Abstract
Effective and eco-friendly technologies are required for the treatment of tannery wastewater as its biological toxicity and large volume leads toground water pollution. Hydrophobic (unmodified carbon felt) and hydrophilic modified carbon felt with Linde Type A zeolite (LTA zeolite) and bentonite were examined for their effects on bacterial attachment, current generation, and tannery wastewater treatment efficiency. Chronoamperometry and cyclic voltammetry confirmed the higher electron transfer obtained with modified anodes. Maximum current densities of 24.5 and 27.9 A/m² were provided with LTA zeolite and bentonite-modified anodes, respectively, while the unmodified carbon felt gave a maximum current density of 16.9 A/m². Compared with hydrophobic unmodified carbon felt, hydrophilic modified electrodes increased the exploitation of the internal surface area of the 3D structure of the carbon felt by the electroactive biofilm. The study revealed 93.8 ± 1.7% and 96.3 ± 2.1% of chemical oxygen demand (COD) reduction for LTA zeolite and bentonite, respectively. Simultaneous chromium removal was achieved with values of 94.6 ± 3.6 and 97.5 ± 2.2 for LTA zeolite and bentonite, respectively. This study shows the potential approach of carbon felt clay modification for the efficient tannery wastewater treatment using bioelectrochemicals systems (BESs) accompanied with high current recovery.
1. Introduction
Microbial fuel cells (MFCs) technologies have a potential application in simultaneous wastewater treatment and electricity generation [,]. In fact, this technology is able to convert the chemical energy contained in organic and inorganic chemical compounds directly into electricity via microbial bioanodes [,]. The performance of MFCs, in terms of current production and effluent treatment, show considerable variation due to the MFC designs and the electrode materials used. Many electrode materials have been tested to treat domestic or industrial wastewater in MFCs []. However, weak anodic performance, primarily limited by the extracellular electron transfer between the exoelectrogenic bacteria and the anode, represents a major problem for the scaling-up and future practical application of MFCs. Thus, efficient modification of anode electrodes is predicted to improve power output in MFCs [,,,]. For instance, carbon felt coating with akaganeite (b-FeOOH) was found to improve the electrochemical performance of a MFC by increasing the peak current density of the oxidation reaction from 0.7 to 6.1 A/m². A maximum power density of 504 mW/m² was obtained with the anode modified MFCs, which was 2.3 times higher than that with the unmodified anode []. Paul et al. (2017) reported a 3.6-times higher power density with carbon felt anodes modified with graphene oxide (GO)-zeolite composite (GZMA), than that of the unmodified anodes. The highly porous structure and increased hydrophilicity of zeolite with a few layers of thin GO offered better adhesion and higher surface area for bacterial cells to attach to the electrode, which improved anodic kinetics resulting in a greater performance of the MFC.
Although considerable progress has been made recently regarding anode modification for the improvement of MFC energy recovery efficiency, most of this research was restricted to synthetic media only. In contrast, the bioanode designed to treat real wastewater still faces practical barriers such as low current density leaving a large gap between real raw wastewater and synthetic media [].
The present study developed practical, easily fabricated, and low-cost modified electrode materials by Linde Type A (LTA) zeolite and bentonite to assist the bioelectrocatalytic oxidation of tannery wastewater by using an acclimated sludge obtained from a wastewater treatment plant. To the best of our knowledge, this was the first approach to combine both acclimation of activated sludge and electrode modification for the treatment of full strength tannery wastewater without any supplements of salt or vitamins in the BESs.
2. Material and Methods
2.1. Activated Sludge Acclimation to Industrial Tannery Wastewater
The tannery wastewater effluent was collected from industrial tanneries of Fez (Morocco). Initial COD of 5680 mg/L, total chromium of 651 mg/L, and pH 4.8 were quantified in the raw wastewater collected. Activated sludge was collected from a domestic wastewater treatment plant (Castanet-Tolosan, France). The synthetic acclimation medium consisted of (g·L−1) KH2PO4: 4.4, K2HPO4: 3.4, NH4Cl: 1.3, NaCl: 0.5, acetate: 1.0, CaCl2: 0.0146, NaHCO3: 1.0, yeast extract: 0.375, peptone: 0.375. Prior to use, the supernatant of the centrifuged activated sludge was replaced with synthetic medium, then incubated for 24 h at 30 °C. After that, the pellet was decanted and fresh synthetic medium with 20% of tannery wastewater was added to recover the original volume. The procedure of settling, decanting, and addition of synthetic medium and tannery wastewater was repeated at 24 h intervals, increasing the proportion of tannery wastewater by 20% each day, until the entire synthetic medium was replaced by tannery wastewater.
2.2. Preparation of Clay-Modified Electrodes
LTA zeolite and bentonite-modified electrodes were prepared as films coated on carbon felt electrodes. LTA zeolite was synthesized following the protocol of Belaabed et al. [] and bentonite was purchased from Sigma–Aldrich (France). Before deposition, the carbon felt electrode surface was washed with acetone and rinsed with water and ethanol. LTA zeolite and bentonite-modified carbon was prepared by hand mixing 1 g of LTA zeolite or bentonite in 100 mL of ethanol solution. Then, the carbon felt (2 cm²) was placed in the solution and sonicated for 30 min. The modified carbon felt was finally dried at 100 °C [].
2.3. Characterization of Unmodified and Modified Electrodes
FTIR absorption spectra of the unmodified and modified carbon anodes were recorded by a VERTEX 70 Optic spectrophotometer (Bruker) in the wave numbers range from 400 to 1400 cm−1. The specific surface areas (SSAs) of the anode materials were determined from an N2 adsorption–desorption experiment with an ASAP 2020 surface area analyzer (BET method). The hydrophobicity of the anode surfaces was determined by contact angle measurement using a goniometer (GBX Instruments, France).
2.4. Electroanalytical Techniques
All the experiments were run in three-electrode (single chamber) bioelectrochemical reactors (600 mL) and were conducted at 30 °C. Each bioreactor was equipped with a 2 cm² projected surface area, working electrodes connected electrically via a thin platinum wire, a saturated calomel electrode (SCE) (SCE, +0.24 V vs. standard hydrogen electrode), and a 6 cm² platinum grid auxiliary electrode. All the working electrodes were polarized at −0.2 V/SCE using a multi-channel potentiostat (Biologic VSP2). The polarization was periodically suspended to perform cyclic voltammetries at 1 mV/s in the potential range from −0.5 to 0.2 V/SCE. After inoculation, each reactor was purged with N2 for 30 min in order to create anaerobic conditions.
2.5. Biofilm Visualization
A series of Environmental Scanning Electron Microscopy (ESEM) and epifluorescence microscopy images were taken to provide a visual characterization of electrode modifications and the surface morphologies of the biofilm growing over the electrode. Prior to ESEM imaging, fixation was performed using phosphate buffer (400 mM, pH = 7.4) with 4% glutaraldehyde. The samples were rinsed in phosphate buffer containing saccharose (0.4 M) and then dehydrated by immersion in increasing concentrations of the following solutions: acetone (50%, 70%, 100%), acetone and hexamethyldisilazane (50:50), 100% hexamethyldisilazane (HMDS). The last batch of HMDS was dried until all the moisture was removed.
Prior to epifluorescence microscopy and in order to eliminate all soluble and solid materials except the attached biofilms, microbial biofilms developed on electrodes were washed with sterile physiological water. Before being left to dry at room temperature, bioelectrodes were colored with 0.03% acridine orange (A6014, Sigma) for 10 min. The biofilms were imaged using a Carl Zeiss Axio Imager-M2 microscope equipped for epifluorescence with an HXP 200 C light source and the Zeiss 09 filter (excitor HP450e 490, reflector FT 10, barrier filter LP520). Images were obtained with a digital camera (Zeiss AxioCamMRm) every 0.5 µm along the Z-axis and the set of images was processed with the Zen® software.
2.6. Wastewater Analysis Techniques
COD and total chromium were analyzed according to standard methods []. COD was measured using a photometric cuvette test (LCK514 kit, DCO measurement 100–2000 mgO2/L). The % COD removal is defined as:
The Coulombic efficiency (CE) is defined as the ratio of total Coulombs actually transferred to the anode from the substrate and is calculated by:
where M stands for the molecular weight of the substrate, F is Faraday’s constant (96,485 C/mol), b is the number of moles of electrons produced per mol of the substrate, V is the volume of liquid in the anode compartment, and ∆COD is the change in COD over time t.
Total chromium was determined by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES). Each test was performed in triplicate and the mean values were recorded with standard deviations.
Student t-test for COD removal, chromium removal, and CEs were conducted between modified and unmodified carbon felt. The significance level of statistical analyses was set at α = 0.05.
3. Results and Discussion
3.1. Effect of Activated Sludge Acclimation on Electroactive Biofilm Formation
Raw industrial tannery wastewater inoculated with 5% (v/v) of acclimated or unacclimated activated sludge was first tested in three-electrode bioelectrochemical reactors. Unmodified carbon felt material polarized at −0.2 V/SCE was used for the working electrodes, and two successive additions of 20 mM acetate were made at t0 and t17days to accelerate the growth kinetics of the biofilm. The bioelectrochemical reactor inoculated with the unacclimated activated sludge did not produce any current during the 40 days of testing (Figure S1).
In contrast, with the progressively acclimated sludge, after a 5 day lag phase, a rapid increase in current density was detected from day 5 to day 9. The current density reached a maximum exceeding 16 A/m² and gradually decreased after day 19 as the acetate availability was limited. The week of progressive acclimation of activated sludge helped the bacterial population growth to resist the usual negative effects of toxic substances present in tannery wastewater.
3.2. Effect of Carbon Felt Modification
3.2.1. Physicochemical Characterization of the Unmodified and Modified Carbon Felt Surfaces
The FTIR spectra of the unmodified carbon felt before and after modification are shown in Figure 1 and confirmed the successful electrode modification. The IR spectrum of LTA zeolite-modified carbon shows new peaks at ≈460 cm−1, 575 cm−1, and 1090 cm−1 corresponding to vibrational modes bending T–O, double six-rings, and asymmetric stretching vibration of internal tetrahedral T–O–T (with T = Al or Si), respectively, which are the dominating building units in the zeolite structure. Similarly, new peaks appeared at 460 cm−1, 525 cm−1, and 1090 cm−1 in modified bentonite carbon felt spectra, which were attributed to the bending vibration of Si–O–Si, bending vibration of Al–O–Si, and stretching vibration of Si–O, respectively. Additionally, the IR bands between 640 and 950 cm−1 were due to the bending vibration of T–OH–T (with T = Al or Fe or Mg).
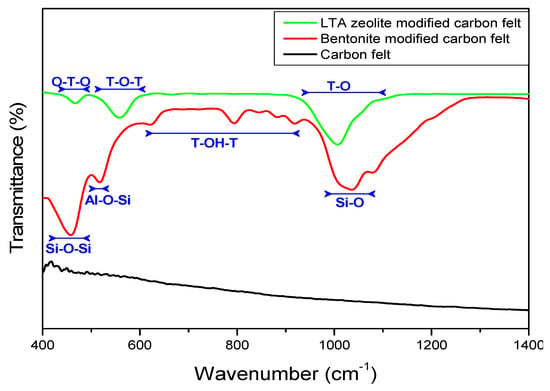
Figure 1.
FTIR analysis of modified and unmodified carbon felt.
The modified electrode mass increased by 0.1 and 0.14 g due to the deposition on carbon felt of LTA zeolite and bentonite, respectively.
As observed by ESEM in Figure 2, the unmodified carbon felt was composed of intermingled tubular fibers with an average diameter of 15–20 µm. The surface of fibers was soft and clean. As a consequence of the carbon felt modification by LTA zeolite and bentonite, the presence of crystalline solid particles was observed, randomly dispersed on the surface of the carbon fibers. More precisely, the ESEM images of LTA zeolite and bentonite-modified carbon felt revealed a particle size ranging from 2 to 4 μm for elementary crystals forming individual deposits or aggregates non-uniformly distributed around carbon fibers.
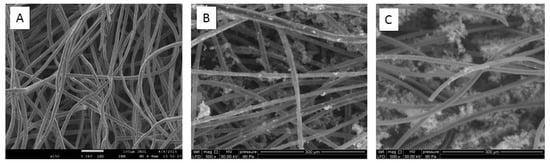
Figure 2.
ESEM images of unmodified and modified carbon felt. (A) Commercial carbon felt, (B) Linde Type A (LTA) zeolite-modified carbon felt, (C) Bentonite-modified carbon felt.
The SSA of the modified carbon felt increased from 6.2 to 13.3 and 16.7 m²/g for LTA zeolite and bentonite, respectively (Table S1). This augmentation of the SSA of the electrode material, by a factor greater than 2, was mainly due to the mesoporous and microporous structures of the clay minerals, which can provide more sites for electroactive biofilm and consequently increases current generation [].
The carbon felt modification by clay mineral coating also had an effect on the surface hydrophobicity. After coating, the surface of the carbon felt changed completely: from superhydrophobic it became superhydrophilic. The carbon hydrophobicity makes the diffusion of the electrolyte toward the felt more difficult than in a hydrophilic architecture and consequently facilitates the biofilm colonization inside the 3D carbon felt [].
3.2.2. Electrochemical Performances of Tannery Wastewater Oxidizing Bioanodes
Chronoamperometry Analysis
The ability to generate a current of oxidation on unmodified electrodes and electrodes modified by clay minerals was evaluated directly in real tannery wastewater completed with acetate (20 mM). The experiments were conducted in parallel and were inoculated at the same time with the same acclimated activated sludge (5% v/v). All the working electrodes were maintained at −0.2 V vs. SCE. The current density on LTA zeolite and bentonite-modified electrodes increased rapidly after the inoculation, reaching 1.5 A/m² and 0.5 A/m², respectively, after only 2 days (Figure 3). Then, the current decreased because of acetate depletion. Successive additions of 20 mM acetate restarted the current generation. After the third addition of acetate, the electroactive biofilm colonizing the modified anodes reached maximum current densities of 24.5 A/m² and 27.9 A/m² for zeolite and bentonite, respectively. In contrast, for unmodified carbon felt, the initial lag time before any current increase was detected was very long. The oxidation current began to increase after 5 days. The highest current density, of 16.9 A/m², was also reached after the third addition of acetate. In sum, coating the carbon felt with clay minerals changed its bioelectrochemical behavior. The start-up time of the current generation using a hydrophilic surface (LTA zeolite and bentonite) was shorter than that required by a hydrophobic surface (unmodified carbon felt). Several groups have reported that microorganisms adhere differently to materials with different hydrophobicity, and it is easier for bacteria to adhere to hydrophilic materials than hydrophobic ones, therefore, hydrophilic materials should be applied as anodes in MFC systems to shorten the start-up time [,].
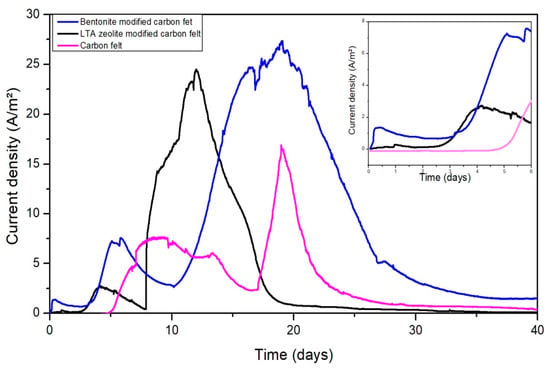
Figure 3.
Evolution of the current density on raw carbon felt and modified carbon felt electrodes polarized at −0.2 V vs. SCE in tannery wastewater and 20 mM of acetate inoculated with acclimated activated sludge. The inset gives a closer look at the first six days of the experiments.
Cyclic Voltammetry
Applying cyclic voltammetry at different phases of the formation of a bioanode and the metabolic activity of electroactive biofilm can provide important information on the mechanism of anodic electron transfer. Figure 4 shows the low scan rate cyclic voltammograms (CVs) recorded at the beginning of the experiment (Figure 4A) and when the current density was at its maximum (Figure 4B) for unmodified and modified electrodes. When the maximum current density was reached, the rate of electron transfer rose rapidly at a potential higher than −0.450 V/SCE.
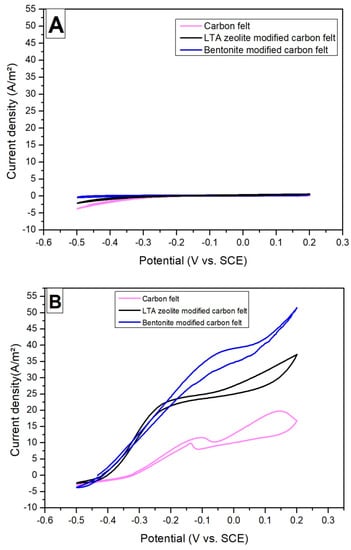
Figure 4.
Cyclic voltammetries recorded on untreated carbon felt and modified carbon felt electrodes in tannery wastewater and 20 mM of acetate inoculated with acclimated activated sludge. (A) At the beginning of the experiment and (B) at the maximum current density measured during the chronoamperometry.
The kinetic behavior of the bioelectrochemical oxidation reaction is clearly different between −0.450 and −0.200 V vs. SCE, on the one hand, and −0.200 and +0.200 V vs. SCE, on the other hand. Between −0.200 and +0.200 V vs. SCE, the kinetics of oxidation slowed down (weaker slope J/E) for bioanodes formed on unmodified and zeolite-modified carbon felt. This slowing down was not as remarkable in the case of the bioanode formed from the bentonite-modified carbon felt, probably because the quantity of bacterial cells ensuring the oxidation activity was greater on this electrode (more biofilm on the electrode where the mineral clay deposit is the highest).Above all, the CV curves showed that LTA zeolite and bentonite-modified carbon felt could provide at least 50 A/m² and 35 A/m², respectively, at a potential of +0.200 V vs. SCE (Figure 4).
3.3. Biofilm Colonization
In order to correlate the maximum current generation obtained with biofilm formation inside the porosity of carbon felt-based electrodes, epifluorescence microscopy was used in conjunction with ESEM imaging to explore the microbial development on and inside the porous carbon felt. Each of the bianodes formed by chronoamperometry on modified and unmodified carbon felt was cross-sectioned so that the external and the internal distribution of the microbial colonization could be imaged. The images of the LTA zeolite and bentonite-modified anodes showed an extensive biofilm covering the complete electrode surface, which was much thicker than the one obtained on unmodified carbon felt (Figure 5).
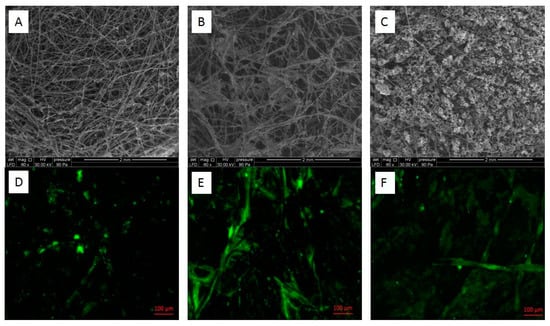
Figure 5.
ESEM and epifluorescence microscopy images of bioanodes formed on unmodified carbon felt electrodes (A,D), LTA zeolite-modified electrode (B,E) and bentonite-modified electrode (C,F) polarized at −0.2 V/ECS for 45 days in tannery wastewater supplemented with 20 mM of acetate and inoculated with acclimated activated sludge.
ESEM imaging of the unmodified electrode presented an open structure with space between colonized fibers, while the modified ones had a very tight network of interwoven threads. An almost uniform biofilm was observable on bentonite-modified carbon felt (Figure 5C), while the LTA zeolite-modified carbon felt surface was only partly clogged (Figure 5B). The images acquired in epifluorescence microscopy confirmed that the colonization was denser on the modified electrodes (Figure 5).
It should be noted that the electrode cross section (Figure 6) shows that the modification of carbon felt allowed a large cross-sectional area for the electroactive biofilm to penetrate into the 3D structure and exploit the internal surface area of the electrode, while unmodified ones are not colonized at all. In contrast, Blanchet et al. attributed the weak biofilm penetration into the 3D porous electrode to the formation of a surface biofilm that clogged the porosity of the felt []. These findings contradict our results because, even when the surface was not totally colonized, biofilm was unable to penetrate into the electrode, which could be explained by the superhydrophobic nature of the carbon felt. However, the superhydrophilic nature of the modified electrode allowed the medium to penetrate into the electrodes, thus making it possible for biofilm colonize the 3D carbon felt.
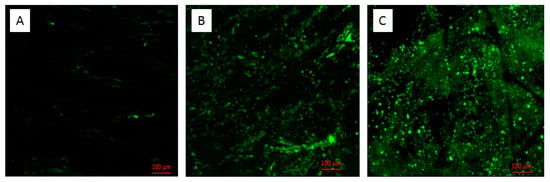
Figure 6.
Epifluorescence microscopy images of cross sectioned anodes formed on carbon felt (A), LTA zeolite-modified anodes (B), and bentonite-modified anodes (C).
3.4. COD, Chromium Removal, and Coulombic Efficiency
3.4.1. COD Removal and Coulombic Efficiency
It was observed that the LTA zeolite and bentonite-catalyzed electrode performances were better in terms of COD removal and CE as compared to the unmodified carbon felt. Table 1 shows the COD reduction and CE for batch runs for unmodified carbon felt, LTA zeolite, and bentonite-modified anodes. The COD removal for bentonite and LTA zeolite-modified carbon felt (96.3 ± 2.1 % and 93.8 ± 1.7%, respectively) was significantly higher (p < 0.001) than that obtained with the unmodified carbon felt (78.7 ± 1.3).

Table 1.
COD removal rate and COD removal rate constant, chromium removal rate, and coulombic efficiency (CE) obtained with unmodified and modified carbon felt bioanodes.
CEs increased significantly (p < 0.001) when the modified anodes were employed. The CE was 29.4 ± 1.5%with the bentonite-modified anode, 21.2 ± 2.1% with the LTA zeolite-modified anode, and 14.4 ± 1.9% with the unmodified carbon felt, even though the same tannery wastewater was used. The relatively higher CE obtained with the modified anodes might be explained by a greater amount of electro-oxidative bacteria with the ability to oxidize more organic substrates (COD) and transfer more electrons.
3.4.2. Chromium Removal
Student t-tests underlined that for each anode the differences in the chromium removal rate were significant (p < 0.001). High chromium removal values (94.6 ± 3.6% and 97.5 ± 2.2%) were found using LTA zeolite and bentonite, respectively (Table 1). Meanwhile, a lower chromium removal value was found using the unmodified carbon felt (72.4 ± 3.1%). These findings could be explained by the adsorption properties of zeolite and bentonite [,]. Also, the microorganisms adhering to LTA zeolite and bentonite were proven to be highly efficient in biological heavy metal removal from wastewater, especially of chromium [,]. There are several mechanisms that might contribute to chromium removal in the BESs. The chromium removal in the unmodified carbon felt with acclimated activated sludge suggests that biosorption is an important mechanism for the heavy metals. The components participating in biosorption are principally the functional groups (carboxylate, hydroxyl, phosphate, amine, and sulfate) of polysaccharides, proteins, and lipids on the microbial cell walls []. Metal precipitation due to the presence of bicarbonate anions in the solution may also contribute to the metal removal in this study, since bicarbonate is created as a byproduct of acetate oxidation at the bioanode. The non-inoculated reactors with activated sludge showed electrodeposition of low concentrations of chromium on the surface of the carbon felt anode, which means that chromium reduction was implicated in the chromium removal process. Besides the mechanisms described above, the LTA zeolite and bentonite particles adsorbed chromium in modified anodes.
4. Conclusions
Bioelectrochemical systems electrodes were effectively modified by LTA zeolite and bentonite. The resulting electrodes were successfully explored for tannery wastewater treatment and proved that the clay modification approach anticipated in this study is reliable and BESs can be fabricated using low-cost electrodes to maximize COD/chromium removal from real wastewater with improved electricity generation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/9/11/2259/s1, Table S1: Characteristics of unmodified and modified carbon felts; Figure S1: Variation of the current density on carbon felt electrodes polarized at −0.2 V vs. SCE in tannery wastewater and 20 mM of acetate inoculated with acclimated activated sludge (dotted line) or non-acclimated activated sludge (solid gray line).
Author Contributions
All authors contributed conception of the manuscript. A.E. and R.E.k. wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version. A.E., B.E., S.E. and R.E.k. contributed to conception and editing of the manuscript, and final approval of the version to be published.
Funding
This work was done as part of the Volubilis project. We are grateful to the organizations responsible for managing this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ezziat, L.; Elabed, A.; Ibnsouda, S.; El Abed, S. Challenges of Microbial Fuel Cell Architecture on Heavy Metal Recovery and Removal From Wastewater. Front. Energy Res. 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Yu, H.-Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2013, 7, 911–924. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Paul, D.; Noori, M.T.; Rajesh, P.P.; Ghangrekar, M.M.; Mitra, A. Modification of carbon felt anode with graphene oxide-zeolite composite for enhancing the performance of microbial fuel cell. Sustain. Energy Technol. Assessments 2018, 26, 77–82. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Li, T.; Dong, Z.; Wang, Y. Modification of carbon felt anodes using double-oxidant HNO3/H2O2 for application in microbial fuel cells. RSC Adv. 2018, 8, 2059–2064. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Ying, M.; Fu, Y.B.; Chen, W. Improving Electrochemical Performance of Carbon Felt Anode by Modifying With Akaganeite in Marine Benthic Microbial Fuel Cells. Fuel Cells 2019, 19, 190–199. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Sayed, E.T.; Cho, H.; Park, M.; Obaid, M.; Kim, H.Y.; Barakat, N.A.M. Effective strategies for anode surface modification for power harvesting and industrial wastewater treatment using microbial fuel cells. J. Environ. Manag. 2018, 206, 228–235. [Google Scholar] [CrossRef]
- Blanchet, E.; Desmond, E.; Erable, B.; Bridier, A.; Bouchez, T.; Bergel, A. Comparison of synthetic medium and wastewater used as dilution medium to design scalable microbial anodes: Application to food waste treatment. Bioresour. Technol. 2015, 185, 106–115. [Google Scholar] [CrossRef]
- Belaabed, R.; Elabed, S.; Addaou, A.; Laajab, A.; Rodr??guez, M.A.; Lahsini, A. Synthesis of LTA zeolite for bacterial adhesion. Bol. la Soc. Esp. Ceram. Vidr. 2016, 55, 152–158. [Google Scholar] [CrossRef]
- Wu, X.Y.; Tong, F.; Song, T.S.; Gao, X.Y.; Xie, J.J.; Zhou, C.C.; Zhang, L.X.; Wei, P. Effect of zeolite-coated anode on the performance of microbial fuel cells. J. Chem. Technol. Biotechnol. 2013, 90, 87–92. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenbaerg, A.E.; Eaton, A.D. Standard Methods for Examination of Water and Wastewater, 20th ed.; APHA American Public Health Association: Washington, DC, USA, 1998; Volume 552, pp. 5–16. [Google Scholar]
- Di Lorenzo, M.; Scott, K.; Curtis, T.P.; Head, I.M. Effect of increasing anode surface area on the performance of a single chamber microbial fuel cell. Chem. Eng. J. 2010, 156, 40–48. [Google Scholar] [CrossRef]
- Thu Pham, H.T.; Jo, C.; Lee, J.; Kwon, Y. MoO2 nanocrystals interconnected on mesocellular carbon foam as a powerful catalyst for vanadium redox flow battery. RSC Adv. 2016, 6, 17574–17582. [Google Scholar] [CrossRef]
- Guo, K.; Freguia, S.; Dennis, P.G.; Chen, X.; Donose, B.C.; Keller, J.; Gooding, J.J.; Rabaey, K. Effects of surface charge and hydrophobicity on anodic biofilm formation, community composition, and current generation in bioelectrochemical systems. Environ. Sci. Technol. 2013, 47, 7563–7570. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Guilizzoni, M.; Correa Baena, J.P.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The effects of carbon electrode surface properties on bacteria attachment and start up time of microbial fuel cells. Carbon N. Y. 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Blanchet, E.; Erable, B.; De Solan, M.L.; Bergel, A. Two-dimensional carbon cloth and three-dimensional carbon felt perform similarly to form bioanode fed with food waste. Electrochem. Commun. 2016, 66, 38–41. [Google Scholar] [CrossRef]
- Basaldella, E.I.; Vázquez, P.G.; Iucolano, F.; Caputo, D. Chromium removal from water using LTA zeolites: Effect of pH. J. Colloid Interface Sci. 2007, 313, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Xi, Y.; Megharaj, M.; Krishnamurti, G.S.R.; Rajarathnam, D.; Naidu, R. Remediation of hexavalent chromium through adsorption by bentonite based Arquad® 2HT-75 organoclays. J. Hazard. Mater. 2010, 183, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadou, I.A.; Papoulis, D.; Chrysikopoulos, C.V.; Panagiotaras, D.; Karakosta, E.; Fardis, M.; Papavassiliou, G. Attachment of Pseudomonas putida onto differently structured kaolinite minerals: A combined ATR-FTIR and 1H NMR study. Colloids Surf. B Biointerfaces 2011, 84, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Erdoan, B.C.; Ülkü, S. Cr(VI) sorption by using clinoptilolite and bacteria loaded clinoptilolite rich mineral. Microporous Mesoporous Mater. 2012, 152, 253–261. [Google Scholar] [CrossRef]
- Vullo, D.L.; Ceretti, H.M.; Daniel, M.A.; Ramírez, S.A.M.; Zalts, A. Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. Bioresour. Technol. 2008, 99, 5574–5581. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).