Highly Graphitic Carbon Nanofibers Web as a Cathode Material for Lithium Oxygen Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Carbon Nanofibers Air Electrode
2.2. Characterization
2.3. Evaluation of Electrochemical Properties
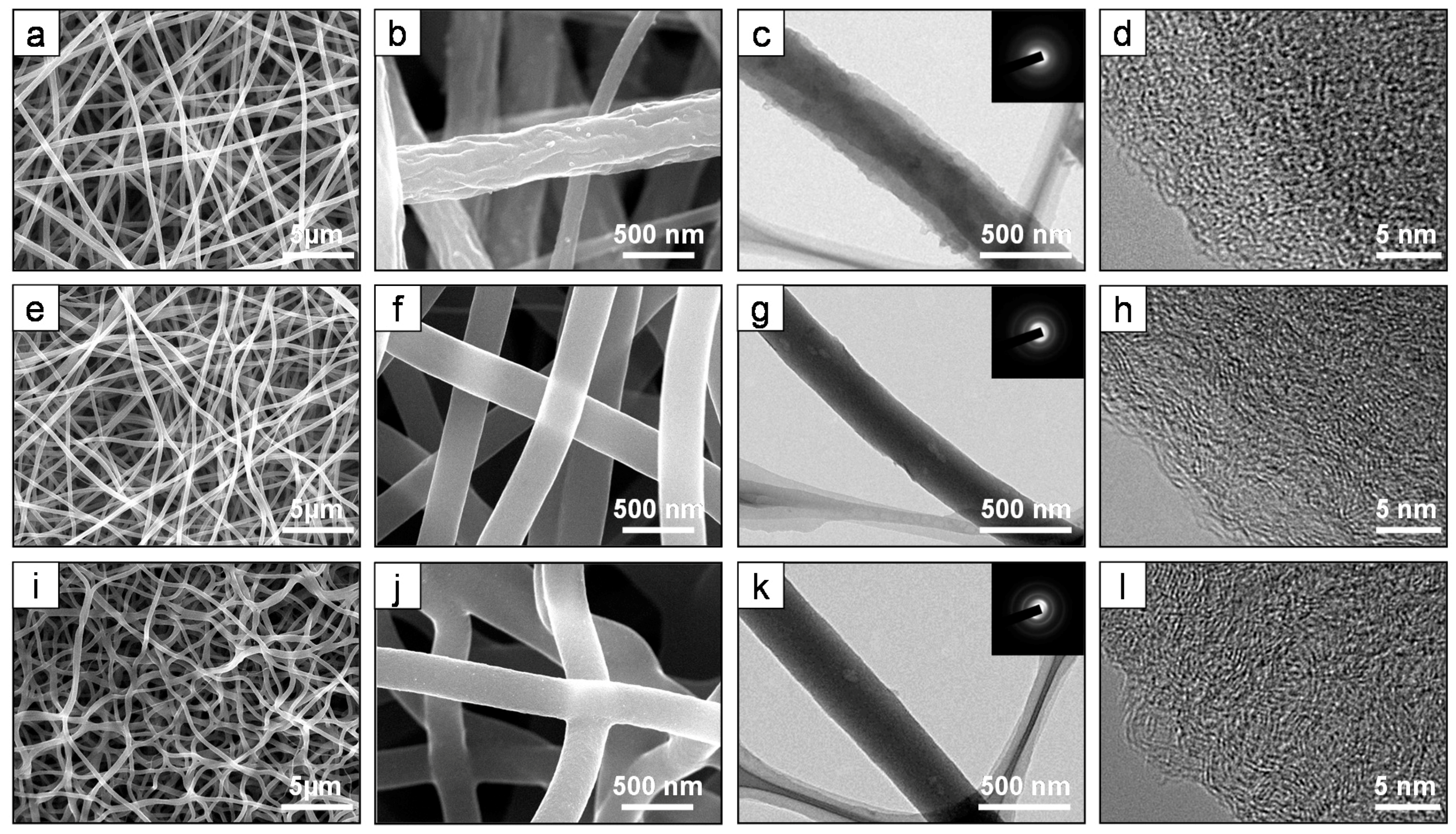
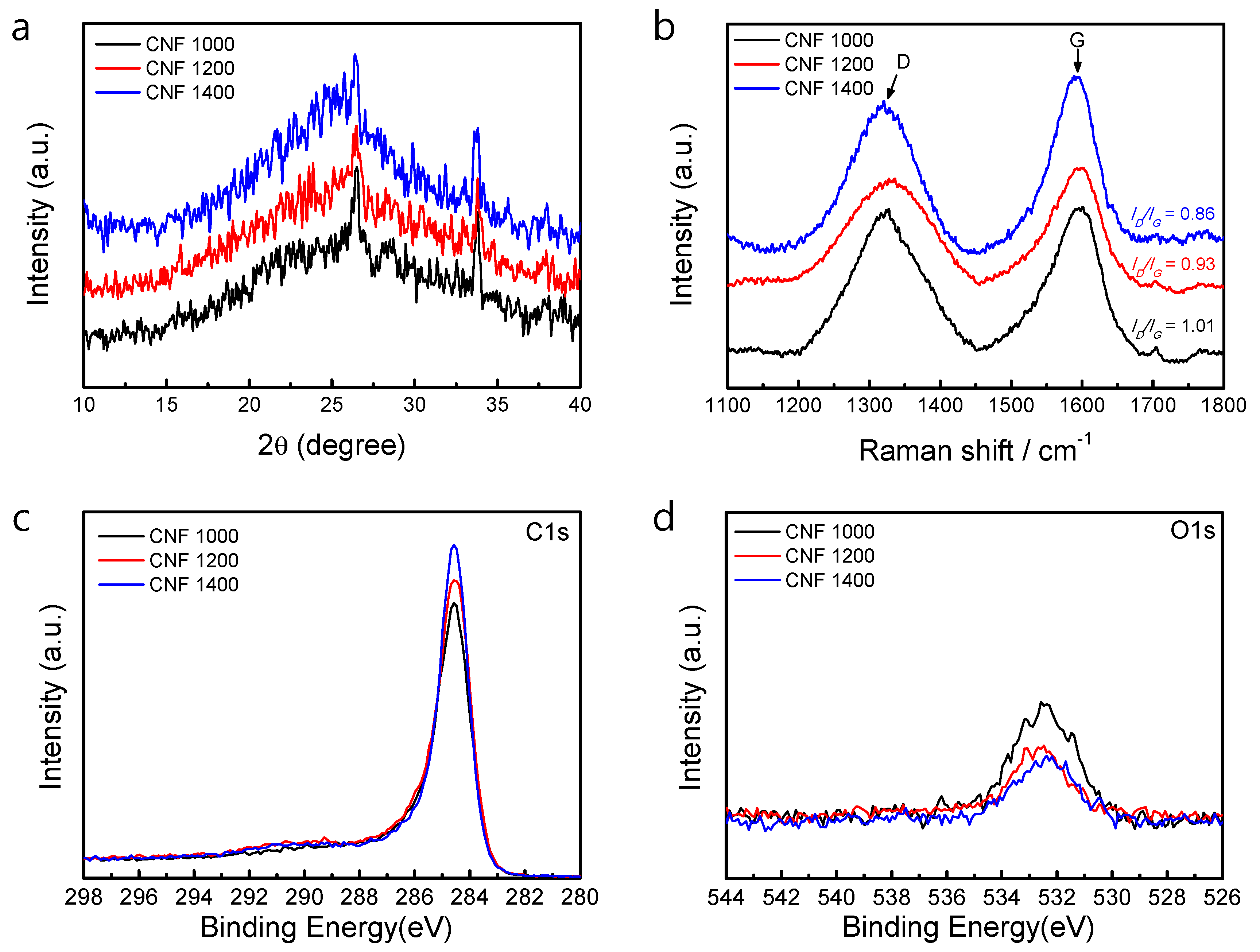
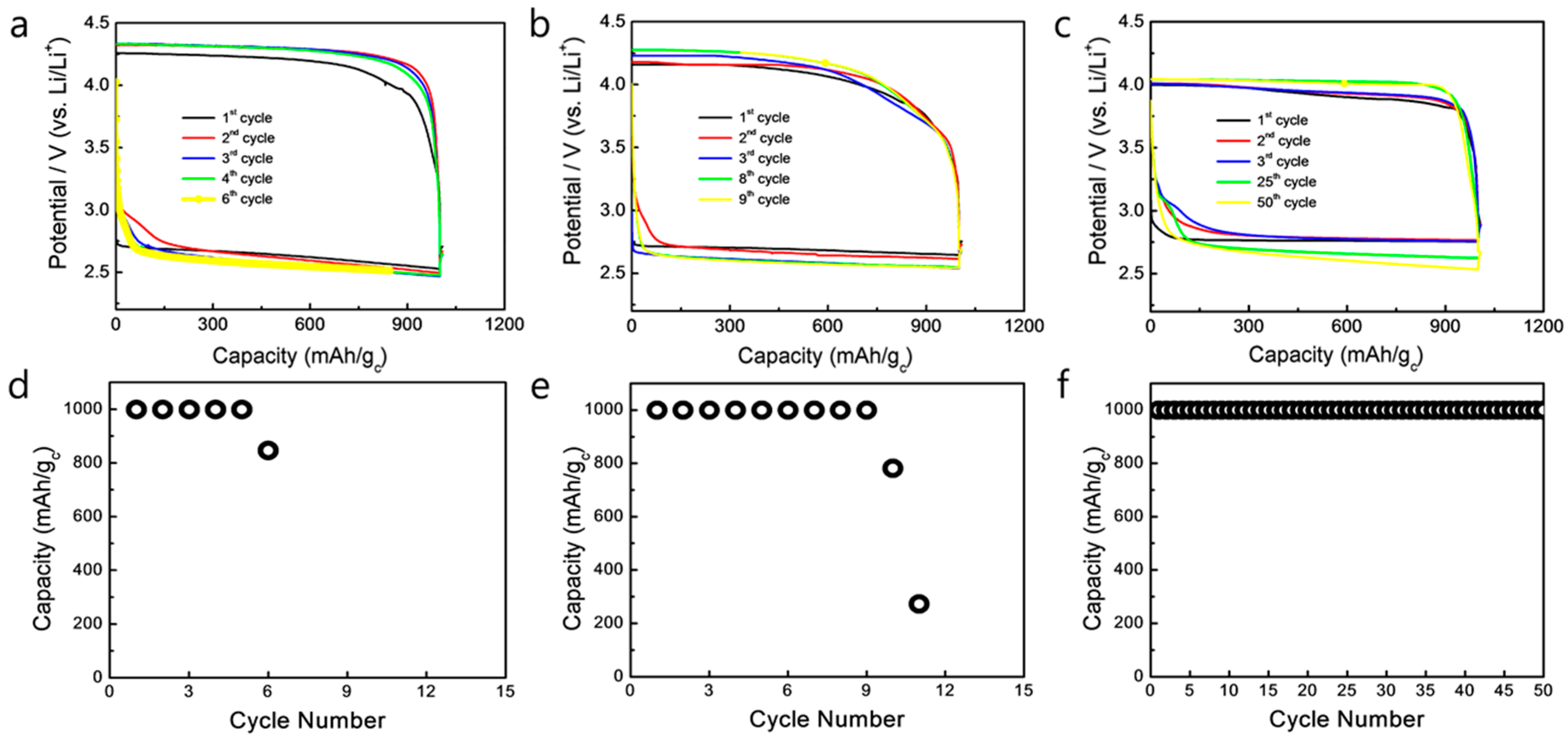
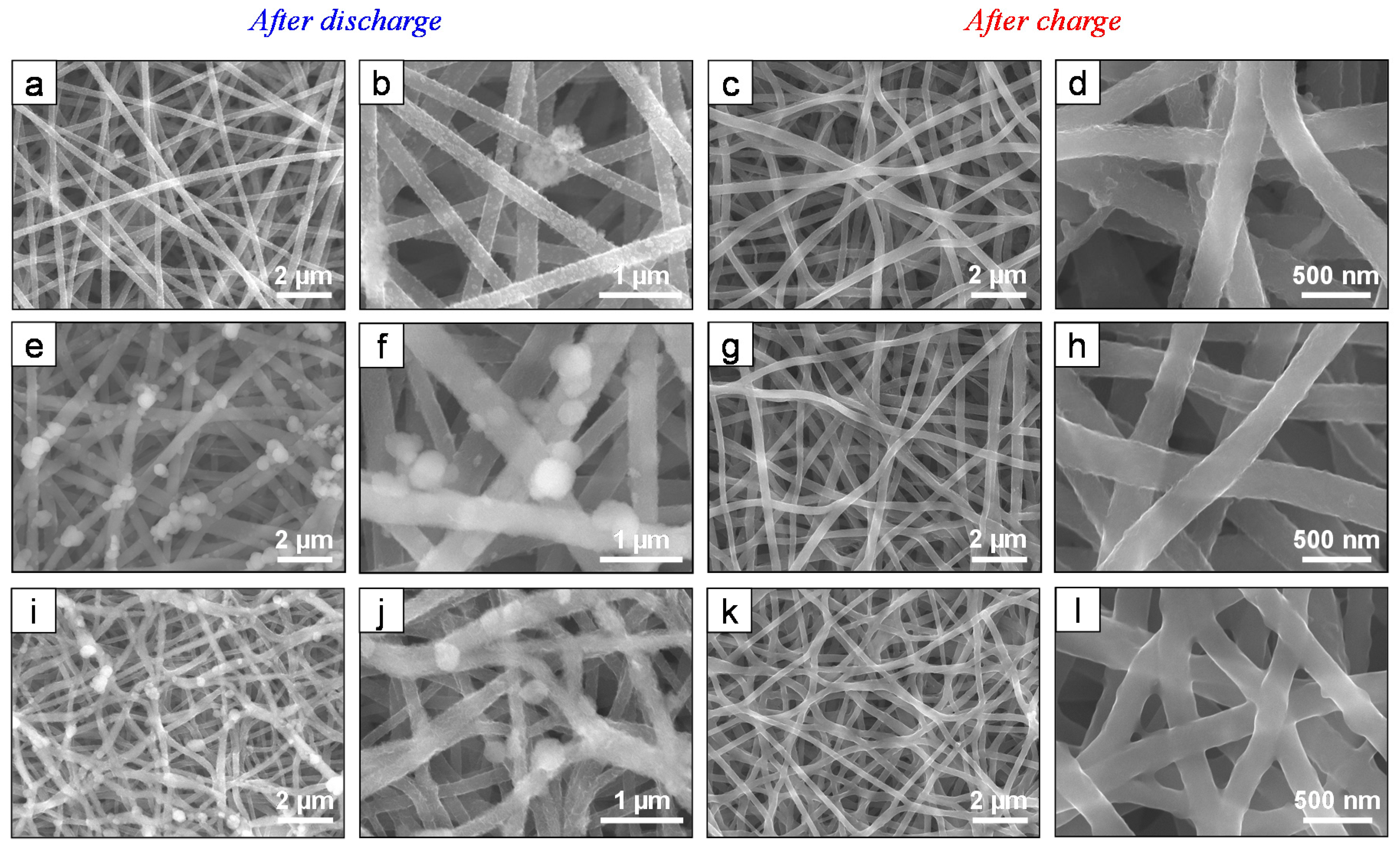
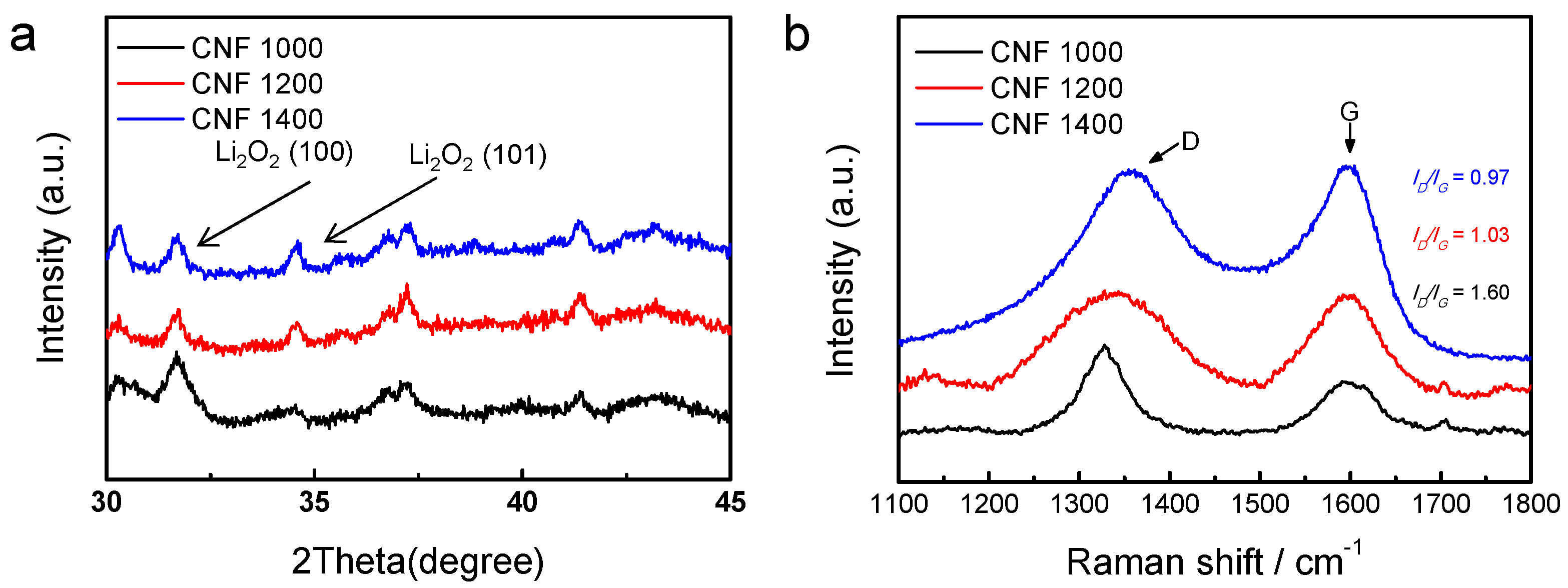
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, Y.C.; Gallant, B.M.; Kwabi, D.G.; Harding, J.R.; Mitchell, R.R.; Whittingham, M.S.; Shao-Horn, Y. Lithium-oxygen batteries: Bridging mechanistic understanding and battery performance. Energy Environ. Sci. 2013, 6, 750–768. [Google Scholar] [CrossRef]
- Li, F.J.; Zhang, T.; Zhou, H.S. Challenges of non-aqueous Li-O2 batteries: Electrolytes, catalysts, and anodes. Energy Environ. Sci. 2013, 6, 1125–1141. [Google Scholar] [CrossRef]
- Jung, H.G.; Kim, H.S.; Park, J.B.; Oh, I.H.; Hassoun, J.; Yoon, C.S.; Scrosati, B.; Sun, Y.K. A Transmission Electron Microscopy Study of the Electrochemical Process of Lithium-Oxygen Cells. Nano Lett. 2012, 12, 4333–4335. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Lim, H.D.; Park, K.Y.; Seo, D.H.; Gwon, H.; Hong, J.; Goddard, W.A.; Kim, H.; Kang, K. Toward a Lithium-“Air” Battery: The Effect of CO2 on the Chemistry of a Lithium-Oxygen Cell. J. Am. Chem. Soc. 2013, 135, 9733–9742. [Google Scholar] [CrossRef] [PubMed]
- Black, R.; Lee, J.H.; Adams, B.; Mims, C.A.; Nazar, L.F. The Role of Catalysts and Peroxide Oxidation in Lithium-Oxygen Batteries. Angew. Chem.-Int. Ed. 2013, 52, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.R.; Gallant, B.M.; Thompson, C.V.; Shao-Horn, Y. All-carbon-nanofiber electrodes for high-energy rechargeable Li-O2 batteries. Energy Environ. Sci. 2011, 4, 2952–2958. [Google Scholar] [CrossRef]
- Xiao, J.; Mei, D.H.; Li, X.L.; Xu, W.; Wang, D.Y.; Graff, G.L.; Bennett, W.D.; Nie, Z.M.; Saraf, L.V.; Aksay, I.A.; et al. Hierarchically Porous Graphene as a Lithium-Air Battery Electrode. Nano Lett. 2011, 11, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Itkis, D.M.; Semenenko, D.A.; Kataev, E.Y.; Belova, A.I.; Neudachina, V.S.; Sirotina, A.P.; Havecker, M.; Teschner, D.; Knop-Gericke, A.; Dudin, P.; et al. Reactivity of Carbon in Lithium-Oxygen Battery Positive Electrodes. Nano Lett. 2013, 13, 4697–4701. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Black, R.; Pomerantseva, E.; Lee, J.H.; Nazar, L.F. Synthesis of a metallic mesoporous pyrochlore as a catalyst for lithium-O2 batteries. Nat. Chem. 2012, 4, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Black, R.; Adams, B.; Nazar, L.F. Non-Aqueous and Hybrid Li-O2 Batteries. Adv. Energy Mater. 2012, 2, 801–815. [Google Scholar] [CrossRef]
- Lee, J.H.; Black, R.; Popov, G.; Pomerantseva, E.; Nan, F.H.; Botton, G.A.; Nazar, L.F. The role of vacancies and defects in Na0.44MnO2 nanowire catalysts for lithium-oxygen batteries. Energy Environ. Sci. 2012, 5, 9558–9565. [Google Scholar] [CrossRef]
- Sun, B.; Wang, B.; Su, D.W.; Xiao, L.D.; Ahn, H.; Wang, G.X. Graphene nanosheets as cathode catalysts for lithium-air batteries with an enhanced electrochemical performance. Carbon 2012, 50, 727–733. [Google Scholar] [CrossRef]
- Laoire, C.O.; Mukerjee, S.; Abraham, K.M.; Plichta, E.J.; Hendrickson, M.A. Influence of Nonaqueous Solvents on the Electrochemistry of Oxygen in the Rechargeable Lithium-Air Battery. J. Phys. Chem. C 2010, 114, 9178–9186. [Google Scholar] [CrossRef]
- Zhu, X.J.; Zhu, Y.W.; Murali, S.; Stollers, M.D.; Ruoff, R.S. Nanostructured Reduced Graphene Oxide/Fe2O3 Composite as a High-Performance Anode Material for Lithium Ion Batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xiao, J.; Zhang, J.; Wang, D.Y.; Zhang, J.G. Optimization of Nonaqueous Electrolytes for Primary Lithium/Air Batteries Operated in Ambient Environment. J. Electrochem. Soc. 2009, 156, A773–A779. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium-Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Duke, A.S.; Galhenage, R.P.; Tenney, S.A.; Ammal, S.C.; Heyden, A.; Sutter, P.; Chen, D.A. In Situ Ambient Pressure X-ray Photoelectron Spectroscopy Studies of Methanol Oxidation on Pt(111) and Pt-Re Alloys. J. Phys. Chem. C 2015, 119, 23082–23093. [Google Scholar] [CrossRef]
- Padbury, R.; Zhang, X.W. Lithium-oxygen batteries-Limiting factors that affect performance. J. Power Sources 2011, 196, 4436–4444. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Holzapfel, M.; Novak, P.; Johnson, C.S.; Kang, S.H.; Thackeray, M.M.; Bruce, P.G. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 2006, 128, 8694–8698. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Wang, Z.L.; Xu, D.; Zhang, L.L.; Zhang, X.B. Tailoring deposition and morphology of discharge products towards high-rate and long-life lithium-oxygen batteries. Nat. Commun. 2013, 4, 2438. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Munroe, P.; Wang, G.X. Ruthenium nanocrystals as cathode catalysts for lithium-oxygen batteries with a superior performance. Sci. Rep. 2013, 3, 2247. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Xu, D.; Wang, Z.L.; Wang, H.G.; Zhang, L.L.; Zhang, X.B. Synthesis of Perovskite-Based Porous La0.75Sr0.25MnO3 Nanotubes as a Highly Efficient Electrocatalyst for Rechargeable LithiumOxygen Batteries. Angew. Chem.-Int. Ed. 2013, 52, 3887–3890. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Xu, Z.C.; Gasteiger, H.A.; Chen, S.; Hamad-Schifferli, K.; Shao-Horn, Y. Platinum-Gold Nanoparticles: A Highly Active Bifunctional Electrocatalyst for Rechargeable Lithium-Air Batteries. J. Am. Chem. Soc. 2010, 132, 12170–12171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Reddy, M.; Chowdari, B. Ramakrishna, Long-Term Cycling Studies on Electrospun Carbon Nanofibers as Anode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 12175–12184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, P.; Reddy, M.; Chowdari, B.; Ramakrishna, S. Maghemite Nanoparticles on Electrospun CNFs Template as Prospective Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2014, 6, 1951. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.J.; Yang, J.M.; Wang, S.P.; Xiong, Z.B.; Cai, W.S.; Liu, J.Y. Facile fabrication of porous carbon nanofibers by electrospun PAN/dimethyl sulfone for capacitive deionization. J. Mater. Chem. A 2015, 3, 13827–13834. [Google Scholar]
- Yi, B.; Rajagopalan, R.; Burket, C.L.; Foley, H.C.; Liu, X.M.; Eklund, P.C. High temperature rearrangement of disordered nanoporous carbon at the interface with single wall carbon nanotubes. Carbon 2009, 47, 2303–2309. [Google Scholar]
- Qi, X.; Ruan, X.F.; Pan, C.X. Graphitization of solid carbon nanofibers at an unexpectedly low temperature. Mater. Lett. 2007, 61, 4272–4275. [Google Scholar] [CrossRef]
- Khai, T.V.; Na, H.G.; Kwak, D.S.; Kwon, Y.J.; Ham, H.; Shim, K.B.; Kim, H.W. Significant enhancement of blue emission and electrical conductivity of N-doped graphene. J. Mater. Chem. 2012, 22, 17992–18003. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Waller, G.H.; Liu, Y.; Liu, M.L.; Wong, C.P. Simple preparation of nanoporous few-layer nitrogen-doped graphene for use as an efficient electrocatalyst for oxygen reduction and oxygen evolution reactions. Carbon 2013, 53, 130–136. [Google Scholar] [CrossRef]
- Ogasawara, T.; Debart, A.; Holzapfel, M.; Novak, P.; Bruce, P.G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 2006, 128, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.M.; Jiang, Z. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 1996, 143, 1–5. [Google Scholar] [CrossRef]
- Mizuno, F.; Nakanishi, S.; Kotani, Y.; Yokoishi, S.; Iba, H. Rechargeable Li-Air Batteries with Carbonate-Based Liquid Electrolytes. Electrochemistry 2010, 78, 403–405. [Google Scholar] [CrossRef]
- Laoire, C.O.; Mukerjee, S.; Plichta, E.J.; Hendrickson, M.A.; Abraham, K.M. Rechargeable Lithium/TEGDME-LiPF6/O2 Battery. J. Electrochem. Soc. 2011, 158, A302–A308. [Google Scholar] [CrossRef]
- Carboni, M.; Brutti, S.; Marrani, A.G. Surface Reactivity of a Carbonaceous Cathode in a Lithium Triflate/Ether Electrolyte-Based Li-O2 Cell. ACS Appl. Mater. Interfaces 2015, 7, 21751–21762. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.G.; Hassoun, J.; Park, J.B.; Sun, Y.K.; Scrosati, B. An improved high-performance lithium-air battery. Nat. Chem. 2012, 4, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.D.; Song, H.; Kim, J.; Gwon, H.; Bae, Y.; Park, K.Y.; Hong, J.; Kim, H.; Kim, T.; Kim, Y.H.; et al. Superior Rechargeability and Efficiency of Lithium-Oxygen Batteries: Hierarchical Air Electrode Architecture Combined with a Soluble Catalyst. Angew. Chem.-Int. Ed. 2014, 53, 3926–3931. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Huang, X.D.; Chen, S.Q.; Munroe, P.; Wang, G.X. Porous Graphene Nanoarchitectures: An Efficient Catalyst for Low Charge-Overpotential, Long Life, and High Capacity Lithium-Oxygen Batteries. Nano Lett. 2014, 14, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Radtke, C.; Black, R.; Trudeau, M.L.; Zaghib, K.; Nazar, L.F. Current density dependence of peroxide formation in the Li-O2 battery and its effect on charge. Energy Environ. Sci. 2013, 6, 1772–1778. [Google Scholar] [CrossRef]
- Tan, P.; Shi, L.; Shyy, W.; Zhao, T.S. Morphology of the Discharge Product in Non-aqueous Lithium-Oxygen Batteries: Furrowed Toroid Particles Correspond to a Lower Charge Voltage. Energy Technol. 2016, 4, 393–400. [Google Scholar] [CrossRef]
- Griffith, L.D.; Sleightholme, A.E.S.; Mansfield, J.F.; Siegel, D.J.; Monroe, C.W. Correlating Li/O2 Cell Capacity and Product Morphology with Discharge Current. ACS Appl. Mater. Interfaces 2015, 7, 7670–7678. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.X.; Han, X.P.; Cheng, F.Y.; Zhao, Q.; Hu, Z.; Chen, J. Size effect of lithium peroxide on charging performance of Li-O2 batteries. Nanoscale 2014, 6, 177–180. [Google Scholar] [PubMed]
- Zhou, J.; Sui, Z.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.; Yuan, W. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785. [Google Scholar]
- Song, S.; Xu, W.; Zheng, J.; Luo, L.; Engelhard, M.; Bowden, M.; Liu, B.; Wang, C.; Zhang, J. Complete Decomposition of Li2CO3 in Li-O2 Batteries Using Ir/B4C as Noncarbon-Based Oxygen Electrode. Nano Lett. 2017, 17, 1417. [Google Scholar] [PubMed]
- Jaber-Ansari, L.; Puntambekar, K.P.; Tavassol, H.; Yildirim, H.; Kinaci, A.; Kumar, R.; Saldana, S.J.; Gewirth, A.A.; Greeley, J.P.; Chan, M.K.Y.; et al. Defect Evolution in Graphene upon Electrochemical Lithiation. ACS Appl. Mater. Interfaces 2014, 6, 17626–17636. [Google Scholar] [CrossRef] [PubMed]
| Carbon Materials (for Oxygen Electrode) | Electrolyte | Differences of Change and Discharge Viltage (V) | Curremt Desity | Capacity (mAhg-c−1) | Ref. |
|---|---|---|---|---|---|
| Super P carbon + α-MnO2 | LiPF6-PC | 1.3 | 70 mAhg-c−1 | 1000 | [32] |
| Chevlon carbon + Co-phthalocyanine | LiPF6-PC-EC-PAN | 0.92 | 0.1 mAcm−2 (discharge)0.05 mAcm−2 (charge) | 520 | [33] |
| Super P carbon + MnO2 | LiTFSi-PC | 1.8 | 0.02 mAhg-c−1 | 800 | [34] |
| BP2000 carbon + MnO2 | LiPF6-TEGDME | 1.4 | 0.13 mAhg-c−1 | 800 | [35] |
| Super P carbon | LiPF6-TEGDME | 1.8 | 70 mAhg-c−1 | 3000 | [36] |
| Carbon on carbon paper | LiCF2SO2-TEGEME | 1.7 | 500 mAhg-c−1 | 3000 | [37] |
| CNT fibril | TEGEME | 1.6 | 2000 mAhg-c−1 | 1000 | [38] |
| All carbon-nanofiber | LiPF6-EC:DMC | ~1.55 | 43 mAhg-c−1 | −5000 | [7] |
| Graphene nanosheets | LiClO4-PC | 1.22 | 0.1 mAhg-c−1 | 2332 | [13] |
| Porous graphene nanoarchitectures | DMSO | 1.579 | 200 mAhg-c−1 | 29,375 | [39] |
| CHF 1000 (this work) | LiTFSi-TEGEME | 1.58 | 50 mAhg-c−1 | 1000 | |
| CHF 1000 (this work) | LiTFSi-TEGEME | 1.42 | 50 mAhg-c−1 | 1000 | |
| CHF 1000 (this work) | LiTFSi-TEGEME | 1.16 | 50 mAhg-c−1 | 1000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Jeon, Y.; Liu, Z.; Song, T. Highly Graphitic Carbon Nanofibers Web as a Cathode Material for Lithium Oxygen Batteries. Appl. Sci. 2018, 8, 209. https://doi.org/10.3390/app8020209
Han H, Jeon Y, Liu Z, Song T. Highly Graphitic Carbon Nanofibers Web as a Cathode Material for Lithium Oxygen Batteries. Applied Sciences. 2018; 8(2):209. https://doi.org/10.3390/app8020209
Chicago/Turabian StyleHan, Hyungkyu, Yeryung Jeon, Zhiming Liu, and Taeseup Song. 2018. "Highly Graphitic Carbon Nanofibers Web as a Cathode Material for Lithium Oxygen Batteries" Applied Sciences 8, no. 2: 209. https://doi.org/10.3390/app8020209
APA StyleHan, H., Jeon, Y., Liu, Z., & Song, T. (2018). Highly Graphitic Carbon Nanofibers Web as a Cathode Material for Lithium Oxygen Batteries. Applied Sciences, 8(2), 209. https://doi.org/10.3390/app8020209







