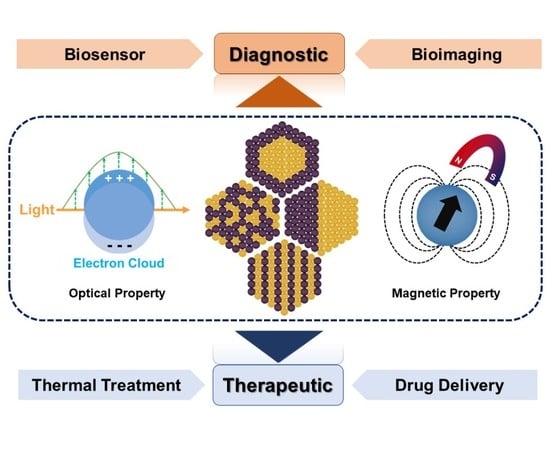
Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications
Abstract
1. Introduction
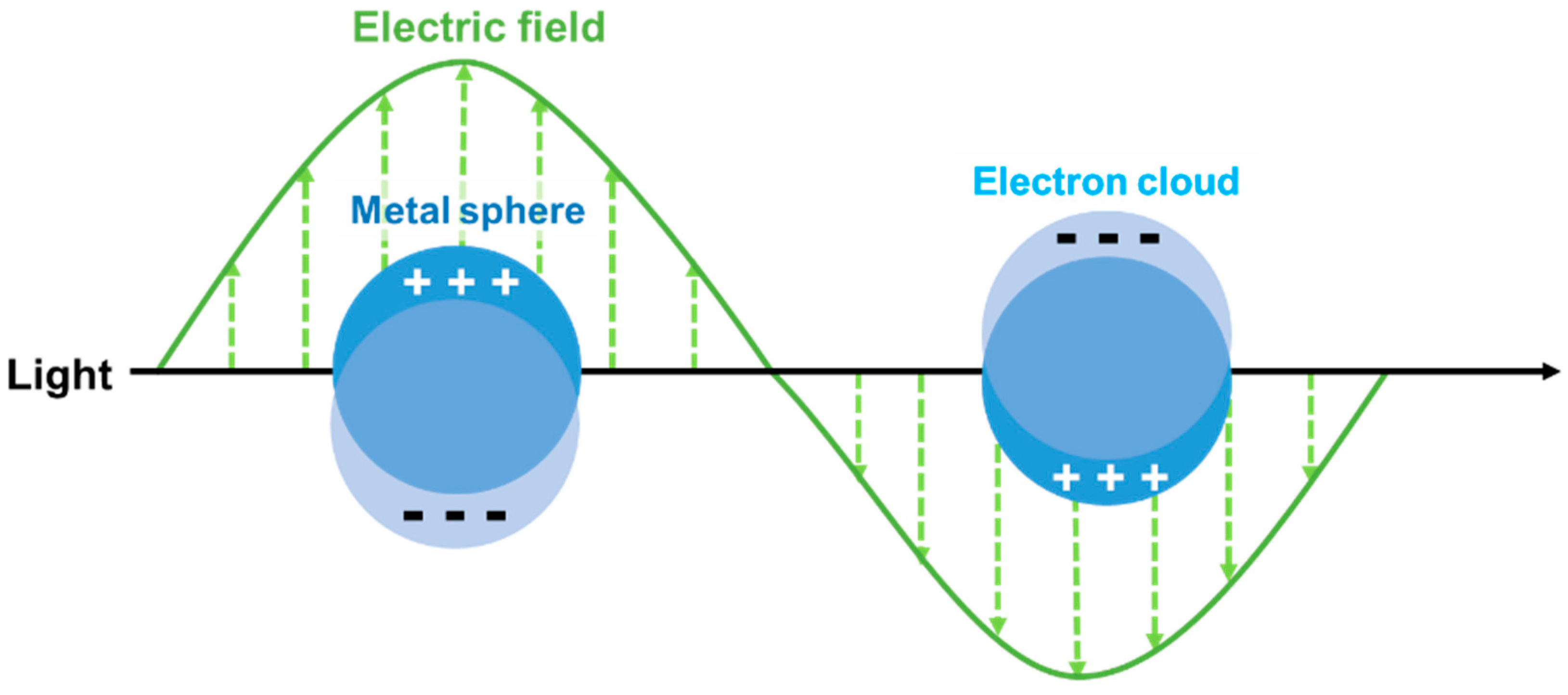
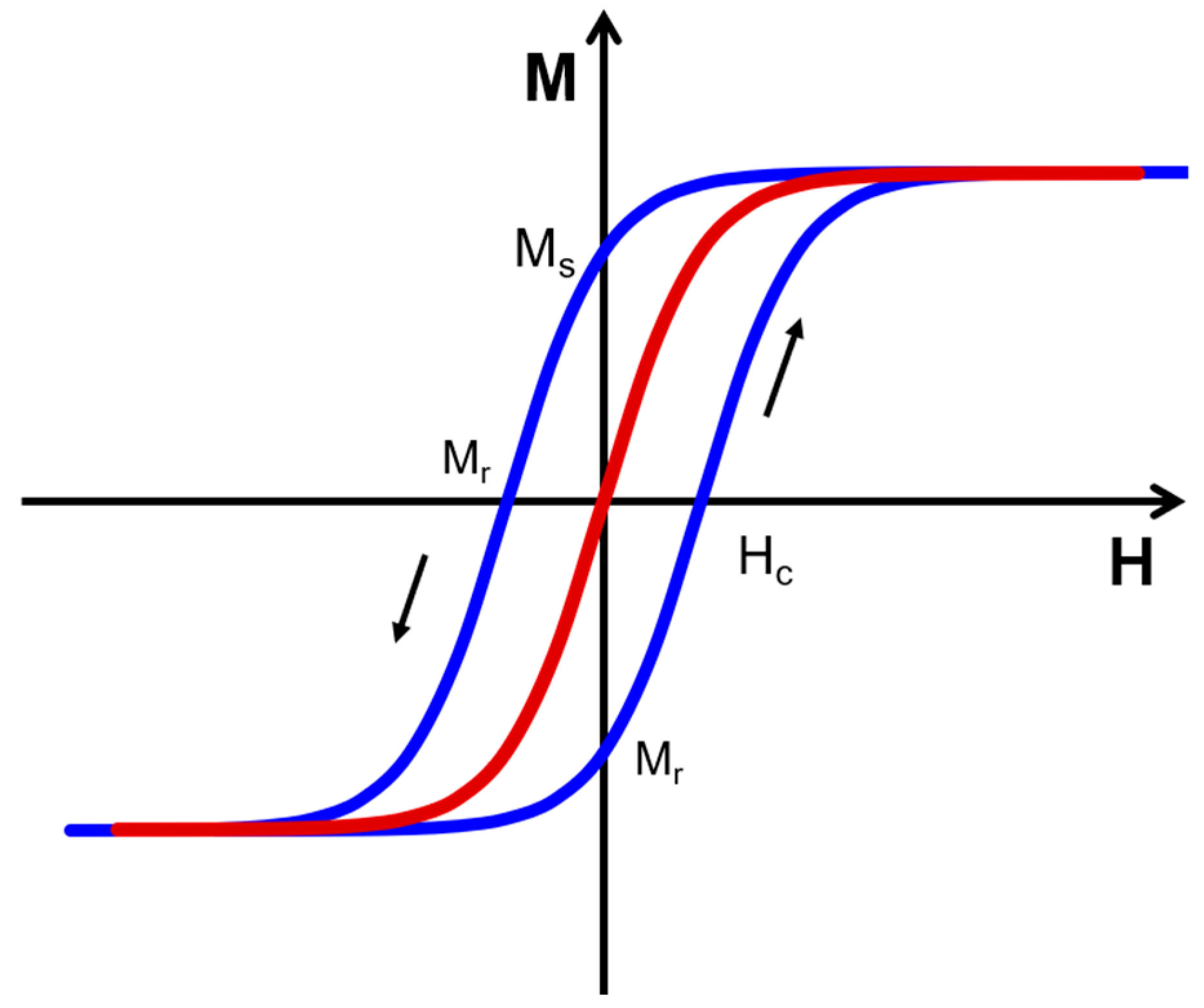
2. Optical and Magnetic Properties of Bimetallic Nanoparticles for Biological Applications.
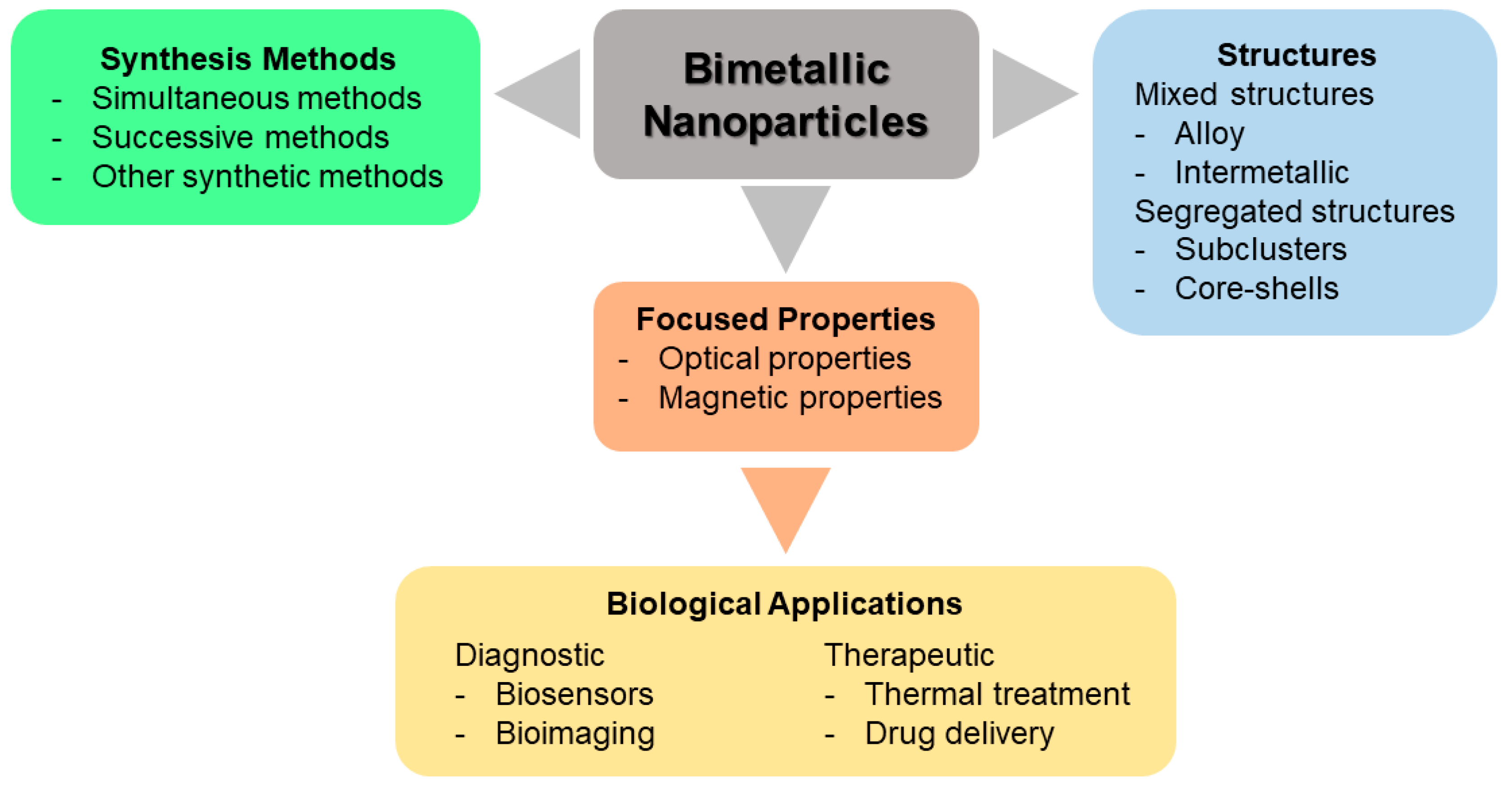
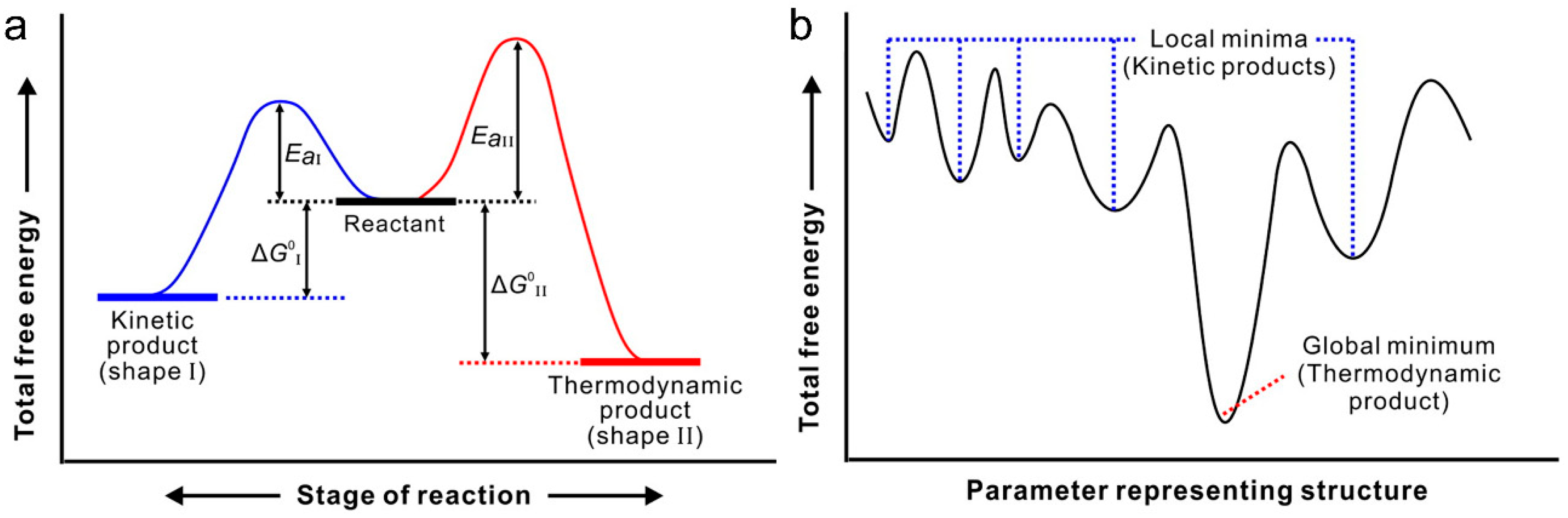
3. Classification and Synthesis of Bimetallic Nanoparticles
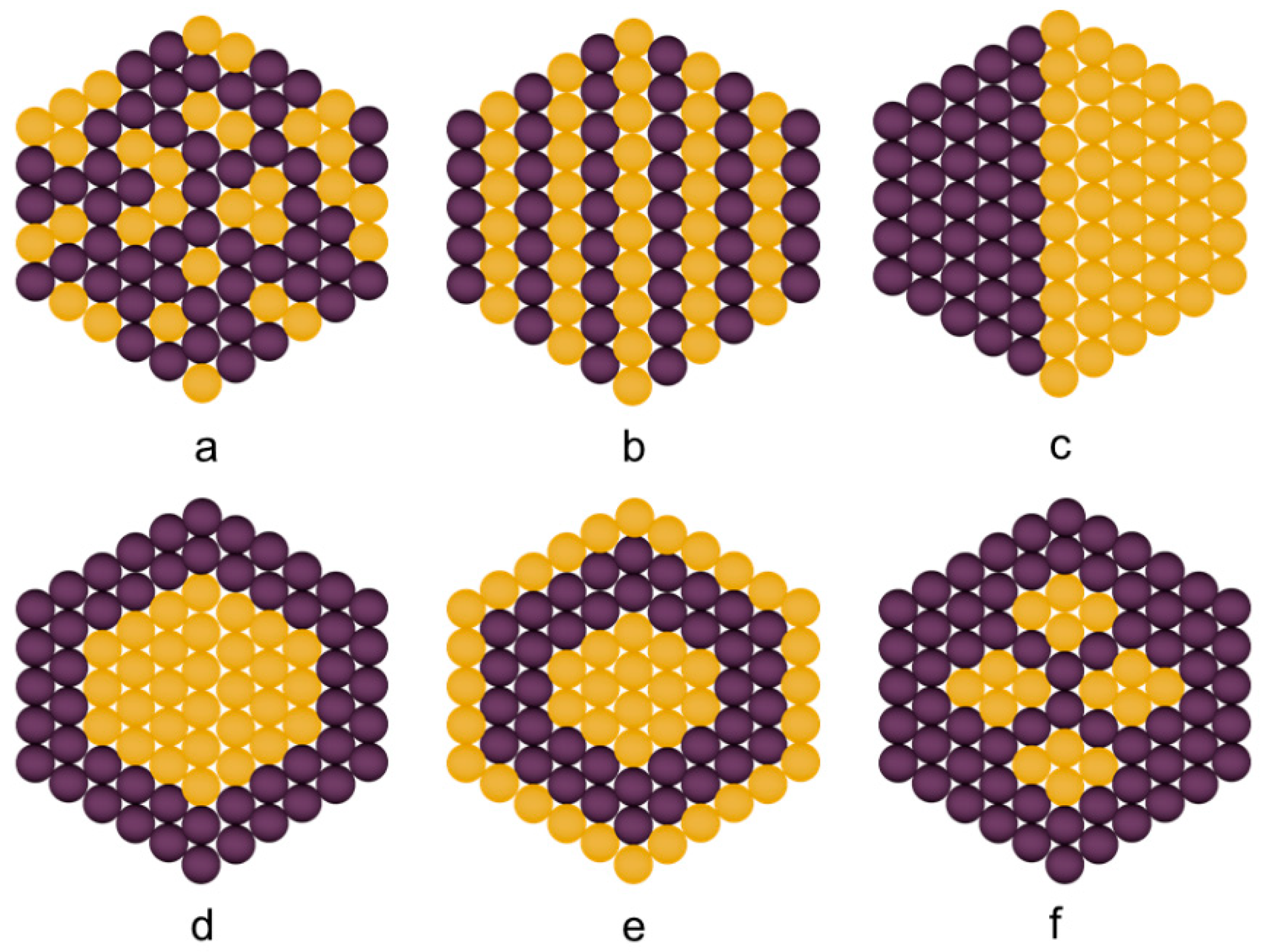
3.1. Classification of Bimetallic Nanoparticles
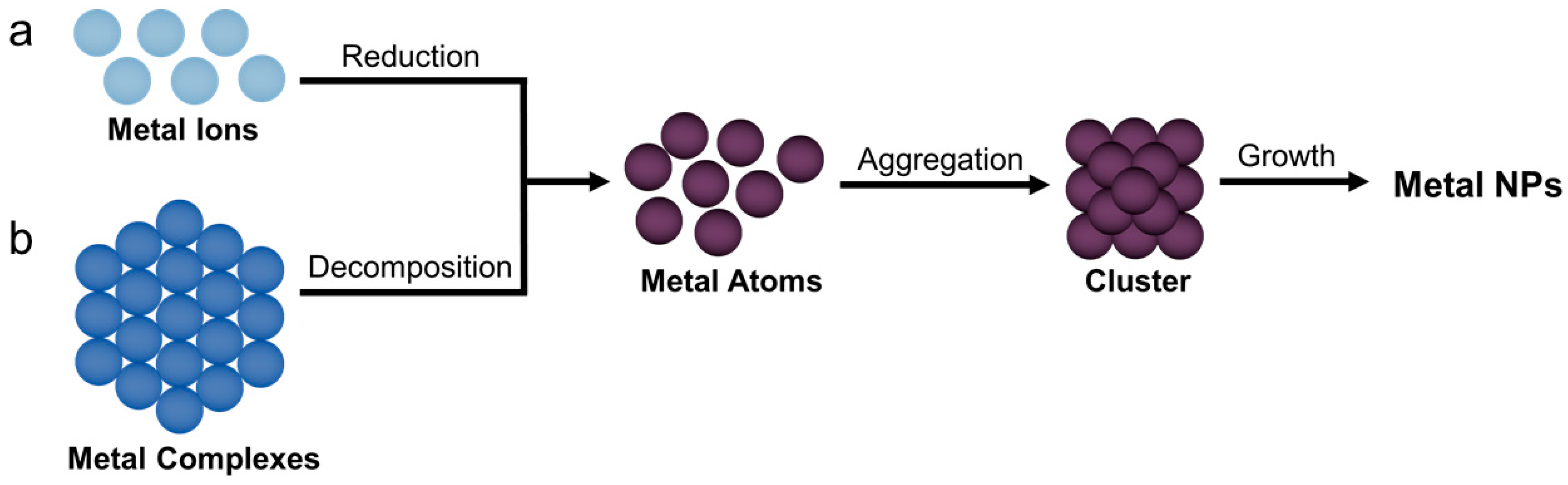
3.2. Synthesis of Bimetallic Nanoparticles
3.3. Simultaneous Methods
3.3.1. Co-Reduction
3.3.2. Thermal Decomposition
3.3.3. Radiolytic Synthesis
3.3.4. Sonochemical Synthesis
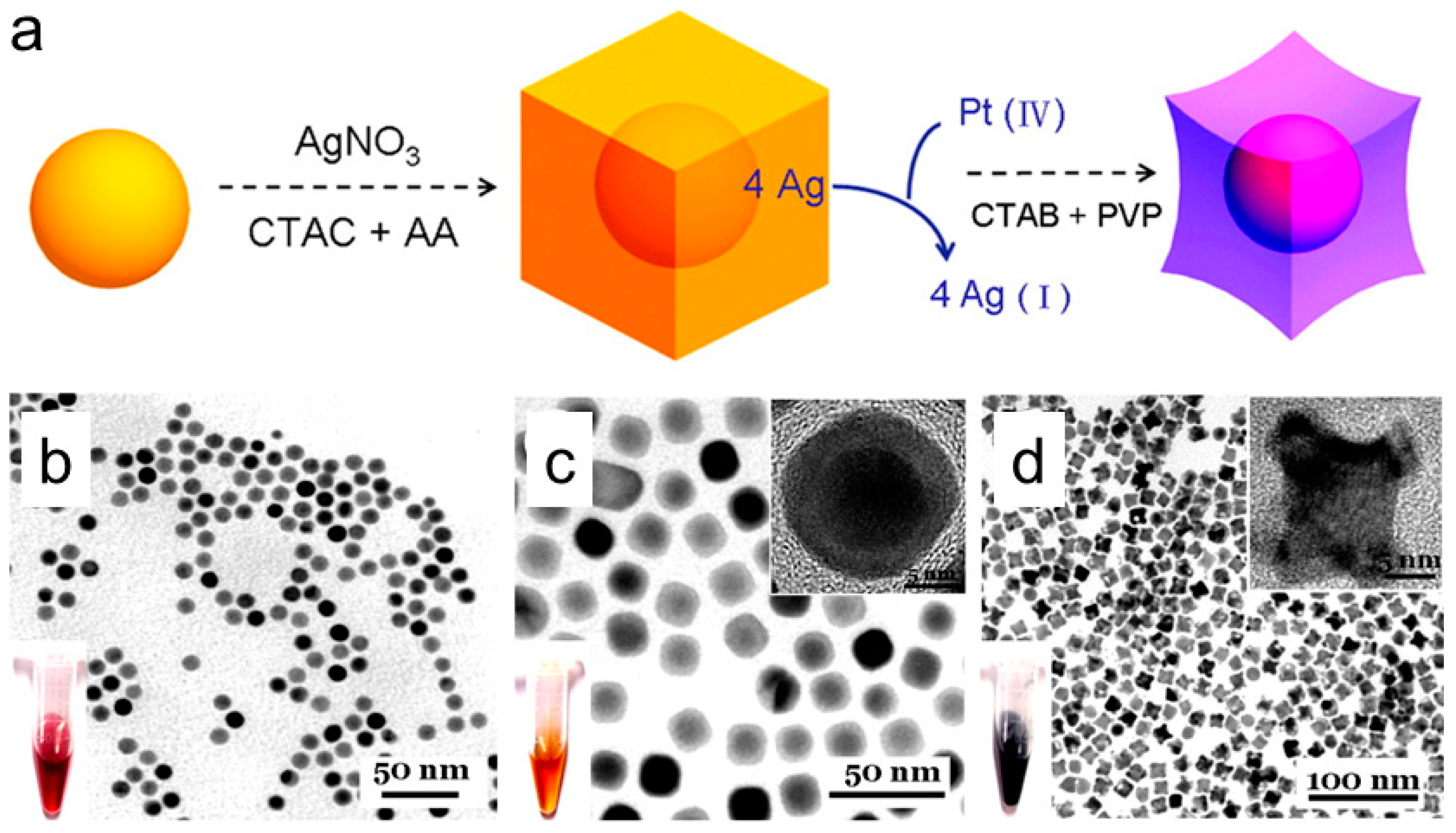
3.4. Successive Methods
Seed-Mediated Growth
3.5. Other Synthetic Methods
3.6. Stabilization Strategies
4. Biological Applications
4.1. Diagnostic Applications
4.1.1. Biosensors
4.1.2. Bioimaging
4.2. Therapeutic Applications
4.2.1. Thermal Treatments
4.2.2. Drug Delivery
5. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Li, C.-H.; Li, M.-C.; Liu, S.-P.; Jamison, A.C.; Lee, D.; Lee, T.R.; Lee, T.-C. Plasmonically enhanced photocatalytic hydrogen production from water: The critical role of tunable surface Plasmon resonance from gold–silver nanoshells. ACS Appl. Mater. Interfaces 2016, 8, 9152–9161. [Google Scholar] [CrossRef] [PubMed]
- Hien Pham, T.T.; Cao, C.; Sim, S.J. Application of citrate-stabilized gold-coated ferric oxide composite nanoparticles for biological separations. J. Magn. Magn. Mater. 2008, 320, 2049–2055. [Google Scholar] [CrossRef]
- Li, J.J.; Peng, X. Photocatalytic activity of gold nanocrystals and its role in determining the stability of surface thiol monolayers. J. Nanosci. Nanotechnol. 2004, 4, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Tessier, P.M.; Velev, O.D.; Kalambur, A.T.; Rabolt, J.F.; Lenhoff, A.M.; Kaler, E.W. Assembly of gold nanostructured films templated by colloidal crystals and use in surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2000, 122, 9554–9555. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, M.; Lee, W.Y.; Hyeon, T. Fabrication of hollow palladium spheres and their successful application as the recyclable heterogeneous catalyst for Suzuki coupling reactions. J. Am. Chem. Soc. 2002, 124, 7642–7643. [Google Scholar] [CrossRef] [PubMed]
- Nicewarner-Peña, S.R.; Freeman, R.G.; Reiss, B.D.; He, L.; Peña, D.J.; Walton, I.D.; Cromer, R.; Keating, C.D.; Natan, M.J. Submicrometer metallic barcodes. Science 2001, 294, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Saeki, F.; Wiley, B.J.; Cang, H.; Cobb, M.J.; Li, Z.-Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X. Gold Nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Mao, K.; Zhou, X.; Hu, J. A novel biosensor based on Au@Ag core-shell nanoparticles for SERS Detection of Arsenic (III). Talanta 2016, 146, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Almalki, M.A.; Ilavenil, S.; Rebecca, J.; Choi, K.C. Silver nanopaticles synthesized using the seed extract of Trigonella Foenum-Graecum L. and their antimicrobial mechanism and anticancer properties. Saudi J. Biol. Sci. 2017, in press. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Liu, S.; Zhang, J.; Chen, W.; Huang, C.; Mao, L. Effect of Pt–Pd hybrid nano-particle on CdS’s Activity for water splitting under visible light. Int. J. Hydrog. Energy 2016, 41, 23015–23021. [Google Scholar] [CrossRef]
- Logunov, S.L.; Ahmadi, T.S.; El-Sayed, M.A.; Khoury, J.T.; Whetten, R.L. Electron dynamics of passivated gold nanocrystals probed by subpicosecond transient absorption spectroscopy. J. Phys. Chem. B 1997, 101, 3713–3719. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Bratescu, M.A.; Cho, S.-P.; Takai, O.; Saito, N. Size-controlled gold nanoparticles synthesized in solution Plasma. J. Phys. Chem. C 2011, 115, 24569–24576. [Google Scholar] [CrossRef]
- Naskar, S.; Freytag, A.; Deutsch, J.; Wendt, N.; Behrens, P.; Köckritz, A.; Bigall, N.C. Porous aerogels from shape-controlled metal nanoparticles directly from nonpolar colloidal solution. Chem. Mater. 2017, 29, 9208–9217. [Google Scholar] [CrossRef]
- Personick, M.L.; Langille, M.R.; Zhang, J.; Mirkin, C.A. Shape control of gold nanoparticles by silver underpotential deposition. Nano Lett. 2011, 11, 3394–3398. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tang, R.; Yu, H.; Gibbons, P.C.; Buhro, W.E. Size- and shape-controlled synthesis of bismuth nanoparticles. Chem. Mater. 2008, 20, 3656–3662. [Google Scholar] [CrossRef]
- Murphy, C.J.; Jana, N.R. Controlling the aspect ratio of inorganic nanorods and nanowires. Adv. Mater. 2002, 14, 80–82. [Google Scholar] [CrossRef]
- Wiley, B.J.; Chen, Y.; Mclellan, J.M.; Xiong, Y.; Li, Z.-Y.; Ginger, D.; Xia, Y. Synthesis and optical properties of silver nanobars and nanorice. Nano Lett. 2007, 7, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Tsukahara, Y.; Yamada, K.; Sakata, T.; Wada, Y. Nucleation and growth of magnetic Ni–Co (Core-shell) nanoparticles in a one-pot reaction under microwave irradiation. Chem. Mater. 2011, 23, 75–84. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Gao, J.; Ren, X.; Chen, D.; Tang, F.; Ren, J. Bimetallic Ag–Pt hollow nanoparticles: Synthesis and tunable surface Plasmon resonance. Scr. Mater. 2007, 57, 687–690. [Google Scholar] [CrossRef]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles-novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Vongsavat, V.; Vittur, B.M.; Bryan, W.W.; Kim, J.-H.; Lee, T.R. Ultrasmall hollow gold–silver nanoshells with extinctions strongly red-shifted to the near-infrared. ACS Appl. Mater. Interfaces 2011, 3, 3616–3624. [Google Scholar] [CrossRef] [PubMed]
- Sra, A.K.; Schaak, R.E. Synthesis of atomically ordered AuCu and AuCu3 nanocrystals from bimetallic nanoparticle precursors. J. Am. Chem. Soc. 2004, 126, 6667–6672. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hight Walker, A.R. Synthesis and characterization of cobalt/gold bimetallic nanoparticles. J. Magn. Magn. Mater. 2007, 311, 31–35. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core-shell nanoparticle-enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Krull, U.J. Localized surface Plasmon resonance: Nanostructures, bioassays and biosensing—A Review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized surface Plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, properties, and applications of hollow micro-/nanostructures. Chem. Rev. 2016, 116, 10983–11060. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, C.; Jurgons, R.; Schmid, R.; Hilpert, A.; Bergemann, C.; Parak, F.; Iro, H. In vitro and in vivo investigations of targeted chemotherapy with magnetic nanoparticles. J. Magn. Magn. Mater. 2005, 293, 389–393. [Google Scholar] [CrossRef]
- Demers, L.M.; Oestblom, M.; Zhang, H.; Jang, N.-H.; Liedberg, B.; Mirkin, C.A. Thermal desorption behavior and binding properties of DNA bases and nucleosides on gold. J. Am. Chem. Soc. 2002, 124, 11248–11249. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Gao, J.; Liang, G.; Cheung, J.S.; Pan, Y.; Kuang, Y.; Zhao, F.; Zhang, B.; Zhang, X.; Wu, E.X.; Xu, B. Multifunctional yolk-shell nanoparticles: A potential MRI contrast and anticancer agent. J. Am. Chem. Soc. 2008, 130, 11828–11833. [Google Scholar] [CrossRef] [PubMed]
- Lane, L.A.; Qian, X.; Nie, S. SERS Nanoparticles in medicine: From label-free detection to spectroscopic tagging. Chem. Rev. 2015, 115, 10489–10529. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Lee, J.-H.; Shin, T.-H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.; Jamison, A.C.; Litvinov, D.; Willson, R.; Lee, T. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Bai, J.; Wang, J.-P. High-magnetic-moment multifunctional nanoparticles for nanomedicine applications. J. Magn. Magn. Mater. 2007, 311, 131–134. [Google Scholar] [CrossRef]
- Yin, S.; Li, Z.; Cheng, L.; Wang, C.; Liu, Y.; Chen, Q.; Gong, H.; Guo, L.; Li, Y.; Liu, Z. Magnetic PEGylated Pt3Co nanoparticles as a novel MR contrast agent: In vivo MR imaging and long-term toxicity study. Nanoscale 2013, 5, 12464–12473. [Google Scholar] [CrossRef] [PubMed]
- Bansmann, J.; Baker, S.H.; Binns, C.; Blackman, J.A.; Bucher, J.P.; Dorantes-Dávila, J.; Dupuis, V.; Favre, L.; Kechrakos, D.; Kleibert, A.; et al. Magnetic and structural properties of isolated and assembled clusters. Surf. Sci. Rep. 2005, 56, 189–275. [Google Scholar] [CrossRef]
- Jeong, U.; Teng, X.; Yong, W.; Yang, H.; Xia, Y. Superparamagnetic colloids: Controlled synthesis and niche applications. Adv. Mater. 2007, 19, 33–60. [Google Scholar] [CrossRef]
- Sharma, G.; Gupta, V.K.; Agarwal, S.; Kumar, A.; Thakur, S.; Pathania, D. Fabrication and characterization of Fe@MoPO Nanoparticles: Ion exchange behavior and photocatalytic activity against malachite green. J. Mol. Liq. 2016, 219, 1137–1143. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble metal-based bimetallic nanoparticles: The effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Daněk, V. Physico-Chemical Analysis of Molten Electrolytes; Elsevier Science: New York, NY, USA, 2006. [Google Scholar]
- Riccardo, F. Symmetry breaking and morphological instabilities in core-shell metallic nanoparticles. J. Phys. Condens. Matter 2015, 27, 013003. [Google Scholar]
- Langlois, C.; Li, Z.L.; Yuan, J.; Alloyeau, D.; Nelayah, J.; Bochicchio, D.; Ferrando, R.; Ricolleau, C. Transition from core-shell to Janus chemical configuration for bimetallic nanoparticles. Nanoscale 2012, 4, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Baletto, F.; Mottet, C.; Ferrando, R. Growth of three-shell Onionlike bimetallic nanoparticles. Phys. Rev. Lett. 2003, 90, 135504. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, D.; Torres-Castro, A.; Gao, X.; Sepulveda-Guzman, S.; Ortiz-Mendez, U.; Jose-Yacaman, M. Three-Layer core/shell structure in Au–Pd bimetallic nanoparticles. Nano Lett. 2007, 7, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xia, X.; Peng, H.-C. Shape-controlled synthesis of colloidal metal nanocrystals: Thermodynamic versus kinetic products. J. Am. Chem. Soc. 2015, 137, 7947–7966. [Google Scholar] [CrossRef] [PubMed]
- Yawen, W.; Jiating, H.; Cuicui, L.; Han, C.W.; Hongyu, C. Thermodynamics versus kinetics in nanosynthesis. Angew. Chem. Int. Ed. 2015, 54, 2022–2051. [Google Scholar]
- Gaffet, E.; Tachikart, M.; El Kedim, O.; Rahouadj, R. Nanostructural materials formation by mechanical alloying: Morphologic analysis based on transmission and scanning electron microscopic observations. Mater. Charact. 1996, 36, 185–190. [Google Scholar] [CrossRef]
- Klabunde, K.J. Nanoscale Materials in Chemistry; Wiley-Interscience: Hoboken, NJ, USA, 2001. [Google Scholar]
- Hu, J.-W.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Sun, S.-G.; Tian, Z.-Q. Palladium-coated gold nanoparticles with a controlled shell thickness used as surface-enhanced Raman scattering substrate. J. Phys. Chem. C 2007, 111, 1105–1112. [Google Scholar] [CrossRef]
- Link, S.; Wang, Z.L.; El-Sayed, M.A. Alloy formation of gold–silver nanoparticles and the dependence of the Plasmon absorption on their composition. J. Phys. Chem. B 1999, 103, 3529–3533. [Google Scholar] [CrossRef]
- Sagitha, P.; Sarada, K.; Muraleedharan, K. One-pot synthesis of Poly Vinyl Alcohol (PVA) supported silver nanoparticles and its efficiency in catalytic reduction of methylene blue. Trans. Nonferr. Met. Soc. China 2016, 26, 2693–2700. [Google Scholar] [CrossRef]
- Toshima, N.; Harada, M.; Yamazaki, Y.; Asakura, K. Catalytic activity and structural analysis of polymer-protected gold-palladium bimetallic clusters prepared by the simultaneous reduction of hydrogen Tetrachloroaurate and palladium dichloride. J. Phys. Chem. 1992, 96, 9927–9933. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Okitsu, K.; Maeda, Y.; Yamamoto, T.A.; Oshima, R.; Nagata, Y. Sonochemical preparation of bimetallic nanoparticles of gold/palladium in aqueous solution. J. Phys. Chem. B 1997, 101, 7033–7037. [Google Scholar] [CrossRef]
- Kan, C.; Cai, W.; Li, C.; Zhang, L.; Hofmeister, H. Ultrasonic synthesis and optical properties of Au/Pd bimetallic nanoparticles in ethylene glycol. J. Phys. D Appl. Phys. 2003, 36, 1609–1614. [Google Scholar] [CrossRef]
- Vasquez, Y.; Luo, Z.; Schaak, R.E. Low-temperature solution synthesis of the non-equilibrium ordered intermetallic compounds Au3Fe, Au3Co, and Au3Ni as nanocrystals. J. Am. Chem. Soc. 2008, 130, 11866–11867. [Google Scholar] [CrossRef] [PubMed]
- Ueji, M.; Harada, M.; Kimura, Y. Synthesis of Pt/Ru bimetallic nanoparticles in high-temperature and high-pressure fluids. J. Colloid Interface Sci. 2008, 322, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Harpeness, R.; Gedanken, A. Microwave synthesis of core-shell gold/palladium bimetallic nanoparticles. Langmuir 2004, 20, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Treguer, M.; De Cointet, C.; Remita, H.; Khatouri, J.; Mostafavi, M.; Amblard, J.; Belloni, J.; De Keyzer, R. Dose rate effects on radiolytic synthesis of gold–silver bimetallic clusters in solution. J. Phys. Chem. B 1998, 102, 4310–4321. [Google Scholar] [CrossRef]
- Hund, J.F.; Bertino, M.F.; Zhang, G.; Sotiriou-Leventis, C.; Leventis, N. Synthesis of homogeneous alloy metal nanoparticles in silica aerogels. J. Non-Cryst. Solids 2004, 350, 9–13. [Google Scholar] [CrossRef]
- Schmid, G.L.A.; Malm, J.-O.; Bovin, J.-O. Ligand-stabilized bimetallic colloids identified by HRTEM and EDX. Angew. Chem. Int. Ed. 1991, 30, 874–876. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. A greener synthesis of core (Fe, Cu)-Shell (Au, Pt, Pd, and Ag) nanocrystals using aqueous vitamin C. Cryst. Growth Des. 2007, 7, 2582–2587. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, J.Y.; Too, H.-P. Core-shell Ag–Au nanoparticles from replacement reaction in organic medium. J. Phys. Chem. B 2005, 109, 19208–19212. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Lau, K.C.; Hsu, J.C.; Hajfathalian, M.; Mian, S.; Chhour, P.; Uppuluri, L.; Mcdonald, E.S.; Maidment, A.D.A.; Cormode, D.P. Gold Silver Alloy Nanoparticles (GSAN): An imaging probe for breast cancer screening with dual-energy mammography or computed tomography. Nanoscale 2016, 8, 13740–13754. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Kim, C.; Zhou, F.; Cobley, C.M.; Song, K.H.; Chen, J.; Li, Z.-Y.; Wang, L.V.; Xia, Y. Measuring the optical absorption cross sections of Au–Ag Nanocages and Au Nanorods by Photoacoustic imaging. J. Phys. Chem. C 2009, 113, 9023–9028. [Google Scholar] [CrossRef] [PubMed]
- Srnova-Sloufova, I.; Lednicky, F.; Gemperle, A.; Gemperlova, J. Core-Shell (Ag)Au bimetallic nanoparticles: Analysis of transmission electron microscopy images. Langmuir 2000, 16, 9928–9935. [Google Scholar] [CrossRef]
- Park, G.; Seo, D.; Jung, J.; Ryu, S.; Song, H. Shape evolution and gram-scale synthesis of Gold@Silver Core-shell Nanopolyhedrons. J. Phys. Chem. C 2011, 115, 9417–9423. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S.; Zhu, J.; Wang, K.; Gao, M.; Wang, X.; Xu, Q. Au–Ag bimetallic nanoparticles decorated multi-amino Cyclophosphazene hybrid microspheres as enhanced activity catalysts for the reduction of 4-nitrophenol. Mater. Chem. Phys. 2018, 207, 315–324. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.-C.; Lee, G.-J.; Park, H.-K.; Lee, A.; Choi, S. Low-cost label-free biosensing bimetallic cellulose strip with SILAR-synthesized silver core–gold shell nanoparticle structures. Anal. Chem. 2017, 89, 6448–6454. [Google Scholar] [CrossRef] [PubMed]
- Dahal, N.; Chikan, V.; Jasinski, J.; Leppert, V.J. Synthesis of water-soluble iron–gold alloy nanoparticles. Chem. Mater. 2008, 20, 6389–6395. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M.; Bakr, O.M.; Riello, P.; Polizzi, S.; Anjum, D.H.; Fiameni, S.; Arosio, P.; Orlando, T.; De Julian Fernandez, C.; Pineider, F.; Sangregorio, C.; Lascialfari, A. Coexistence of Plasmonic and magnetic properties in Au89Fe11 nanoalloys. Nanoscale 2013, 5, 5611–5619. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Scaramuzza, S.; Litti, L.; Meneghetti, M.; Zuccolotto, G.; Rosato, A.; Nicolato, E.; Marzola, P.; Fracasso, G.; Anselmi, C.; Pinto, M.; Colombatti, M. Magneto-Plasmonic Au–Fe alloy nanoparticles designed for multimodal SERS-MRI-CT Imaging. Small 2014, 10, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Prouty, M.D.; Guo, Z.; Golub, V.O.; Kumar, C.S.S.R.; Lvov, Y.M. Magnetic switch of permeability for polyelectrolyte microcapsules embedded with Co@Au nanoparticles. Langmuir 2005, 21, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.; Ménard, D.; Meunier, M. Nanoclustered Co–Au particles fabricated by femtosecond laser fragmentation in liquids. J. Phys. Chem. C 2010, 114, 13497–13500. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Koga, K.; Kawaguchi, K.; Wang, H.; Pyatenko, A.; Koshizaki, N. Pulsed laser irradiation of colloidal nanoparticles: A new synthesis route for the production of non-equilibrium bimetallic alloy submicrometer spheres. RSC Adv. 2013, 3, 79–83. [Google Scholar] [CrossRef]
- Salem, A.K.; Searson, P.C.; Leong, K.W. Multifunctional nanorods for gene delivery. Nat. Mater. 2003, 2, 668. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Sheu, H.-S.; Lin, C.-Y.; Huang, C.-C.; Lo, Y.-W.; Pu, Y.-C.; Weng, J.-C.; Shieh, D.-B.; Chen, J.-H.; Yeh, C.-S. Nanoshell magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2007, 129, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Belousov, O.V.; Belousova, N.V.; Sirotina, A.V.; Solovyov, L.A.; Zhyzhaev, A.M.; Zharkov, S.M.; Mikhlin, Y.L. Formation of Bimetallic Au–Pd and Au–Pt nanoparticles under hydrothermal conditions and microwave irradiation. Langmuir 2011, 27, 11697–11703. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, Y.; Fujimoto, T.; Nagata, Y.; Oshima, R.; Maeda, Y. Characterization and catalytic activity of core-shell structured gold/palladium bimetallic nanoparticles synthesized by the sonochemical method. J. Phys. Chem. B 2000, 104, 6028–6032. [Google Scholar] [CrossRef]
- Okitsu, K.; Murakami, M.; Tanabe, S.; Matsumoto, H. Catalytic behavior of Au Core/Pd shell bimetallic nanoparticles on silica prepared by sonochemical and sol-gel processes. Chem. Lett. 2000, 29, 1336–1337. [Google Scholar] [CrossRef]
- Hu, J.-W.; Zhang, Y.; Li, J.-F.; Liu, Z.; Ren, B.; Sun, S.-G.; Tian, Z.-Q.; Lian, T. Synthesis of Au@Pd core-shell nanoparticles with controllable size and their application in surface-enhanced Raman spectroscopy. Chem. Phys. Lett. 2005, 408, 354–359. [Google Scholar] [CrossRef]
- Zhan, G.; Huang, J.; Du, M.; Abdul-Rauf, I.; Ma, Y.; Li, Q. Green synthesis of Au–Pd bimetallic nanoparticles: Single-step bioreduction method with plant extract. Mater. Lett. 2011, 65, 2989–2991. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, J.; Li, J.; Zhang, N.; Hong, R.; Deng, X.; Tang, P.; Li, D. Hydrogen sensing properties of Pt–Au bimetallic nanoparticles loaded on ZnO Nanorods. Sens. Actuators B Chem. 2017, 241, 895–903. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt–Au dendrimer-like nanoparticles supported on Polydopamine-functionalized Graphene and their high performance toward 4-Nitrophenol reduction. Appl. Catal. B Environ. 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Zhai, Y.; Xu, X.; Jin, Y. Synthesis of monodisperse plasmonic Au Core–Pt shell concave Nanocubes with superior catalytic and Electrocatalytic activity. ACS Catal. 2013, 3, 2045–2051. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Sastry, M. Hollow gold and platinum nanoparticles by a transmetallation reaction in an organic solution. Chem. Commun. 2005, 1684–1686. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tian, W.; Ding, Y.; Ma, Y.-Q.; Wang, Z.L.; Markovic, N.M.; Stamenkovic, V.R.; Daimon, H.; Sun, S. Rational synthesis of heterostructured nanoparticles with morphology control. J. Am. Chem. Soc. 2010, 132, 6524–6529. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Xiao, Y.; Li, W.; Tan, L.; Xu, X.; Qian, Z. Porous Au@Pt Nanoparticles: Therapeutic platform for tumor chemo-photothermal Co-Therapy and alleviating doxorubicin-induced oxidative damage. ACS Appl. Mater. Interfaces 2018, 10, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Prokhorov, E.; Bahena, D.; Esparza, R.; Meyyappan, M. Chitosan-Covered Pd@Pt core-shell nanocubes for direct electron transfer in electrochemical enzymatic glucose biosensor. ACS Omega 2017, 2, 1896–1904. [Google Scholar] [CrossRef]
- Fujimoto, T.; Terauchi, S.-Y.; Umehara, H.; Kojima, I.; Henderson, W. Sonochemical preparation of single-dispersion metal nanoparticles from metal salts. Chem. Mater. 2001, 13, 1057–1060. [Google Scholar] [CrossRef]
- Chen, J.; Wiley, B.; Mclellan, J.; Xiong, Y.; Li, Z.-Y.; Xia, Y. Optical Properties of Pd–Ag and Pt–Ag nanoboxes synthesized via galvanic replacement reactions. Nano Lett. 2005, 5, 2058–2062. [Google Scholar] [CrossRef] [PubMed]
- Doudna, C.M.; Bertino, M.F.; Blum, F.D.; Tokuhiro, A.T.; Lahiri-Dey, D.; Chattopadhyay, S.; Terry, J. Radiolytic synthesis of bimetallic Ag–Pt nanoparticles with a high aspect ratio. J. Phys. Chem. B 2003, 107, 2966–2970. [Google Scholar] [CrossRef]
- Jiang, B.; Kani, K.; Iqbal, M.; Abe, H.; Kimura, T.; Hossain, M.S.A.; Anjaneyulu, O.; Henzie, J.; Yamauchi, Y. Mesoporous bimetallic RhCu alloy nanospheres using a sophisticated soft-templating strategy. Chem. Mater. 2018, 30, 428–435. [Google Scholar] [CrossRef]
- Mancier, V.; Rousse-Bertrand, C.; Dille, J.; Michel, J.; Fricoteaux, P. Sono and electrochemical synthesis and characterization of copper core–silver shell nanoparticles. Ultrason. Sonochem. 2010, 17, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-W.; Liu, C.-L.; Liu, T.-M.; Shen, Y.-F.; Kuo, L.-C.; Wu, C.-H.; Hsieh, T.-Y.; Wu, P.-C.; Tsai, M.-R.; Yang, C.-C.; et al. Infrared-active quadruple contrast FePt nanoparticles for multiple scale molecular imaging. Biomaterials 2016, 85, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Maenosono, S.; Suzuki, T.; Saita, S. Superparamagnetic FePt nanoparticles as excellent MRI contrast agents. J. Magn. Magn. Mater. 2008, 320, 79–83. [Google Scholar] [CrossRef]
- Chen, C.-L.; Kuo, L.-R.; Lee, S.-Y.; Hwu, Y.-K.; Chou, S.-W.; Chen, C.-C.; Chang, F.-H.; Lin, K.-H.; Tsai, D.-H.; Chen, Y.-Y. Photothermal cancer therapy via femtosecond-laser-excited FePt nanoparticles. Biomaterials 2013, 34, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, J.; Tian, Q.; Hu, H.; Fang, Y.; Wu, H.; Yang, S. One-pot synthesis of amphiphilic superparamagnetic FePt nanoparticles and magnetic resonance imaging in vitro. J. Magn. Magn. Mater. 2010, 322, 973–977. [Google Scholar] [CrossRef]
- Chou, S.-W.; Shau, Y.-H.; Wu, P.-C.; Yang, Y.-S.; Shieh, D.-B.; Chen, C.-C. In vitro and in vivo studies of FePt nanoparticles for dual modal CT/MRI molecular imaging. J. Am. Chem. Soc. 2010, 132, 13270–13278. [Google Scholar] [CrossRef] [PubMed]
- Fuchigami, T.; Kawamura, R.; Kitamoto, Y.; Nakagawa, M.; Namiki, Y. Ferromagnetic FePt-nanoparticles/polycation hybrid capsules designed for a magnetically guided drug delivery system. Langmuir 2011, 27, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Loh, K.P.; Zhong, Y.L.; Lin, M.; Ding, J.; Foo, Y.L. Bifunctional FePt core-shell and hollow Spheres: Sonochemical preparation and self-assembly. Chem. Mater. 2007, 19, 2566–2572. [Google Scholar] [CrossRef]
- Sahu, N.K.; Gupta, J.; Bahadur, D. PEGylated FePt-Fe3O4 Composite Nanoassemblies (CNAs): In vitro hyperthermia, drug delivery and generation of Reactive Oxygen Species (ROS). Dalton Trans. 2015, 44, 9103–9113. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Yu, Y.; Li, X.; Liu, W.; Yang, H.; Wu, D.; Yang, S. Dextran-coated Superparamagnetic amorphous Fe–Co nanoalloy for magnetic resonance imaging applications. Mater. Res. Bull. 2014, 49, 285–290. [Google Scholar] [CrossRef]
- Seo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; Mcconnell, M.V.; Nishimura, D.G.; Dai, H. FeCo/graphitic-shell Nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Srinivasan, B.; Jing, Y.; Yao, X.; Hugger, M.A.; Wang, J.-P.; Xing, C. Nanomagnetic competition assay for low-abundance protein biomarker quantification in unprocessed human sera. J. Am. Chem. Soc. 2010, 132, 4388–4392. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Yuanpeng, L.; Ying, J.; Yunhao, X.; Xiaofeng, Y.; Chengguo, X.; Jian-Ping, W. A detection system based on giant magnetoresistive sensors and high-moment magnetic nanoparticles demonstrates Zeptomole sensitivity: Potential for personalized medicine. Angew. Chem. Int. Ed. 2009, 48, 2764–2767. [Google Scholar]
- Cui, B.Z.; Marinescu, M.; Liu, J.F. High magnetization Fe–Co and Fe–Ni submicron and nanosize particles by thermal decomposition and hydrogen reduction. J. Appl. Phys. 2014, 115, 17A315. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Zhou, H.; Zhuang, Y.; Hu, H.; Wu, H.; Yang, S. Monodisperse water-soluble Fe–Ni nanoparticles for magnetic resonance imaging. J. Alloys Compd. 2011, 509, 1217–1221. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Schnablegger, H.; Kornowski, A.; Festin, O.; Svedlindh, P.; Haase, M.; Weller, H. Study of nucleation and growth in the organometallic synthesis of magnetic alloy nanocrystals: The role of nucleation rate in size control of CoPt3 Nanocrystals. J. Am. Chem. Soc. 2003, 125, 9090–9101. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Qi, C.; Li, Y.; Han, Q.; Yang, R.; Wang, C. PtCo Bimetallic nanoparticles with high oxidase-like catalytic activity and their applications for magnetic-enhanced colorimetric biosensing. J. Mater. Chem. B 2016, 4, 1869–1877. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Liu, B.; Ren, B.; Zheng, N. Enhancing the photothermal stability of plasmonic metal nanoplates by a core-shell architecture. Adv. Mater. 2011, 23, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Ban, I.; Stergar, J.; Drofenik, M.; Ferk, G.; Makovec, D. Synthesis of copper-nickel nanoparticles prepared by mechanical milling for use in magnetic hyperthermia. J. Magn. Magn. Mater. 2011, 323, 2254–2258. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.-C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y. Preparation of a gold organosol in chloroform and its discolouration by photoirradiation. J. Chem. Soc. Chem. Commun. 1994, 2067–2068. [Google Scholar] [CrossRef]
- Nath, S.; Jana, S.; Pradhan, M.; Pal, T. Ligand-stabilized metal nanoparticles in organic solvent. J. Colloid Interface Sci. 2010, 341, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef]
- Radjala, T.R.H.; Apostolescu, G.; Mostafavi, M.; Thomazeau, C.; Uzio, D. Bimetallic Au-Pd and Ag-Pd Clusters Synthesised by γ or Electron Beam Radiolysis and Study of the Reactivity/Structure Relationships in the Selective Hydrogenation of Buta-1,3-Diene. Oil Gas J. 2006, 61, 789. [Google Scholar] [CrossRef]
- Mulvaney, P.; Giersig, M.; Henglein, A. Electrochemistry of multilayer colloids: Preparation and absorption spectrum of gold-coated silver particles. J. Phys. Chem. 1993, 97, 7061–7064. [Google Scholar] [CrossRef]
- Henglein, A. Colloidal palladium nanoparticles. Reduction of Pd(II) by H2; PdcoreAushellAgshell Particles. J. Phys. Chem. B 2000, 104, 6683–6685. [Google Scholar] [CrossRef]
- Wang, H.; Pyatenko, A.; Kawaguchi, K.; Li, X.; Swiatkowska-Warkocka, Z.; Koshizaki, N. Selective pulsed heating for the synthesis of semiconductor and metal Submicrometer spheres. Angew. Chem. Int. Ed. 2010, 49, 6361–6364. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hughes, T.; Beck, S.; Vakil, S.; Li, S.; Pantano, P.; Draper, R.K. Generation of toxic degradation products by sonication of Pluronic® Dispersants: Implications for nanotoxicity testing. Nanotoxicology 2013, 7, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Niu, H.; Wu, M.; Ning, M.; Zhu, H.; Chen, Q. Sonochemical preparation of bimetallic Co/Cu nanoparticles in aqueous solution. Mater. Res. Bull. 2005, 40, 1623–1629. [Google Scholar] [CrossRef]
- Xia, Y.; Gilroy, K.D.; Peng, H.-C.; Xia, X. Seed-mediated growth of colloidal metal nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 60–95. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Zhang, L.; Xu, G. Seed-mediated growth of noble metal nanocrystals: Crystal growth and shape control. Nanoscale 2013, 5, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Mcgilvray, K.L.; Fasciani, C.; Bueno-Alejo, C.J.; Schwartz-Narbonne, R.; Scaiano, J.C. Photochemical strategies for the seed-mediated growth of gold and gold–silver nanoparticles. Langmuir 2012, 28, 16148–16155. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Roduner, E. Size Matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Chen, N.; Hou, Y.; Wang, Z.C.; Lv, S.H.; Fujita, T.; Jiang, J.H.; Hirata, A.; Chen, M.W. Geometrically controlled nanoporous PdAu bimetallic catalysts with tunable Pd/Au ratio for direct ethanol fuel cells. ACS Catal. 2013, 3, 1220–1230. [Google Scholar] [CrossRef]
- Dai, L.; Song, L.; Huang, Y.; Zhang, L.; Lu, X.; Zhang, J.; Chen, T. Bimetallic Au/Ag core-shell superstructures with tunable surface plasmon resonance in the near-infrared region and high performance surface-enhanced Raman scattering. Langmuir 2017, 33, 5378–5384. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Cassidy, C.; Grammatikopoulos, P.; Djurabekova, F.; Nordlund, K.; Sowwan, M. Heterogeneous gas-phase synthesis and molecular dynamics modeling of Janus and core-satellite Si–Ag nanoparticles. J. Phys. Chem. C 2014, 118, 13869–13875. [Google Scholar] [CrossRef]
- Wilson, O.M.; Scott, R.W.J.; Garcia-Martinez, J.C.; Crooks, R.M. Synthesis, characterization, and structure-selective extraction of 1–3-nm diameter AuAg Dendrimer-encapsulated bimetallic nanoparticles. J. Am. Chem. Soc. 2005, 127, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Sheny, D.S.; Mathew, J.; Philip, D. Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of anacardium occidentale. Spectrochim. Acta A 2011, 79, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A 2009, 73, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Meena Kumari, M.; Jacob, J.; Philip, D. Green synthesis and applications of Au–Ag bimetallic nanoparticles. Spectrochim. Acta A 2015, 137, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Raju, D.; Mendapara, R.; Mehta, U.J. Protein mediated synthesis of Au–Ag bimetallic nanoparticles. Mater. Lett. 2014, 124, 271–274. [Google Scholar] [CrossRef]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora Crassa. Colloids Surf. B. Biointerfaces 2011, 83, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.-X.; Wang, A.-L.; Li, G.-R.; Liu, Z.-Q.; Zhao, W.-X.; Su, C.-Y.; Tong, Y.-X. Porous Pt–Ni–P Composite Nanotube Arrays: Highly electroactive and durable catalysts for methanol electrooxidation. J. Am. Chem. Soc. 2012, 134, 5730–5733. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.; Turkoglu, V.; Bulut, A.; Zahmakiran, M. Electrochemical sensing of hydrogen peroxide using Pd@Ag bimetallic nanoparticles decorated functionalized reduced graphene oxide. Electrochim. Acta 2018, 263, 118–126. [Google Scholar] [CrossRef]
- Hierrezuelo, J.; Sadeghpour, A.; Szilagyi, I.; Vaccaro, A.; Borkovec, M. Electrostatic stabilization of charged colloidal particles with adsorbed polyelectrolytes of opposite charge. Langmuir 2010, 26, 15109–15111. [Google Scholar] [CrossRef] [PubMed]
- Faraday, M. The bakerian lecture: experimental relations of gold (and other metals) to light. Philos. Trans. 1857, 147, 145. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.-Y.; Zhong, Z.; Gates, B.; Xia, Y.; Venkateswaran, S. Synthesis and characterization of stable aqueous dispersions of silver nanoparticles through the Tollens process. J. Mater. Chem. 2002, 12, 522–527. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures & Nanometerials Synthesis, Properties & Applications; Imperial College Press: London, UK, 2004. [Google Scholar]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Herricks, T.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 2004, 4, 1733–1739. [Google Scholar] [CrossRef]
- Zheng, P.; Jiang, X.; Zhang, X.; Zhang, W.; Shi, L. Formation of Gold@Polymer core-shell particles and gold particle clusters on a template of thermoresponsive and pH-responsive coordination Triblock copolymer. Langmuir 2006, 22, 9393–9396. [Google Scholar] [CrossRef] [PubMed]
- Mcnamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Dutta, D.; Chattopadhyay, A.; Ghosh, S.S. Cationic BSA Templated Au–Ag bimetallic nanoclusters as a theranostic gene delivery vector for Hela cancer cells. ACS Biomater. Sci. Eng. 2016, 2, 2090–2098. [Google Scholar] [CrossRef]
- Rick, J.; Tsai, M.-C.; Hwang, B. Biosensors incorporating bimetallic nanoparticles. Nanomaterials 2016, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Lodewijks, K.; Van Roy, W.; Borghs, G.; Lagae, L.; Van Dorpe, P. Boosting the Figure-of-Merit of LSPR-based refractive index sensing by phase-sensitive measurements. Nano Lett. 2012, 12, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Li, E.Q.; Vakarelski, I.U.; Chan, D.Y.C.; Thoroddsen, S.T. Stabilization of thin liquid films by repulsive van der Waals force. Langmuir 2014, 30, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D.A.; Haes, A.J.; Yonzon, C.R.; Hicks, E.M.; Duyne, R.P.V. Biological applications of localised surface plasmonic phenomenae. IEE Proc. Nanobiotechnol. 2005, 152, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Csete, M.; Szalai, A.; Csapó, E.; Tóth, L.; Somogyi, A.; Dékány, I. Collective Plasmonic resonances on arrays of cysteine-functionalized silver nanoparticle aggregates. J. Phys. Chem. C 2014, 118, 17940–17955. [Google Scholar] [CrossRef]
- Aili, D.; Selegård, R.; Baltzer, L.; Enander, K.; Liedberg, B. Colorimetric protein sensing by controlled assembly of gold nanoparticles functionalized with synthetic receptors. Small 2009, 5, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.K.; Rosenzweig, Z. Development of an aggregation-based immunoassay for anti-protein a using gold nanoparticles. Anal. Chem. 2002, 74, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, J.; Li, J.-J.; Zhao, J.-W. A promising direct visualization of an Au@Ag nanorod-based colorimetric sensor for trace detection of alpha-fetoprotein. J. Mater. Chem. C 2015, 3, 6035–6045. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, B.; Chen, L. Sers Tags: Novel optical Nanoprobes for Bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zong, S.; Wu, L.; Zhu, D.; Cui, Y. SERS-activated platforms for immunoassay: Probes, encoding methods, and applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ni, H.; Zhang, D.; Wang, D.; Fu, D.; Chen, H.; Gu, Z.; Zhao, X. ultrasensitive detection of protein with wide linear dynamic range based on core-shell SERS nanotags and photonic crystal beads. ACS Sens. 2017, 2, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing using magnetic particle detection techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Li, Y.; Jing, Y.; Xing, C.; Slaton, J.; Wang, J.-P. A three-layer competition-based giant magnetoresistive assay for direct quantification of endoglin from human urine. Anal. Chem. 2011, 83, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Nekrashevich, I.; Litvinov, D.; Willson, R.C.; Lee, T.R. Cubic silica-coated and amine-functionalized FeCo nanoparticles with high saturation magnetization. Chem. Mater. 2013, 25, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ho, P.-L.; Wt Tsang, K.; Yu, C.-W.; Xu, B. Using biofunctional magnetic nanoparticles to capture gram-negative bacteria at an ultra-low concentration. Chem. Commun. 2003, 1966–1967. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.-L.; Tsang, K.W.T.; Wang, L.; Xu, B. Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration. J. Am. Chem. Soc. 2003, 125, 15702–15703. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Kim, C.; Cobley, C.M.; Xia, Y.; Wang, L.V. Near-infrared gold nanocages as a new class of tracers for photoacoustic sentinel lymph node mapping on a rat model. Nano Lett. 2009, 9, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.-W.; Lee, J.-H.; Cheon, J. Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew. Chem. Int. Ed. 2008, 47, 5122–5135. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.-W.; Huh, Y.-M.; Choi, J.-S.; Lee, J.-H.; Song, H.-T.; Kim, S.; Yoon, S.; Kim, K.-S.; Shin, J.-S.; Suh, J.-S.; Cheon, J. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef] [PubMed]
- Rittikulsittichai, S.; Kolhatkar, A.G.; Sarangi, S.; Vorontsova, M.A.; Vekilov, P.G.; Brazdeikis, A.; Randall Lee, T. Multi-responsive hybrid particles: Thermo-, pH-, Photo-, and magneto-responsive magnetic hydrogel cores with gold nanorod optical triggers. Nanoscale 2016, 8, 11851–11861. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.; Duce, S.L.; Brown, S.; Lee, S.; Melzer, A.; Cuschieri, S.A.; André, P. Engineered biocompatible nanoparticles for in vivo imaging applications. J. Am. Chem. Soc. 2010, 132, 15022–15029. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Jun, Y.-W.; Yeon, S.-I.; Kim, H.C.; Shin, J.-S.; Cheon, J. Biocompatible heterostructured nanoparticles for multimodal biological detection. J. Am. Chem. Soc. 2006, 128, 15982–15983. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.S.R.; Hormes, J.; Leuschner, C. Nanofabrication towards Biomedical Applications; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Paulides, M.M.; Stauffer, P.R.; Neufeld, E.; Maccarini, P.; Kyriakou, A.; Canters, R.A.M.; Diederich, C.; Bakker, J.F.; Van Rhoon, G.C. Simulation techniques in hyperthermia treatment planning. Int. J. Hyperth. Off. J. Eur. Soc. Hyperth. Oncol. N. Am. Hyperth. Group 2013, 29, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Baffou, G.; Quidant, R. Thermo-Plasmonics: Using metallic nanostructures as nano-sources of heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Prevo, B.G.; Esakoff, S.A.; Mikhailovsky, A.; Zasadzinski, J.A. Scalable routes to gold nanoshells with tunable sizes and response to near-infrared pulsed-laser irradiation. Small 2008, 4, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Erickson, T.A.; Tunnell, J.W. Gold nanoshells in biomedical applications. In Nanomaterials for the Life Sciences; Kumar, C.S.S.R., Ed.; Wiley-Vch Verlag Gmbh & Co.: Weinheim, Germany, 2009; Volume 3. [Google Scholar]
- Torchi, A.; Simonelli, F.; Ferrando, R.; Rossi, G. Local enhancement of lipid membrane permeability induced by irradiated gold nanoparticles. ACS Nano 2017, 11, 12553–12561. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Chen, J.; Au, L.; Lu, X.; Li, X.; Xia, Y. Gold nanocages for biomedical applications. Adv. Mater. 2007, 19, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Paneque, A.F.; Rodríguez-González, B.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Shape-templated growth of Au@Cu nanoparticles. J. Phys. Chem. C 2013, 117, 2474–2479. [Google Scholar] [CrossRef]
- Kuznetsov, A.A.; Leontiev, V.G.; Brukvin, V.A.; Vorozhtsov, G.N.; Kogan, B.Y.; Shlyakhtin, O.A.; Yunin, A.M.; Tsybin, O.I.; Kuznetsov, O.A. Local radiofrequency-induced hyperthermia using CuNi nanoparticles with therapeutically suitable curie temperature. J. Magn. Magn. Mater. 2007, 311, 197–203. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, X.; Zhao, Z.; Fang, W.; Chen, M.; Huang, Y.; Chen, X. Photothermally enhanced photodynamic therapy based on mesoporous Pd@Ag@mSiO2 nanocarriers. J. Mater. Chem. B 2013, 1, 1133–1141. [Google Scholar] [CrossRef]
- Shin, S.; Song, I.; Um, S. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Mcnamara, K.; Tofail, S.A.M. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Thanh, N.K.T.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001–224015. [Google Scholar] [CrossRef]
- Obaidat, I.; Issa, B.; Haik, Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, F.; Lévy, M.; Wilhelm, C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine 2008, 3, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Afifi, M.M.; Austin, L.A.; El-Sayed, M.A. Exploiting the nanoparticle Plasmon effect: Observing drug delivery dynamics in single cells via Raman/fluorescence imaging spectroscopy. ACS Nano 2013, 7, 7420–7427. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Bai, Y.; Zhang, L.; Yang, Z.; Fan, Q.; Zheng, H.; Yin, Y.; Gao, C. Porous Au–Ag nanospheres with high-density and highly accessible hotspots for SERS analysis. Nano Lett. 2016, 16, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Gai, Z.; Hong, K.; Banerjee, P.; Zhou, S. Multi-functional core-shell hybrid nanogels for pH-dependent magnetic manipulation, fluorescent pH-sensing, and drug delivery. Biomaterials 2011, 32, 9876–9887. [Google Scholar] [CrossRef] [PubMed]
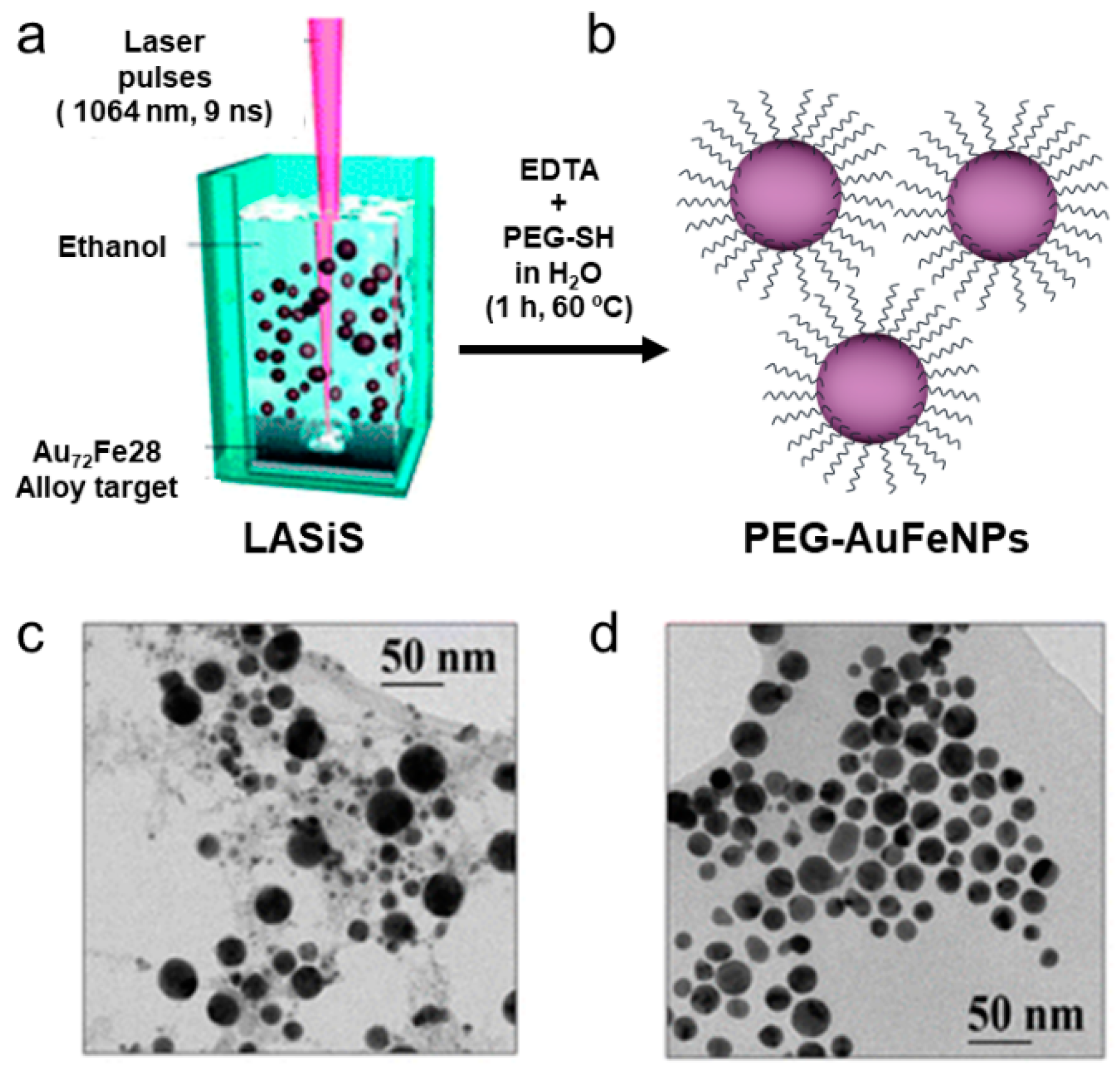
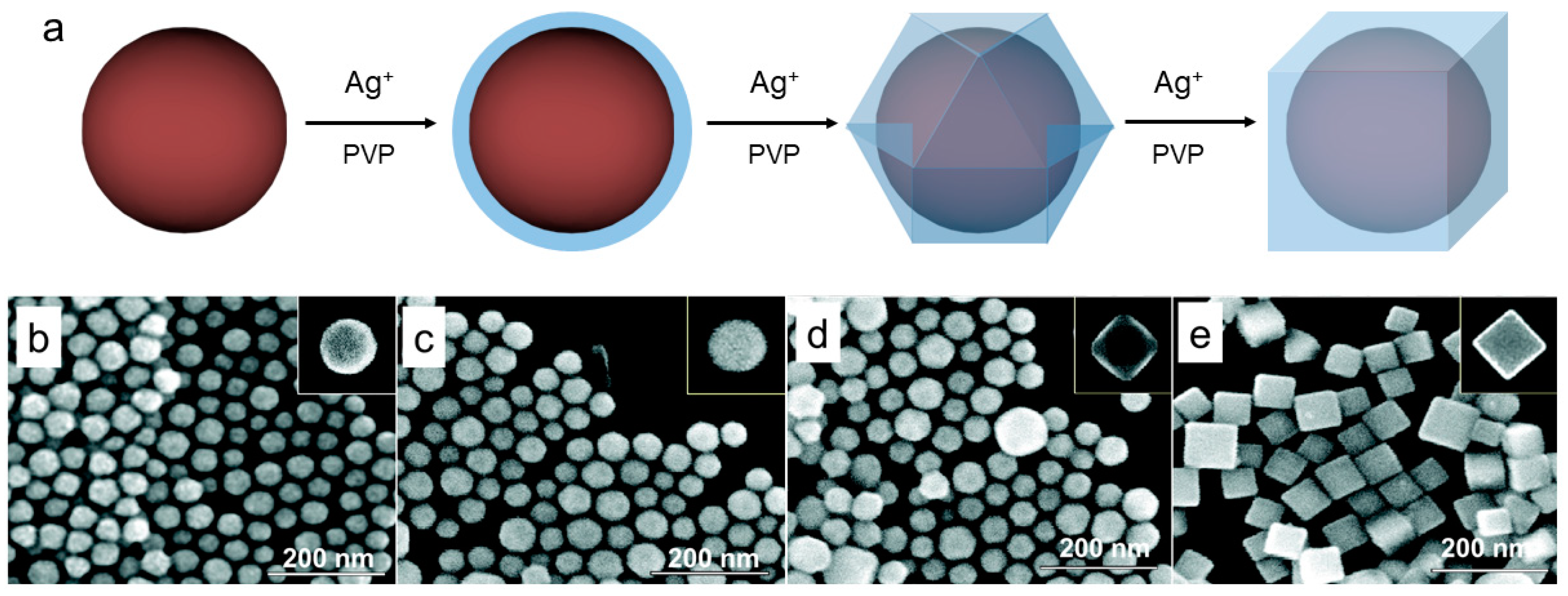

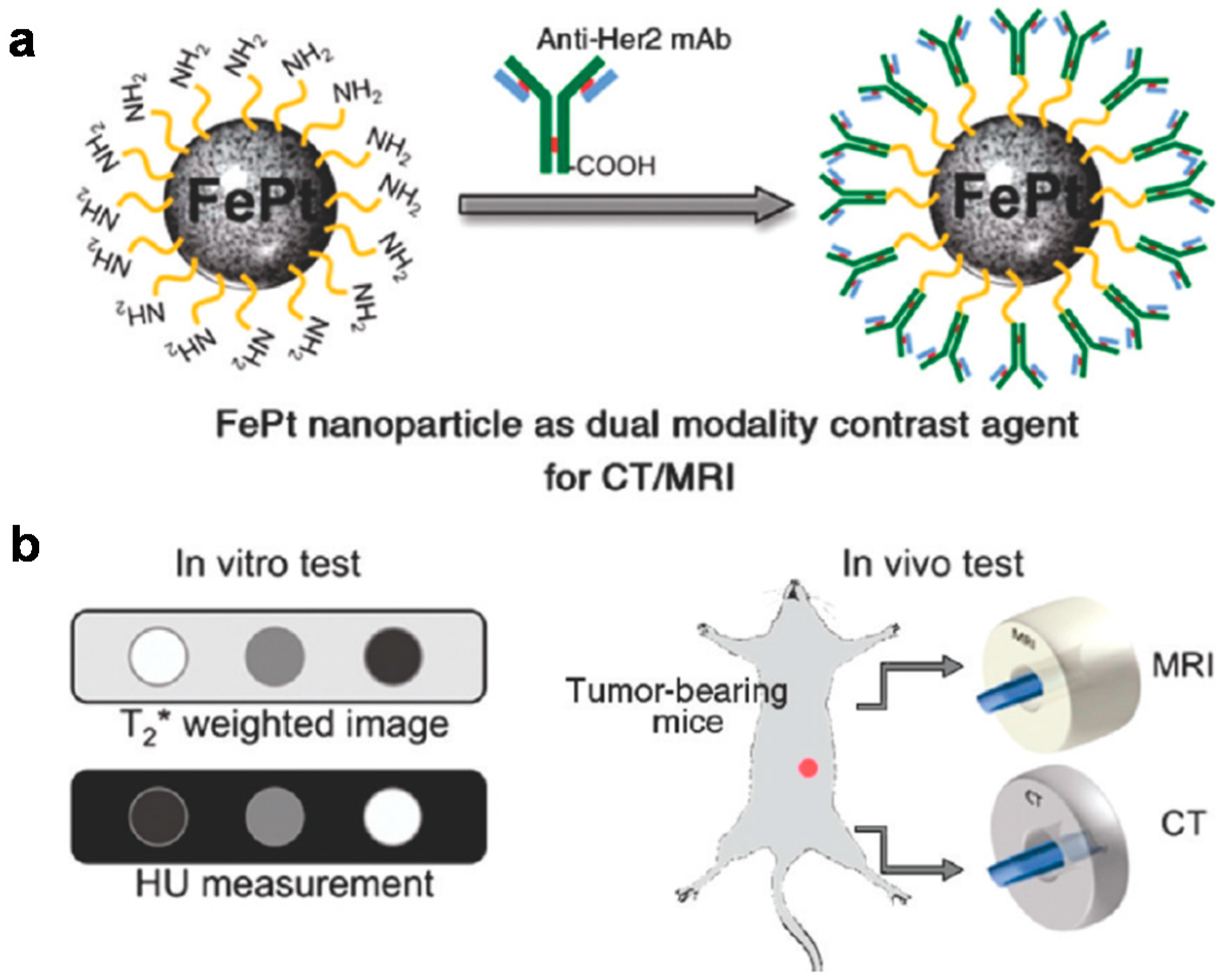
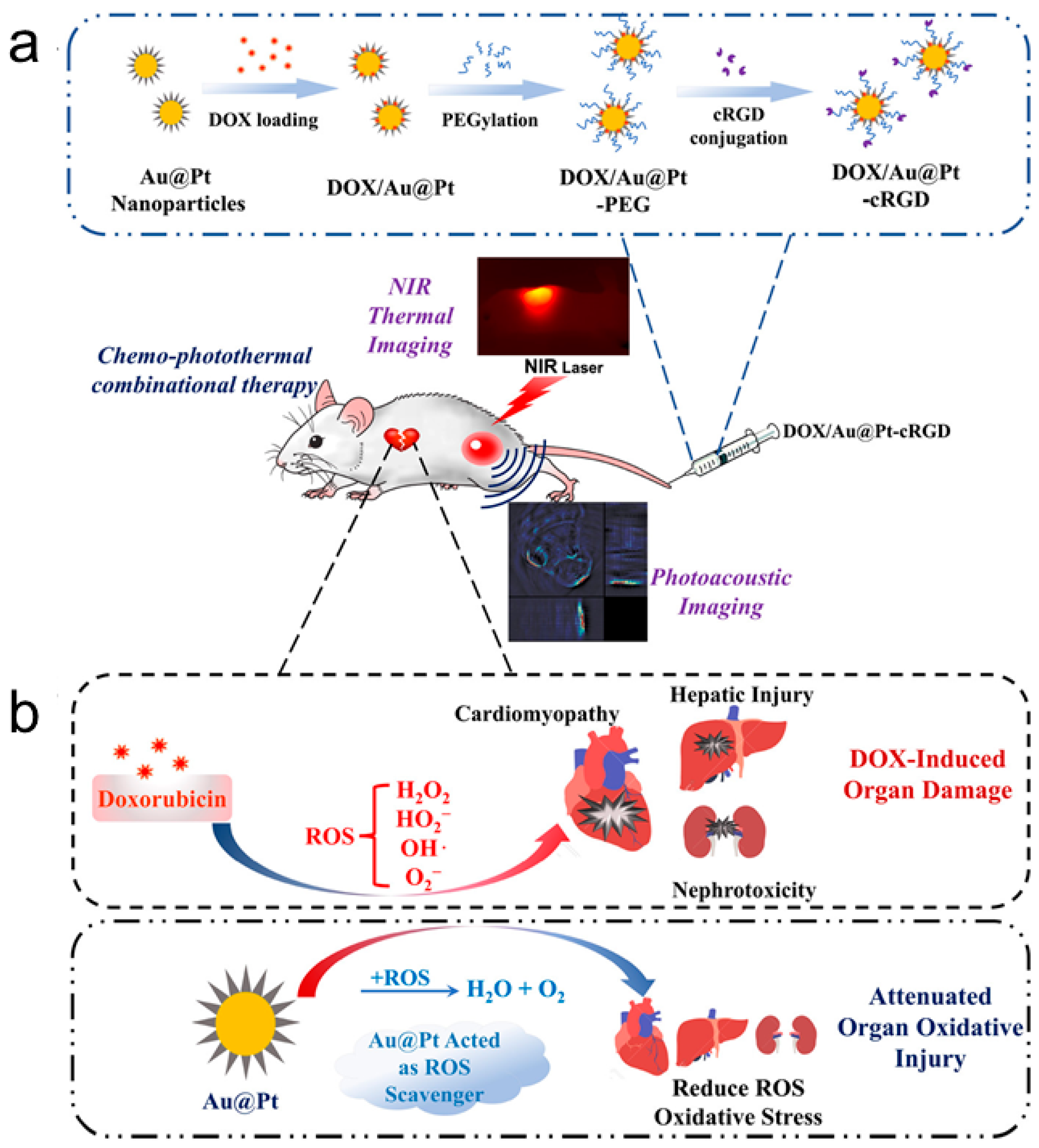
| Material | Synthetic Method | Size | Reference |
|---|---|---|---|
| AuAg | Polyol reduction Galvanic replacement | 50 nm | [69] |
| Co-reduction Galvanic replacement | 10.2 nm | [70] | |
| Chemical reduction | 5 nm | [71] | |
| 32 and 45 nm | [72] | ||
| Radiolysis | 1.5–12 nm | [64] | |
| Seed-mediated growth | 10–16 nm | [73] | |
| Seed-mediated polyol process | 52 nm | [74] | |
| In situ co-reduction method | 2.6 nm | [75] | |
| Successive ionic layer absorption and reaction (SILAR) | 10–20 nm | [76] | |
| AuFe | Chemical reduction | 15–30 nm | [61,77] |
| Laser ablation of a bulk alloy | 30–60 nm | [78,79] | |
| AuCo | Chemical reduction | 3–5 nm | [80] |
| 14.2 nm | [26] | ||
| 15–30 nm | [61] | ||
| Pulsed laser processes | 11 nm, 230 nm | [81,82] | |
| AuNi | Chemical reduction | 15–30 nm | [61] |
| Electrodeposition | 100 nm and 200 nm | [83] | |
| AuCu | Chemical reduction | 8.3 nm, 20–25 nm | [25,84] |
| Chemical reduction using green synthesis with Vitamin C | 5–50 nm | [68] | |
| AuPd | Hydrothermal conditions and microwave irradiation | 40 to 65 nm, 150–200 nm | [85] |
| Chemical reduction | 1.6 nm | [58] | |
| Sonochemical | 5–9 nm | [59,60,86,87] | |
| Microwave-assisted polyol | 9 nm Au core, 3 nm Pd shell | [63] | |
| Seed-mediated growth | 5 nm | [50] | |
| 35–100 nm | [88] | ||
| Green synthesis | 7 nm | [89] | |
| AuPt | Chemical reduction | 3 nm, 6 nm | [90,91] |
| Successive/reduction | 30 nm | [66] | |
| Galvanic replacement | The apex–apex length of the concave nanocubes is 12.81 nm | [92] | |
| Transmetalation reaction/replacement | 10–20 nm | [93] | |
| Seed-mediated growth | 3–8 nm pearlike 5–8 nm peanutlike 7–10 nm cloverlike | [94] | |
| Ultrasonochemical reduction | 39.5–101.6 nm | [95] | |
| Hydrothermal conditions and microwave irradiation | 30 nm | [85] | |
| PdCu | Chemical reduction using green synthesis with Vitamin C | 5–50 nm | [68] |
| PtCu | Chemical reduction using green synthesis with Vitamin C | 50–60 nm | [68] |
| PtRu | Hydrothermal (thermal decomposition) | 2.5 nm | [62] |
| PtPd | Chemical reduction | 5–8 nm, 13 nm | [10,96] |
| Sonochemical | 2–3.6 nm | [97] | |
| PdAg | Galvanic replacement | Nanobox 63 nm in edge length | [98] |
| PtAg | Galvanic replacement | Nanobox 63 nm in edge length | [98] |
| Radiolytic synthesis | 3–20 nm | [99] | |
| RhCu | Soft-templating strategy | 74–135 nm | [100] |
| CuAg | Sono and electrochemical synthesis | 10–80 nm | [101] |
| FePt | Chemical reduction | 3 nm, 12 nm | [34,102] |
| Pyrolysis (thermal decomposition) | 9 nm | [103] | |
| Thermal decomposition | 12 nm | [104] | |
| Polyol (chemical reduction) | 3–12 nm | [23,105,106,107] | |
| Sonochemical | 3–5 nm | [108] | |
| Hydrothermal | 9.4 nm | [109] | |
| FeCo | Chemical reduction | 9 nm | [110] |
| Physical vapor nanoparticle-deposition technique | 3–100 nm | [39] | |
| Thermal decomposition | 4,7 nm | [111] | |
| Sputtering gas condensation technique | 12.8 nm | [112,113] | |
| FeNi | Chemical reduction | 12.2 nm | [114] |
| Pyrolysis chemical reduction | 9 nm | [115] | |
| NiCo | Microwave irradiation | 71 nm | [19] |
| PtCo | Chemical reduction Thermal decomposition | 3–18 nm | [116] |
| Thermal decomposition | 6 nm | [40] | |
| Chemical reduction | 5.3 nm | [117] | |
| CoCu | Sonochemical | 50 nm | [118] |
| CuNi | Mechanical milling | 10 nm | [119] |
| Application | Principle | Materials | Reference |
|---|---|---|---|
| Sensing | LSPR shift | AuAg | [163] |
| Surface-enhanced Raman scattering (SERS) | AuAg | [76] | |
| AuAg | [166] | ||
| GMR sensor; competition assay | FeCo | [112,113,187] | |
| Magnetic capture | FePt | [171] | |
| Magnetically-enhanced colorimetric biosensing | PtCo | [117] | |
| QCM with magnetically controlled permeability | AuCo | [80] | |
| Electrochemical biosensor | PtPd | [96] | |
| Imaging | An imaging probe screening with dual energy mammography or computed tomography | AuAg | [71] |
| The optical absorption cross sections | AuAg | [72] | |
| MRI contrast agent (no imaging) | FePt | [103] | |
| MRI in vitro | FePt | [105] | |
| FeNi | [115] | ||
| PtCo | [40] | ||
| MRI in vivo | AuCu | [84] | |
| FeCo | [110] | ||
| FePt | [177] | ||
| Dual modal CT/MRI molecular imaging in vitro and in vivo | FePt | [132] | |
| MRI and near-infrared agents | FeCo | [111] | |
Multimodal imaging
| FePt | [102] | |
| in vivo Multimodal SERS-MRI-CT imaging | AuFe | [79] | |
| in vivo NIR thermal imaging and photoacoustic imaging | AuPt | [95] | |
| Thermal treatment | in vitro hyperthermia | FePt | [109] |
| Photothermal | FePt | [104] | |
| Hyperthermia | CuNi | [119,188] | |
| Tumor chemo-photothermal therapy | AuPt | [95] | |
| Drug delivery | Drug carrier by pore structure and release by photothermal conversion | FePt | [109] |
| Gene delivery by functionalized surface | AuNi | [83] | |
| Drug delivery by pore structure | AuPt | [95] | |
| Photothermally enhanced photodynamic therapy | PdAg | [118,189] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Appl. Sci. 2018, 8, 1106. https://doi.org/10.3390/app8071106
Srinoi P, Chen Y-T, Vittur V, Marquez MD, Lee TR. Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Applied Sciences. 2018; 8(7):1106. https://doi.org/10.3390/app8071106
Chicago/Turabian StyleSrinoi, Pannaree, Yi-Ting Chen, Varadee Vittur, Maria D. Marquez, and T. Randall Lee. 2018. "Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications" Applied Sciences 8, no. 7: 1106. https://doi.org/10.3390/app8071106
APA StyleSrinoi, P., Chen, Y.-T., Vittur, V., Marquez, M. D., & Lee, T. R. (2018). Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Applied Sciences, 8(7), 1106. https://doi.org/10.3390/app8071106