Effect of Various Temperatures on Bletillae Rhizoma Polysaccharide Extraction and Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Fractional RBP from RBF
2.3. Glucomannan Content Analysis
2.4. Rheological Properties
2.5. Gel Permeation Chromatography (GPC) Analysis
2.6. Scanning Electron Microscopy Analysis
2.7. Thermogravimetric (TG) Analysis
2.8. Differential Scanning Calorimetry (DSC) Analysis
2.9. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.10. X-ray Diffraction (XRD) Analysis
2.11. Elemental Analysis
3. Results and Discussion
3.1. GM Content, Yield, and Extraction Rate Analysis of Fractional RBPs
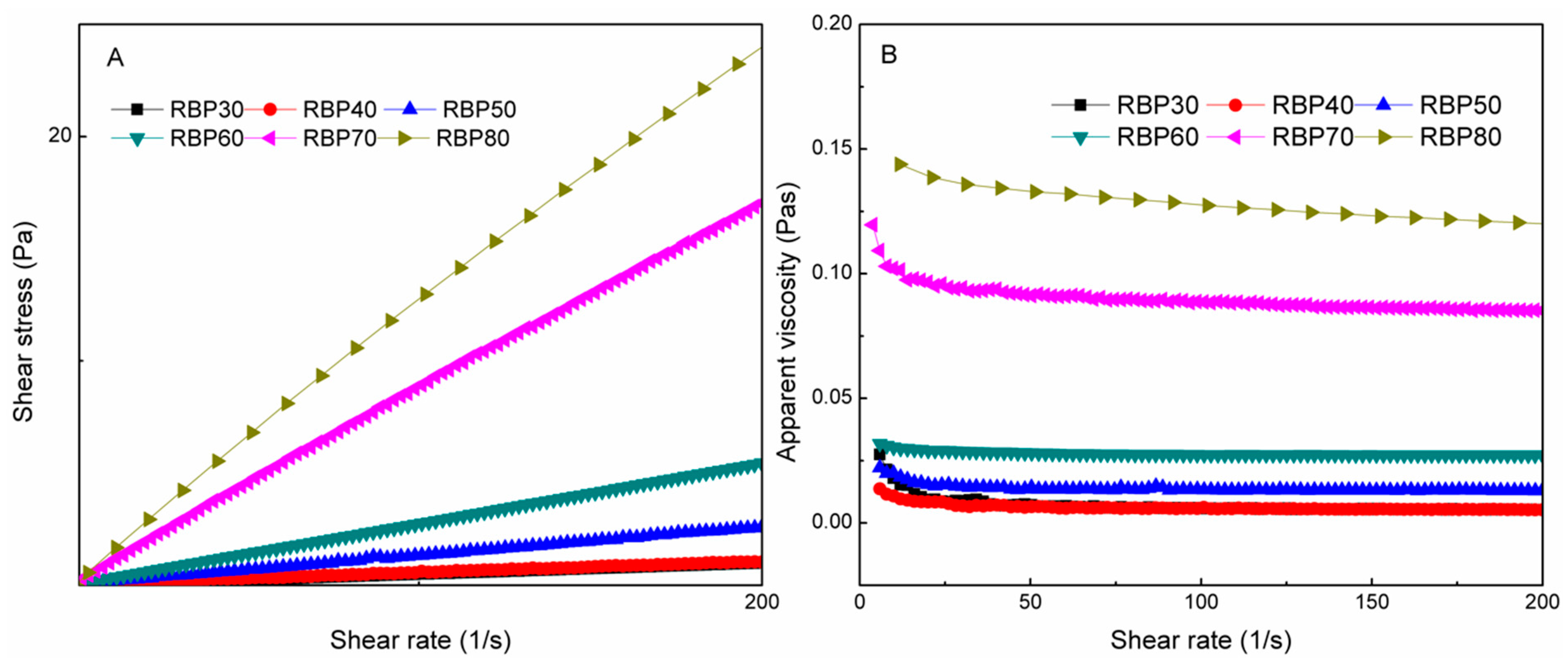
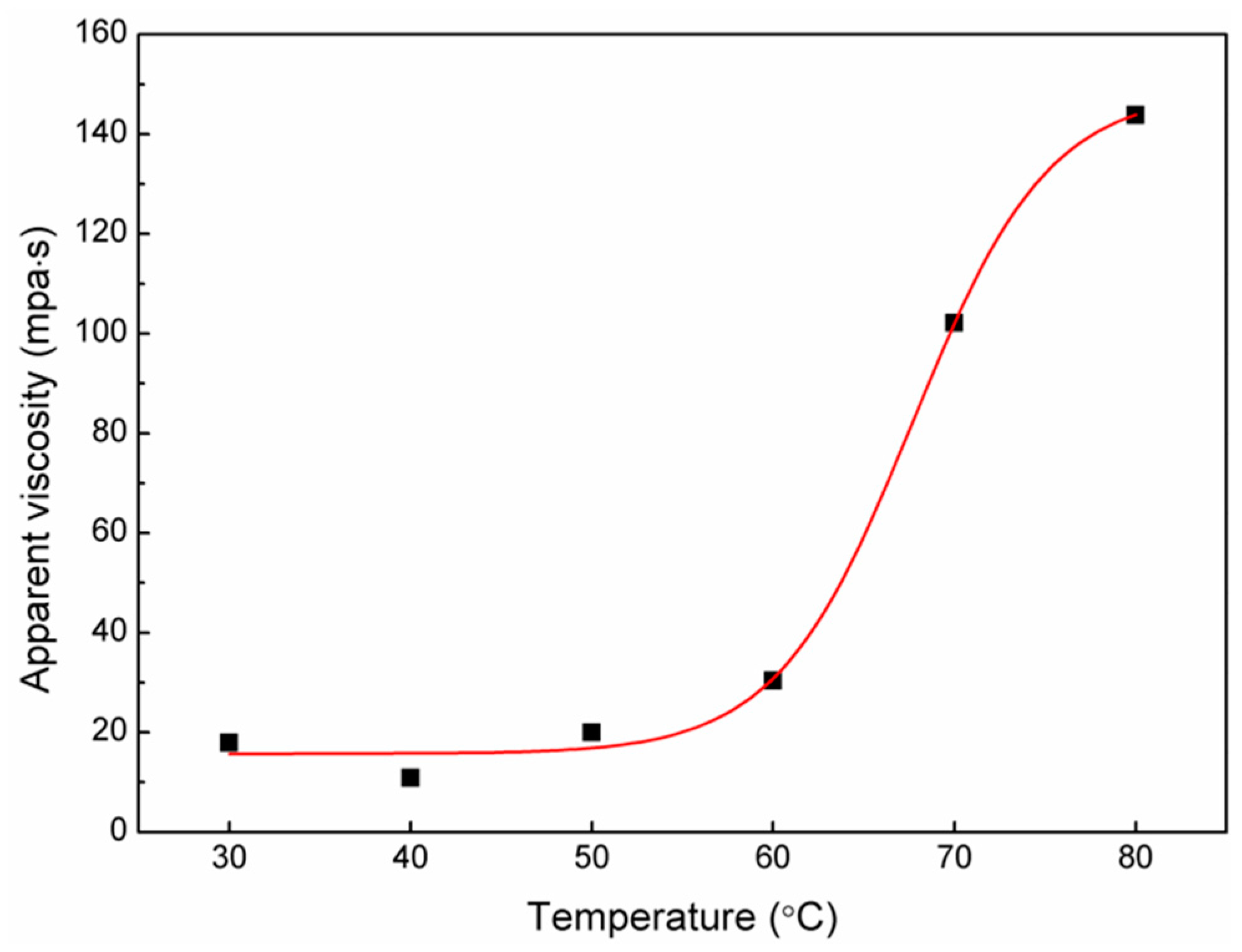
3.2. Rheological Properties of Fractional RBPs
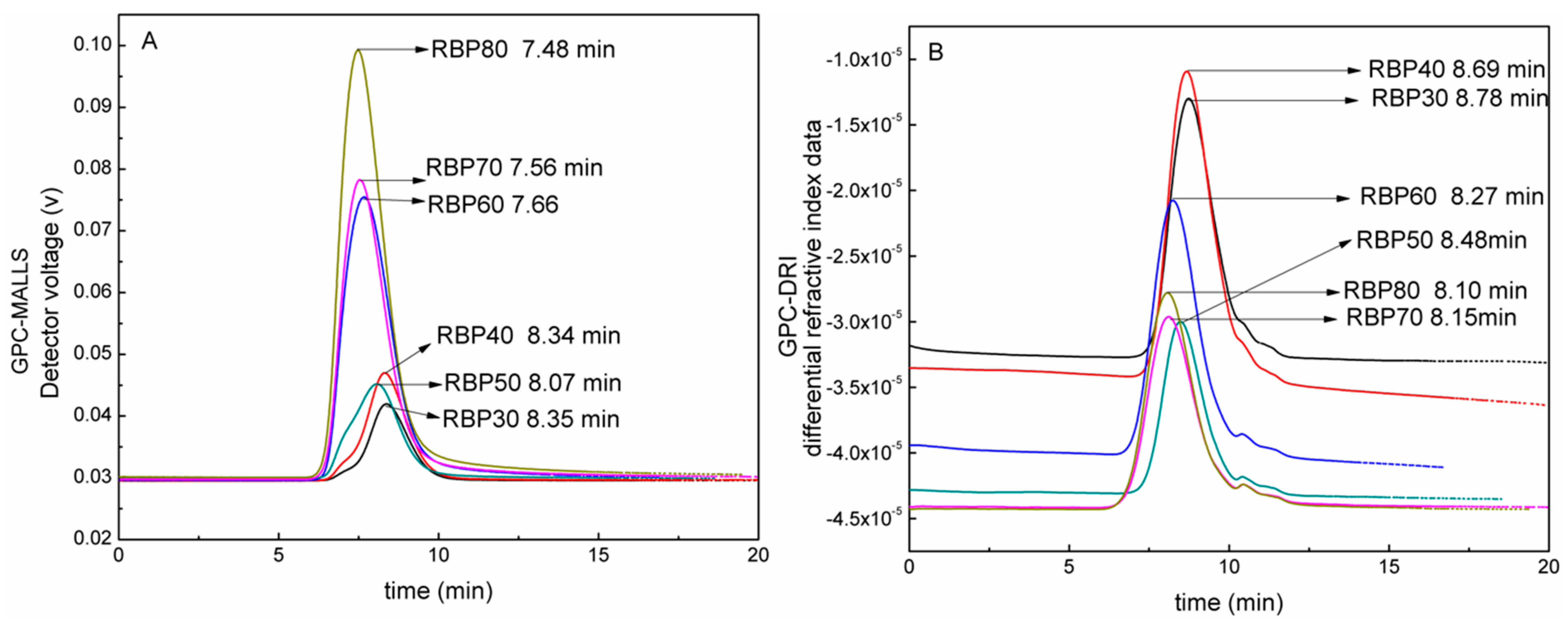
3.3. GPC Analysis
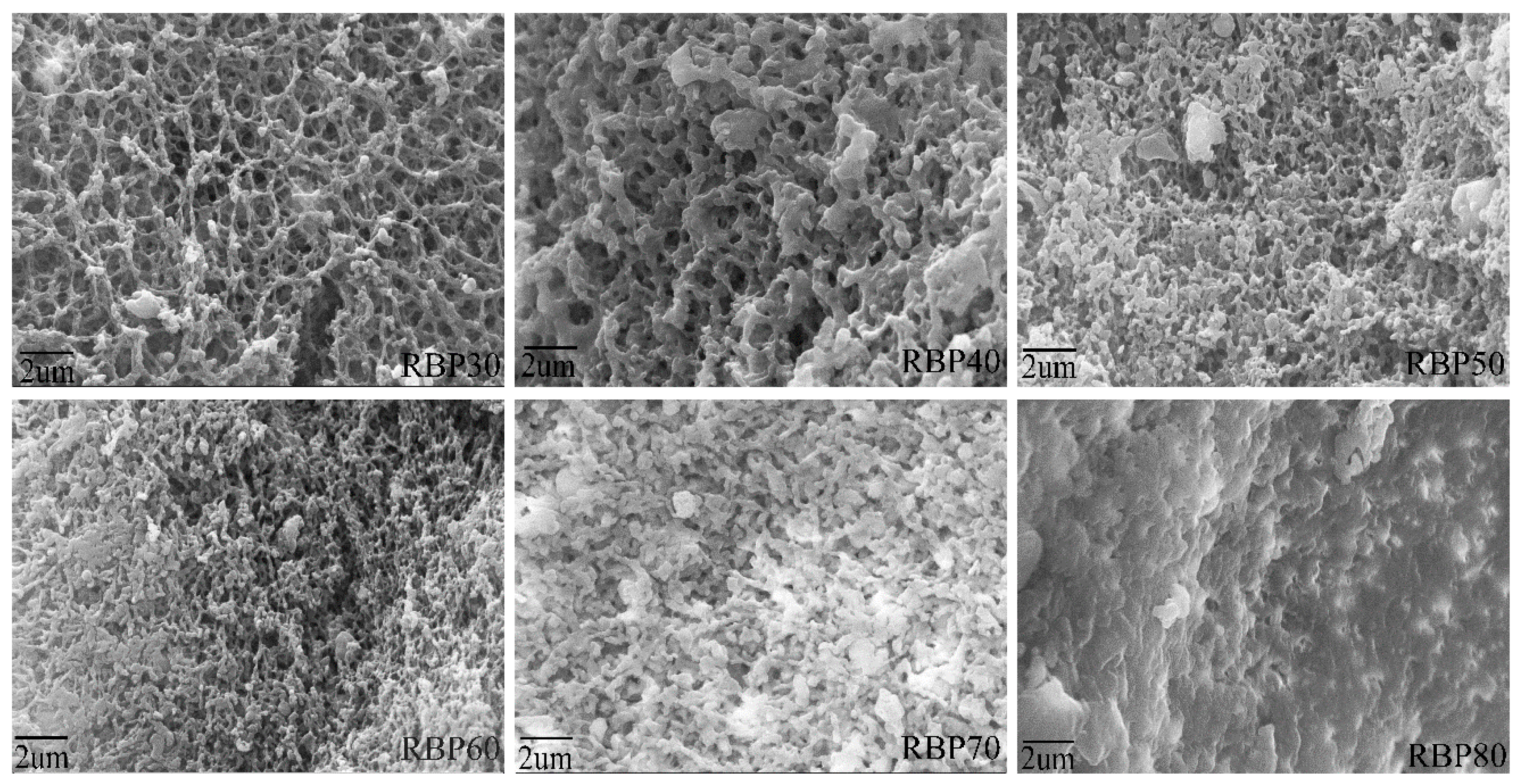
3.4. Morphology of RBPs
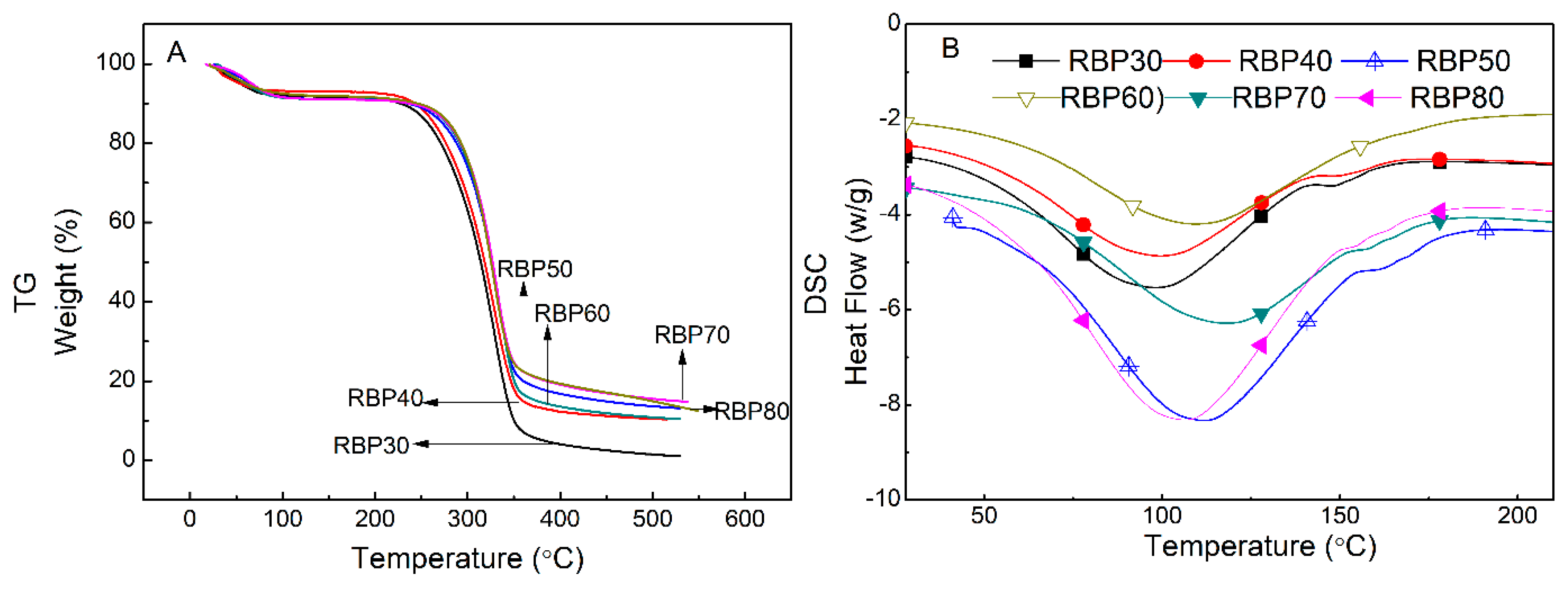
3.5. Thermal Analysis
3.6. FTIR Spectroscopy
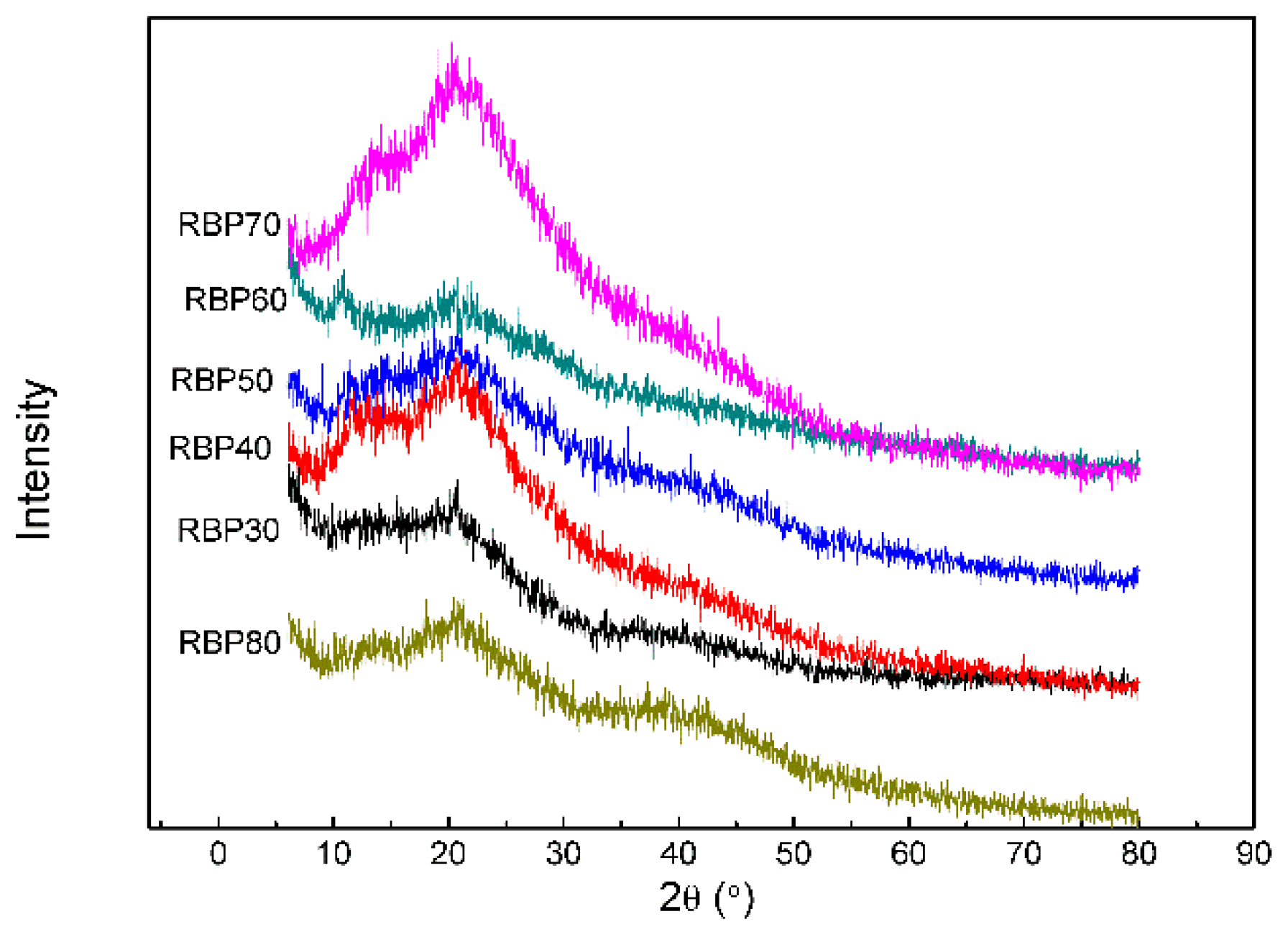
3.7. X-ray Diffraction
3.8. Elemental Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ke, Y.; Wu, Y.; Cui, X.; Liu, X.; Yu, M.; Yang, C.; Li, X. Polysaccharide Hydrogel Combined with Mesenchymal Stem Cells Promotes the Healing of Corneal Alkali Burn in Rats. PLoS ONE 2015, 10, e0119725. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Diao, H.; Xia, S.; Dong, L.; Chen, J.; Zhang, J. A physiologically active polysaccharide hydrogel promotes wound healing. J. Biomed. Mater. Res. Part A 2010, 94A, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moral, N.; Gonzalez, M.C.; Parker, R. Preparation of iron-loaded alginate gel beads and their release characteristics under simulated gastrointestinal conditions. Food Hydrocoll. 2013, 31, 114–120. [Google Scholar] [CrossRef]
- Han, M.; Du, C.; Xu, Z.-Y.; Qian, H.; Zhang, W.-G. Rheological properties of phosphorylated exopolysaccharide produced by Sporidiobolus pararoseus JD-2. Int. J. Biol. Macromol. 2016, 88 (Suppl. C), 603–613. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yu, L.; Feng, T.; Yin, X.; Liu, T.; Dong, L. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydr. Polym. 2015, 125, 1–8. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Lay, H.-L. Effect of growth stages, culture media, and processing methods on the component variations of Bletilla formosana and comparison of its component contents to commercial Rhizoma Bletillae crude drugs. J. Food Drug Anal. 2013, 21, 404–413. [Google Scholar] [CrossRef]
- Dong, L.; Xia, S.; Luo, Y.; Diao, H.; Zhang, J.; Chen, J.; Zhang, J. Targeting delivery oligonucleotide into macrophages by cationic polysaccharide from Bletilla striata successfully inhibited the expression of TNF-alpha. J. Control. Release 2009, 134, 214–220. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Chen, S.J.; Wang, Y.; Jiang, H.X.; Yin, H.P. A new glucomannan from Bletilla striata: Structural and anti-fibrosis effects. Fitoterapia 2014, 92, 72–78. [Google Scholar] [CrossRef]
- Qu, Y.; Li, C.; Zhang, C.; Zeng, R.; Fu, C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016, 148, 345–353. [Google Scholar] [CrossRef]
- Yoon, J.H.; Park, S.G.; Lee, M.J.; Park, J.Y.; Seo, K.S.; Woo, K.C.; Lee, C.E. Antioxidant and Anti-inflammatory Effects of Bletilla striata Reichenbach fil. Fractions as Cosmetic. J. Life Sci. 2013, 23. [Google Scholar] [CrossRef]
- Urrutia, P.; Bernal, C.; Escobar, S.; Santa, C.; Mesa, M.; Wilson, L.; Illanes, A. Influence of Chitosan Derivatization on Its Physicochemical Characteristics and Its Use as Enzyme Support. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Luo, Y.; Xue, W.; Diao, H.; Dong, L.; Chen, J.; Zhang, J. A polysaccharide isolated from the medicinal herb Bletilla striata induces endothelial cells proliferation and vascular endothelial growth factor expression in vitro. Biotechnol. Lett. 2006, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Manikandan, S.; Thirugnanasambandham, K.; Nivetha, C.V.; Dinesh, R. Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Kahyaoglu, T. Purification of glucomannan from salep: Part 2. Structural characterization. Carbohydr. Polym. 2017, 169, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.; Chan, K.; Hocking, T.J.; Williams, P.A.; Perry, C.J.; Baldwin, T.C. Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr. Polym. 2012, 87, 2202–2210. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Purification of glucomannan from salep: Part 1. Detailed rheological characteristics. Carbohydr. Polym. 2017, 168, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, D.; Winkworth-Smith, C.G.; Foster, T.J.; Nirasawa, S.; Tatsumi, E.; Cheng, Y. Effect of a small amount of sodium carbonate on konjac glucomannan-induced changes in wheat starch gel. Carbohydr. Polym. 2015, 116, 182–188. [Google Scholar] [CrossRef]
- Singh, V.; Sethi, R.; Tiwari, A. Structure elucidation and properties of a non-ionic galactomannan derived from the Cassia pleurocarpa seeds. Inte. J. Biol. Macromol. 2009, 44, 9–13. [Google Scholar] [CrossRef]
- Yanuriati, A.; Marseno, D.W.; Harmayani, E. Characteristics of glucomannan isolated from fresh tuber of Porang (Amorphophallus muelleri Blume). Carbohydr. Polym. 2017, 156, 56–63. [Google Scholar] [CrossRef]
- Lee, M.E.; Lee, H.D.; Suh, H.-H. Production and Characterization of Extracellular Polysaccharide Produced by Pseudomonas sp. GP32. J. Life Sci. 2015, 25, 1027–1035. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Zhu, W.; Wang, Z. Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Res. Int. 2013, 50, 633–640. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.-T.; Li, S.-R.; Liu, X.-P.; Wang, J.-S.; Liu, C.-M. Synthesis and Properties of Konjac Glucomannan- graft-poly(acrylic acid-co-trimethylallyl ammonium chloride) as a Novel Polyampholytic Superabsorbent. Adv. Polym. Technol. 2013, 32, E131–E140. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wang, Y.; Anjum, N.; Ahmad, H.; Ahmad, A.; Raza, M. Characterization of new exopolysaccharides produced by coculturing of L. kefiranofaciens with yoghurt strains. Int. J. Biol. Macromol. 2013, 59, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, Q.; Luo, X.; Liu, F.; Luo, X.; He, P. Effect of degree of acetylation on thermoplastic and melt rheological properties of acetylated konjac glucomannan. Carbohydr. Polym. 2010, 82, 167–172. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z. Novel potentially biocompatible nanoporous hydrogel based on poly ((2-dimethylaminoethyl) methacrylate) grafted onto salep: Synthesis, swelling behavior and drug release study. J. Polym. Res. 2013, 20. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Li, X.; Yu, P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2015, 117, 1021–1027. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Rheological properties and structural characterization of salep improved by ethanol treatment. Carbohydr. Polym. 2015, 133, 654–661. [Google Scholar] [CrossRef]
- Zhang, M.S.; Sun, L.; Zhao, W.C.; Peng, X.X.; Liu, F.Q.; Wang, Y.P.; Bi, Y.J.; Zhang, H.B.; Zhou, Y.F. Cholesteryl-Modification of a Glucomannan from Bletilla striata and Its Hydrogel Properties. Molecule 2014, 19, 9089–9100. [Google Scholar] [CrossRef]
- Zeng, W.-C.; Zhang, Z.; Gao, H.; Jia, L.-R.; Chen, W.-Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 2012, 89, 694–700. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Gao, Y.; Teng, X.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M.; et al. Structural elucidation of an extracellular polysaccharide produced by the marine fungus Aspergillus versicolor. Carbohydr. Polym. 2013, 93, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Fan, D.; Ma, W.; Wang, L.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W. Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch-Starke 2012, 64, 598–606. [Google Scholar] [CrossRef]

| Sample Code | GM Content (%) | RBP Yield (%) |
|---|---|---|
| RBF | 60.77 ± 0.02 | – |
| RBP30 | 93.82 ± 0.82 a | 47.47 ± 0.42 |
| RBP40 | 93.29 ± 0.43 a | 49.31 ± 0.95 |
| RBP50 | 92.88 ± 0.38 a | 50.28 ± 0.58 b |
| RBP60 | 93.10 ± 0.84 a | 51.31 ± 0.95 b |
| RBP70 | 94.67 ± 1.29 a | 53.20 ± 0.68 b |
| RBP80 | 94.58 ± 0.80 a | 54.35 ± 0.72 b |
| Sample | RBP30 | RBP40 | RBP50 | RBP60 | RBP70 | RBP80 |
|---|---|---|---|---|---|---|
| MALLS RT (min) | 8.35 | 8.34 | 8.07 | 7.66 | 7.56 | 7.48 |
| DRI RT (min) | 8.78 | 8.69 | 8.48 | 8.27 | 8.15 | 8.1 |
| Mw (g/mol) | 3.598 × 104 | 4.188 × 104 | 8.632 × 104 | 8.850 × 104 | 2.372 × 105 | 3.081 × 105 |
| Mn (g/mol) | 2.539 × 104 | 2.672 × 104 | 5.263 × 104 | 5.181 × 104 | 1.387 × 105 | 1.767 × 105 |
| D | 1.417 | 1.567 | 1.64 | 1.708 | 1.711 | 1.744 |
| Sample | TGA | DSC | ||||
|---|---|---|---|---|---|---|
| First Mass Loss Stage | Second Mass Loss Stage | Endothermic Peak | ||||
| Mass Loss (%) | Onset Temperature (%) | DTG (°C) | Mass Loss (%) | Temperature (°C) | Heat Flow (W/g) | |
| RBP30 | –7.80 | 282.5 | 328.3 | –87.60 | 98.42 | –1.750 |
| RBP40 | –6.83 | 281.2 | 328.5 | –80.76 | 99.34 | –1.539 |
| RBP50 | –8.84 | 296.1 | 333.5 | –77.73 | 106.45 | –1.411 |
| RBP60 | –8.79 | 298.5 | 331.2 | –74.22 | 109.37 | –1.373 |
| RBP70 | –8.69 | 298.3 | 330.8 | –71.74 | 111.88 | –1.299 |
| RBP80 | –7.69 | 295.2 | 327.9 | –72.73 | 118.41 | –1.257 |
| Sample | Mass Percentage, wt% | Protein Content (%) | ||
|---|---|---|---|---|
| C | N | H | ||
| RBP30 | 40.33 | 0.33 | 7.16 | 2.05 |
| RBP40 | 40.22 | 0.30 | 7.17 | 1.89 |
| RBP50 | 40.15 | 0.29 | 7.21 | 1.82 |
| RBP60 | 40.45 | 0.29 | 7.25 | 1.79 |
| RBP70 | 40.19 | 0.26 | 7.33 | 1.63 |
| RBP80 | 40.57 | 0.17 | 7.99 | 1.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Yan, Q.; Peng, L.; Liu, X.; Luo, X. Effect of Various Temperatures on Bletillae Rhizoma Polysaccharide Extraction and Physicochemical Properties. Appl. Sci. 2019, 9, 116. https://doi.org/10.3390/app9010116
Long X, Yan Q, Peng L, Liu X, Luo X. Effect of Various Temperatures on Bletillae Rhizoma Polysaccharide Extraction and Physicochemical Properties. Applied Sciences. 2019; 9(1):116. https://doi.org/10.3390/app9010116
Chicago/Turabian StyleLong, Xiaoyan, Quan Yan, Linwei Peng, Xinyue Liu, and Xuegang Luo. 2019. "Effect of Various Temperatures on Bletillae Rhizoma Polysaccharide Extraction and Physicochemical Properties" Applied Sciences 9, no. 1: 116. https://doi.org/10.3390/app9010116
APA StyleLong, X., Yan, Q., Peng, L., Liu, X., & Luo, X. (2019). Effect of Various Temperatures on Bletillae Rhizoma Polysaccharide Extraction and Physicochemical Properties. Applied Sciences, 9(1), 116. https://doi.org/10.3390/app9010116




