Effect of Nanoparticles Addition on the Microstructure and Properties of Lead-Free Solders: A Review
Abstract
1. Introduction
2. SnAgCu-Based Solders
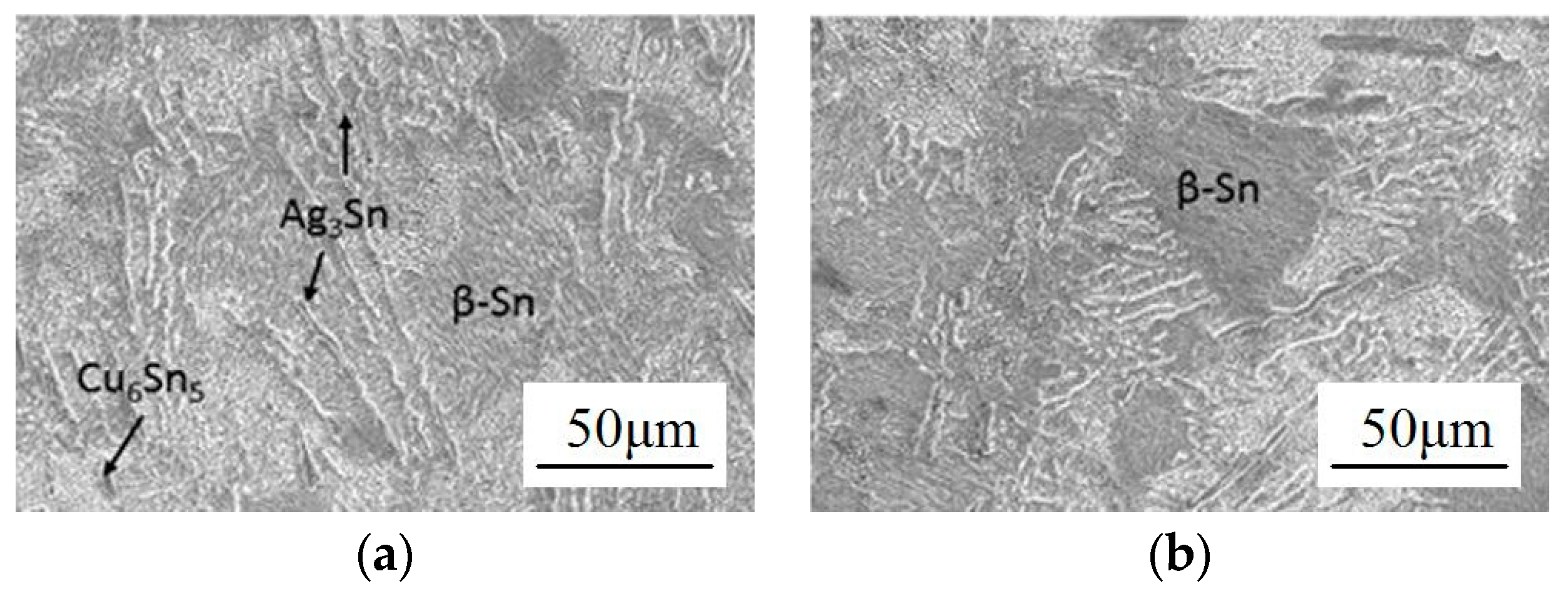
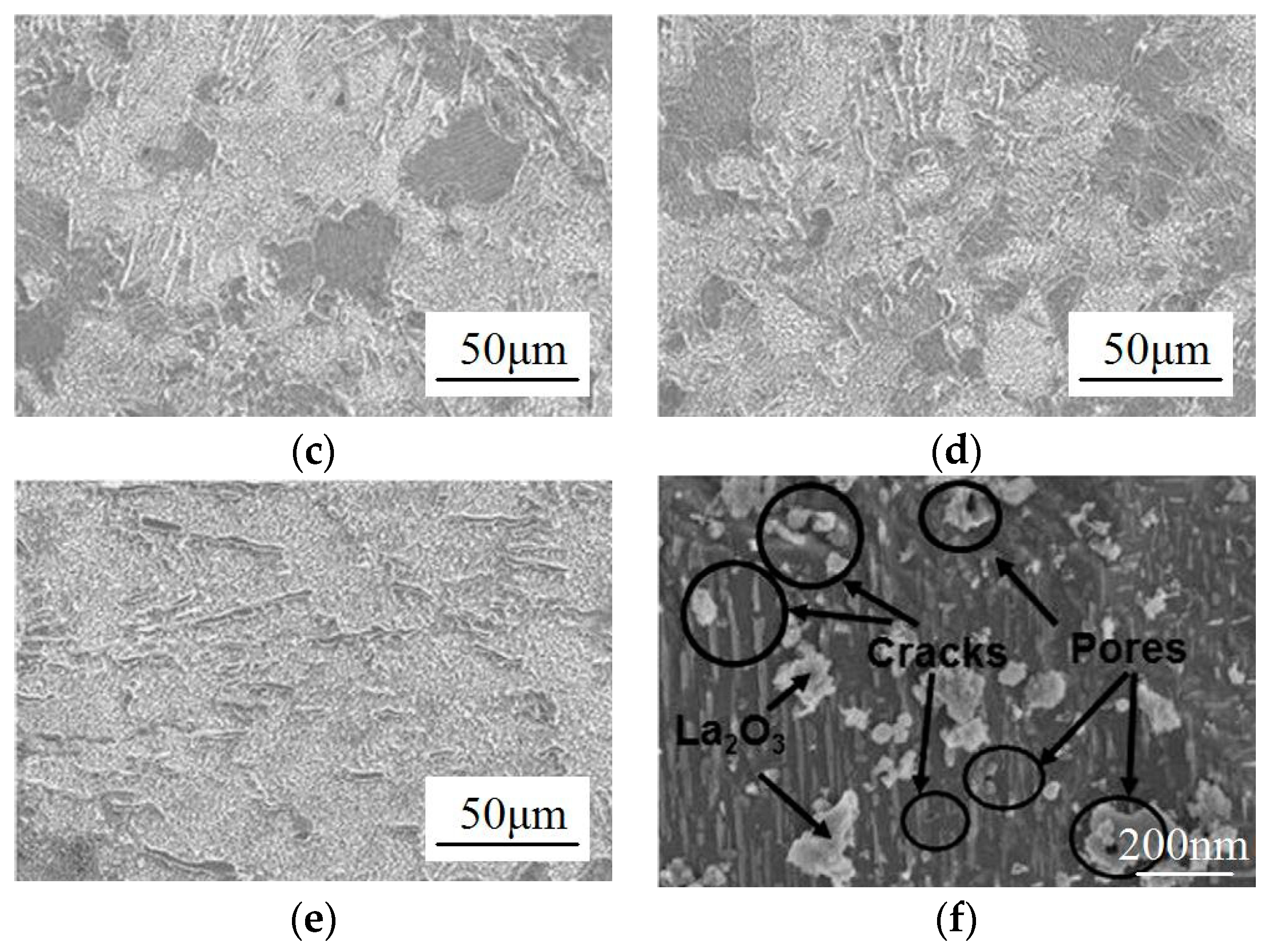
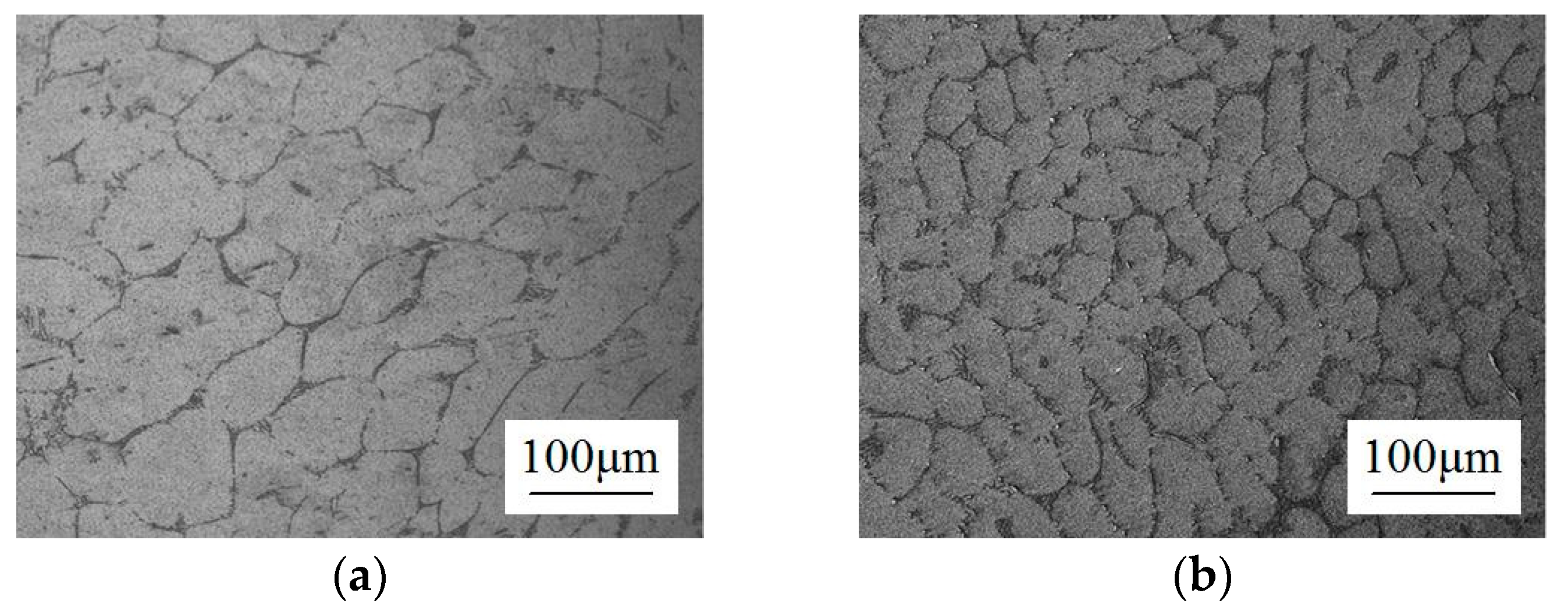
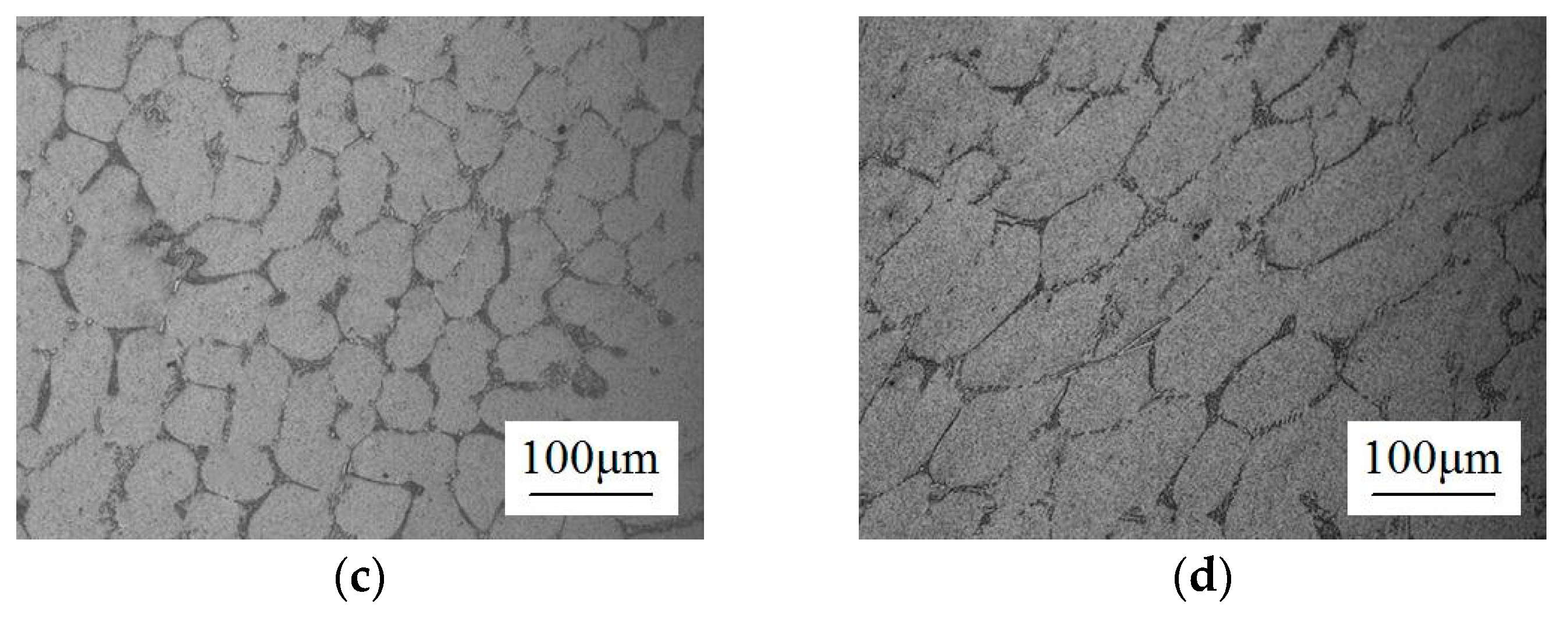
2.1. Microstructure
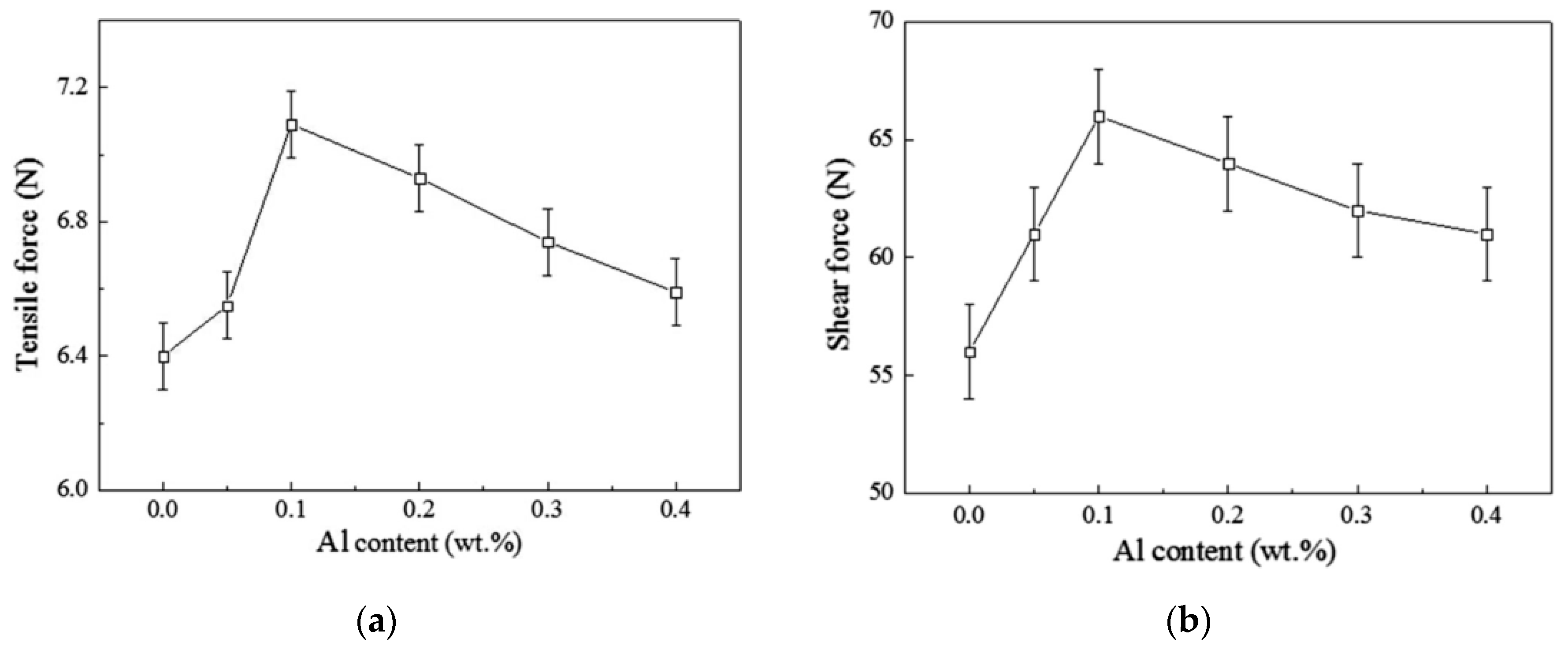
2.2. Mechanical Properties
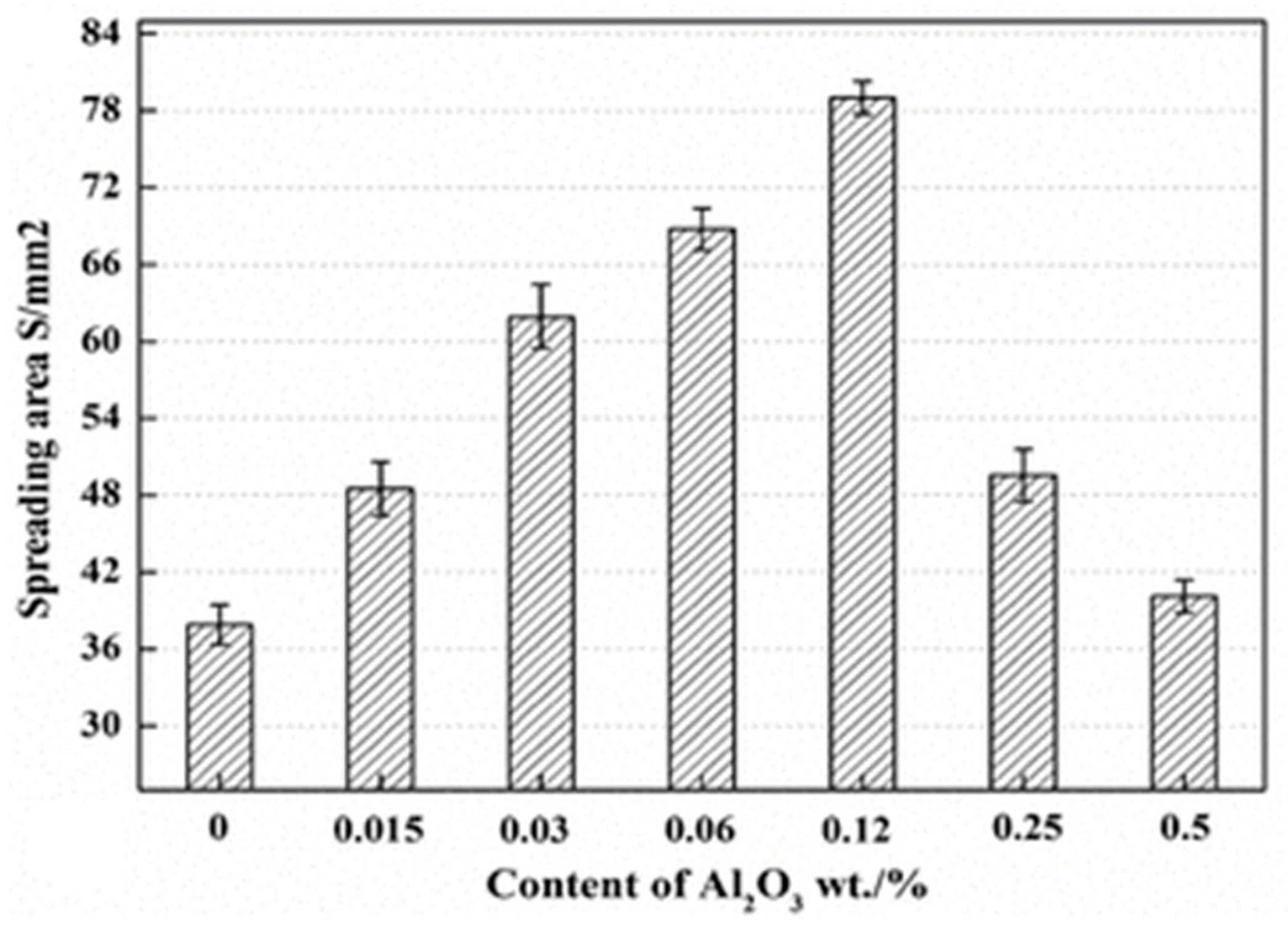
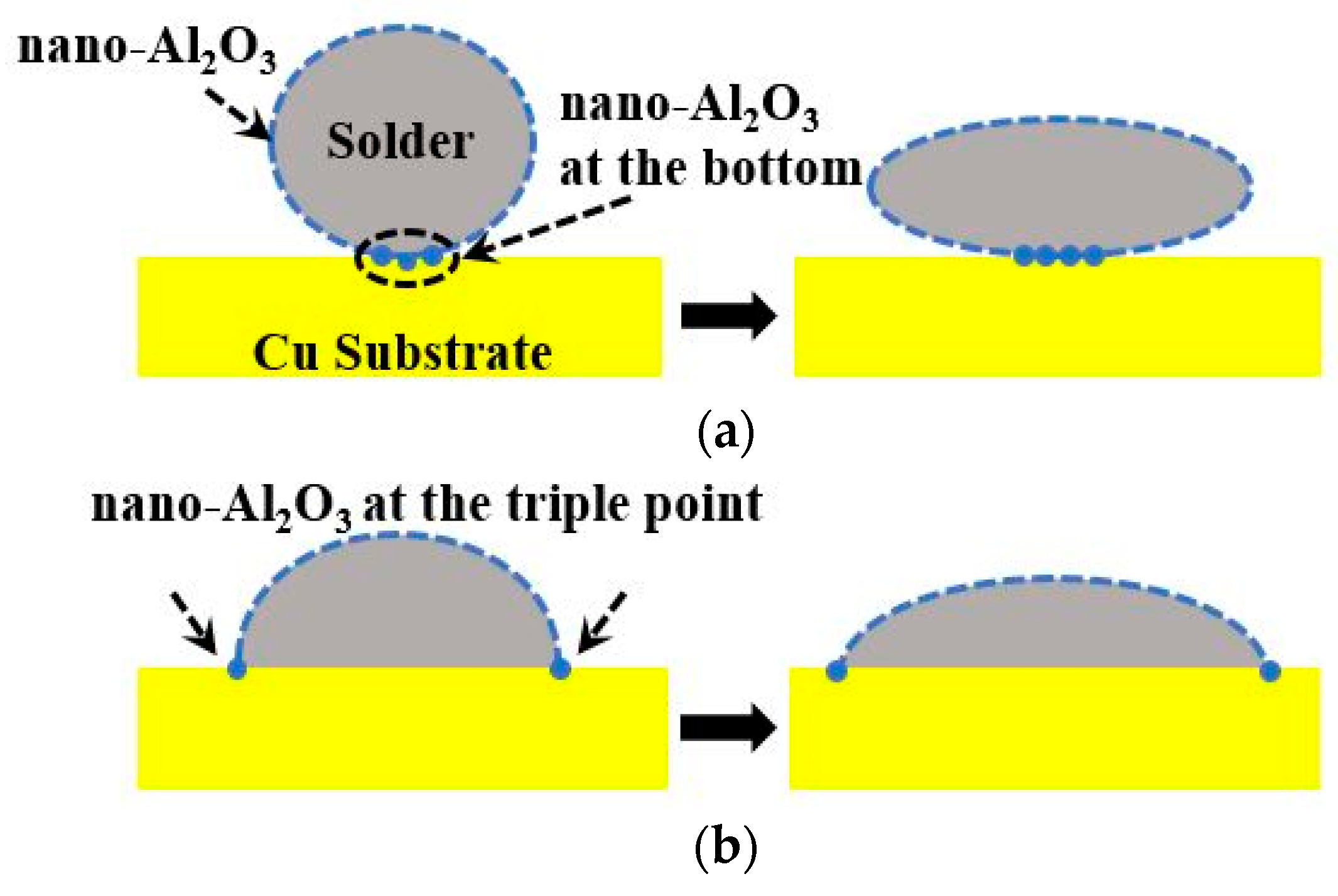
2.3. Wettability
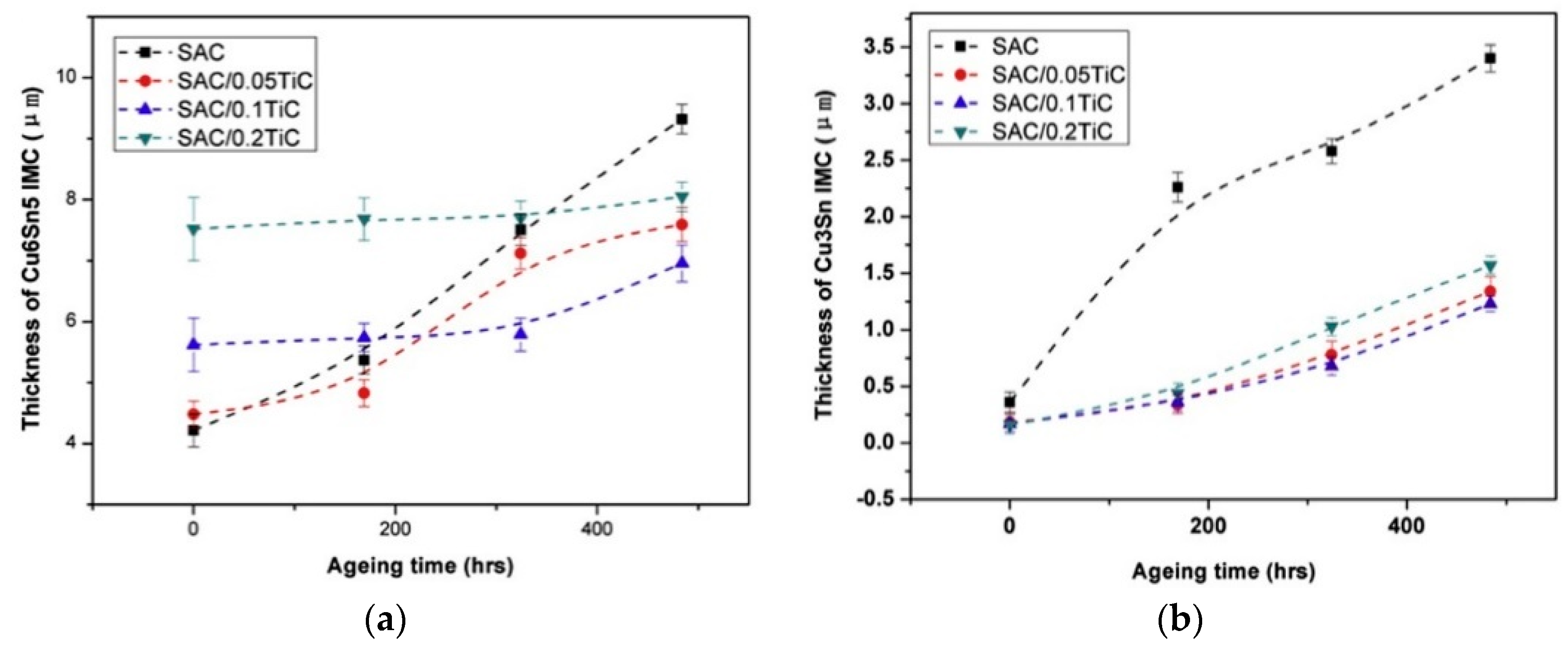
2.4. Reliability
3. SnBi-Based Solders
3.1. Microstructure
3.2. Mechanical Properties
3.3. Wettability
3.4. Reliability
4. SnZn-Based Solders
4.1. Microstructure
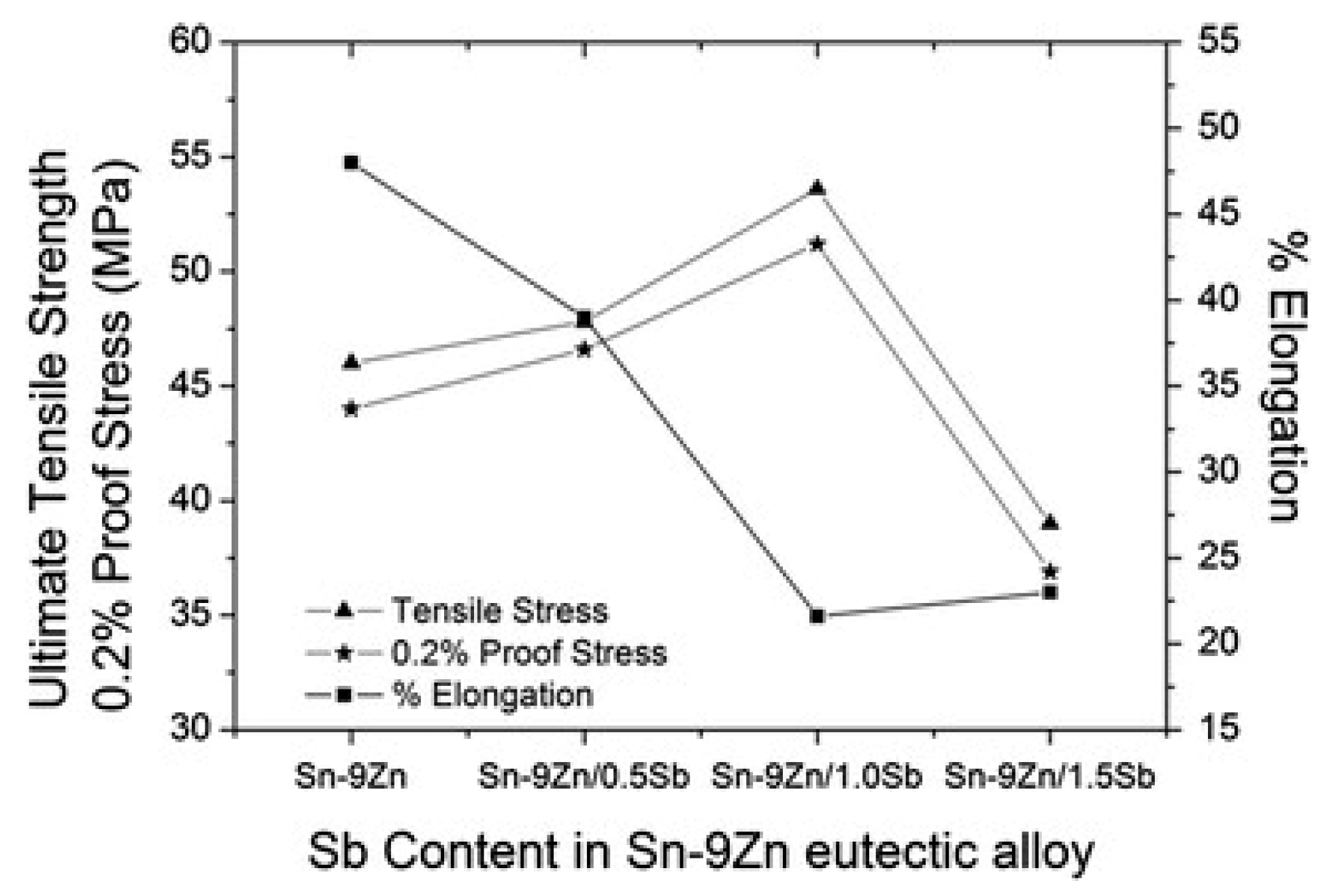
4.2. Mechanical Properties
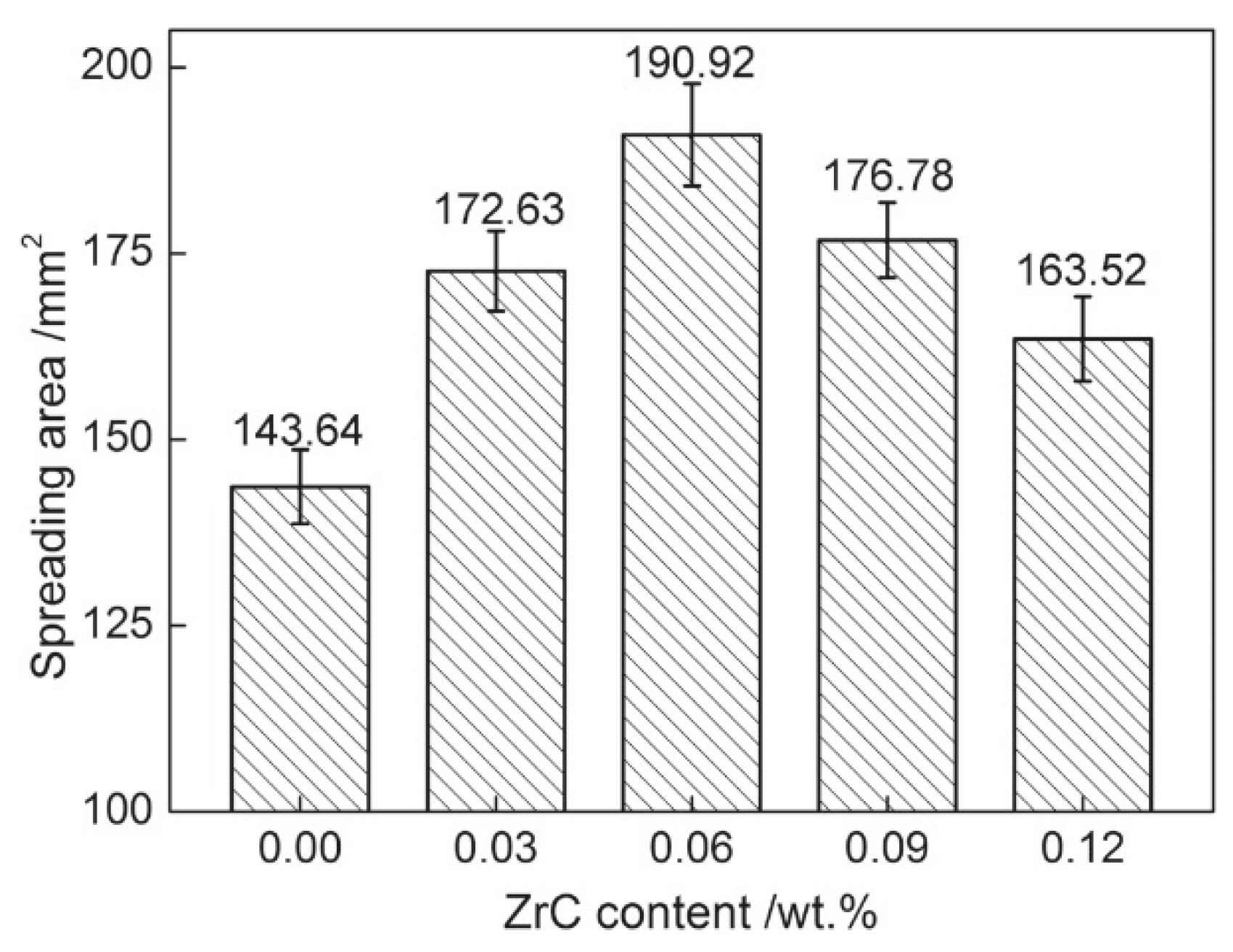
4.3. Wettability
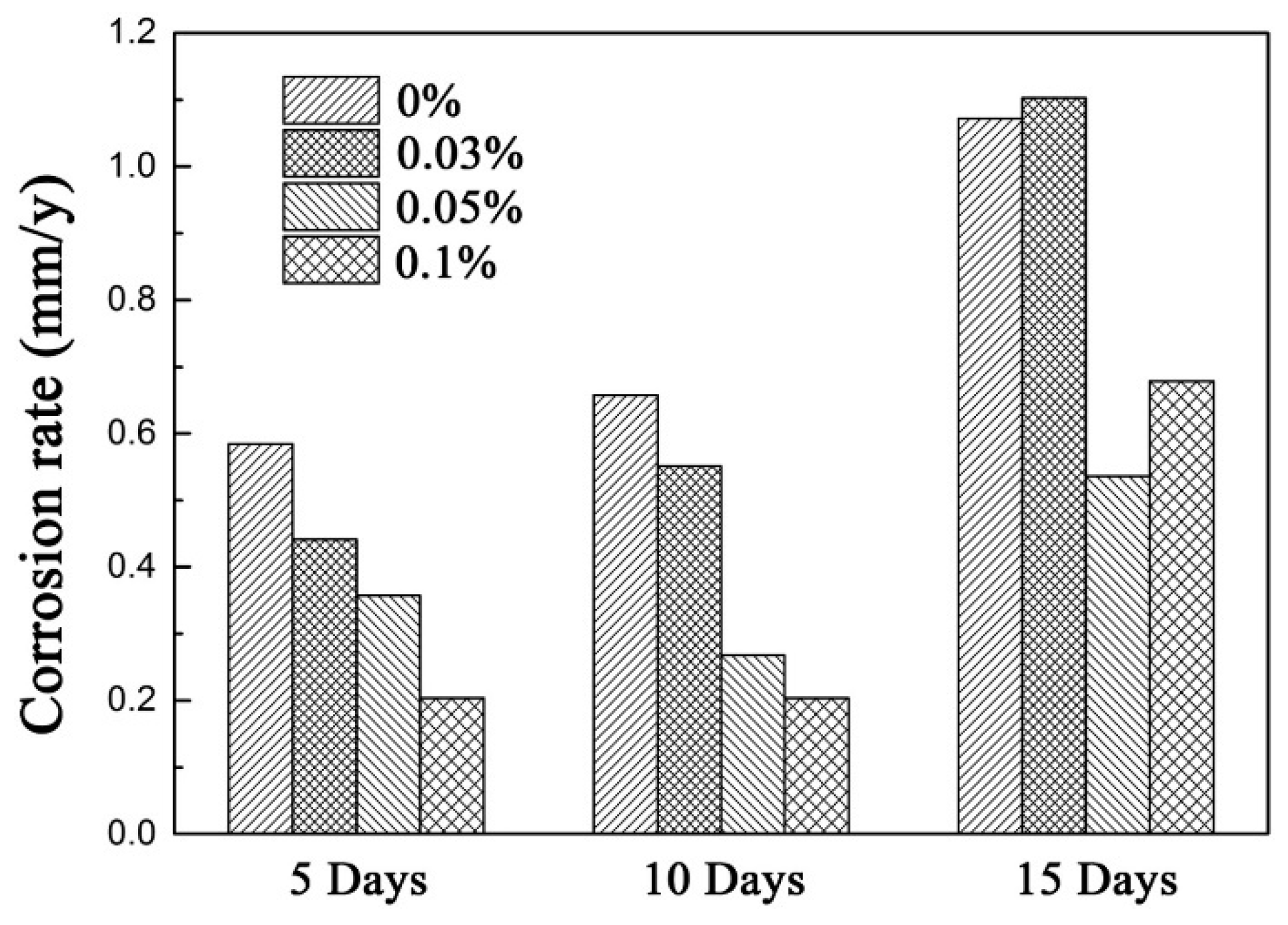
4.4. Reliability
5. Conclusions
- With the development of electronic devices towards miniaturization and high performance, stricter requirements have been put forward for the reliability of lead-free solders. The current research mainly focuses on the effect of thermal stress and current stress on the performance of the solders. However, more and more electronic devices are applied to harsh environments such as cryogenic temperatures [93,94] and irradiation [95]. Therefore, research on the reliability of the solder bearing nanoparticles in these harsh environments will become a hot spot in this subject.
- It has been proven that the coupling effects of RE and nanoparticles can further refine the microstructure and improve the mechanical properties of lead-free solders [96]. Thus, the combined addition of RE and nanoparticles may become a new reinforcement method to improve the performance of the solders.
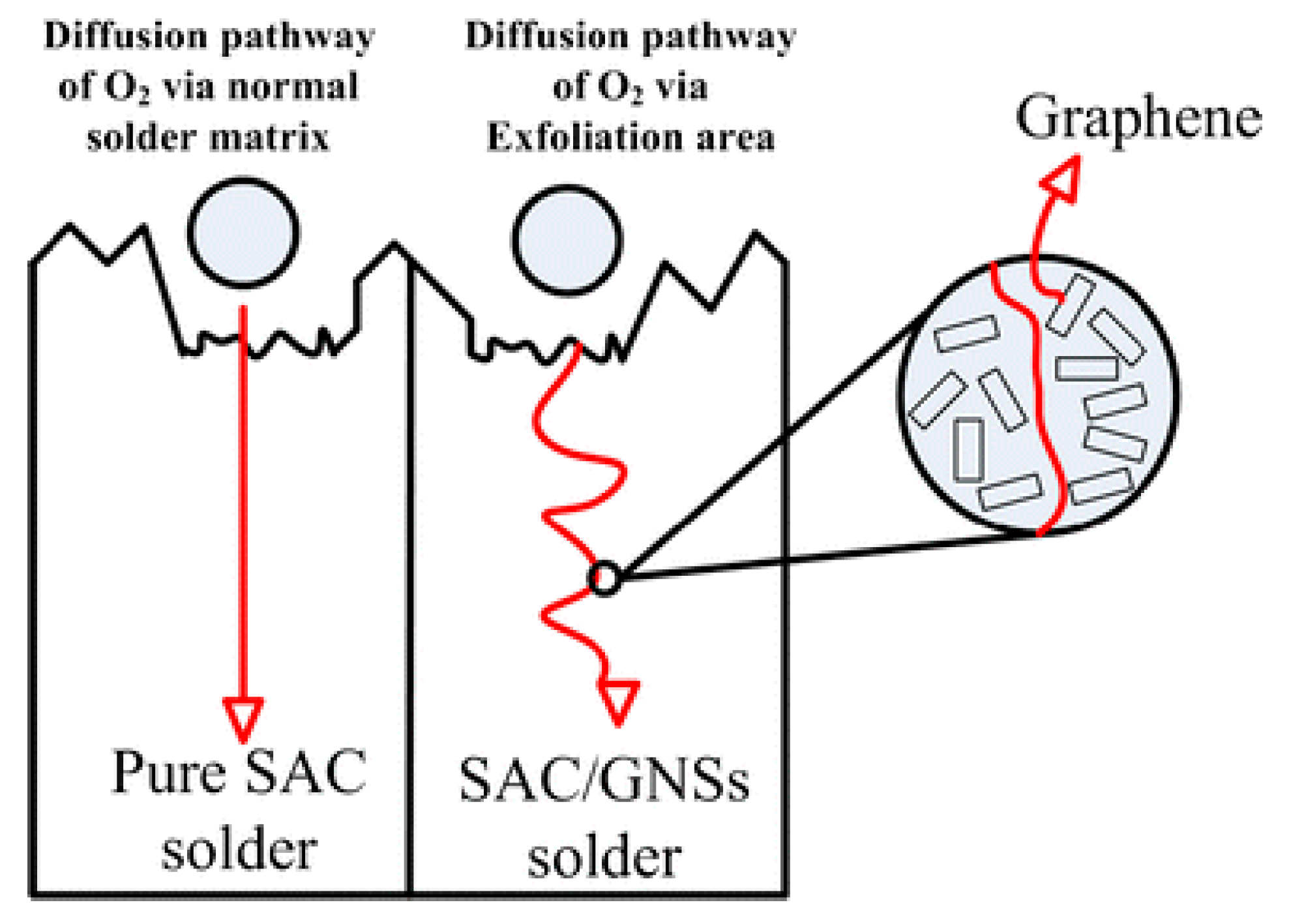
- Due to small tendency of agglomeration and fine physical and chemical properties, carbon-based nanomaterials can effectively improve the microstructure of lead-free solders and improve the properties [97,98]. Consequently, the development of new carbon-based nanomaterial-reinforced solders with better performance may become a new direction for the future research.
- At present, lead-free solders bearing nanoparticles are mainly fabricated by mechanical mixing of the solder particles and nanoparticles. It is claimed that the nanoparticle-reinforced lead-free solders can also be directly prepared by adding nanoparticles though flux doping [37,99] and in-situ formation [100]. Therefore, exploring new preparation methods with simpler processes and low cost attracts wide attention from researchers.
Author Contributions
Funding
Conflicts of Interest
References
- Seong Jun, K.; Coskun, K.; Taner, O.; Moonsub, S.; Ninad, P.; Alam, M.A.; Rotkin, S.V.; Rogers, J.A. High-performance electronics using dense, perfectly aligned arrays of single-walled carbon nanotubes. Nat. Nanotechnol. 2007, 2, 230–236. [Google Scholar]
- Ramli, M.I.I.; Saud, N.; Salleh, M.A.A.M.; Derman, M.N.; Said, R.M. Effect of TiO2 additions on Sn-0.7Cu-0.05Ni lead-free composite solder. Microelectron. Reliab. 2016, 65, 255–264. [Google Scholar] [CrossRef]
- Singh, A.K.; Panda, B.P.; Mohanty, S.; Nayak, S.K.; Gupta, M.K. Recent Developments on Epoxy-Based Thermally Conductive Adhesives (TCA): A Review. Polym. Plast. Technol. Eng. 2017, 57, 903–934. [Google Scholar] [CrossRef]
- Li, Y.; Wong, C.P. Recent advances of conductive adhesives as a lead-free alternative in electronic packaging: Materials, processing, reliability and applications. Mater. Sci. Eng. R 2006, 51, 1–35. [Google Scholar] [CrossRef]
- Shuang, L.; Xue, S.B.; Peng, X.; Luo, D.X. Present status of Sn–Zn lead-free solders bearing alloying elements. J. Mater. Sci. Mater. Electron. 2015, 26, 4389–4411. [Google Scholar]
- Dudek, M.A.; Chawla, N. Effect of Rare-Earth (La, Ce, and Y) Additions on the Microstructure and Mechanical Behavior of Sn-3.9Ag-0.7Cu Solder Alloy. Metall. Mater. Trans. A 2010, 41, 610–620. [Google Scholar] [CrossRef]
- Peng, X.; Xue, S.B.; Shen, Y.F.; Fei, L.; Hong, Z. Wettability and interfacial whiskers of Sn–9Zn–0.5Ga–0.08Nd solder with Sn, SnBi and Au/Ni coatings. J. Mater. Sci. Mater. Electron. 2014, 25, 3520–3525. [Google Scholar]
- Chen, K.I.; Cheng, S.C.; Wu, S.; Lin, K.L. Effects of small additions of Ag, Al, and Ga on the structure and properties of the Sn–9Zn eutectic alloy. J. Alloy. Compd. 2006, 416, 98–105. [Google Scholar] [CrossRef]
- Xu, C.; Feng, X.; Jian, Z.; Yao, Y. Effect of In on microstructure, thermodynamic characteristic and mechanical properties of Sn–Bi based lead-free solder. J. Alloy. Compd. 2015, 633, 377–383. [Google Scholar]
- Gao, F.; Takemoto, T.; Nishikawa, H. Effects of Co and Ni addition on reactive diffusion between Sn–3.5Ag solder and Cu during soldering and annealing. Mater. Sci. Eng. A 2006, 420, 39–46. [Google Scholar] [CrossRef]
- Tian, S.; Li, S.; Zhou, J.; Xue, F.; Cao, R.; Wang, F. Effect of indium addition on interfacial IMC growth and bending properties of eutectic Sn–0.7Cu solder joints. J. Mater. Sci. Mater. Electron. 2017, 28, 16120–16132. [Google Scholar] [CrossRef]
- Xue, P.; Wang, K.H.; Zhou, Q.; Huang, J.; Long, W.M.; Zhang, Q.K. Effect of Nd on tin whisker growth in Sn–Zn soldered joint. J. Mater. Sci. Mater. Electron. 2016, 27, 3742–3747. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, F.; Zhong, S.-J. Whisker growth on SnAgCu–xPr solders in electronic packaging. J. Mater. Sci. Mater. Electron. 2016, 27, 5618–5621. [Google Scholar] [CrossRef]
- Fawzy, A.; Fayek, S.A.; Sobhy, M.; Nassr, E.; Mousa, M.M.; Saad, G. Tensile creep characteristics of Sn–3.5Ag–0.5Cu (SAC355) solder reinforced with nano-metric ZnO particles. Mater. Sci. Eng. A 2014, 603, 1–10. [Google Scholar] [CrossRef]
- Salleh, M.A.A.M.; Mcdonald, S.D.; Gourlay, C.M.; Yasuda, H.; Nogita, K. Suppression of Cu6Sn5 in TiO2 reinforced solder joints after multiple reflow cycles. Mater. Des. 2016, 108, 418–428. [Google Scholar] [CrossRef]
- Yi, L.; Luo, K.; Lim, A.B.Y.; Zhong, C.; Wu, F.; Chan, Y.C. Improving the mechanical performance of Sn57.6Bi0.4Ag solder joints on Au/Ni/Cu pads during aging and electromigration through the addition of tungsten (W) nanoparticle reinforcement. Mater. Sci. Eng. A 2016, 669, 291–303. [Google Scholar]
- Fathian, Z.; Maleki, A.; Niroumand, B. Synthesis and characterization of ceramic nanoparticles reinforced lead-free solder. Ceram. Int. 2017, 43, 5302–5310. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, K.N. Structure and properties of lead-free solders bearing micro and nano particles. Mater. Sci. Eng. R 2014, 82, 1–32. [Google Scholar] [CrossRef]
- Huang, Y.; Xiu, Z.; Wu, G.; Tian, Y.; He, P. Sn–3.0Ag–0.5Cu nanocomposite solders reinforced by graphene nanosheets. J. Mater. Sci. Mater. Electron. 2016, 27, 6809–6815. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, H.; Wu, F.; Chan, Y.C. Comparative study of the microstructure and mechanical strength of tin-copper (Sn0.7Cu) solder modified with silver (Ag) by both alloying and doping methods. J. Mater. Sci. Mater. Electron. 2016, 27, 1–10. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C.; Yung, W.K.C. Effect of additions of ZrO2 nano-particles on the microstructure and shear strength of Sn–Ag–Cu solder on Au/Ni metallized Cu pads. Microelectron. Reliab. 2011, 51, 2306–2313. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Elmosalami, T.A.; Desoky, W.M.; El-Shaarawy, M.G.; Abdraboh, A.M. Tensile deformation behavior and melting property of nano-sized ZnO particles reinforced Sn–3.0Ag–0.5Cu lead-free solder. Mater. Sci. Eng. A 2014, 618, 389–397. [Google Scholar] [CrossRef]
- Sharma, A.; Baek, B.G.; Jung, J.P. Influence of La2O3 nanoparticle additions on microstructure, wetting, and tensile characteristics of Sn–Ag–Cu alloy. Mater. Des. 2015, 87, 370–379. [Google Scholar] [CrossRef]
- Ma, H.; Kunwar, A.; Liu, Z.; Chen, J.; Wang, Y.; Huang, M.; Zhao, N.; Ma, H. Shielding effect of Ag3Sn on growth of intermetallic compounds in isothermal heating and cooling during multiple reflows. J. Mater. Sci. Mater. Electron. 2018, 29, 4383–4390. [Google Scholar] [CrossRef]
- Fouzder, T.; Shafiq, I.; Chan, Y.C.; Sharif, A.; Yung, W.K.C. Influence of SrTiO3 nano-particles on the microstructure and shear strength of Sn–Ag–Cu solder on Au/Ni metallized Cu pads. J. Alloy. Compd. 2011, 509, 1885–1892. [Google Scholar] [CrossRef]
- Chan, Y.H.; Arafat, M.M.; Haseeb, A.S.M.A. Effects of reflow on the interfacial characteristics between Zn nanoparticles containing Sn-3.8Ag-0.7Cu solder and copper substrate. Solder Surf. Mt. Tech. 2013, 25, 91–98. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, S.M.; Li, G.Y.; Yang, Z.; Hou, C.J. Ripening Growth Kinetics of Cu6Sn5 Grains in Sn-3.0Ag-0.5Cu-xTiO2/Cu Solder Joints During the Reflow Process. J. Electron. Packag. 2018, 140. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, Y.; Li, Y.; Liu, Y.; Wang, Y. Effect of γ-Fe2O3 nanoparticles size on the properties of Sn-1.0Ag–0.5Cu nano-composite solders and joints. J. Alloy. Compd. 2016, 662, 272–282. [Google Scholar] [CrossRef]
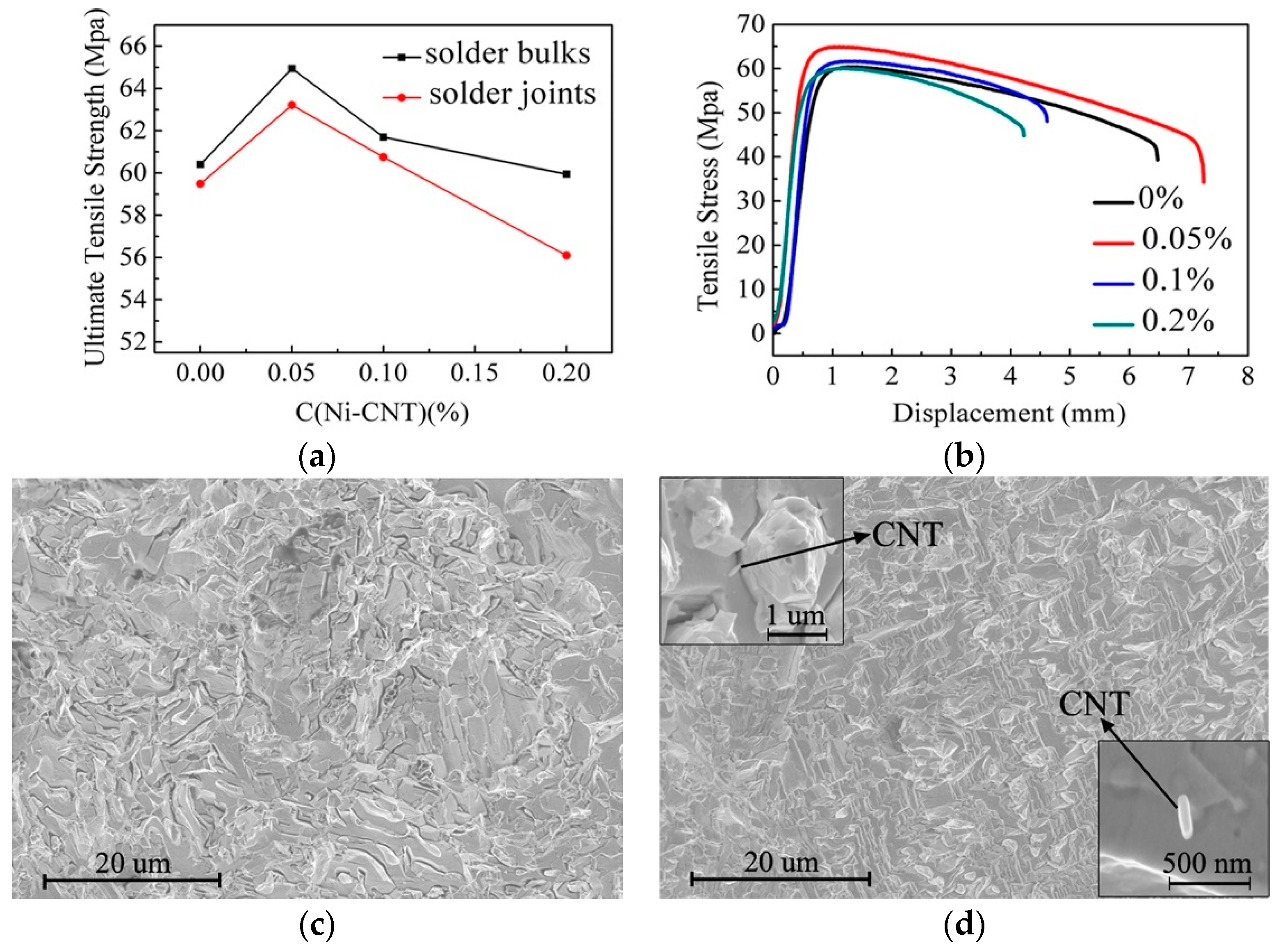
- Zhu, Z.; Chan, Y.C.; Zhong, C.; Gan, C.L.; Wu, F. Effect of the size of carbon nanotubes (CNTs) on the microstructure and mechanical strength of CNTs-doped composite Sn0.3Ag0.7Cu-CNTs solder. Mater. Sci. Eng. A 2018, 727, 160–169. [Google Scholar] [CrossRef]
- Lei, S.; Liang, Z.; Le, X.; Zhong, S.J.; Jia, M.; Li, B. Effect of nano-Al addition on properties and microstructure of low-Ag content Sn–1Ag–0.5Cu solders. J. Mater. Sci. Mater. Electron. 2016, 27, 7665–7673. [Google Scholar]
- Tang, Y.; Luo, S.M.; Huang, W.F.; Pan, Y.C.; Li, G.Y. Effects of Mn nanoparticles on tensile properties of low-Ag Sn-0.3Ag-0.7Cu-xMn solder alloys and joints. J. Alloy. Compd. 2017, 719, 365–375. [Google Scholar] [CrossRef]
- Tsao, L.C.; Chang, S.Y. Effects of Nano-TiO2 additions on thermal analysis, microstructure and tensile properties of Sn3.5Ag0.25Cu solder. Mater. Des. 2010, 31, 990–993. [Google Scholar] [CrossRef]
- Zhang, L.; Han, J.; Liu, F.; Guo, Y.; He, C.; University, J.N. Effect of Nano-particles TiO2 on the Microstructures and Properties of SnAgCu Solders. Rare Metal Mat. Eng. 2013, 42, 1897–1900. [Google Scholar]
- Jing, H.Y.; Guo, H.J.; Wang, L.X.; Wei, J.; Xu, L.Y.; Han, Y.D. Influence of Ag-modified graphene nanosheets addition into Sn–Ag–Cu solders on the formation and growth of intermetallic compound layers. J. Alloy. Compd. 2017, 702, 669–678. [Google Scholar] [CrossRef]
- Du, C.H.; Li, Z.K.; Liu, B.; Li, C.T. The Frontier Analysis of RCS for Micro-Nano Particle. Adv. Mater. Res. 2011, 337, 526–531. [Google Scholar] [CrossRef]
- Fu, G. Composite lead-free electronic solders. J. Mater. Sci. Mater. Electron. 2007, 18, 129–145. [Google Scholar]
- Shen, J.; Chan, Y.C. Effect of metal/ceramic nanoparticle-doped fluxes on the wettability between Sn–Ag–Cu solder and a Cu layer. J. Alloy. Compd. 2009, 477, 909–914. [Google Scholar] [CrossRef]
- Yue, G.; Zhao, X.; Yi, L.; Ying, L.; Yong, W.; Li, Z. Effect of nano-Fe2O3 additions on wettability and interfacial intermetallic growth of low-Ag content Sn–Ag–Cu solders on Cu substrates. J. Alloy. Compd. 2015, 627, 39–47. [Google Scholar]
- Jie, W.; Xue, S.; Wang, J.; Wu, M.; Wang, J. Effects of α-Al2O3 nanoparticles-doped on microstructure and properties of Sn–0.3Ag–0.7Cu low-Ag solder. J. Mater. Sci. Mater. Electron. 2018, 29, 7372–7387. [Google Scholar]
- Sharma, A.; Sohn, H.-R.; Jung, J.P. Effect of Graphene Nanoplatelets on Wetting, Microstructure, and Tensile Characteristics of Sn-3.0Ag-0.5Cu (SAC) Alloy. Metall. Mater. Trans. A 2015, 47, 494–503. [Google Scholar] [CrossRef]
- Li, Y.; Ge, J.; Zhang, Y.; Dai, J. Interfacial IMC Layer and Tensile Properties of Ni-Reinforced Cu/Sn–0.7Cu–0.05Ni/Cu Solder Joint: Effect of Aging Temperature. Trans. Indian Inst. Met. 2017, 70, 2429–2439. [Google Scholar]
- Tang, Y.; Li, G.Y.; Chen, D.Q.; Pan, Y.C. Influence of TiO2 nanoparticles on IMC growth in Sn–3.0Ag–0.5Cu–xTiO2 solder joints during isothermal aging process. J. Mater. Sci. Mater. Electron. 2014, 25, 981–991. [Google Scholar] [CrossRef]
- Li, H.; An, R.; Wang, C. In situ quantitative study of microstructural evolution at the interface of Sn3.0Ag0.5Cu/Cu solder joint during solid state aging. J. Alloy. Compd. 2015, 634, 94–98. [Google Scholar] [CrossRef]
- Chen, J.-S.; Wang, K.-Y.; Chen, J.-Q.; Yu, L. Suppression effect of Cu and Ag on Cu3Sn layer in solder joints. J. Mater. Sci. Mater. Electron. 2013, 24, 4630–4635. [Google Scholar]
- Wen, Y.; Zhao, X.; Chen, Z.; Gu, Y.; Wang, Y.; Chen, Z.; Wang, X. Reliability enhancement of Sn-1.0Ag-0.5Cu nano-composite solders by adding multiple sizes of TiO2 nanoparticles. J. Alloy. Compd. 2017, 696, 799–807. [Google Scholar] [CrossRef]
- Tsao, L.C. Suppressing effect of 0.5 wt.% nano-TiO2 addition into Sn-3.5Ag-0.5Cu solder alloy on the intermetallic growth with Cu substrate during isothermal aging. J. Alloy. Compd. 2011, 509, 8441–8448. [Google Scholar] [CrossRef]
- Chen, G.; Peng, H.; Silberschmidt, V.V.; Chan, Y.C.; Liu, C.; Wu, F. Performance of Sn–3.0Ag–0.5Cu composite solder with TiC reinforcement: Physical properties, solderability and microstructural evolution under isothermal ageing. J. Alloy. Compd. 2016, 685, 680–689. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Z.; Zhou, S.; Chen, L. Stress relaxation and failure behavior of Sn–3.0Ag–0.5Cu flip-chip solder bumps undergoing electromigration. J. Mater. Res. 2014, 29, 2556–2564. [Google Scholar] [CrossRef]
- An, R.; Tian, Y.; Zhang, R.; Wang, C. Electromigration-induced intermetallic growth and voids formation in symmetrical Cu/Sn/Cu and Cu/Intermetallic compounds (IMCs)/Cu joints. J. Mater. Sci. Mater. Electron. 2015, 26, 2674–2681. [Google Scholar] [CrossRef]
- Sharma, A.; Di, E.X.; Chow, J.; Mayer, M.; Sohn, H.R.; Jung, J.P. Electromigration of composite Sn-Ag-Cu solder bumps. Electron. Mater. Lett. 2015, 11, 1072–1077. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, Z.; Ping, W.U. Effects of Ni-coated Carbon Nanotubes addition on the electromigration of Sn-Ag-Cu solder joints. J. Alloy. Compd. 2013, 581, 202–205. [Google Scholar] [CrossRef]
- Sha, X.U.; Yan, C.C.; Zhang, K.; Yung, K.C. Interfacial intermetallic growth and mechanical properties of carbon nanotubes reinforced Sn3.5Ag0.5Cu solder joint under current stressing. J. Alloy. Compd. 2014, 595, 92–102. [Google Scholar]
- Bui, Q.V.; Nam, N.D.; Noh, B.I.; Kar, A.; Kim, J.G.; Jung, S.B. Effect of Ag addition on the corrosion properties of Sn-based solder alloys. Mater. Corros. 2015, 61, 30–33. [Google Scholar] [CrossRef]
- Xu, L.Y.; Zhang, Z.K.; Jing, H.Y.; Wei, J.; Han, Y.D. Effect of graphene nanosheets on the corrosion behavior of Sn–Ag–Cu solders. J. Mater. Sci. Mater. Electron. 2015, 26, 5625–5634. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Interfacial microstructure, wettability and material properties of nickel (Ni) nanoparticle doped tin–bismuth–silver (Sn–Bi–Ag) solder on copper (Cu) substrate. J. Mater. Sci. Mater. Electron. 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Sun, H.; Li, Q.; Chan, Y.C. A study of Ag additive methods by comparing mechanical properties between Sn57.6Bi0.4Ag and 0.4 wt% nano-Ag-doped Sn58Bi BGA solder joints. J. Mater. Sci. Mater. Electron. 2014, 25, 4380–4390. [Google Scholar] [CrossRef]
- Yi, L.; Chan, Y.C. Effect of silver (Ag) nanoparticle size on the microstructure and mechanical properties of Sn58Bi–Ag composite solders. J. Alloy. Compd. 2015, 645, 566–576. [Google Scholar]
- Yang, L.; Hao, Z.; Sun, F. Solderability of SnBi-nano Cu solder pastes and microstructure of the solder joints. J. Mater. Sci. Mater. Electron. 2016, 27, 2235–2241. [Google Scholar]
- Li, X.; Yong, M.; Wei, Z.; Ping, W. Effects of nanoscale Cu6Sn5 particles addition on microstructure and properties of SnBi solder alloys. Mater. Sci. Eng. A 2017, 684, 328–334. [Google Scholar] [CrossRef]
- Peng, H.E.; Xiao-Chun, L.; Lin, T.S.; Hai-Xin, L.I.; Jing, A.N.; Xin, M.A.; Feng, J.C.; Yan, Z.; Qi, L.I.; Qian, Y.Y. Improvement of mechanical properties of Sn-58Bi alloy with multi-walled carbon nanotubes. T. Nonferr. Metal. Soc. 2012, 22, s692–s696. [Google Scholar]
- Sun, H.; Chan, Y.C.; Wu, F. Effect of CNTs and Ni coated CNTs on the mechanical performance of Sn57.6Bi0.4Ag BGA solder joints. Mater. Sci. Eng. A 2016, 656, 249–255. [Google Scholar] [CrossRef]
- Yang, L.; Wei, Z.; Liang, Y.; Cui, W.; Ping, W. Improved microstructure and mechanical properties for Sn58Bi solder alloy by addition of Ni-coated carbon nanotubes. Mater. Sci. Eng. A 2015, 642, 7–15. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Zhou, W.; Yang, L.; Wu, P. Reinforcement of graphene nanosheets on the microstructure and properties of Sn58Bi lead-free solder. Mater. Des. 2017, 113, 264–272. [Google Scholar] [CrossRef]
- Ma, D.; Wu, P. Improved microstructure and mechanical properties for Sn58Bi0.7Zn solder joint by addition of graphene nanosheets. J. Alloy. Compd. 2016, 671, 127–136. [Google Scholar] [CrossRef]
- Yang, L.; Dai, J.; Zhang, Y.; Jing, Y.; Ge, J.; Liu, H. Influence of BaTiO3 Nanoparticle Addition on Microstructure and Mechanical Properties of Sn-58Bi Solder. J. Electron. Mater. 2015, 44, 2473–2478. [Google Scholar] [CrossRef]
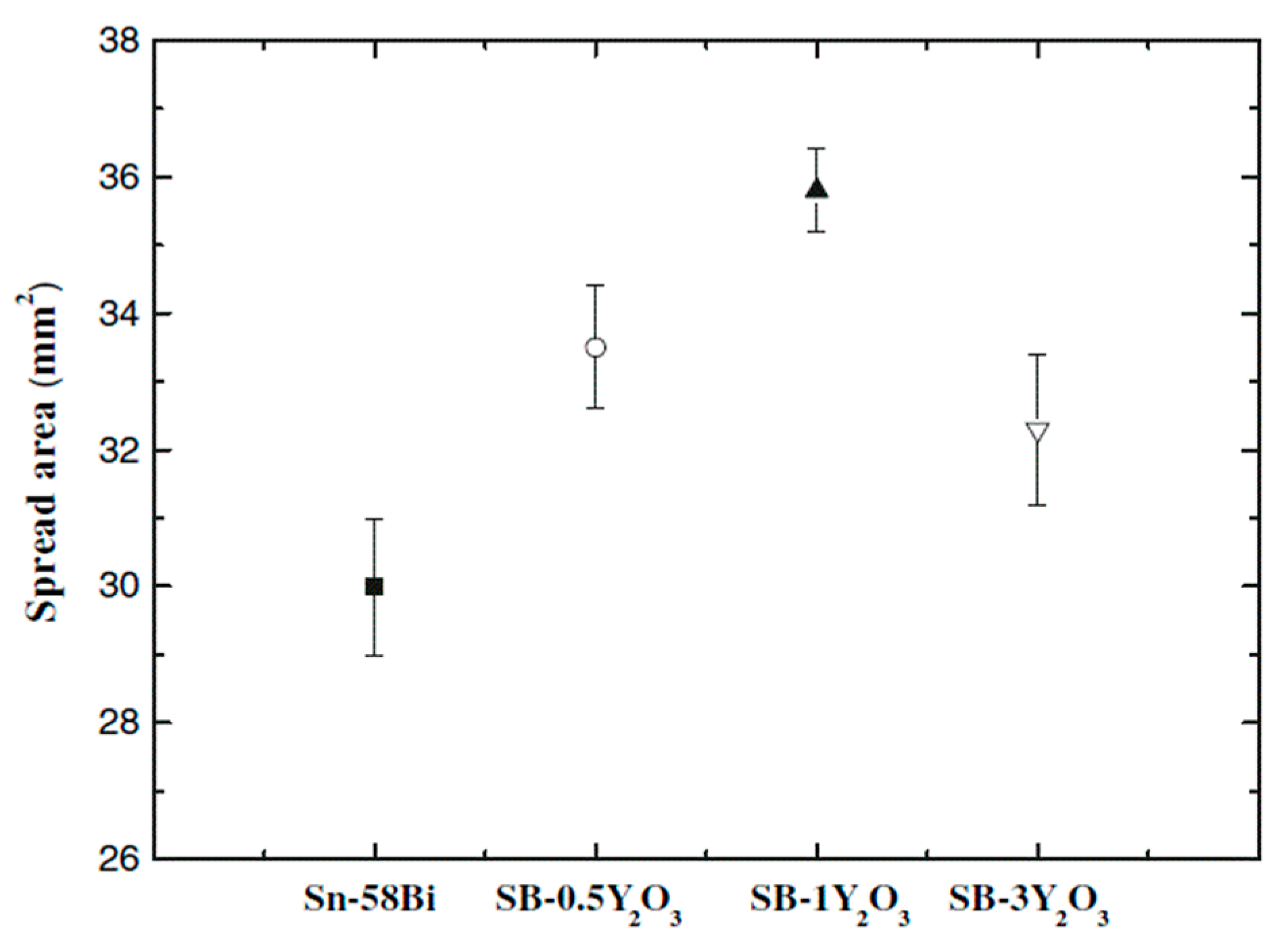
- Liu, X.; Huang, M.; Wu, C.M.L.; Lai, W. Effect of Y2O3 particles on microstructure formation and shear properties of Sn-58Bi solder. J. Mater. Sci. Mater. Electron. 2010, 21, 1046–1054. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Sun, F.; Ban, G.; Fan, J. Effects of nano-copper particles on the properties of Sn58Bi composite solder pastes. Microelectron. Int. 2017, 34, 40–44. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Huang, Y.; Liu, L.; Zhang, Z. Recent progress on the development of Sn–Bi based low-temperature Pb-free solders. J. Mater. Sci. Mater. Electron. 2019, 30, 3222–3243. [Google Scholar] [CrossRef]
- Yang, Q.L.; Shang, J.K. Interfacial segregation of Bi during current stressing of Sn-Bi/Cu solder interconnect. J. Electron. Mater. 2005, 34, 1363–1367. [Google Scholar] [CrossRef]
- Gu, X.; Yang, D.; Chan, Y.C.; Wu, B.Y. Effects of electromigration on the growth of intermetallic compounds in Cu/SnBi/Cu solder joints. J. Mater. Res. 2008, 23, 2591–2596. [Google Scholar] [CrossRef]
- Ismathullakhan, S.; Lau, H.; Chan, Y.C. Enhanced electromigration reliability via Ag nanoparticles modified eutectic Sn–58Bi solder joint. Microsyst. Technol. 2013, 19, 1069–1080. [Google Scholar] [CrossRef]
- Hu, T.; Yi, L.; Chan, Y.C.; Wu, F. Effect of nano Al2O3 particles doping on electromigration and mechanical properties of Sn–58Bi solder joints. Microelectron. Reliab. 2015, 55, 1226–1233. [Google Scholar] [CrossRef]
- Li, L.F.; Cheng, Y.K.; Xu, G.L.; Wang, E.Z.; Zhang, Z.H.; Wang, H. Effects of indium addition on properties and wettability of Sn–0.7Cu–0.2Ni lead-free solders. Mater. Des. 2014, 64, 15–20. [Google Scholar] [CrossRef]
- Liang, Z.; Junhua, C.; Jiguang, H.; Yonghuan, G.; Chengwen, H.E. Microstructures and properties of SnZn-xEr lead-free solders. J. Rare Earth. 2012, 30, 790–793. [Google Scholar]
- Peng, X.; Xue, S.B.; Shen, Y.F.; Hong, Z. Interfacial microstructures and mechanical properties of Sn–9Zn–0.5Ga–xNd on Cu substrate with aging treatment. Mater. Des. 2014, 60, 1–6. [Google Scholar]
- Gain, A.K.; Zhang, L. Microstructure, thermal analysis and damping properties of Ag and Ni nano-particles doped Sn-8Zn-3Bi solder on OSP-Cu substrate. J. Alloy. Compd. 2014, 617, 779–786. [Google Scholar] [CrossRef]
- Fouzder, T.; Li, Q.; Chan, Y.C.; Chan, D.K. Microstructure and kinetic analysis of the properties and behavior of nickel (Ni) nano-particle doped tin–zinc–bismuth (Sn–8Zn–3Bi) solders on immersion silver (Ag)-plated copper (Cu) substrates. J. Mater. Sci. Mater. Electron. 2014, 25, 2529–2539. [Google Scholar] [CrossRef]
- Xing, W.Q.; Yu, X.Y.; Li, H.; Le, M.; Wei, Z.; Peng, D.; Wang, W.X.; Min, D. Microstructure and mechanical properties of Sn-9Zn-xAl2O3 nanoparticles (x = 0–1) lead-free solder alloy: first-principles calculation and experimental research. Mater. Sci. Eng. A 2016, 678, 252–259. [Google Scholar] [CrossRef]
- Peng, C.; Shen, J.; Yin, H. Influences of ZrO2 nano-particles on the microstructures and microhardness of Sn8Zn1Bi-xZrO2/Cu solder joints. J. Mater. Sci. Mater. Electron. 2013, 24, 203–210. [Google Scholar] [CrossRef]
- Al-Ganainy, G.S.; El-Daly, A.A.; Fawzy, A.; Hussein, N. Effect of adding nanometric ZnO particles on thermal, microstructure and tensile creep properties of Sn–6.5 wt%Zn–3 wt%In solder alloy. J. Mater. Sci. Mater. Electron. 2017, 28, 13303–13312. [Google Scholar] [CrossRef]
- Shafiq, I.; Chan, Y.C.; Yung, W.K.C. Influence of small Sb nanoparticles additions on the microstructure, hardness and tensile properties of Sn–9Zn binary eutectic solder alloy. J. Mater. Sci. Mater. Electron. 2012, 23, 1427–1434. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C.; Sharif, A.; Wong, N.B.; Yung, W.K.C. Interfacial microstructure and shear strength of Ag nano particle doped Sn-9Zn solder in ball grid array packages. Microelectron. Reliab. 2009, 49, 746–753. [Google Scholar] [CrossRef]
- Liu, X.D.; Han, Y.D.; Jing, H.Y.; Wei, J.; Xu, L.Y. Effect of graphene nanosheets reinforcement on the performance of Sn-Ag-Cu lead-free solder. Mater. Sci. Eng. A 2013, 562, 25–32. [Google Scholar] [CrossRef]
- Chen, G.; Wu, F.; Liu, C.; Silberschmidt, V.V.; Chan, Y.C. Microstructures and properties of new Sn–Ag–Cu lead-free solder reinforced with Ni-coated graphene nanosheets. J. Alloy. Compd. 2016, 656, 500–509. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Jing, H.; Wang, L.; Wei, J.; Han, Y. Design and performance of Ag nanoparticle-modified graphene/SnAgCu lead-free solders. Mater. Sci. Eng. A 2016, 667, 87–96. [Google Scholar] [CrossRef]
- Kumar, K.M.; Kripesh, V.; Tay, A.A.O. Single-wall carbon nanotube (SWCNT) functionalized Sn–Ag–Cu lead-free composite solders. J. Alloy. Compd. 2008, 450, 229–237. [Google Scholar] [CrossRef]
- Ahmed, M.; Fouzder, T.; Sharif, A.; Gain, A.K.; Chan, Y.C. Influence of Ag micro-particle additions on the microstructure, hardness and tensile properties of Sn–9Zn binary eutectic solder alloy. Microelectron. Reliab. 2010, 50, 1134–1141. [Google Scholar] [CrossRef]
- Li, Y.; Song, B.; Zhang, Y.; Xiong, Y.; Shi, X.; Zhang, K. Microstructure, interfacial IMC layer and mechanical properties of Cu/Sn-9Zn-xZrC/Cu solder joints. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Xing, W.-Q.; Yu, X.-Y.; Li, H.; Ma, L.; Zuo, W.; Dong, P.; Wang, W.-X.; Ding, M. Effect of nano Al2O3 additions on the interfacial behavior and mechanical properties of eutectic Sn-9Zn solder on low temperature wetting and soldering of 6061 aluminum alloys. J. Alloy. Compd. 2017, 695, 574–582. [Google Scholar] [CrossRef]
- Xue, P.; Xue, S.-B.; Shen, Y.-F.; Zhu, H. Inhibiting the growth of Sn whisker in Sn-9Zn lead-free solder by Nd and Ga. J. Mater. Sci. Mater. Electron. 2014, 25, 2671–2675. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C.; Yung, W.K.C. Effect of nano Ni additions on the structure and properties of Sn–9Zn and Sn–Zn–3Bi solders in Au/Ni/Cu ball grid array packages. Mater. Sci. Eng. B 2009, 162, 92–98. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.S.; Inoue, M.; Jiang, J.; Suganuma, K. Effects of Ag and Cu addition on microstructural properties and oxidation resistance of Sn–Zn eutectic alloy. J. Alloy. Compd. 2008, 454, 310–320. [Google Scholar] [CrossRef]
- Tian, R.; Tian, Y.; Wang, C.; Zhao, L. Mechanical properties and fracture mechanisms of Sn-3.0Ag-0.5Cu solder alloys and joints at cryogenic temperatures. Mater. Sci. Eng. A 2017, 684, 697–705. [Google Scholar] [CrossRef]
- Yao, Y.; Li, X.; He, X. Effect of deep cryogenic treatment on mechanical properties and microstructure of Sn3.0Ag0.5Cu solder. J. Mater. Sci. Mater. Electron. 2018, 29, 4517–4525. [Google Scholar] [CrossRef]
- Wang, J.; Xue, S.; Lv, Z.; Wang, L.; Liu, H.; Wen, L. Effect of gamma-ray irradiation on microstructure and mechanical property of Sn63Pb37 solder joints. J. Mater. Sci. Mater. Electron. 2018, 29, 20726–20733. [Google Scholar] [CrossRef]
- Wu, J.; Xue, S.; Wang, J.; Wu, M. Coupling effects of rare-earth Pr and Al2O3 nanoparticles on the microstructure and properties of Sn-0.3Ag-0.7Cu low-Ag solder. J. Alloy. Compd. 2019, 784, 471–487. [Google Scholar] [CrossRef]
- Chen, G.; Li, L.; Du, J.; Silberschmidt, V.V.; Chan, Y.C.; Liu, C.; Wu, F. Thermo-migration behavior of SAC305 lead-free solder reinforced with fullerene nanoparticles. J. Mater. Sci. 2016, 51, 10077–10091. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Sayyadi, R.; Javid, N.S. Lead free Sn-Ag-Cu solders reinforced by Ni-coated graphene nanosheets prepared by mechanical alloying: Microstructural evolution and mechanical durability. Mater. Sci. Eng. A 2017, 702, 371–385. [Google Scholar] [CrossRef]
- Bashir, M.N.; Haseeb, A.S.M.A.; Rahman, A.Z.M.S.; Fazal, M.A.; Kao, C.R. Reduction of electromigration damage in SAC305 solder joints by adding Ni nanoparticles through flux doping. J. Mater. Sci. 2015, 50, 6748–6756. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Y.C.; Gao, H.X. In situ nanoparticulate-reinforced lead-free Sn–Ag composite prepared by rapid solidification. J. Mater. Sci. Mater. Electron. 2006, 18, 463–468. [Google Scholar] [CrossRef]

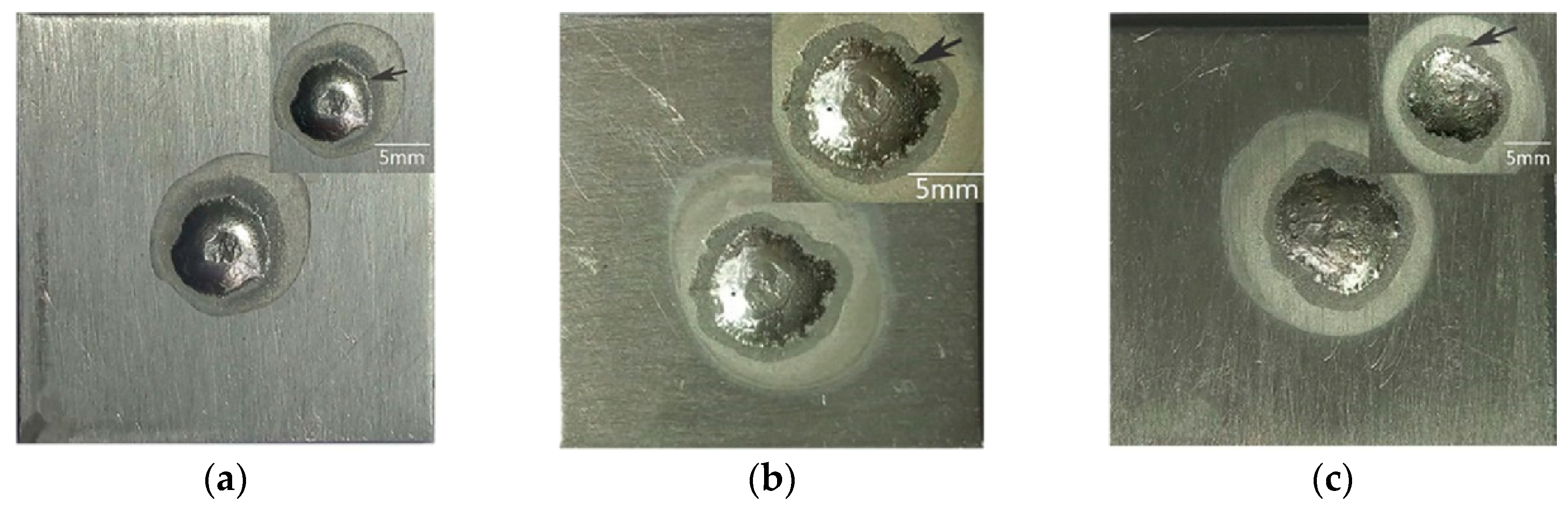
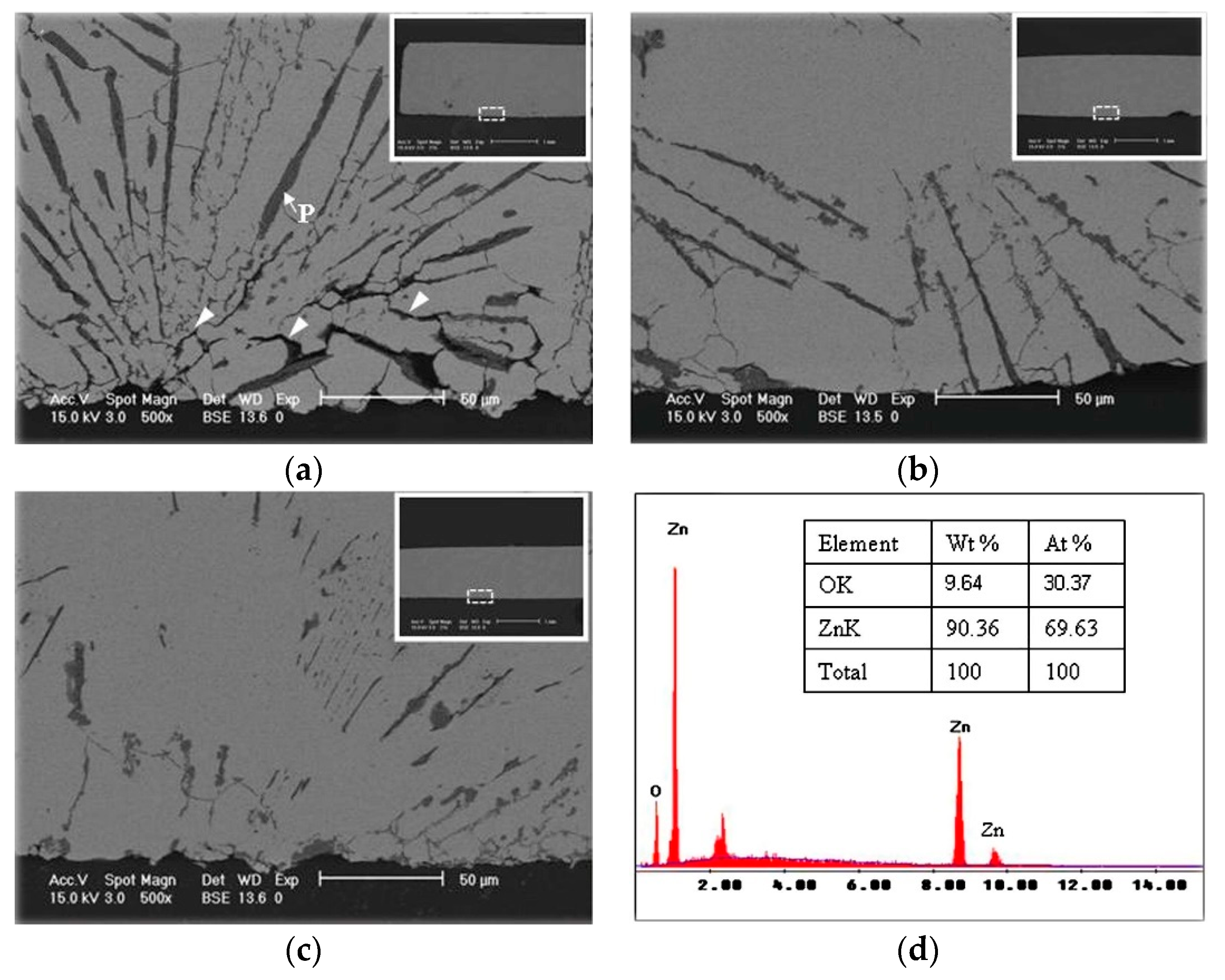
| Solder | Maximal Size (μm) | Minimal Size (μm) | Average Size (μm) | Average Spacing (μm) |
|---|---|---|---|---|
| SnZnBi | 10.5 | 1.2 | 4.6 ± 0.09 | 2.2 ± 0.1 |
| SnZnBi–0.25ZrO2 | 2.5 | 0.5 | 1.5 ± 0.1 | 1.3 ± 0.04 |
| SnZnBi–0.5ZrO2 | 6.6 | 0.4 | 2.8 ± 0.3 | 1.1 ± 0.06 |
| SnZnBi–1ZrO2 | 8.3 | 0.6 | 3.4 ± 0.5 | 2.1 ± 0.08 |
| Solders | UTS (MPa) | Elongation (%) | Microhardness | References |
|---|---|---|---|---|
| Sn3.0Ag0.5Cu | 46.0 | 29.5 | / | [83] |
| Sn0.3Ag-0.5Cu/0.1Mn | 56.5 | / | / | [31] |
| Sn3.5Ag0.25Cu/1TiO2 | 70.1 | 25.2 | 18.5 HV | [32] |
| Sn3.0Ag0.5Cu/0.05La2O3 | 79.0 | 14.1 | 13 HV | [23] |
| Sn3.0Ag0.5Cu/0.03GNSs | 50.3 | 23.5 | / | [83] |
| 3Sn3.0Ag0.5Cu/0.1GNSs | 50.7 | 21.5 | / | [83] |
| Sn3.0Ag0.5Cu/0.2Ni-GNSs | 58.4 | / | 14.6 HV | [84] |
| Sn3.0Ag0.5Cu/0.05Ag-GNSs | 50.1 | 14.7 | / | [85] |
| Sn3.8Ag0.7Cu/1CNTs | 56.7 | 24.4 | / | [86] |
| Sn58Bi | 81 | 21 | 0.30 GPa | [63] |
| Sn58Bi/0.01GNSs | 84 | 32 | 0.26 GPa | [63] |
| Sn58Bi/0.1GNSs | 93 | 17 | 0.41 GPa | [63] |
| Sn58Bi/0.03CNTs | 94.2 | 21.7 | / | [60] |
| Sn9Zn | 41.0 | 43.4 | 13.6 BHN | [87] |
| Sn9Zn/1.0Ag | 43.6 | 19.4 | 16 BHN | [87] |
| Sn9Zn/1.0Sb | 53.6 | 21.6 | 15.9 BHN | [81] |
| Sn9Zn/1.0Al2O3 | 43.4 | 44.9 | 27 HV | [78] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Xue, S.; Wang, J.; Xue, P.; Zhong, S.; Long, W. Effect of Nanoparticles Addition on the Microstructure and Properties of Lead-Free Solders: A Review. Appl. Sci. 2019, 9, 2044. https://doi.org/10.3390/app9102044
Zhang P, Xue S, Wang J, Xue P, Zhong S, Long W. Effect of Nanoparticles Addition on the Microstructure and Properties of Lead-Free Solders: A Review. Applied Sciences. 2019; 9(10):2044. https://doi.org/10.3390/app9102044
Chicago/Turabian StyleZhang, Peng, Songbai Xue, Jianhao Wang, Peng Xue, Sujuan Zhong, and Weimin Long. 2019. "Effect of Nanoparticles Addition on the Microstructure and Properties of Lead-Free Solders: A Review" Applied Sciences 9, no. 10: 2044. https://doi.org/10.3390/app9102044
APA StyleZhang, P., Xue, S., Wang, J., Xue, P., Zhong, S., & Long, W. (2019). Effect of Nanoparticles Addition on the Microstructure and Properties of Lead-Free Solders: A Review. Applied Sciences, 9(10), 2044. https://doi.org/10.3390/app9102044





