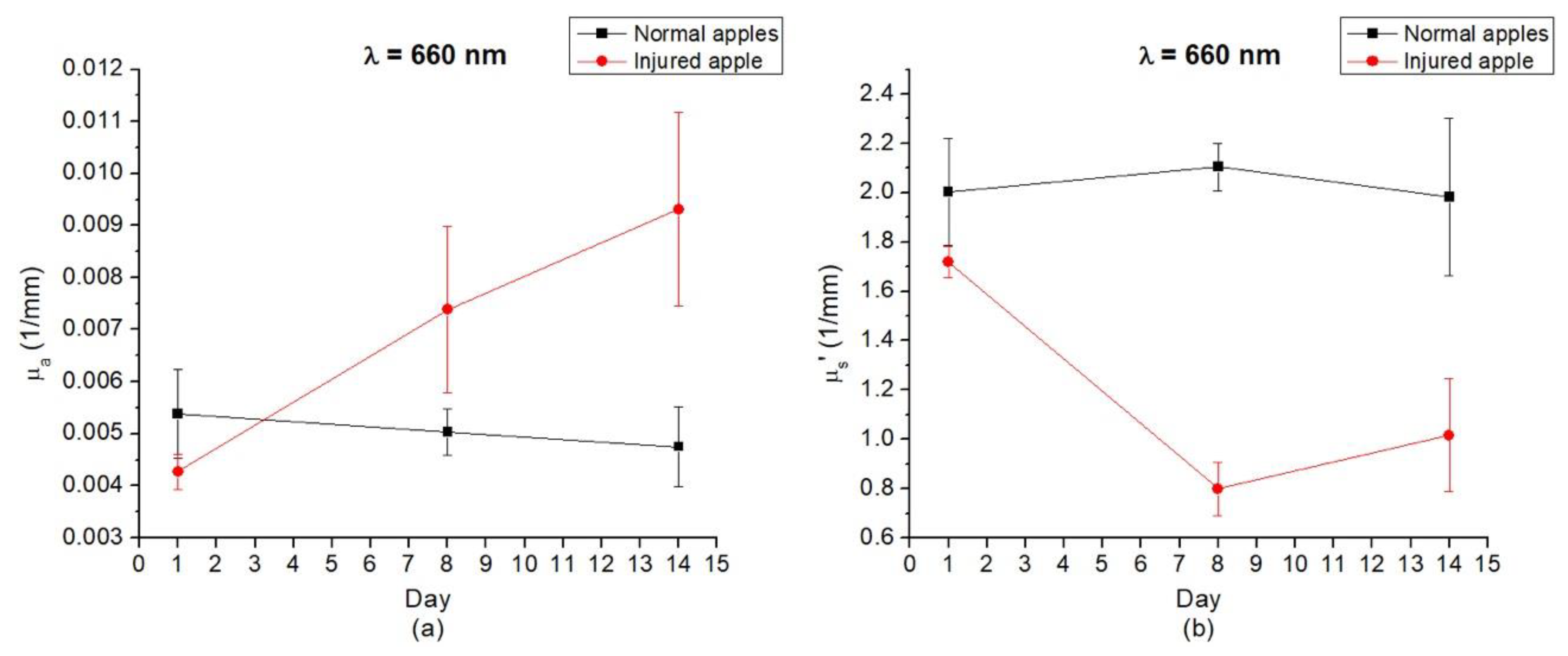
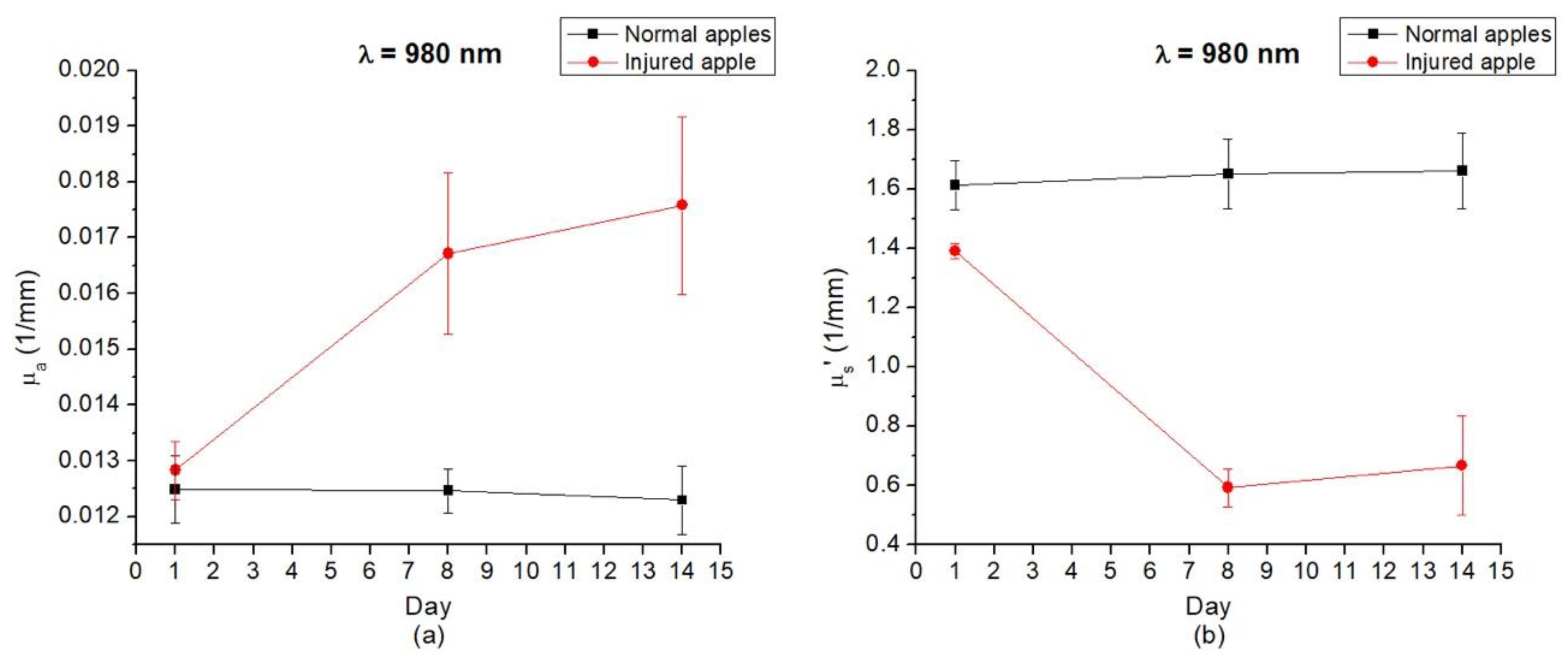
3.3. Optical Properties of Gala Apples that had Irreversible Damage during Storage in Refrigerator
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao-bo, Z.; Jie-wen, Z.; Yanxiao, L.; Holmes, M. In-line detection of apple defects using three color cameras system. Comput. Electron. Agric. 2010, 70, 129–134. [Google Scholar] [CrossRef]
- Lu, R. Detection of bruise on apples using near-infrared hyperspectral imaging. Trans. ASAE 2003, 46, 523–530. [Google Scholar]
- Ariana, D.; Guyer, D.E.; Shrestha, B. Integrating multispectral reflectance and fluorescence imaging for defect detection on apples. Comput. Electron. Agric. 2006, 50, 148–161. [Google Scholar] [CrossRef]
- Moallem, P.; Serajoddin, A.; Pourghassem, H. Computer vision-based apple grading for golden delicious apples based on surface features. Inf. Process. Agric. 2017, 4, 33–40. [Google Scholar] [CrossRef]
- Kleynen, O.; Leemans, V.; Destain, M.-F. Development of a multi-spectral vision system for the detection of defects on apples. J. Food Eng. 2005, 69, 41–49. [Google Scholar] [CrossRef]
- Wang, W.; Li, C. Measurement of the light absorption and scattering properties of onion skin and flesh at 633 nm. Postharvest Biol. Technol. 2013, 86, 494–501. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R.; Mendoza, F.; Beaudry, R.M. Relationship of the optical absorption and scattering properties with mechanical and structural properties of apple tissue. Postharvest Biol. Technol. 2013, 85, 30–38. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Li, T.; Wu, X.; Svanberg, S.; Svanberg, K. Studies of tropical fruit ripening using three different spectroscopic techniques. J. Biomed. Opt. 2014, 19, 067001. [Google Scholar] [CrossRef]
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Dover, C.; Johnson, D.; Ruiz-Altisent, M.; Valero, C. Nondestructive quantification of chemical and physical properties of fruits by time-resolved reflectance spectroscopy in the wavelength range 650–1000 nm. Appl. Opt. 2001, 40, 538–543. [Google Scholar] [CrossRef]
- Saeys, W.; Velazco-Roa, M.A.; Thennadil, S.N.; Ramon, H.; Nicolaï, B.M. Optical properties of apple skin and flesh in the wavelength range from 350 to 2200 nm. Appl. Opt. 2008, 47, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.R.; Cuccia, D.J.; Durkin, A.J. Detection of bruises on golden delicious apples using spatial-frequency-domain imaging. In Advanced Biomedical and Clinical Diagnostic Systems V; 64301O; International Society for Optics and Photonics: San Jose, CA, USA, 2007. [Google Scholar]
- Strain, H.H.; Thomas, M.R.; Katz, J.J. Spectral absorption properties of ordinary and fully deuteriated chlorophylls a and b. Biochim. Biophys. Acta 1963, 75, 306–311. [Google Scholar] [CrossRef]
- Curcio, J.A.; Petty, C.C. The near infrared absorption spectrum of liquid water. JOSA 1951, 41, 302–304. [Google Scholar] [CrossRef]
- Cybulska, J.; Vanstreels, E.; Ho, Q.T.; Courtin, C.M.; Van Craeyveld, V.; Nicolaï, B.; Zdunek, A.; Konstankiewicz, K. Mechanical characteristics of artificial cell walls. J. Food Eng. 2010, 96, 287–294. [Google Scholar] [CrossRef]
- Haskell, R.C.; Svaasand, L.O.; Tsay, T.-T.; Feng, T.-C.; McAdams, M.S.; Tromberg, B.J. Boundary conditions for the diffusion equation in radiative transfer. JOSA A 1994, 11, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Chen, C.-C.; Huang, P.-J.; Tseng, S.-H. Artificial neural networks for retrieving absorption and reduced scattering spectra from frequency-domain diffuse reflectance spectroscopy at short source-detector separation. Biomed. Opt. Express 2016, 7, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Peng, Y. Hyperspectral scattering for assessing peach fruit firmness. Biosyst. Eng. 2006, 93, 161–171. [Google Scholar] [CrossRef]
- Hertog, M.L.; Ben-Arie, R.; Róth, E.; Nicolaï, B.M. Humidity and temperature effects on invasive and non-invasive firmness measures. Postharvest Biol. Technol. 2004, 33, 79–91. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 977–988. [Google Scholar] [CrossRef]
- Rowe, P.I.; Künnemeyer, R.; McGlone, A.; Talele, S.; Martinsen, P.; Seelye, R. Relationship between tissue firmness and optical properties of ‘Royal Gala’ apples from 400 to 1050 nm. Postharvest Biol. Technol. 2014, 94, 89–96. [Google Scholar] [CrossRef]
- Bobelyn, E.; Serban, A.-S.; Nicu, M.; Lammertyn, J.; Nicolai, B.M.; Saeys, W. Postharvest quality of apple predicted by NIR-spectroscopy: Study of the effect of biological variability on spectra and model performance. Postharvest Biol. Technol. 2010, 55, 133–143. [Google Scholar] [CrossRef]
- Bevilacqua, F.; Berger, A.J.; Cerussi, A.E.; Jakubowski, D.; Tromberg, B.J. Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl. Opt. 2000, 39, 6498–6507. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Brackmann, A.; Streif, J.; Bangerth, F. Controlled atmosphere storage of apples: Cell wall composition and fruit softening. J. Hortic. Sci. Biotechnol. 1996, 71, 613–620. [Google Scholar] [CrossRef]
- Murata, M.; Tsurutani, M.; Tomita, M.; Homma, S.; Kaneko, K. Relationship between apple ripening and browning: Changes in polyphenol content and polyphenol oxidase. J. Agric. Food. Chem. 1995, 43, 1115–1121. [Google Scholar] [CrossRef]
- Hashim, N.; Janius, R.B.; Baranyai, L.; Rahman, R.A.; Osman, A.; Zude, M. Kinetic model for colour changes in bananas during the appearance of chilling injury symptoms. Food Bioprocess Technol. 2012, 5, 2952–2963. [Google Scholar] [CrossRef]
- Paull, R. Effect of temperature and relative humidity on fresh commodity quality. Postharvest Biol. Technol. 1999, 15, 263–277. [Google Scholar] [CrossRef]
- Jha, P.K.; Xanthakis, E.; Chevallier, S.; Jury, V.; Le-Bail, A. Assessment of freeze damage in fruits and vegetables. Food Res. Int. 2018, 121, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Ruiz-Altisent, M.; Valero, C.; Ortiz, C.; Dover, C.; et al. Time-resolved reflectance spectroscopy applied to the nondestructive monitoring of the internal optical properties in apples. Appl. Spectrosc. 2001, 55, 1368–1374. [Google Scholar] [CrossRef]
| Normal Apples (N = 5) | Injured Apple (N = 1) | |||
|---|---|---|---|---|
| Day 1 | 0.0054 | 2.004 | 0.0043 | 1.718 |
| Day 7 | 0.0050 | 2.105 | 0.0074 | 0.798 |
| Day 14 | 0.0048 | 1.982 | 0.0093 | 1.017 |
| Normal Apples (N = 5) | Injured Apple (N = 1) | |||
|---|---|---|---|---|
| Day 1 | 0.0125 | 1.613 | 0.0128 | 1.390 |
| Day 7 | 0.0125 | 1.650 | 0.0167 | 0.592 |
| Day 14 | 0.0122 | 1.661 | 0.0176 | 0.667 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, N.-Y.; Chen, C.-C.; Liang, B.-J.; Tseng, S.-H. Nondestructive Evaluation of Apple Fruit Quality by Frequency-Domain Diffuse Reflectance Spectroscopy: Variations in Apple Skin and Flesh. Appl. Sci. 2019, 9, 2355. https://doi.org/10.3390/app9112355
Cheng N-Y, Chen C-C, Liang B-J, Tseng S-H. Nondestructive Evaluation of Apple Fruit Quality by Frequency-Domain Diffuse Reflectance Spectroscopy: Variations in Apple Skin and Flesh. Applied Sciences. 2019; 9(11):2355. https://doi.org/10.3390/app9112355
Chicago/Turabian StyleCheng, Nan-Yu, Chien-Chih Chen, Bo-Jian Liang, and Sheng-Hao Tseng. 2019. "Nondestructive Evaluation of Apple Fruit Quality by Frequency-Domain Diffuse Reflectance Spectroscopy: Variations in Apple Skin and Flesh" Applied Sciences 9, no. 11: 2355. https://doi.org/10.3390/app9112355
APA StyleCheng, N.-Y., Chen, C.-C., Liang, B.-J., & Tseng, S.-H. (2019). Nondestructive Evaluation of Apple Fruit Quality by Frequency-Domain Diffuse Reflectance Spectroscopy: Variations in Apple Skin and Flesh. Applied Sciences, 9(11), 2355. https://doi.org/10.3390/app9112355




