Chemical and Molecular Variations in Commercial Epoxide Photoresists for X-ray Lithography
Abstract
:Featured Application
Abstract
1. Introduction
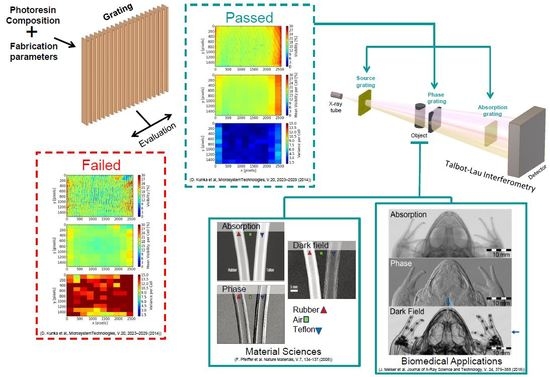
1.1. Deep X-ray Lithography (DXRL)
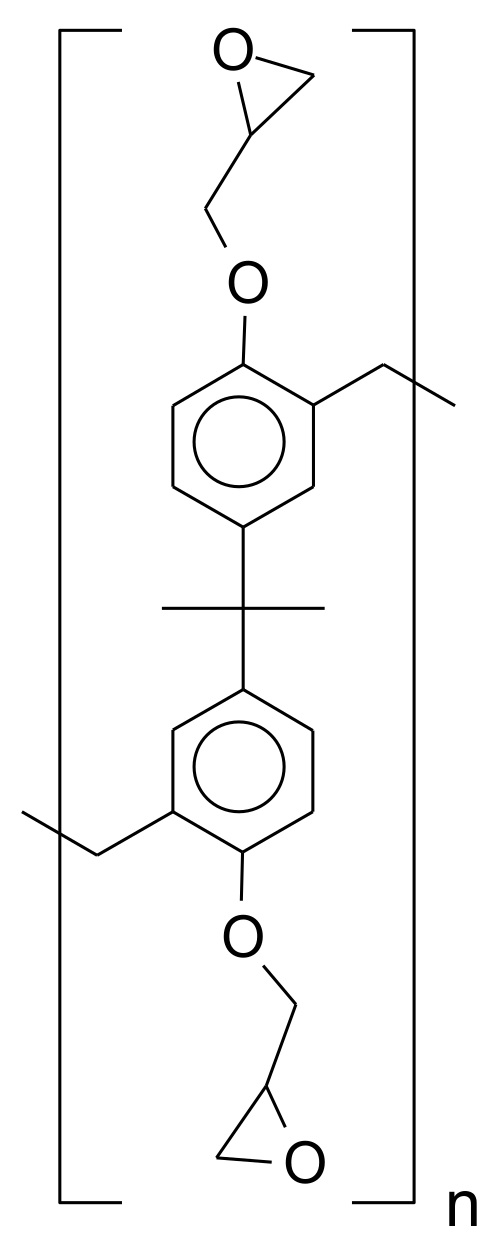
1.2. Epoxy-Based Photoresists
2. Materials and Methods
2.1. Thermogravimetric Analysis
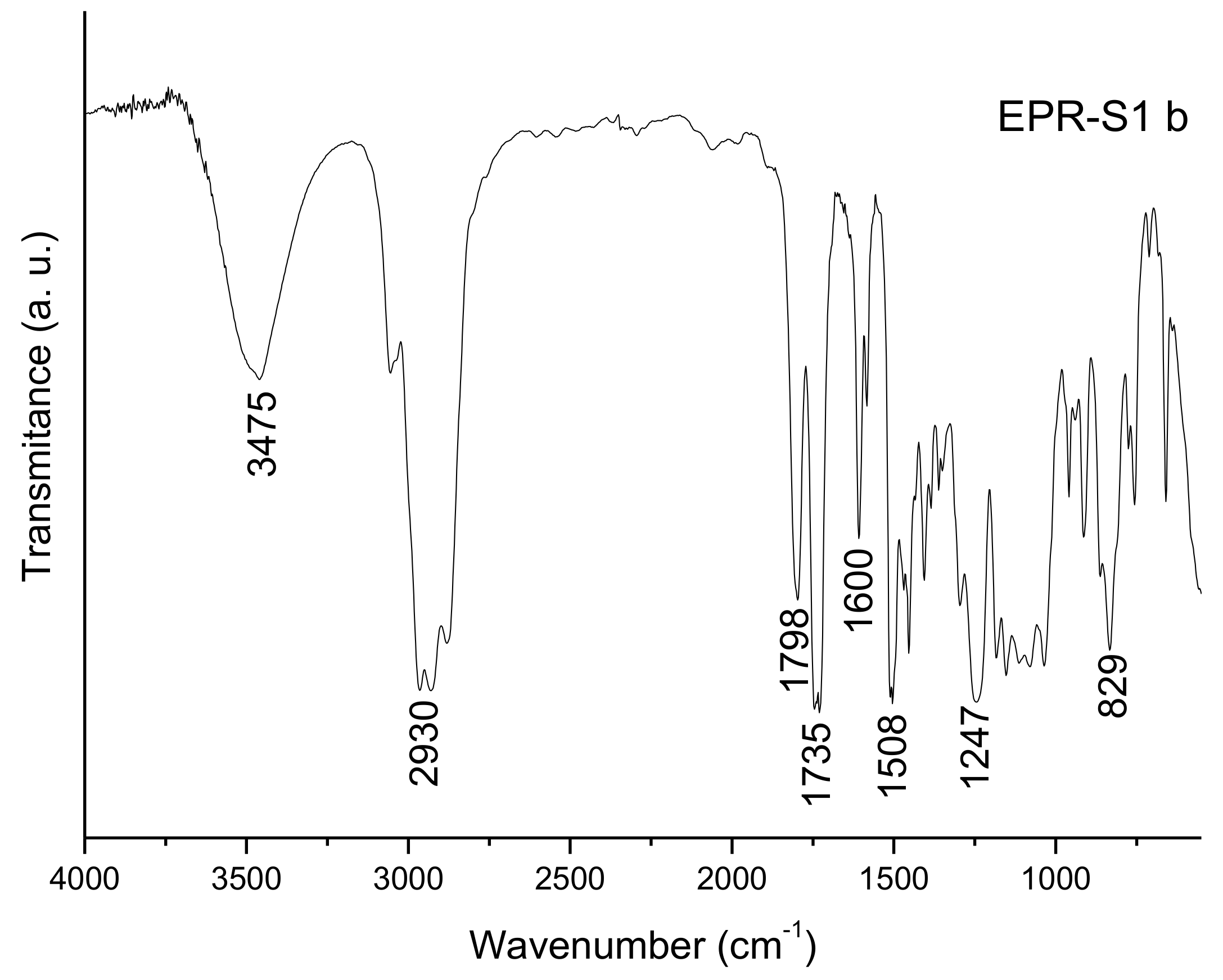
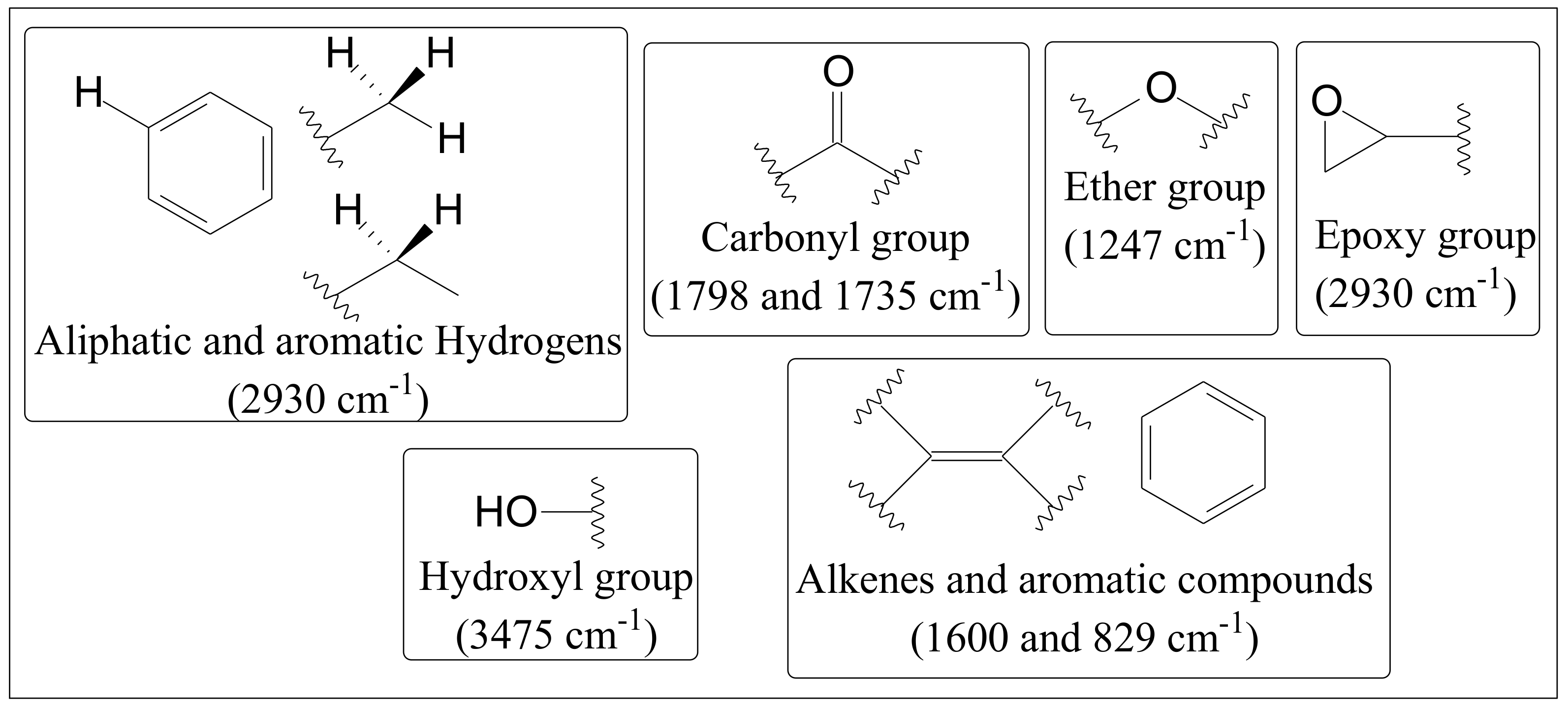
2.2. Fourier-Transform Infra-Red Spectroscopy
2.3. Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectroscopy
3. Results and Discussion
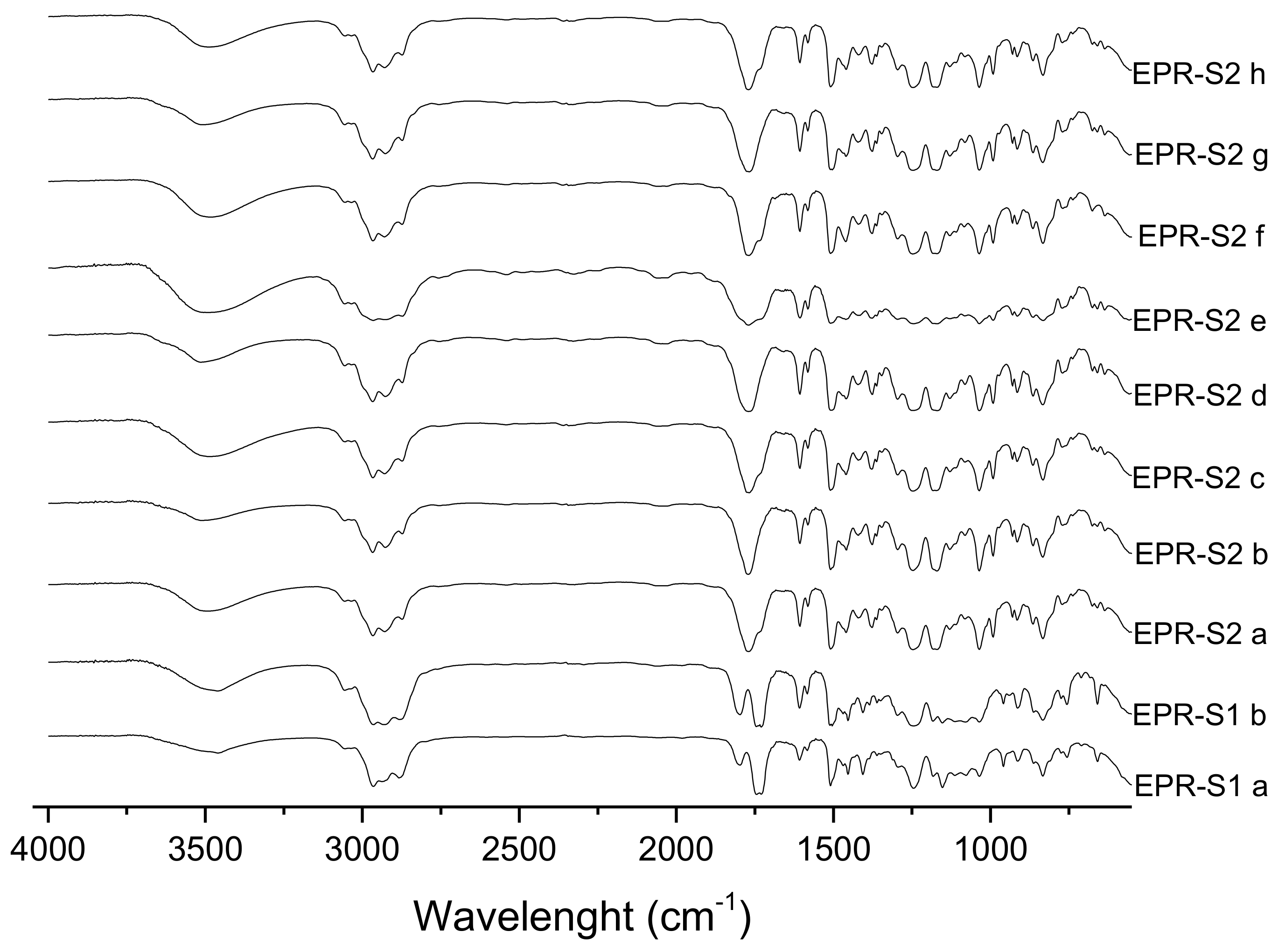
3.1. FTIR Spectra Evaluation
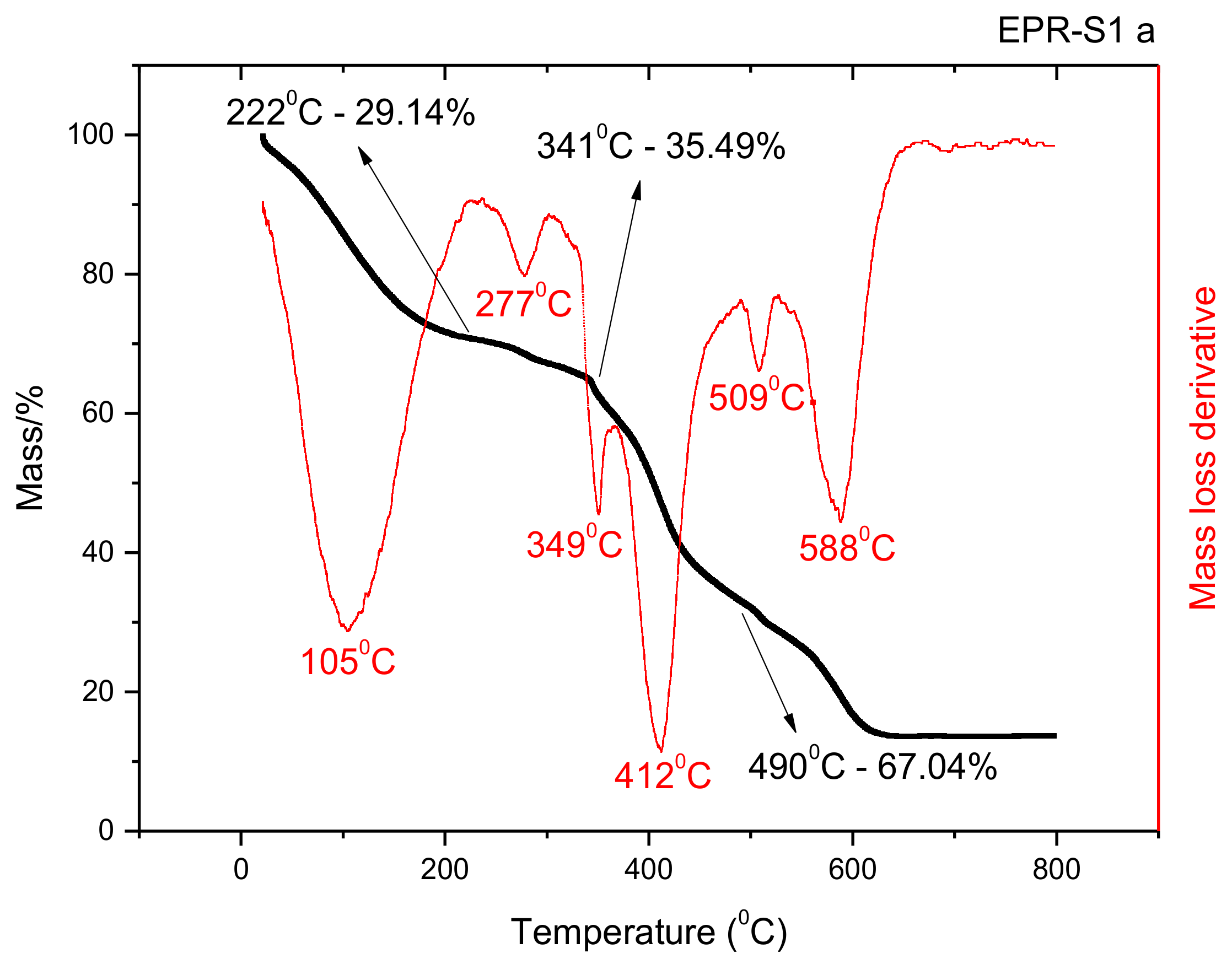
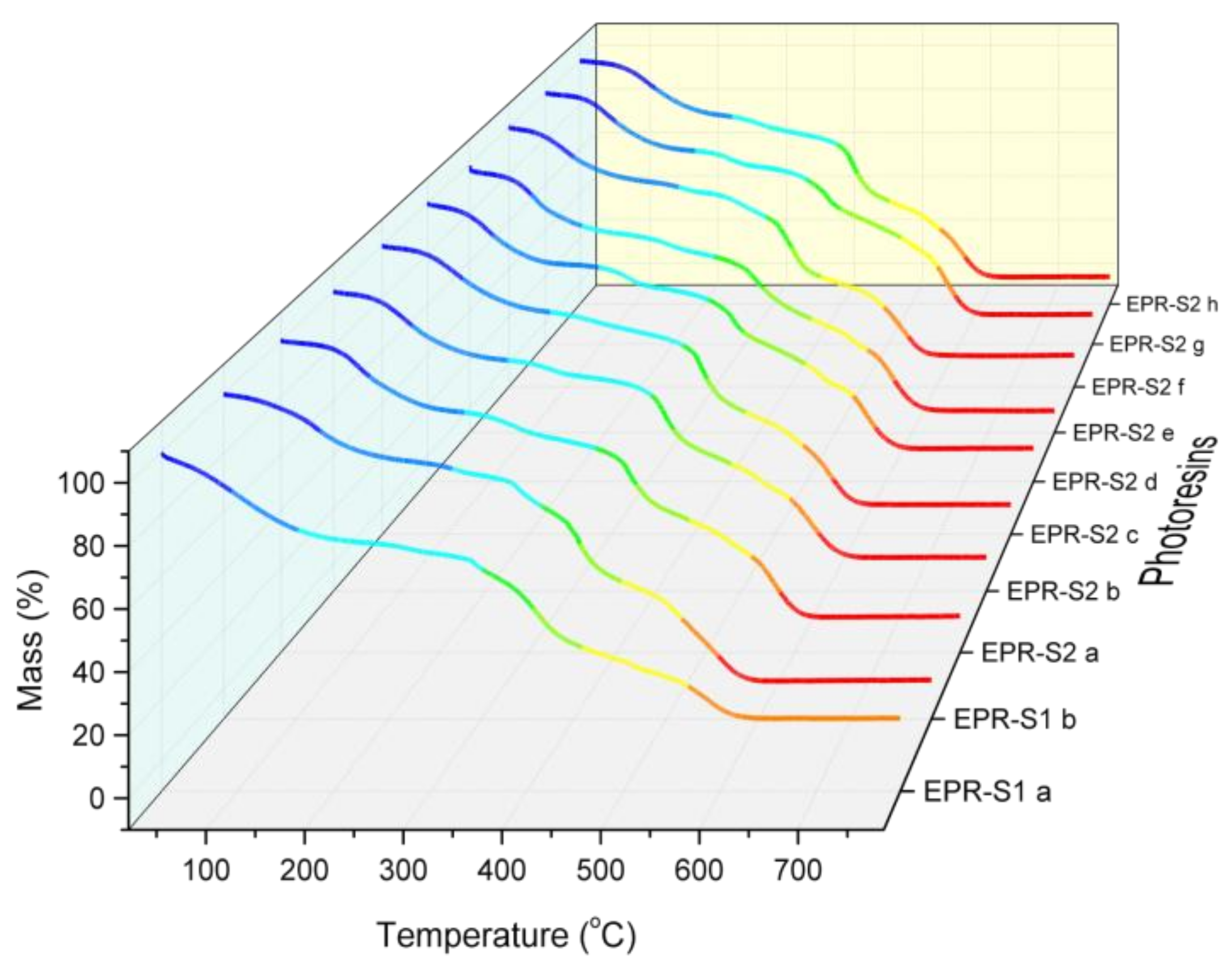
3.2. TGA Analysis
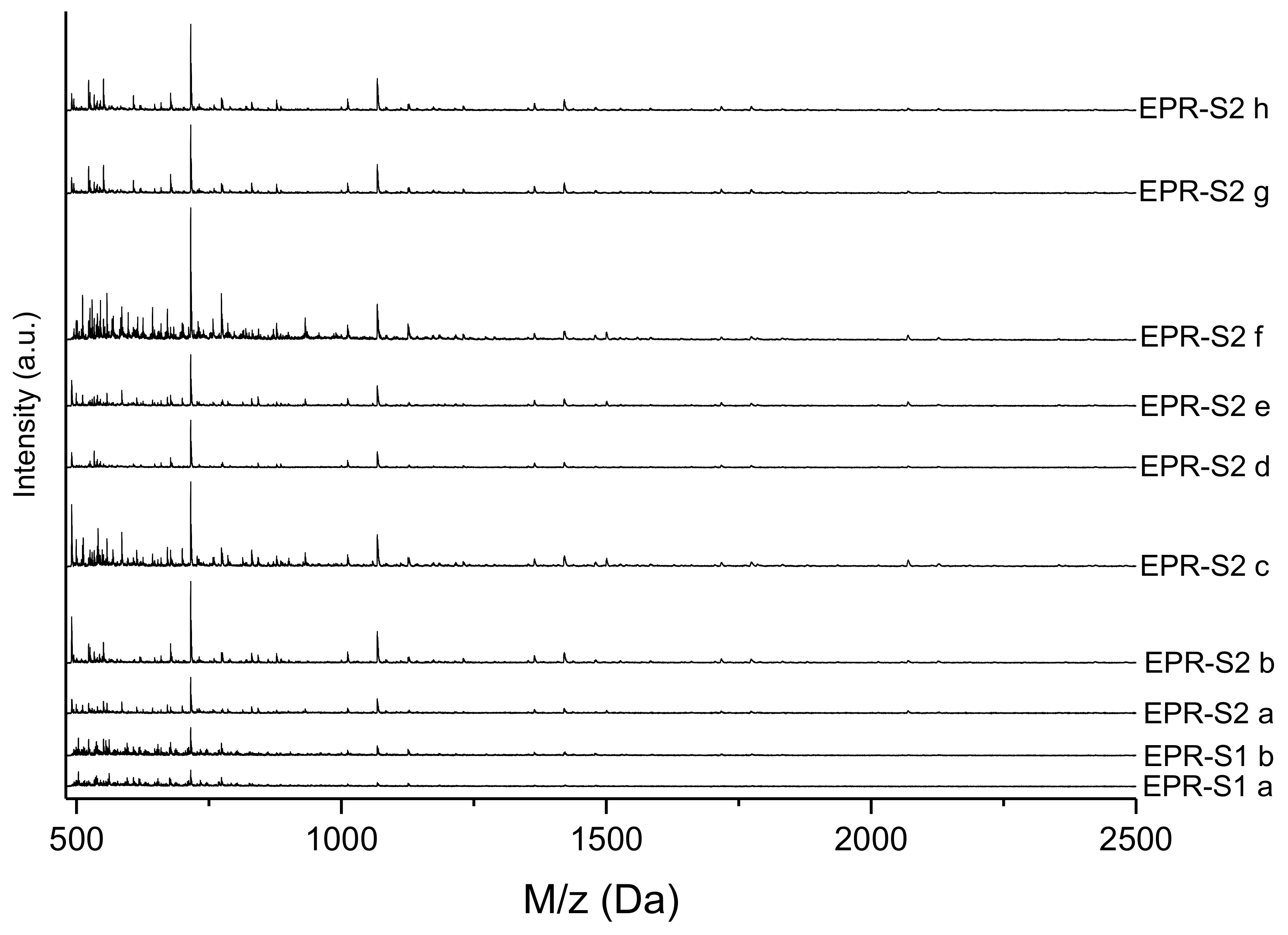
3.3. MALDI-TOF Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kunka, D.; Mohr, J.; Nazmov, V.; Meiser, J.; Meyer, P.; Amberger, M.; Koch, F.; Schulz, J.; Walter, M.; Duttenhofer, T.; et al. Characterization method for new resist formulations for HAR patterns made by X-ray lithography. Microsyst. Technol. 2014, 20, 2023–2029. [Google Scholar] [CrossRef]
- Koukharenko, E.; Kraft, M.; Ensell, G.J.; Hollinshead, N. A comparative study of different thick photoresists for MEMS applications. J. Mater. Sci. Mater. Electron. 2005, 16, 741–747. [Google Scholar] [CrossRef]
- Cheng, C.-M.; Chen, R.-H. Key issues in fabricating microstructures with high aspect ratios by using deep X-ray lithography. Microelectron. Eng. 2004, 71, 335–342. [Google Scholar] [CrossRef]
- Ruilin, Z.; Wei, S.; Xuyuan, C. Characterizing and smoothing of striated sidewall morphology on UV-exposed thick SU-8 structures for micromachining millimeter wave circuits. J. Micromech. Microeng. 2010, 20, 035007. [Google Scholar]
- Seidemann, V.; Bütefisch, S.; Büttgenbach, S. Fabrication and investigation of in-plane compliant SU8 structures for MEMS and their application to micro valves and micro grippers. Sens. Actuators A Phys. 2002, 97–98, 457–461. [Google Scholar] [CrossRef]
- Toshihiko, T.; Mitsuaki, M.; Hiroaki, O.; Taro, O. Freeze-Drying Process to Avoid Resist Pattern Collapse. Jpn. J. Appl. Phys. 1993, 32, 5813. [Google Scholar]
- Toshiyuki, M.; Yoshinori, K.; Masatoshi, I.; Takuya, M.; Takaaki, S.; Fumikazu, O.; Shozo, I.; Takahiro, N. Influences of pretreatment and hard baking on the mechanical reliability of SU-8 microstructures. J. Micromech. Microeng. 2013, 23, 105016. [Google Scholar]
- Hamlett, C.A.E.; McHale, G.; Newton, M.I. Lithographically fabricated SU8 composite structures for wettability control. Surf. Coat. Technol. 2014, 240, 179–183. [Google Scholar] [CrossRef]
- Tanaka, T.; Morigami, M.; Atoda, N. Mechanism of Resist Pattern Collapse during Development Process. Jpn. J. Appl. Phys. 1993, 32, 6059. [Google Scholar] [CrossRef]
- Khan Malek, C.G. SU8 resist for low-cost X-ray patterning of high-resolution, high-aspect-ratio MEMS. Microelectron. J. 2002, 33, 101–105. [Google Scholar] [CrossRef]
- Namatsu, H. Supercritical Drying for Nanostructure Fabrication. J. Photopolym. Sci. Technol. 2002, 15, 381–388. [Google Scholar] [CrossRef]
- Namatsu, H.; Yamazaki, K.; Kurihara, K. Supercritical resist dryer. J. Vac. Sci. Technol. B 2000, 18, 780–784. [Google Scholar] [CrossRef]
- Micro-chem–SU-8 3000–Data Sheet. Available online: http://microchem.com/pdf/SU-8%203000%20Data%20Sheet.pdf (accessed on 17 September 2017).
- Chiamori, H.C.; Brown, J.W.; Adhiprakasha, E.V.; Hantsoo, E.T.; Straalsund, J.B.; Melosh, N.A.; Pruitt, B.L. Suspension of nanoparticles in SU-8: Processing and characterization of nanocomposite polymers. Microelectron. J. 2008, 39, 228–236. [Google Scholar] [CrossRef]
- Jiguet, S.; Judelewicz, M.; Mischler, S.; Hofmann, H.; Bertsch, A.; Renaud, P. SU-8 nanocomposite coatings with improved tribological performance for MEMS. Surf. Coat. Technol. 2006, 201, 2289–2295. [Google Scholar] [CrossRef]
- Ruano-López, J.M.; Aguirregabiria, M.; Tijero, M.; Arroyo, M.T.; Elizalde, J.; Berganzo, J.; Aranburu, I.; Blanco, F.J.; Mayora, K. A new SU-8 process to integrate buried waveguides and sealed microchannels for a Lab-on-a-Chip. Sens. Actuators B Chem. 2006, 114, 542–551. [Google Scholar] [CrossRef]
- Bednorz, M.; Urbańczyk, M.; Pustelny, T.; Piotrowska, A.; Papis, E.; Sidor, Z.; Kamińska, E. Application of SU8 polymer in waveguide interferometer ammonia sensor. Mol. Quantum Acoust. 2006, 27, 31–40. [Google Scholar]
- Gut, K. Bimodal Layers of the Polymer SU8 as Refractometer. Procedia Eng. 2012, 47, 326–329. [Google Scholar] [CrossRef]
- Hill, G.C.; Melamud, R.; Declercq, F.E.; Davenport, A.A.; Chan, I.H.; Hartwell, P.G.; Pruitt, B.L. SU-8 MEMS Fabry-Perot pressure sensor. Sens. Actuators A Phys. 2007, 138, 52–62. [Google Scholar] [CrossRef]
- Brien, J.O.; Hughes, P.J.; Brunet, M.; Neill, B.O.; Alderman, J.; Lane, B.; Riordan, A.O.; Driscoll, C.O. Advanced photoresist technologies for microsystems. J. Micromech. Microeng. 2001, 11, 353. [Google Scholar] [CrossRef]
- Gelorme, J.D.; Cox, R.J.; Gutierrez, S.A. Photoresist Composition and Printed Circuit Boards and Packages Made Therewith. U.S. Patent 4,882,245, 21 November 1989. [Google Scholar]
- Tung, K.K.; Wong, W.H.; Pun, E.Y.B. Polymeric optical waveguides using direct ultraviolet photolithography process. Appl. Phys. A 2005, 80, 621–626. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Triarylsulfonium salts as photoinitiators of free radical and cationic polymerization. J. Polym. Sci. Polym. Lett. Ed. 1979, 17, 759–764. [Google Scholar] [CrossRef]
- Dickson, L.W.; Singh, A. Radiation curing of epoxies. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1988, 31, 587–593. [Google Scholar] [CrossRef]
- Rath, S.K.; Boey, F.Y.C.; Abadie, M.J.M. Cationic electron-beam curing of a high-functionality epoxy: Effect of post-curing on glass transition and conversion. Polym. Int. 2004, 53, 857–862. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, K.L.; Hong, G.D.; Yang, L.J.; Gong, H.Q. Polymerization optimization of SU-8 photoresist and its applications in microfluidic systems and MEMS. J. Micromech. Microeng. 2001, 11, 20. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, K.L.; Gong, H.Q. Characterization of the polymerization of SU-8 photoresist and its applications in micro-electro-mechanical systems (MEMS). Polym. Test. 2001, 20, 693–701. [Google Scholar] [CrossRef]
- Jiguet, S.; Judelewicz, M.; Mischler, S.; Bertch, A.; Renaud, P. Effect of filler behavior on nanocomposite SU8 photoresist for moving micro-parts. Microelectron. Eng. 2006, 83, 1273–1276. [Google Scholar] [CrossRef]
- Yeh, W.-M. Pattern Collapse in Lithographic Nanostructures: Quantifying Photoresist Nanostructure Behavior and Novel Methods for Collapse Mitigation. Ph.D. Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2013. [Google Scholar]
- Bogunovic, L.; Anselmetti, D.; Regtmeier, J. Photolithographic fabrication of arbitrarily shaped SU-8 microparticles without sacrificial release layers. J. Micromech. Microeng. 2011, 21, 027003. [Google Scholar] [CrossRef]
- Harutaka, M. Relationship between aspect ratio and narrowing of reflowed photoresist structures. Jpn. J. Appl. Phys. 2015, 54, 06FM01. [Google Scholar]
- Huang, Y.T.; Hsu, W. A simulation model on photoresist SU-8 thickness after development under partial exposure with reflection effect. Jpn. J. Appl. Phys. 2014, 53, 036505. [Google Scholar] [CrossRef]
- Xu, M.-J.; Ma, Y.; Hou, M.-J.; Li, B. Synthesis of a cross-linked triazine phosphine polymer and its effect on fire retardancy, thermal degradation and moisture resistance of epoxy resins. Polym. Degrad. Stab. 2015, 119, 14–22. [Google Scholar] [CrossRef]
- Tirta, A.; Baek, E.R.; Chang, S.S.; Kim, J.H. Fabrication of porous material for micro component application by direct X-ray lithography and sintering. Microelectron. Eng. 2012, 98, 297–300. [Google Scholar] [CrossRef]
- Mautjana, N.A.; Pasch, H. Matrix-Assisted Laser Desorption Ionization Mass Spectrometry of Synthetic Polymers. Macromol. Symp. 2012, 313–314, 157–161. [Google Scholar] [CrossRef]
- Puglisi, C.; Samperi, F.; Carroccio, S.; Montaudo, G. MALDI−TOF Investigation of Polymer Degradation. Pyrolysis of Poly(bisphenol A carbonate). Macromolecules 1999, 32, 8821–8828. [Google Scholar] [CrossRef]
- Bahr, U.; Deppe, A.; Karas, M.; Hillenkamp, F.; Giessmann, U. Mass spectrometry of synthetic polymers by UV-matrix-assisted laser desorption/ionization. Anal. Chem. 1992, 64, 2866–2869. [Google Scholar] [CrossRef]
- Esser, E.; Keil, C.; Braun, D.; Montag, P.; Pasch, H. Matrix-assisted laser desorption/ionization mass spectrometry of synthetic polymers. 4. Coupling of size exclusion chromatography and MALDI-TOF using a spray-deposition interface. Polymer 2000, 41, 4039–4046. [Google Scholar] [CrossRef]
- Du, G.; Lei, H.; Pizzi, A.; Pasch, H. Synthesis–structure–performance relationship of cocondensed phenol–urea–formaldehyde resins by MALDI-ToF and 13C NMR. J. Appl. Polym. Sci. 2008, 110, 1182–1194. [Google Scholar] [CrossRef]
- Murgasova, R.; Hercules, D.M. MALDI of synthetic polymers—An update. Int. J. Mass Spectrom. 2003, 226, 151–162. [Google Scholar] [CrossRef]
- Schrod, M.; Rode, K.; Braun, D.; Pasch, H. Matrix-assisted laser desorption/ionization mass spectrometry of synthetic polymers. VI. Analysis of phenol–urea–formaldehyde cocondensates. J. Appl. Polym. Sci. 2003, 90, 2540–2548. [Google Scholar] [CrossRef]
- Belu, A.M.; DeSimone, J.M.; Linton, R.W.; Lange, G.W.; Friedman, R.M. Evaluation of matrix-assisted laser desorption ionization mass spectrometry for polymer characterization. J. Am. Soc. Mass Spectrom. 1996, 7, 11–24. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Pizzi, A.; Abdulkhani, A.; Doosthoseini, K.; Shakeri, A.; Lagel, M.C.; Delmotte, L. MALDI-TOF, 13C NMR and FT-MIR analysis and strength characterization of glycidyl ether tannin epoxy resins. Ind. Crops Prod. 2016, 83, 177–185. [Google Scholar] [CrossRef]
- Hoong, Y.B.; Pizzi, A.; Chuah, L.A.; Harun, J. Phenol–urea–formaldehyde resin co-polymer synthesis and its influence on Elaeis palm trunk plywood mechanical performance evaluated by 13C NMR and MALDI-TOF mass spectrometry. Int. J. Adhes. Adhes. 2015, 63, 117–123. [Google Scholar] [CrossRef]

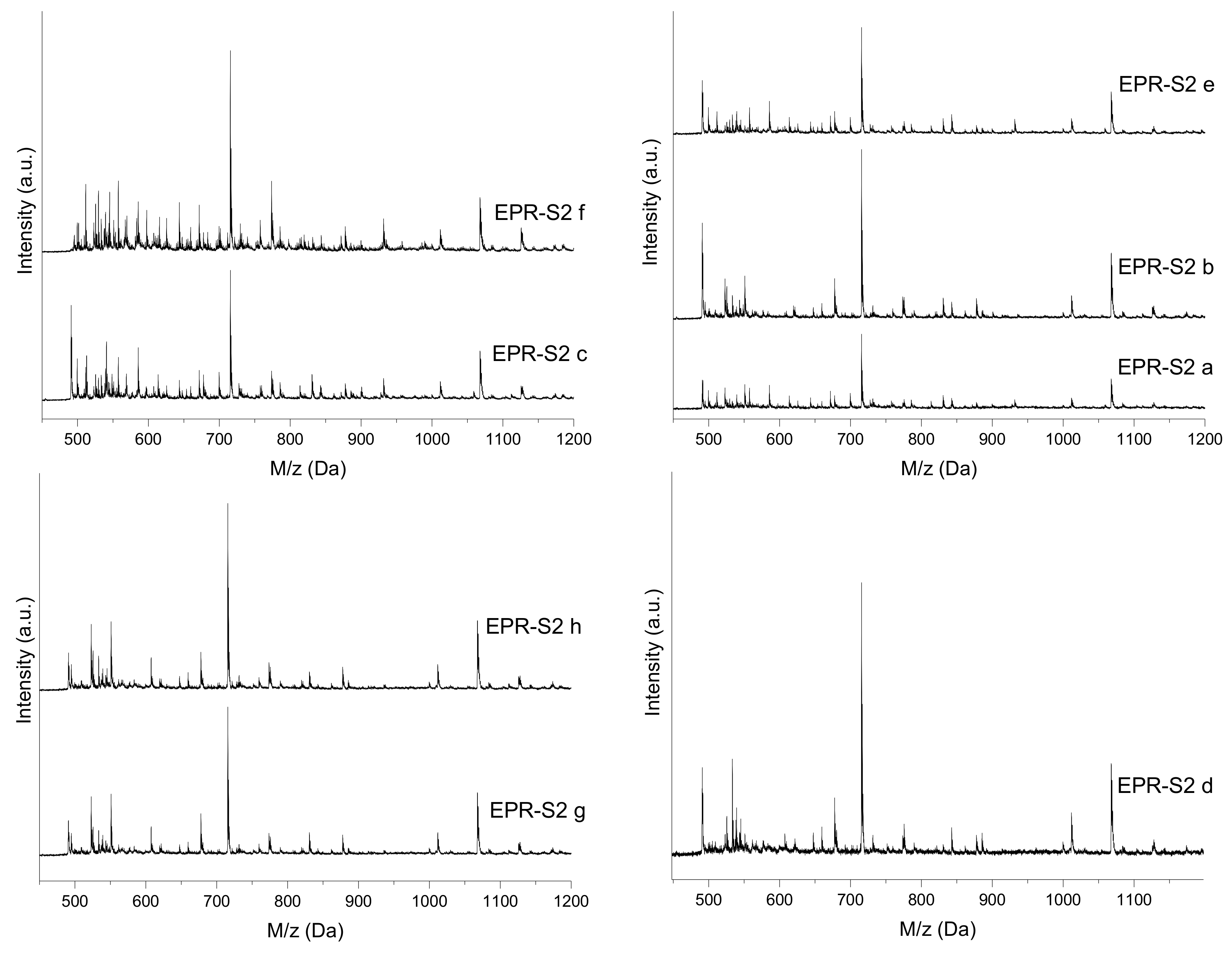
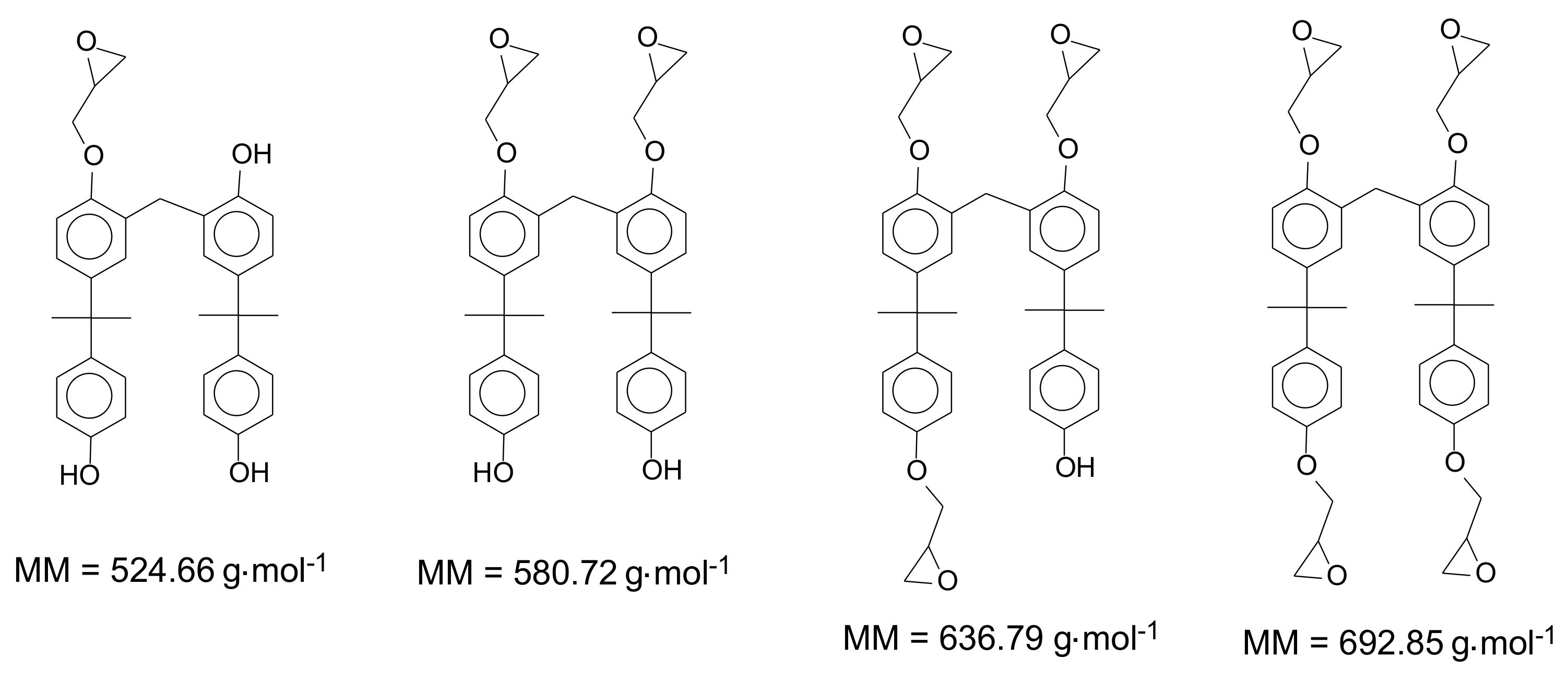
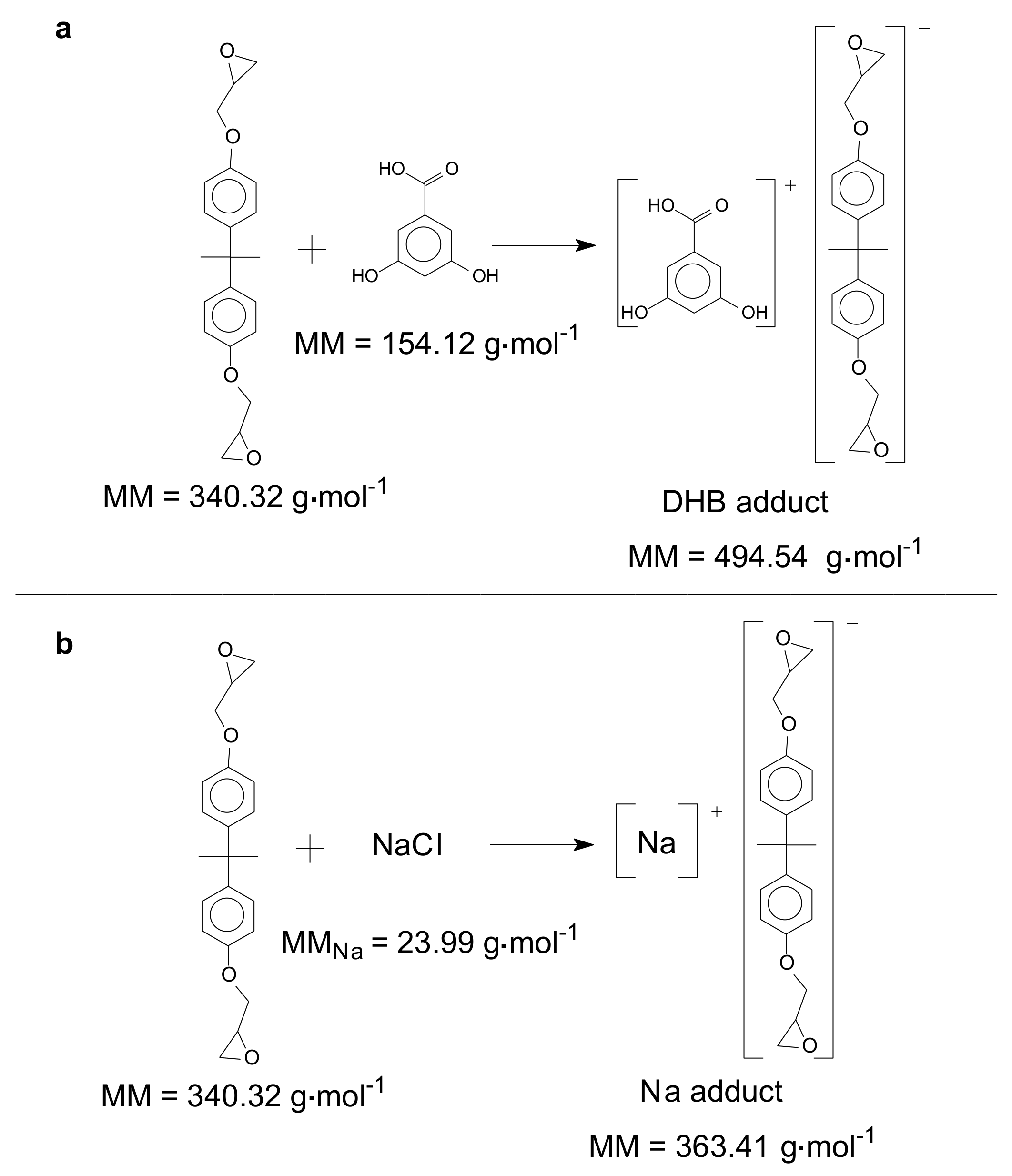
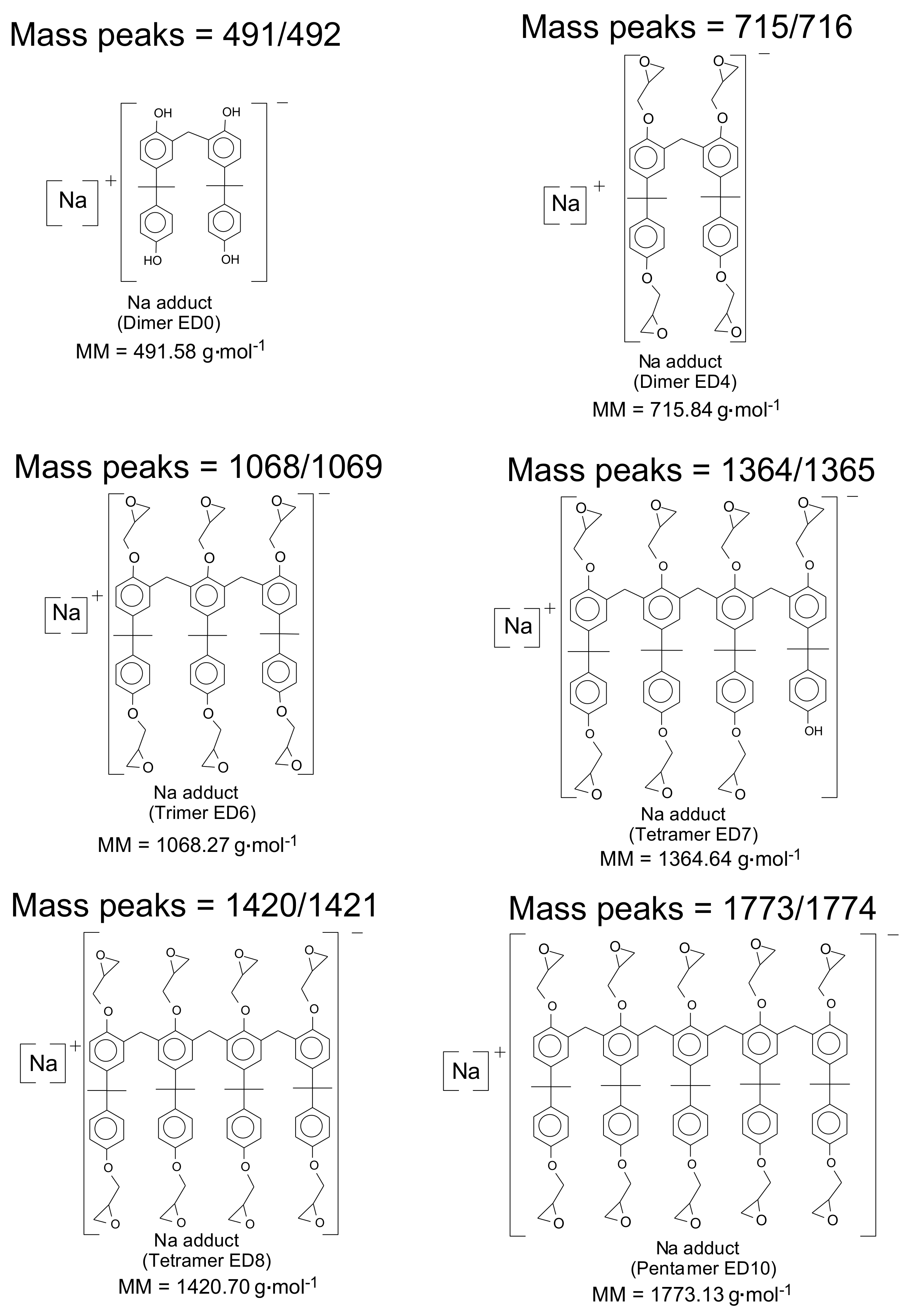
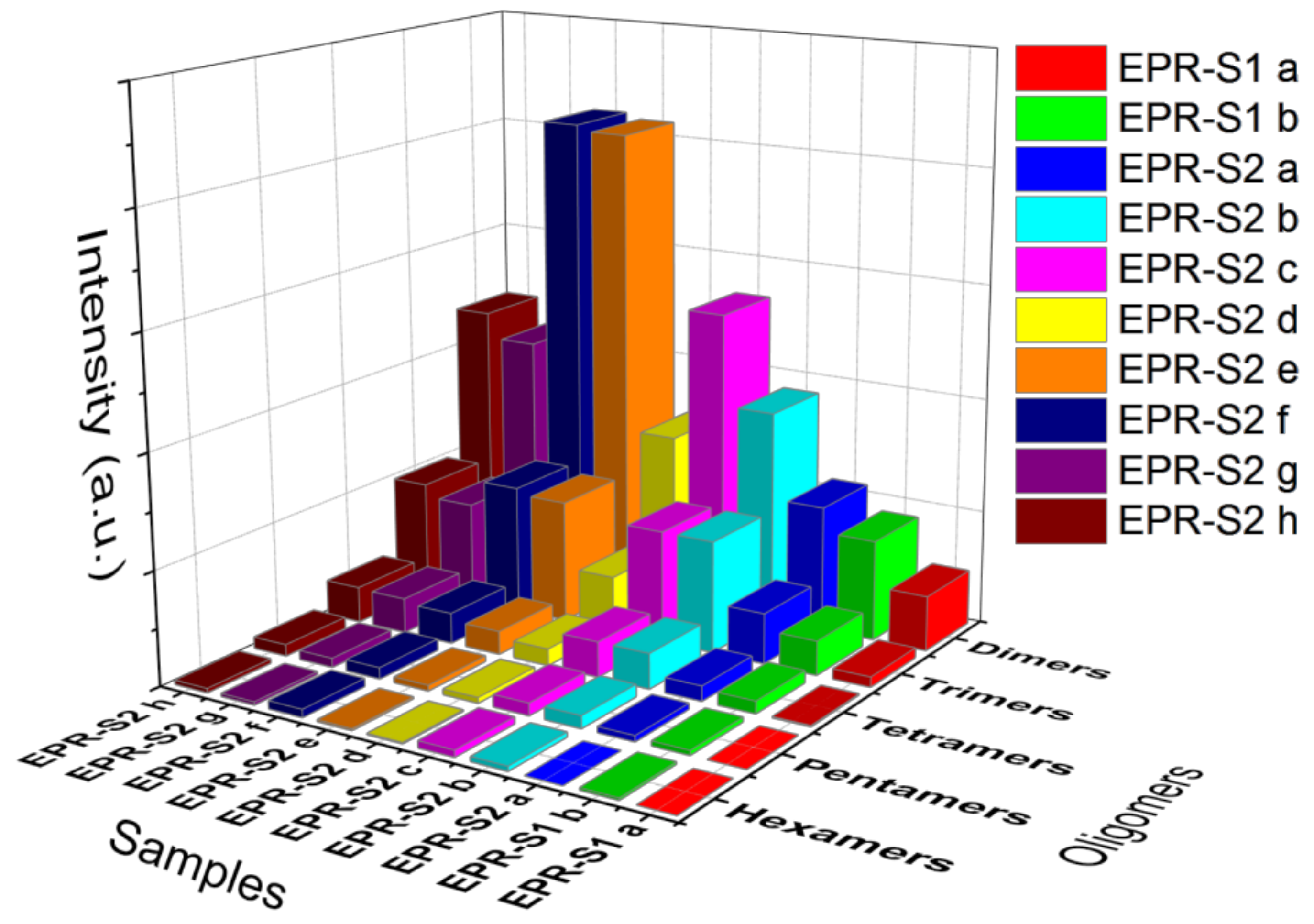
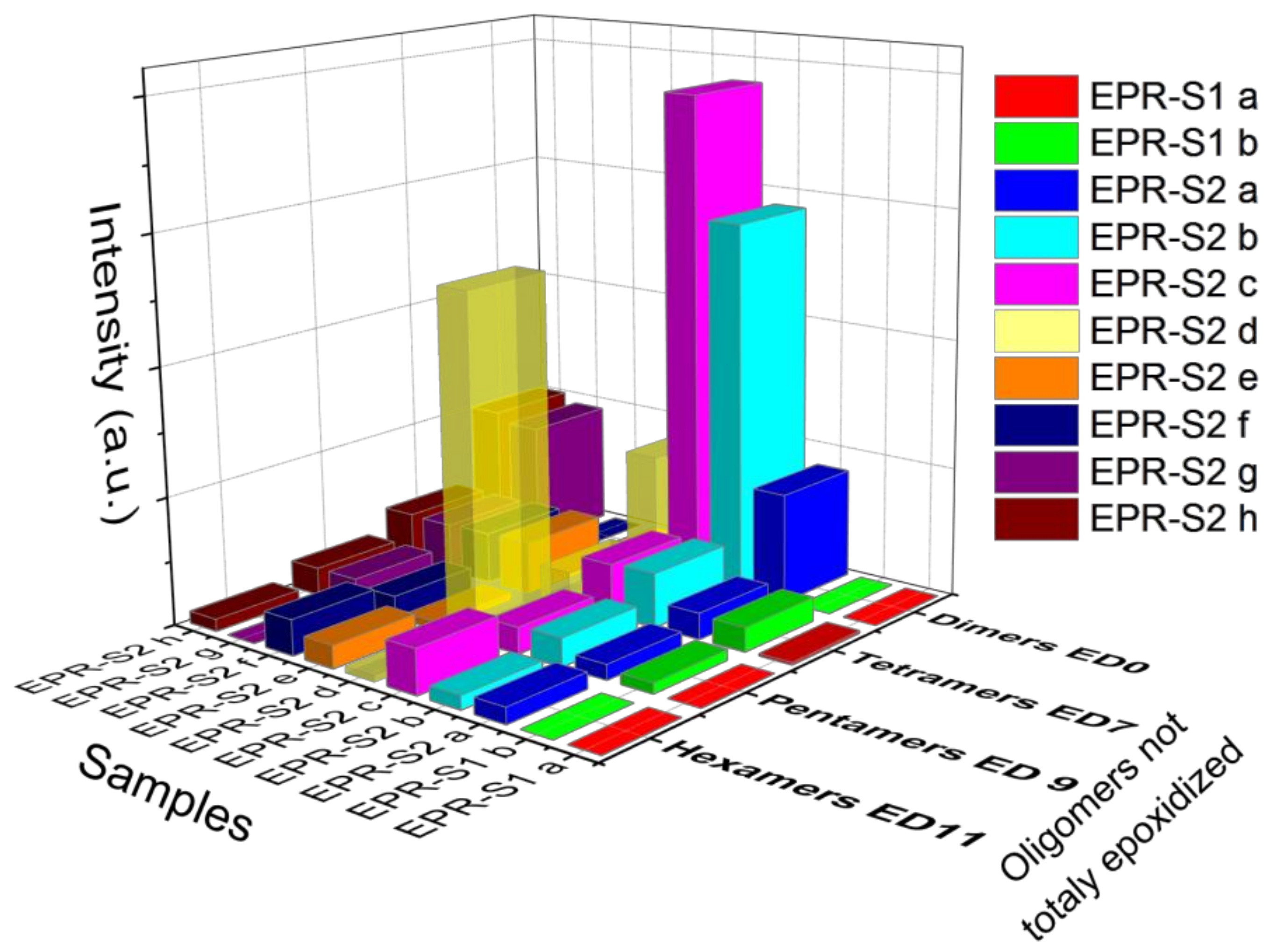
| Oligomer | ED0 | ED1 | ED2 | ED3 | ED4 | ED5 | ED6 | ED7 | ED8 | ED9 | ED10 | ED11 | ED12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monomer | 228.29 | 284.36 | 340.32 | - | - | - | - | - | - | - | - | - | - |
| Dimer | 468.59 | 524.66 | 580.72 | 636.79 | 692.85 | - | - | - | - | - | - | - | - |
| Trimer | 708.89 | 764.96 | 821.02 | 877.09 | 933.15 | 989.22 | 1045.28 | - | - | - | - | - | - |
| Tetramer | 949.20 | 1005.26 | 1061.32 | 1117.39 | 1173.45 | 1229.52 | 1285.58 | 1341.65 | 1397.71 | - | - | - | - |
| Pentamer | 1213.49 | 1343.62 | 1269.55 | 1399.68 | 1325.62 | 1455.75 | 1381.68 | 1511.81 | 1437.74 | 1567.87 | 1493.81 | - | - |
| Hexamer | 1429.80 | 1485.86 | 1541.93 | 1597.99 | 1654.06 | 1710.12 | 1766.19 | 1822.25 | 1878.31 | 1937.38 | 1988.43 | 2046.51 | 2102.57 |
| Epoxidation Degree | ED0 | ED1 | ED2 | ED3 | ED4 | ED5 | ED6 | |||||||
| Oligomer | aNa | aDHB | aNa | aDHB | aNa | aDHB | aNa | aDHB | aNa | aDHB | aNa | aDHB | aNa | aDHB |
| Monomer | 251.28 | 382.41 | 307.35 | 438.48 | 363.31 | 494.44 | - | - | - | - | - | - | - | - |
| Dimer | 491.58 | 622.71 | 547.65 | 678.78 | 603.71 | 734.84 | 659.78 | 790.91 | 715.84 | 846.97 | - | - | - | - |
| Trimer | 731.88 | 863.01 | 787.95 | 919.08 | 844.01 | 975.14 | 900.08 | 1031.21 | 956.14 | 1087.27 | 1012.21 | 1143.34 | 1068.27 | 1199.4 |
| Tetramer | 972.19 | 1103.32 | 1028.25 | 1159.38 | 1084.31 | 1215.44 | 1140.38 | 1271.51 | 1196.44 | 1327.57 | 1252.51 | 1383.64 | 1308.57 | 1439.7 |
| Pentamer | 1212.49 | 1343.62 | 1268.55 | 1399.68 | 1324.62 | 1455.75 | 1380.68 | 1511.81 | 1436.74 | 1567.87 | 1492.81 | 1623.94 | 1548.87 | 1680 |
| Hexamer | 1452.79 | 1583.92 | 1508.85 | 1639.98 | 1564.92 | 1696.05 | 1620.98 | 1752.11 | 1677.05 | 1808.18 | 1733.11 | 1864.24 | 1789.18 | 1920.31 |
| Epoxidation Degree | ED7 | ED8 | ED9 | ED10 | ED11 | ED12 | ||||||||
| Oligomer | aNa | aDHB | aNa | aDHB | aNa | aDHB | aNa | aDHB | aNa | aDHB | aNa | aDHB | ||
| Tetramer | 1364.64 | 1495.77 | 1420.7 | 1551.83 | - | - | - | - | - | - | - | - | ||
| Pentamer | 1604.94 | 1736.07 | 1661 | 1792.13 | 1717.07 | 1848.2 | 1773.13 | 1904.26 | - | - | - | - | ||
| Hexamer | 1845.24 | 1976.37 | 1901.3 | 2032.43 | 1960.37 | 2091.5 | 2011.42 | 2142.55 | 2069.5 | 2200.63 | 2125.56 | 2256.69 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlnieska, V.; Zakharova, M.; Börner, M.; Bade, K.; Mohr, J.; Kunka, D. Chemical and Molecular Variations in Commercial Epoxide Photoresists for X-ray Lithography. Appl. Sci. 2018, 8, 528. https://doi.org/10.3390/app8040528
Vlnieska V, Zakharova M, Börner M, Bade K, Mohr J, Kunka D. Chemical and Molecular Variations in Commercial Epoxide Photoresists for X-ray Lithography. Applied Sciences. 2018; 8(4):528. https://doi.org/10.3390/app8040528
Chicago/Turabian StyleVlnieska, Vitor, Margarita Zakharova, Martin Börner, Klaus Bade, Jürgen Mohr, and Danays Kunka. 2018. "Chemical and Molecular Variations in Commercial Epoxide Photoresists for X-ray Lithography" Applied Sciences 8, no. 4: 528. https://doi.org/10.3390/app8040528
APA StyleVlnieska, V., Zakharova, M., Börner, M., Bade, K., Mohr, J., & Kunka, D. (2018). Chemical and Molecular Variations in Commercial Epoxide Photoresists for X-ray Lithography. Applied Sciences, 8(4), 528. https://doi.org/10.3390/app8040528