Abstract
This paper discusses the effect of doping of electro-insulating liquids with nanoparticle materials on the thermal properties of the obtained nanoliquids and heat transport in the transformer. Mineral oil, synthetic ester, and natural ester were used as base liquids. The effectiveness of doping base liquids with nanoparticles was supported by ultraviolet-visible (UV/VIS) measurements. In turn, Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) confirmed the absence of intermolecular interactions (i.e., hydrogen bonding). The influence of modification of electro-insulating liquids with fullerene C60 and titanium dioxide TiO2 nanoparticles on such thermal properties as thermal conductivity, specific heat, kinematic viscosity, density, and thermal expansion was investigated. Based on these properties and the theory of similarity, the cooling efficiency of the transformer filled with the analyzed nanofluids was determined. Nanofluids’ cooling effectiveness was compared with the cooling effectiveness of the base liquids. This comparison was supported by an analysis of Grashof, Prandtl, and Nusselt numbers. It has been shown that the modification of electro-insulating liquids with nanoparticles widely used in order to improve their dielectric properties, such as C60 and TiO2, does not have a significant influence on their thermal properties. The addition of fullerene C60 caused an increase in kinematic viscosity, which was compensated by the increase in specific heat. In the case of TiO2, the addition of this nanoparticle resulted in an increase in kinematic viscosity and a decrease in specific heat, which were balanced out by the increase in thermal conductivity. In summary, the heat exchange-capacity of liquids did not change due to doping with nanoparticles.
1. Introduction
Ensuring proper electrical insulation and heat dissipation out of devices are the main tasks for electro-insulating liquids which are used in electrical power equipment [1,2]. In the case of transformers, heat transport takes place along the heat source (core, windings), insulation system (paper impregnated with electro-insulating liquid and electro-insulating liquid), tank, and air. The fact that the electro-insulating liquid has the largest volume in the transformer cooling system makes it essential in heat transport. The heat generated in the windings arises as a result of Joule’s losses, while the heat generated in the core is related to magnetic hysteresis and eddy currents. The electro-insulating liquid that flows through the oil channels cools the windings and core, taking away the heat from them. The heat generated by the core and the winding through the liquid is transferred to the ladle, from where it goes to the surroundings. The main purpose of the cooling system is to prevent excessive temperature increases in the windings. The areas characterized by the highest temperature occur in places where windings are in contact with solid insulation (paper impregnated with electro-insulating liquid). Due to the fact that this insulation is very sensitive to temperature (elevated temperature accelerates the aging processes [3,4,5] and causes its degradation [6,7]), it is necessary to use materials that ensure an adequate level of cooling, thus reducing the risk of sudden transformer damage [8,9,10].
At present, the most commonly used electro-insulating liquid in transformers around the world is mineral oil. The key success of mineral oil is primarily due to its three advantages: good dielectric properties, low costs associated with its availability, and very good recognition of this liquid [11,12]. However, the growing requirements regarding fire safety and environmental protection, as well as extending the lifetime of transformers, has forced their producers and users to search for alternative electro-insulating liquids for mineral oil [13,14]. To a large extent, the alternative to mineral oil is considered to be natural and synthetic esters. So far, these liquids have been used mainly in distribution transformers. Recently, however, they are increasingly being used in power transformers [15,16,17,18,19]. This is mainly due to the properties of esters, such as high biodegradability [20,21,22], low toxicity [15,23], and high flash-point and fire-point values [24,25,26,27,28]. These properties allow for the use of esters in places where, previously, they were only used in dry transformers—in densely populated areas, near water reservoirs, or in petrochemical plants or mines [29,30].
Recently, an increasing amount of research related to the search for alternative electro-insulating liquids has focused on the use of nanotechnology [31]. By dispersing nanometric materials (nanoparticles, nanofibers, nanotubes, nonowires, nonorods, nonosheets) in base fluids, a new class of dielectric liquids called nanofluids can be obtained [32,33]. Choi invented this term in 1995 [34]. Nanofluids can be defined as nanoscale colloidal suspensions containing concentrated nanomaterials. They are therefore two-phase systems, with one phase (solid phase) being suspended in the other (liquid phase) [32]. Currently, it is believed that nanofluids are next-generation fluids, which, compared to ordinary liquids, offer greater possibilities, both in terms of dielectric properties and improved heat-exchange efficiency [35]. The area of potential application of nanofluids is very wide and covers many important areas, such as electronics, medicine, and electrical power engineering [36,37,38,39].
In the case of nanofluids’ applications in transformers, the main research is related to the improvement of the dielectric properties of the base liquids. There are many publications confirming the positive influence of electro-insulating liquids’ modifications, with nanomaterials on their dielectric properties and phenomena such as breakdown voltage [40,41,42,43], streamer propagation [44], electrical conductivity, and the dissipation factor [45,46,47,48,49]. In addition, as reported in the literature [50,51], fullerene C60 can also be used to absorb free radicals arising from the aging of vegetable oils. Bearing in mind that the electro-insulating liquid fills the transformer’s interior, in addition to ensuring adequate electrical insulation, it also has the task of cooling the device, and seems fully justified in that the use of nanotechnology can also contribute to the improvement of heat transport and thus the reliability of transformers.
As is well-known in the context of the general improvement of cooling efficiency, liquids’ properties, such as thermal conductivity, viscosity, density, specific heat, and thermal expansion, are important. Therefore, in many scientific publications, scientists are improving these properties by dispersing nanomaterials in the base liquids (water, oils, ethanol). It was observed that the improvement of thermal properties of the resulting nanofluids, in relation to the base liquids, occurs in most cases of the applied nanomaterials [52,53,54,55,56,57,58,59,60]. Das et al. [53] observed that the thermal conductivity of nanofluids based on water and nanoparticles Al2O3 and CuO increased by about 30% compared to base liquids. However, such a large increase was observed only in the case where the concentration of nanoparticles ranged from 4–10% of the volume. In the case of concentrations below 2% of the volume, the low thermal conductivity of the analyzed nanoparticles resulted in low thermal conductivity of the analyzed nanofluids. The same authors have also observed that in the case of ethylene glycol and CuO nanoparticles, the thermal conductivity of the resulting nanofluids, in which the concentration of nanoparticles is 10–15% by volume, increases by about 40–50% relative to the base liquids. In turn, Ozernic et al. in [54] presented the results of studies that showed that the thermal conductivity of nanofluids based on Al2O3, CuO, Cu, SiO2, and TiO2 nanoparticles increased with decreasing thermal conductivity of the base liquid. In [58], Shukla et al. presented the results of studies in which they applied Newtonian nanoparticles using functionalized nanodiamonds (NDs). The authors observed that NDs were a good type of nanofiller, because without the deterioration of electro-insulating properties and viscosity (viscosity increased by about 1% in relation to the base liquid), they improve the thermal conductivity of the oil by about 14.5%. Du et al. in [59] found that doping vegetable oil with nanoparticles of boron nitride resulted in a significant improvement in heat transfer with respect to the base liquid. In turn, Raymon et al. in [60] showed that, depending on the type of nanoparticles used, they had different (both positive and negative) effects on the viscosity of the analyzed nanofluids. On this basis, it can be concluded that the effect of nanoparticles on the thermal properties of liquids is not linear and requires detailed testing for each potential nanofluid.
As can be seen from the literature, in the case of electro-insulating nanofluids, their dielectric properties are more important. Only few papers concern the thermal properties of nanofluids for applications in the transformer insulation system. Therefore, in this paper, the authors decided to analyze the impact of nanoparticles modification of the most commonly used insulating liquids on the efficiency of the transformer cooling system. Modifying the base liquids with fullerene C60 and titanium dioxide TiO2 nanoparticles was what made the insulating nanofluids. The produced nanofluids were characterized by adequate dispersion stability and thermal properties that differed from the properties of the base liquids. Methods for obtaining stable solutions and colloids using the presented nanoparticles are presented. The chemical structures of the tested liquids and nanofluids were examined using Fourier transform infrared spectroscopy. On the basis of the investigated thermal properties, the ability of manufactured nanofluids to transport heat was determined. The results of our research and discussion are presented in the following chapters.
2. Materials and Methods
2.1. Used Materials
Three of the most commonly applied electro-insulating liquids in electrical power devices were used for the tests. The base liquids used for the research were Nytro Draco mineral oil (MO) produced by Nynas (Stockholm, Sweden), MIDEL 7131 synthetic ester (SE) produced by M&I Materials (Manchester, UK), and Envirotemp FR3 natural ester (NE) produced by Cargil (Minneapolis, MN, USA). The characteristic properties of the electro-insulating liquids used are given in Table 1. Table 2 shows the level of moisture (WCO: Water Content in Oil; and RS: Relative Saturation) and aging parameters (acid number and surface tension) of the investigated liquids.

Table 1.
Some physicochemical and dielectric properties of the electro-insulating liquids used [15].

Table 2.
Moisture levels and acid numbers of the electro-insulating liquids used.
The electro-insulating liquids were modified using fullerene C60 and titanium dioxide TiO2 nanoparticles. The average particle size of fullerene C60 (Sigma-Aldrich, CAS 99685-96-8) was 0.7 nm. In turn, the average size of titanium dioxide TiO2 nanoparticles (Sigma-Aldrich, CAS 13463-67-7, St. Louis, MO, USA) was 21 nm. To produce stable colloids based on titanium dioxide nanoparticles, it was necessary to use a surfactant (monolaurate sorbitan C18H34O6) with the trade name Span® 20 (Sigma-Aldrich, CAS 1338-39-2). The chemical structure of surfactant Span® 20 is shown in Figure 1.
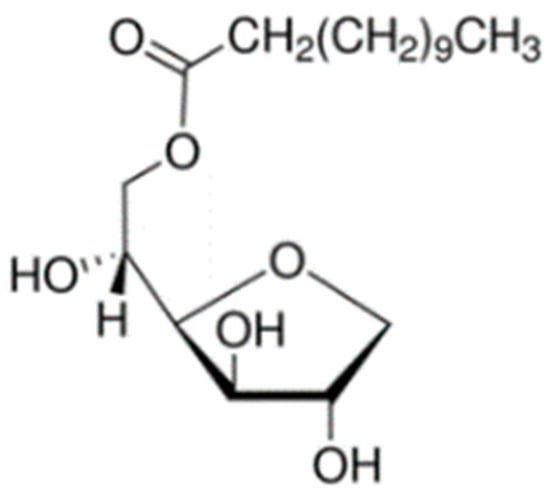
Figure 1.
Chemical structure of surfactant Span® 20.
2.2. Preparation of Nanofluids Based on Fullerene C60
As is known from the literature, one of the main difficulties associated with the preparation of stable electro-insulating nanofluids is the fact that nanoparticles are hard-soluble compounds, or insoluble in base liquids [33,61]. It is difficult to obtain a stable dispersion of nanoparticles in electro-insulating liquids. Therefore, in most cases, a two-step method is used to create nanoliquids [32,38]. In the first stage of this method, the nanofiller is prepared in the form of a dry powder (through the use of chemical or physical methods). Then, in the second stage, the prepared nanofiller is dispersed in the base fluid (e.g., by using mechanical mixing, either ultrasonic or homogenization).
The preparation of stable nanofluids based on mineral oil or esters and fullerene C60 was carried out in accordance with the procedures presented in [62,63]. The addition of fullerene C60 to the base liquid results in the sedimentation and agglomeration of molecules [32]. This, in turn, may cause a reduction or deterioration of selected properties of nanofluids. In order to prevent these phenomena, it is necessary to use mechanical methods to obtain the true solutions [38]. Therefore, electro-insulating liquids after the addition of fullerene C60 were mechanically mixed (for 72 h) and then, as shown by Peppas et al. [47], were placed in an ultrasonic bath. The sonication process was carried out for 5 h at a constant temperature of 60 °C [63,64]. After the sonication process, the prepared samples were allowed to stand for several hours and degassed to remove air bubbles.
The concentration of fullerene C60 in the base liquids was 0.1 g·L−1, which corresponds to the concentrations used to improve the dielectric properties of electro-insulating liquids [65,66]. Figure 2 presents a comparison of prepared samples based on fullerene C60 with samples of base liquids. As can be seen, there is a noticeable change in the color of the electro-insulating liquids as a result of doping with fullerene C60. Mineral oil and natural ester doped with fullerene C60 are characterized by a yellow-brown color, while the synthetic ester is characterized by a violet color. The yellow-brown color is most probably associated with the effect of unsaturated bonding of liquid with fullerene C60, as indicated by the presence of a peak at 433 nm in the UV/VIS spectrum of the analyzed samples (Figure 3). As reported in [67], a peak in the range of 430–450 nm indicates the addition of 1.2 double bonds to the fullerene cage. UV/VIS spectra were taken on a Jasco V-530 apparatus (Jasco Corporation, Tokyo, Japan). As a reference, pure electro-insulating liquids were used.

Figure 2.
Comparison of prepared samples of fullerene C60-based nanofluids with samples of base liquids; from the left: MO—mineral oil, MO + C60—mineral oil doped with fullerene C60, SE—synthetic ester, SE + C60—synthetic ester doped with fullerene C60, NE—natural ester, NE + C60—natural ester doped with fullerene C60.
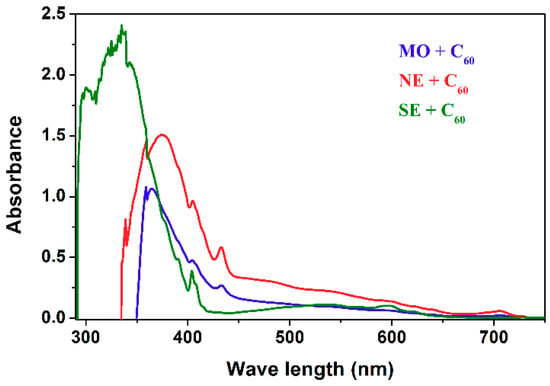
Figure 3.
UV-VIS spectra of dielectric liquids modified by fullerene C60.
2.3. Preparation of Nanofluids Based on Titanium Dioxide TiO2
Titanium dioxide TiO2 nanoparticles belong to the group of nanoparticles which are insoluble in base liquids. Due to the large surface area and high surface activity, they also tend to aggregate to form larger agglomerates [32,39]. Agglomeration of nanoparticles, in addition to the severity of sedimentation, may also reduce the thermal conductivity of nanofluids [68]. Therefore, as in the previous case, it is necessary to use a two-step method to prepare nanofluids based on titanium dioxide TiO2. In addition, a surfactant (dispersant) is also used to reduce the repulsive forces between the nanoparticles and the base liquid [38,69,70]. This facilitates the formation of stable colloids, and also reduces the likelihood of the agglomeration of nanoparticles.
In the process of producing nanofluids based on titanium dioxide TiO2 nanoparticles, several different surfactants are used, such as hexadecyl trimethyl ammonium bromide [71], silane coupling agent [72], nonionic surfactants [73], and oleic acid [74]. In the present study, the low molecular weight surfactant Span® 20 was used to prepare the nanofluids with a titanium dioxide TiO2 nanoparticles. This allows the creation of secondary forces (van der Waals forces, electrostatic, hydrogen bonds) between the applied nanoparticles and the dispersant [75]. The polar groups of the dispersant interact with the high-energy surface of the titanium dioxide nanoparticles, while the long hydrocarbon chains are soluble in electro-insulating liquids; this enables the adsorption and accumulation of the dispersant molecules at the oil–nanoparticles interface, which allow for obtaining a stable dispersion of the nanoparticle in base liquids [64,76]. The base liquids doped with the surfactant were subjected to 3 h of sonication in an ultrasonic bath.
In the next step, titanium dioxide TiO2 nanoparticles were added to the prepared liquids (electro-insulating oils with surfactant). Bearing in mind that the potential of the applied nanoparticles can be released in a situation where they are evenly spread in the base liquids, the prepared nanofluids were subjected to sonication and homogenization. The sonication process was carried out for 5 h at a constant temperature of 60 °C. In turn, the homogenization process, similarly as in [77], was carried out for one hour (24 kHz and 400 W). After this time, stable colloidal solutions were obtained. Similarly to the nanofluids based on fullerene C60, once the sonication process was completed, the prepared samples were allowed to stand for several hours, and degassed to remove air bubbles.
The concentration of surfactant Span® 20 in the base liquids was 5.00 g·L−1. Having concentrations of surfactants which are too high may cause deterioration of the properties of the base liquids [75]. The concentration of titanium dioxide nanoparticles was 0.82 g·L−1. The selected concentrations correspond to the concentrations used to improve the dielectric properties of insulating electro-liquids [49,73]. Figure 4 presents a comparison of prepared samples of nanofluids based on surfactant and TiO2 nanoparticles with samples of base liquids. Similarly to the previous case, there was a noticeable change in the color of the insulating liquids as a result of doping with titanium dioxide nanoparticles.

Figure 4.
Comparison of prepared samples of Span® 20 and titanium dioxide TiO2-based nanofluids with samples of base liquids; from the left: MO—mineral oil, MO + TiO2—mineral oil doped with TiO2, SE—synthetic ester, SE + TiO2—synthetic ester doped with TiO2, NE—natural ester, NE + TiO2—natural ester doped with TiO2.
2.4. Measurement of Thermal Properties of Obtained Nanofluids
Thermal conductivity, specific heat, kinematic viscosity, density, and the thermal expansion coefficient of the investigated electro-insulating nanofluids were analyzed. These properties were determined on the basis of measurements. The thermal conductivity coefficient was examined using a measurement system designed and built by the authors [78]. The specific heat was determined using a Mettler Toledo DSC1 differential scanning calorimeter (Mettler Toledo, Greifensee, Switzerland). The kinematic viscosity of the tested nanofluids was determined according to the standard [79]. The density was determined in accordance with the standard [80], and the thermal expansion coefficient was based on the standard [81]. Tests of all the mentioned properties were carried out at four temperatures: 25, 40, 60, and 80 °C.
2.5. Determination of Heat Transfer in the Analyzed Nanofluids
As previously mentioned, effective cooling is one of the basic functions that must be provided by the electro-insulating liquid. This function is performed by thermal conductivity and convection, which depends on such liquid properties as specific heat, viscosity, density, and thermal expansion.
In order to determine the heat transfer in the analyzed electrical insulating nanofluids, the Newton equation should be used:
where q is heat flux density [W·m−2], α is the heat transfer coefficient [W·m−2·K−1], and ΔT is the temperature drop between the solid surface and liquid [K]. After transformation, the dependence is obtained by:
In order to determine the heat transfer coefficient, the theory of similarity and dimensional analysis is used, which is based on the convergence of physical phenomena with empirical studies. It allows reducing a significant number of properties and parameters determining the heat transfer coefficient.
The heat transfer process by liquid can be determined using the similarity criteria (dimensionless modules). However, one must distinguish between natural and forced convection, since in both cases, the method of determining the heat transfer coefficient varies. In the analyzed case, the considerations were limited to the case of heat transfer with free flow of liquids (natural convection), which is characteristic for most transformers.
In the convective heat transfer analysis, the heat transfer coefficient is determined on the basis of the Nusselt criterion. This criterion combines the movement of heat in the fluid stream with the penetration of heat through the boundary surface. It defines the ratio of the heat transfer rate through convection to the heat transfer rate due to thermal conductivity. This criterion is defined by the dependence:
where δ is the characteristic dimension [m] and λ is the thermal conductivity coefficient [W·m−1·K−1].
Before determining the similarity criteria, it is necessary to determine the quantities that affect the course of free convection. In the case of transformers, heat transport will depend on the temperature difference (ΔT) between the heat transfer surface (winding, core) and the temperature of the electro-insulating liquid. In addition, convection is caused by buoyancy forces, which depend on the difference in density, ρ. In turn, the difference in density results from the temperature and the thermal expansion coefficient β. The gravity acceleration, g also plays an important role, which, together with the difference in density, affects the specific gravity. In the case of transformers, the characteristic height, δ is the height of the heating surface. In addition, when considering the boundary layer, it should be noted that the factor causing its increase is the kinematic viscosity, υ. In the case of heat transfer by convection, the viscosity of the liquid causes the movement of particles in the boundary layer to slow down.
Bearing in mind the values characterizing the phenomenon of convection, the Grashof number can be taken as the criterion of similarity, which determines the ratio of buoyancy forces to the forces of internal friction (viscosity) of the fluid:
Due to the fact that in the analyzed case, heat transport is determined for transformers with natural oil circulation (natural convection), that the heat transfer as a result of taking convection in geometrically convergent systems was an equivalent criterion for Nusselt must be a function of thermal and mechanical similarity numbers. Therefore, assuming a steady flow of heat, this criterion takes the form:
where Pr is the Prandtl number characterizing the similarity of the fluid type. This number is determined by the dependence:
where cp is the specific heat [J·kg−1·K−1].
Taking into account the dimensional analysis and experimental research, the criterion equation for natural convection takes the form:
where c, n are constants depending on the type of heat transfer.
The product of the Grashof and Prandtl numbers is called the Rayleigh number:
The values of c and n constants change with the applicability range of Equation (8).
Taking into account Equations (1)–(8), the temperature decrease can be determined from the dependence:
2.6. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectra Acquisition
A Nexus Nicolet 5700 Fourier Transform Infrared Spectrophotometer (Thermo Electron Scientific Instruments Corporation, Madison, WI, USA) equipped with an attenuated total reflection (ATR) accessory with a ZnSe crystal was used to take infrared spectra of investigated samples. All spectra, both for pure electro-insulating liquids and modified liquids (with the addition of nanoparticles and/or surfactant) were recorded at room temperature (25 °C) in the range of 4000–600 cm−1. The resolution was 4 cm−1 at 64 scans. Before every scan of an investigated sample, a background scan (reference air spectrum) was measured. After every measurement of an oil sample, ATR crystal was cleaned thoroughly with tissue soaked in chloroform. All spectra were treated using spectral software Omnic (Thermo Electron Scientific Instruments Corporation).
3. Results and Discussion
3.1. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Measurements
The typical ATR-FTIR spectra for pure mineral and ester oils are shown in Figure 5. Also, the FTIR spectra of pure and modified liquids with two types of nanofillers, C60 and TiO2, are presented in Figure 6. Additionally, spectra of liquids modified with surfactant Span® 20 are shown.
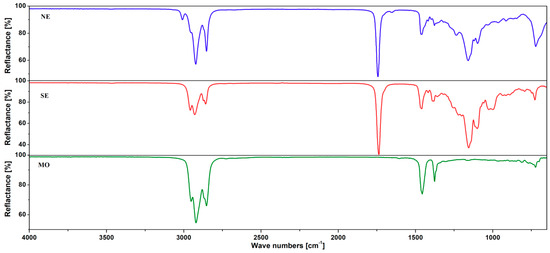
Figure 5.
Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectra of investigated dielectric liquids at room temperature (25 °C).
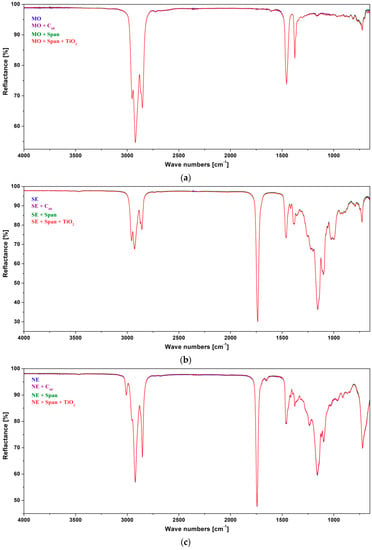
Figure 6.
ATR-FTIR spectra of investigated base dielectric liquids and dielectric liquids modified with C60, TiO2, surfactant Span, or Span with TiO2 at room temperature: (a) Mineral oil; (b) synthetic ester; (c) natural ester.
The infrared spectra of investigated liquids (Figure 5) contain fundamental and characteristic bands whose frequencies and intensities can determine the relevant functional groups in the investigated liquids [82,83,84].
Two of the studied liquids were esters (synthetic and natural esters), and absorption bands attributed to vibrations of esters were present at their spectra. Spectral bands in the regions of 1770–1715 cm−1 (C=O stretching vibration of the ester carbonyl functional groups of the triglycerides), 1300–1100 cm−1 (C–O stretching vibration of ester groups) and a peak with a maximum around 3460 cm−1 (overtone of C=O of ester group) are attributed to vibrations of esters. Bands derived from aliphatic hydrocarbons vibrations were present in the spectra, too: asymmetric stretching vibration of C–H of aliphatic –CH3 groups due to the alkyl rest of triglycerides (2953 cm−1 in NE oil and 2958 cm−1 in SE oil), C–H asymmetric stretching vibration of the aliphatic CH2 group (2923 cm−1 and 2929 cm−1 for NE and SE oil, respectively), and C–H symmetric stretching vibration of the aliphatic CH2 group (2858 cm−1 and 2853 cm−1 for NE and SE oil, respectively), C–H bending vibration of CH2 and CH3 aliphatic groups (1466 cm−1 and 1459 cm−1 for NE and SE oil), C–H bending symmetric vibration of CH2 groups (1397 cm−1 and 1389 cm−1 for NE and SE oil), and overlapping of C-H rocking vibration of CH2 and the out-of-plane vibration of the cis –HC=CH– group of disubstituted olefins (722 cm−1 and 727 cm−1 for NE and SE oil). In addition, the NE ester is an unsaturated ester, as evidenced by the presence of absorption bands with a maximum at 3009 cm−1 (=C–H stretching symmetric vibration of the cis double bonds) and 1655 cm−1 (C=C stretching vibration of cis-disubstituted olefins, RHC=CHR) on its spectrum.
Mineral oils are high-boiling fractions obtained from the refining of crude oil. These oils mainly contain compounds with a ring structure, i.e., that which are unsaturated-aromatic and saturated-naphthenic, which may have additional saturated side-chains—paraffins. Depending on the predominance of a given structure, they are properly classified as aromatic, naphthenic, or paraffinic. The straight-chain paraffinic components are normal-alkanes, whereas the naphthenic group constitutes of cycloalkanes. Aromatics contain at least one ring of six-carbon atoms, with alternating single and double bonds [85]. On the mineral oil spectrum (Figure 5), absorption bands from saturated hydrocarbons are observed: 2952, 2921, 2853, 1456, and 1376 cm−1, which indicates a naphthenic or paraffinic oil.
The introduction of nanofillers (C60, TiO2) or surfactant (Span® 20) did not cause any changes in the spectra of investigated liquids; the intensity or location of the bands of characteristic oil or modifier functional groups did not change. This result suggests the lack of interactions between the investigated liquids and the introduced modifiers.
3.2. Thermal Properties of Electro-Insulating Liquids Modified by Fullerene C60
Table 3 presents the measured values of thermal properties of the analyzed electro-insulating liquids and nanofluids based on fullerene C60. On the basis of the presented measurement results, it can be stated that doping the liquids with fullerene C60 did not cause any changes in thermal conductivity, density, and thermal expansion. Synthetic ester and mineral oil have a similar value of specific heat, while natural ester has a higher value of specific heat. It is most probably associated with the presence of unsaturated bonds.

Table 3.
Thermal properties of pure electro-insulating liquids and nanofluids based on fullerene C60 [62,63,64].
The addition of fullerene caused an increase in the specific heat of all nanofluids. This increase is due to the presence of hydrophobic bonds between fullerene C60 and hydrocarbon molecules contained in the analyzed electro-insulating liquids [64]. This also explains the obtaining of appropriate stable solutions on the basis of fullerene C60 and the analyzed liquids. Hydrophobic bonds are important from the point of view of particle absorption on the surface of carbon nanotubes [86]. These bonds are so strong that, despite the small concentration of fullerene in the analyzed liquids (0.1 g·L−1) and more than twice the lower values of specific heat [87,88,89], they manage to increase the specific heat of analyzed nanofluids.
A minimal increase in kinematic viscosity of the nanofluids based on mineral oil and synthetic ester was also visible. This increase may negatively affect the heat transfer coefficient, which will consequently affect the cooling effectiveness of the device. Other researchers also observed the negative influence of nanoparticles on the viscosity of the analyzed nanofluids [59].
Table 4 presents the values of the criterion numbers of the analyzed liquids calculated on the basis of Equations (4), (6), and (7), which correspond to the numbers of Grashof, Prandtl, and Nusselt, respectively.

Table 4.
Calculation results of the Grashof, Prandtl, and Nusselt numbers of pure electro-insulating liquids and nanofluids based on fullerene C60.
As mentioned earlier, the Grashof number expresses the ratio of the buoyancy force to the fluid viscosity. This number defines the boundary between laminar and turbulent flow during natural convection. It is assumed that this limit occurs for around 109. As can be seen from the presented calculation results, for a temperature range of 40–80 °C and for both mineral oil and mineral oil modified with fullerene C60, it is possible to have turbulent flows. On the other hand, in the case of synthetic ester, the occurrence of turbulent flows is possible in the temperature range of 60–80 °C. For the other analyzed insulating liquids (synthetic ester modified by C60, natural ester, and natural ester modified by C60), turbulent flow will probably occur at temperatures above 80 °C. In the case of C60 fullerene nanofluids, the occurrence of turbulent flows can also have its advantages—these flows are accompanied by a mixing phenomenon, which can have a positive effect on the uniform dispersion of fullerene in the base liquid. The modification of the liquids with fullerene C60 does not significantly change the value of the Grashof number.
The Prandtl number expresses the ratio of the viscosity of the fluid to its thermal conductivity. A high value of the Prandtl number indicates that the convection is dominant in the spread of heat in the liquid medium, whereas a low Prandtl value means that heat transport mainly occurs through thermal conductivity at a molecular level. In the case of the analyzed liquids, it can be seen that modification with fullerene C60 did not cause significant changes in the value of the Prandtl number. On the basis of the obtained results, it can only be stated that in comparison with pure and modified mineral oil, and in the case of pure and modified esters, convection plays a greater role in heat transport. The greater role of convection in the case of esters and nanofluids is mainly due to their higher viscosity and density.
The Nusselt number expresses the ratio of the heat transfer rate due to convection of the heat transfer rate, which is caused by thermal conductivity at the fluid boundary and the heat transfer surface. The closer the Nusselt number is to a value equal to 1, the heat transfer in the fluid (at the border of the centers) is more conducive to conduction. In turn, the higher the Nusselt number is, the higher the heat transfer takes place by convection. Analyzing the data in Table 4, it can be seen that the highest value of the Nusselt number is characterized by pure mineral oil and mineral oil modified with fullerene C60. In turn, the lowest value of the Nusselt number is characterized by pure natural ester and natural ester modified with fullerene C60. This means that in the case of mineral oil, the transfer of heat in the boundary layer will take place to a greater extent by convection. On the other hand, in the case of esters, the transfer in the thermal conductivity will have greater significance in this layer. This is mainly due to the higher thermal conductivity of the esters compared to mineral oil. Modification of electro-insulating liquids with fullerene C60 does not significantly change the Nusselt number.
Table 5 presents the results of calculations of temperature decrease in pure electro-insulating liquids and nanofluids based on fullerene C60. The temperature decrease was determined based on Equation (9). Analyzing the calculated temperature decreases, it can be concluded that the modification of electro-insulating liquids with fullerene C60 does not significantly affect the heat transport in the obtained nanofluids. Bearing in mind that fullerene C60 is mainly used to improve dielectric properties [65,66] and to absorb free radicals and to slow the acidification of vegetable oils [50,51], it can be concluded that it does not impair the cooling efficiency of the device.

Table 5.
Calculation results of the temperature decrease in pure electro-insulating liquids and nanofluids based on fullerene C60.
3.3. Thermal Properties of Electro-Insulating Liquids Modified by Surfactant Span® 20 and Titanium Dioxide (TiO2)
Table 6 presents measured values of the thermal properties of analyzed electro-insulating liquids and nanofluids based on surfactant Span® 20 and titanium dioxide TiO2 nanoparticles.

Table 6.
Thermal properties of pure electro-insulating liquids and nanofluids based on surfactant Span® 20 and titanium dioxide (TiO2) [62,63,64].
In order to eliminate the influence of surfactant on the thermal properties of the resulting nanofluids, the results of the measurements of thermal properties of base liquids modified with surfactant are also presented. Similarly to the previous case, it was noticed that the modification of electro-insulating liquids with a surfactant did not cause changes in thermal conductivity, density, or thermal expansion. The increase in kinematic viscosity was clearly observable, which was due to the higher viscosity of the surfactant compared to the viscosity of the analyzed electro-insulating liquids. On the other hand, when analyzing the results of specific heat measurements, it can be noticed that in the case of mineral oil and synthetic ester, the modification of the surfactant caused the increase of the specific heat. On the other hand, in the case of a natural ester modified with a surfactant, the specific heat decreased.
When analyzing the effect of modifying electro-insulating liquids with titanium dioxide TiO2 nanoparticles, an increase in the thermal conductivity of the generated nanofluids relative to the base liquids can be observed. Other researchers also observed such results [53]. The increase in the thermal conductivity of the resulting nanofluids should be related to the high thermal conductivity of titanium dioxide TiO2 nanoparticles (approximately 22 W·m−1·K−1) [64].
Taking into account the results of measurements of specific heat of electro-insulating liquids modified with TiO2 nanoparticles, it can be noticed that in the case of mineral oil and natural ester, the value of specific heat in relation to the base liquids was reduced. On the other hand, in the case of a synthetic ester, an increase in the value of specific heat relative to the base liquid is noticeable. The obtained results may be related to the size of the nanoparticles. According to literature [90], the smaller the particle size is, the specific heat is higher. The obtained results suggest that in the case of a synthetic ester, a dispersion containing the smallest-sized particles was obtained. This effect is attributed to the surface energy, distortion, and lattice energy of the nanoparticles [91].
Based on the obtained results, it was found that modifying the analyzed liquids with nanoparticles of titanium dioxide increases their viscosity in relation to the base liquids. The increase in viscosity is directly related to the addition of solid materials to the liquid. As in the previous case, this may result in a deterioration of the cooling efficiency.
There was no influence of modification of base liquids with TiO2 nanoparticles on their density and thermal expansion.
Table 7 presents the calculated values based on Equations (4), (6), and (7) of the criterion numbers of the analyzed liquids.

Table 7.
Calculation results of Grashof, Prandtl, and Nusselt numbers of pure electro-insulating liquids and nanofluids based on surfactant Span® 20 and titanium dioxide (TiO2).
As can be seen from the calculations, for temperatures in the range of 40–80 °C, for both mineral oils modified with surfactant and mineral oils modified with surfactant and titanium dioxide TiO2 nanoparticles, turbulent flows are possible. In turn, for all ester-derived nanofluids analyzed (both synthetic and natural), turbulent flow may occur at a temperature of 80 °C. As in the previous case, the turbulent nature of the flow can positively influence the dispersion of titanium dioxide TiO2 nanoparticles in the analyzed nanofluids. Modification of electro-insulating liquids with surfactant Span® 20 and titanium dioxide TiO2 nanoparticles does not significantly change the value of the Grashof number.
In the case of the analyzed liquids, it can be noticed that modifying the mineral oil and the synthetic ester with the surfactant Span® 20 causes a minimal increase in the Prandtl number. In turn, the addition of titanium dioxide TiO2 nanoparticles to the resulting liquids decreases the value of this criterion number. In the case of a natural ester, it can be seen that modifying with dispersant causes a reduction in the value of the Prandtl number. The addition of titanium dioxide TiO2 nanoparticles further enhances this change. On the basis of the obtained results, it can only be stated that in the case of mineral oil and synthetic ester modified with titanium dioxide TiO2 nanoparticles, as in the case of base fluids, convection plays a greater role in heat transport. On the other hand, in the case of natural ester modified with nanoparticles of titanium dioxide, the role of thermal conductivity in heat transport has increased.
Table 8 presents the results of calculations of the temperature drop between pure electro-insulating liquids and nanofluids based on surfactant Span® 20 and titanium dioxide TiO2. The temperature decrease was determined based on Equation (9). Analyzing the calculated temperature decreases, it can be concluded that the modification of electro-insulating liquids with surfactant Span® 20 and titanium dioxide TiO2 nanoparticles does not significantly affect the heat transport in the obtained nanofluids. Bearing in mind that these nanoparticles are mainly used to improve dielectric properties [41,46,49], it can be concluded that they will not cause deterioration in the cooling efficiency of the device.

Table 8.
Calculation results of temperature decrease in pure electro-insulating liquids and nanofluids based on surfactant Span® 20 and titanium dioxide (TiO2).
Analyzing the effect of modifying electro-insulating liquids with surfactant Span® 20 on the Nusselt number value, it can be noticed that for all the analyzed liquids, there are no changes in the value of this criterion number. The decrease in the Nusselt number is observable only after the base liquids have been modified with titanium dioxide TiO2 nanoparticles. This means that, compared to base fluids, the heat transfer in the boundary layer will occur to a greater extent in the thermal conductivity of the analyzed nanofluids. This is due to the greater thermal conductivity of the resulting nanoliquids compared to the base liquids.
4. Conclusions
The doping of electro-insulating liquids (mineral oil, and synthetic and natural esters) with analyzed nanoparticles (C60, TiO2) did not in any way affect the heat exchange capacity of the obtained nanofluids. This is a very important conclusion in the context of doping electro-insulating liquids with nanoparticles to improve their other properties, such as electro-insulating properties (breakdown voltage, electrical resistivity, electrical permittivity) or aging (dielectric loss factor, the ability to absorb water, release of sludge and sediments).
The modification of electro-insulating liquids with fullerene C60 did not cause changes in thermal conductivity, density, and thermal expansion. The addition of fullerene C60 caused an increase in kinematic viscosity, which may adversely affect the heat exchange capacity of the obtained nanoliquids. The increase in viscosity was compensated by the increase in specific heat. As a consequence, the heat exchange capacity of insulating liquids did not change due to the addition of nanoparticles to them.
The modification of electro-insulating liquids with titanium dioxide TiO2 nanoparticles (along with the surfactant) did not cause changes in density and thermal expansion. The addition of TiO2, on the other hand, resulted in an increase in the kinematic viscosity of all liquids and a decrease in specific heat (with the exception of synthetic ester), which may have had a negative impact on the heat exchange capacity of the obtained nanofluids. The increase in viscosity and the decrease in specific heat have been balanced out by the increase in thermal conductivity. In conclusion, the heat exchange capacity of liquids did not change due to the doping with nanoparticles.
We recommend that future research concerning the use of electro-insulating nanofluids in electrical power equipment should be conducted in two directions.
The first of these is the analysis of the impact of other nanoparticles, used sporadically to improve dielectric properties of electro-insulating liquids on their thermal properties. Such nanoparticles include nanodiamonds (NDs), which improve thermal conductivity [57], and boron nitride nanoparticles [58].
The second direction is the use of well-recognized and widely used nanoparticles in electro-insulating liquids, which significantly improve the thermal properties of other liquids. As it is commonly known, Al2O3 or CuO nanoparticles improve the thermal conductivity of water [52]. In turn, in [53], the beneficial effect of Cu and SiO2 nanoparticles on the thermal conductivity of other liquids has been proved. It should therefore be assumed that these nanoparticles also improve the thermal properties of insulating liquids, such as mineral oil or synthetic and natural esters. Therefore, it would be necessary to check how the mentioned nanoparticles affect the dielectric properties of the resulting nanoliquids. Thus, this is the opposite approach in relation to that described in this paper.
Author Contributions
Section 1 was prepared by G.D. Used materials (Section 2.1) were described by G.D. and Z.N. Preparation of nanofluids based on fullerene C60 (Section 2.2) and nanofluids based on titanium dioxide TiO2 (Section 2.3) were described by G.D. and A.M. Measurements of thermal properties of obtained nanofluids (Section 2.4) and determination on heat transfer in analyzed nanofluids (Section 2.5) were described by G.D. Section 2.5 regarding Attenuated Total Reflection_Fourier Transform Infrared Spectra Acquisition was prepared by A.M. Attenuated Total Reflection_Fourier Transform Infrared results were described in Section 3.1 by A.M. The measurements of thermal properties (Section 3.2 and Section 3.3) were carried out by G.D. Thermal properties of electro-insulating liquids modified by fullerene C60 and titanium dioxide were described jointly by all the authors. The temperature decreases in pure electro-insulating liquids and obtained nanofluids was calculated by Z.N. G.D., Z.N., and A.M. conducted the experiment. Z.N. prepared conclusions.
Funding
This research was funded by Ministry of Science and Higher Education, grant number 04/41/DSPB/4350.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ortiz, A.; Delgado, F.; Ortiz, F.; Fernandez, I.; Santisteban, A. The aging impact on the cooling capacity of a natural ester used in power transformers. Appl. Therm. Eng. 2018, 144, 797–803. [Google Scholar] [CrossRef]
- Ariffin, M.M.; Ishak, M.T.; Hamid, M.H.A.; Katim, N.J.A.; Ishak, A.M.; Azis, N. Ageing effect of vegetable oils impregnated paper in transformer application. In Proceedings of the IEEE International Conference on High Voltage Engineering and Power System (ICHVEPS), Sanur, Indonesia, 2–5 October 2017; pp. 183–187. [Google Scholar]
- Oommen, T.V. Vegetable oils for liquid-filled transformers. IEEE Electr. Insul. Mag. 2002, 18, 6–11. [Google Scholar] [CrossRef]
- Khelfane, I.; Debche, A.; Nacer, A.; Beldjilali, A.; Toudja, T.; Moulai, H. Moisture and electrical discharges effect on naphthenic mineral oil properties. IET Sci. Meas. Technol. 2014, 8, 588–594. [Google Scholar] [CrossRef]
- Przybylek, P. The influence of temperature and aging of cellulose on water distribution in oil-paper insulation. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 552–556. [Google Scholar] [CrossRef]
- Hohlein, I.; Kachler, A.J. Aging of cellulose at transformer service temperatures. Part 2. Influence of moisture and temperature on degree of polymerization and formation of furanic compounds in free-breathing systems. IEEE Electr. Insul. Mag. 2005, 21, 20–24. [Google Scholar] [CrossRef]
- Fernandez, O.H.A.; Fofana, I.; Jalbert, J.; Gagnon, S.; Rodriguez-Celis, E.; Duchesne, S.; Ryadi, M. Aging characterization of electrical insulation papers impregnated with synthetic ester and mineral oil: Correlations between mechanical properties, depolymerization and some chemical markers. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 217–227. [Google Scholar] [CrossRef]
- Dombek, G.; Nadolny, Z.; Przybyłek, P. The study of thermal properties of mineral oil and synthetic ester modified by nanoparticles TiO2 and C60. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application (ICHVE), Poznan, Poland, 8–11 September 2014; pp. 1–4. [Google Scholar]
- Lelekakis, N.; Guo, W.; Martin, D.; Wijaya, J.; Susa, D. A field study of aging in paper-oil insulation systems. IEEE Electr. Insul. Mag. 2012, 28, 12–19. [Google Scholar] [CrossRef]
- Sikorski, W.; Szymczak, C.; Siodla, K.; Polak, F. Hilbert curve fractal antenna for detection and on-line monitoring of partial discharges in power transformers. Eksploat. Niezawodn. 2018, 20, 343–351. [Google Scholar] [CrossRef]
- Dombek, G.; Goscinski, P.; Nadolny, Z. Comparison of mineral oil and esters as cooling liquids in high voltage transformer in aspect of environment protection. E3S Web. Conf. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Makmud, M.Z.H.; Illias, H.A.; Chee, C.Y.; Sarjadi, M.S. Influence of conductive and semi-conductive nanoparticles on the dielectric response of natural ester-based nanofluid insulation. Energies 2018, 11, 333. [Google Scholar] [CrossRef]
- Hamadi, A.; Fofana, I.; Djillali, M. Stability of mineral oil and oil-ester mixtures under thermal aging and electrical discharges. IET Gener. Transm. Distrib. 2017, 11, 2384–2392. [Google Scholar] [CrossRef]
- Toudja, T.; Chetibi, F.; Beldjilali, A.; Moulai, H.; Neroual, A. Electrical and physiochemical properties of mineral and vegetable oils mixtures. In Proceedings of the IEEE 18th International Conference on Dielectric Liquids (ICDL), Bled, Slovenia, 29 June–3 July 2014; pp. 1–4. [Google Scholar]
- Experiences in Service with New Insulating Liquids; Cigré Technical Brochure 436; International Council on Large Electric Systems (CIGRE): Paris, France, 2010.
- Malinowski, G.; Moranda, H.; Siodla, K. Dependence of creeping partial discharge activity in a synthetic esters-paper insulating system on the temperature. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application (ICHVE), Poznan, Poland, 8–11 September 2014; pp. 1–3. [Google Scholar]
- McShane, C. New safety dielectric coolants for distribution and power transformers. IEEE Ind. Appl. Mag. 2000, 6, 24–32. [Google Scholar] [CrossRef]
- Yang, L.J.; Liao, R.J.; Sun, C.X.; Zhu, M.Z. Influence of vegetable oil on the thermal aging of transformer paper and its mechanism. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 692–700. [Google Scholar] [CrossRef]
- Li, J.; Yao, S.H.; Du, B.; Yao, W. Analysis to principle problems and future prospect of research on vegetable insulating oils and their applications. High Volt. Eng. 2015, 2, 353–363. [Google Scholar]
- Perrier, C.; Beroual, A. Experimental investigations on mineral and ester oils for power transformers. In Proceedings of the IEEE International Symposium on Electrical Insulation, Vancouver, BC, Canada, 18 July 2008; pp. 178–181. [Google Scholar]
- Fofana, I. 50 years in the development of insulating liquids. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- Stockton, D.P.; Bland, J.R.; McClanahan, T.; Wilson, J.; Harris, D.L.; McShane, P. Natural ester transformer fluids: Safety, reliability & environmental performance. In Proceedings of the IEEE Petroleum and Chemical Industry Technical Conference, Calgary, AB, Canada, 17–19 September 2007; pp. 1–7. [Google Scholar]
- Shinde, R. Condition monitoring of a retro-filled power transformer by natural ester Envirotemp FR3 fluid. In Proceedings of the 3rd International Conference on Condition Assessment Techniques in Electrical Systems (CATCON), Rupnagar, India, 16–18 November 2017; pp. 265–269. [Google Scholar]
- Mazzaro, M.; De Bartolomeo, D.; Bemporad, E.; Beradi, S.; Ledda, A.; Calcara, L.; Pompili, M.; Falconi, M.; Vecchio, A.; Scatiggio, F.; et al. Power transformer fire and environmental risk reduction by using natural esters. In Proceedings of the IEEE 19th International Conference on Dielectric Liquid (ICDL), Manchester, UK, 25–29 June 2017; pp. 1–4. [Google Scholar]
- Mehta, D.M.; Kundu, P.; Chowdhury, A.; Lakhiani, V.K.; Jhala, A.S. A review on critical evaluation of natural ester vis-a-vis mineral oil insulating liquid for use in transformers: Part 1. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 873–880. [Google Scholar] [CrossRef]
- Mehta, D.M.; Kundu, P.; Chowdhury, A.; Lakhiani, V.K.; Jhala, A.S. A review on critical evaluation of natural ester vis-a-vis mineral oil insulating liquid for use in transformers: Part II. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1705–1712. [Google Scholar] [CrossRef]
- Bertrand, Y.; Lauzevis, P. Development of a low viscosity insulating liquid based on natural ester for distribution transformers. In Proceedings of the 22nd International Conference and Exhibition on Electricity Distribution (CIRED), Stockholm, Sweden, 10–13 June 2013; pp. 1–4. [Google Scholar]
- Dombek, G.; Gielniak, J.; Wroblewski, R. Fire safety and electrical properties of mineral oil/synthetic ester mixtures. In Proceedings of the IEEE International Symposium on Electrical Insulating Materials (ISEIM), Toyohashi, Japan, 11–15 September 2017; pp. 227–230. [Google Scholar]
- Rozga, P.; Stanek, M.; Pasternak, B. Characteristics of negative streamer development in ester liquids and mineral oil in a point-to-sphere electrode system with a pressboard barrier. Energies 2018, 11, 1088. [Google Scholar] [CrossRef]
- Pukel, G.J.; Fleck, G.; Pregartner, H.; Fritsche, R. Safe and environmentally friendly large power transformers with ester successful introduction of ester liquids at the 420 kV transmission level. In Proceedings of the IEEE Electrical Insulation Conference (EIC), Montreal, QC, Canada, 19–22 August 2016; pp. 134–137. [Google Scholar]
- Saidur, R.; Leong, K.Y.; Mohammad, H.A. A review on applications and challenges of nanofludis. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A review of nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 1–17. [Google Scholar] [CrossRef]
- Ali, H.M.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.; Javed, S. Preparation techniques of TiO2 nanofluids and challenges: A review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the ASME International Mechanical Engineering Congress Exposition, San Francisco, CA, USA, 12–17 November 1995; pp. 99–105. [Google Scholar]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Kang, K.; Cho, Y.; Yu, K.J. Novel nano-materials and nano-fabrication techniques for flexible electronic systems. Micromachines 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Kantaria, T.; Kantaria, T.; Kabauri, S.; Ksovreli, M.; Kachlishvili, T.; Kulikova, N.; Tugushi, D.; Katsarava, R. Biodegradable nanoparticles made of amino-acid-based ester polymers: Preparation, characterization, and in vitro biocompatibility study. Appl. Sci. 2016, 6, 444. [Google Scholar] [CrossRef]
- Primo, V.A.; Garcia, B.; Albarracin, R. Improvement of transformer liquid insulation using nanodielectric fluids: A review. IEEE Electr. Insul. Mag. 2018, 34, 13–26. [Google Scholar] [CrossRef]
- Lv, Y.; Zhou, Y.; Li, C.; Wang, Q.; Qi, B. Recent progress in nanofluids based on transformer oil: Preparation and electrical insulation properties. IEEE Electr. Insul. Mag. 2014, 30, 23–32. [Google Scholar] [CrossRef]
- Lv, Y.; Ge, Y.; Wang, L.; Sun, L.; Zhou, Y.; Huag, M.; Li, C.; Yuan, J.; Qi, B. Effects of nanoparticle materials on prebreakdown and breakdown properties of transformer oil. Appl. Sci. 2018, 8, 601. [Google Scholar] [CrossRef]
- Du, Y.F.; Lv, Y.Z.; Zhou, J.Q.; Li, X.X.; Li, C.R. Breakdown properties of transformer oil-based TiO2 nanofluid. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), West Lafayette, IN, USA, 17–20 October 2010; pp. 1–4. [Google Scholar]
- Jin, H.; Andritsch, T.; Morshuis, P.H.F.; Smit, J.J. AC breakdown voltage and viscosity of mineral oil based SiO2 nanofluids. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Montreal, QC, Canada, 14–17 December 2012; pp. 1–4. [Google Scholar]
- Nazari, M.; Rasoulifard, M.H.; Hosseini, H. Dielectric breakdown strength of magnetic nanofluids based on insulation oil after impulse test. J. Magn. Magn. Mater. 2016, 399, 1–4. [Google Scholar] [CrossRef]
- Velasco, J.; Frascella, R.; Albarracin, R.; Burgos, J.C.; Dong, M.; Ren, M.; Yang, L. Comparison of positive streamers in liquid dielectrics with and without nanoparticles simulated with finite-element software. Energies 2018, 11, 361. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Huang, B.; Hao, J.; Chen, G. Review of research progress on the electrical properties and modification of mineral insulating oils used in power transformers. Energies 2018, 11, 487. [Google Scholar] [CrossRef]
- Mentlik, V.; Trnka, P.; Hornak, J.; Totzauer, P. Development of a biodegradable electro-insulating liquid and its subsequent modification by nanoparticles. Energies 2018, 11, 508. [Google Scholar] [CrossRef]
- Peppas, G.D.; Bakandritsos, A.; Charalampakos, V.P.; Pyrgioti, E.C.; Tucek, J.; Zboril, R.; Gonos, I.F. Ultrastable natural ester-based nanofluids for high voltage insulation applications. ASC Appl. Mater. Interfaces 2016, 8, 25202–25209. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, Y.; Yang, Y.; Zhang, L.; Jin, F. Characterization of high performance AlN nanoparticle-based transformer oil nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2757–2767. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zou, P.; Grzybowski, S.; Zahn, M. Preparation of a vegetable oil-based nanofluid and investigation of its breakdown and dielectric properties. IEEE Electr. Insul. Mag. 2012, 28, 43–50. [Google Scholar] [CrossRef]
- Kadish, K.M.; Ruoff, R.S. Fullerenes: Chemistry, Physics and Technology, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000; pp. 467–479. ISBN 0-471-29089-0. [Google Scholar]
- Zeynalov, D.B.; Allen, N.S.; Salmanova, N.I. Radical scavenging efficiency of different fullerenes C60-C70 and fullerene soot. Polym. Degrad. Stabil. 2009, 94, 1183–1189. [Google Scholar] [CrossRef]
- Ding, Y.; Alias, H.; Wen, D.; Williams, R.A. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int. J. Heat Mass Transf. 2006, 49, 240–250. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. J. Heat Transf. 2003, 125, 567–574. [Google Scholar] [CrossRef]
- Ozerinc, S.; Kakac, S.; Yazicioglu, A.G. Enhanced thermal conductivity of nanofluids: A state-of-the-art review. Microfluid. Nanofluid. 2010, 8, 145–170. [Google Scholar] [CrossRef]
- Kristiawan, B.; Santoso, B.; Wijayanta, A.T.; Aziz, M.; Miyazaki, T. Heat transfer enhancement of TiO2/water nanofluids at laminar and turbulent flows: A numerical approach for evaluating the effect of nanoparticle loadings. Energies 2018, 11, 1584. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S.U.S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Du, B.; Zhang, Z. Study on the stability and viscosity of Fe3O4 nano-particles vegetable insulating oils. In Proceedings of the International Conference on High Voltage Engineering and Application, Shanghai, China, 17–20 August 2012; pp. 307–310. [Google Scholar]
- Shukla, G.; Aiyer, H. Thermal conductivity enhancement of transformer oil using functionalized nanodiamonds. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2185–2190. [Google Scholar] [CrossRef]
- Du, B.X.; Li, X.L.; Li, J.; Tao, X.Y. Effects of BN nanoparticles on thermal conductivity and breakdown strength of vegetable oil. In Proceedings of the IEEE 11th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Sydney, Australia, 19–22 July 2015; pp. 476–479. [Google Scholar]
- Raymon, A.; Sakthibalan, S.; Cinthal, C.; Subramaniaraja, R.; Yuvaraj, M. Enhancement and comparison of nano-ester insulating fluids. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 892–900. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Mohammed, H.A.; Alawi, O.A.; Samion, S.A. A review on preparation methods and challenges of nanofluids. Int. Commun. Heat Mass Transf. 2014, 54, 115–125. [Google Scholar] [CrossRef]
- Nadolny, Z.; Dombek, G.; Przybylek, P.; Przadka, D. Thermal properties of mineral oil admixed with C60 and TiO2 nanoparticles. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Toronto, ON, Canada, 16–19 October 2016; pp. 538–541. [Google Scholar]
- Dombek, G.; Nadolny, Z.; Przybylek, P. Cooling properties of natural ester modified by nanopowders fullerene C60 and TiO2 for high voltage insulation applications. In Proceedings of the IEEE International Symposium on Electrical Insulating Materials (ISEIM), Toyohashi, Japan, 11–15 September 2017; pp. 442–445. [Google Scholar]
- Nadolny, Z.; Dombek, G. Electro-insulating nanofluids based on synthetic ester and TiO2 or C60 nanoparticles in power transformer. Energies 2018, 11, 1953. [Google Scholar] [CrossRef]
- Aksamit, P.; Zmarzly, D.; Boczar, T. Electrostatic properties of aged fullerene-doped mineral oil. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1459–1462. [Google Scholar] [CrossRef]
- Aksamit, P.; Zmarzly, D. Dielectric properties of fullerene-doped insulating liquids. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Virginia Beach, VA, USA, 18–21 October 2009; pp. 212–215. [Google Scholar]
- Cataldo, F.; Braun, F. The solubility of C60 fullerene in long chain fatty acids esters. Fuller. Nanotub. Carbon. Nanostruct. 2007, 15, 331–339. [Google Scholar] [CrossRef]
- Parameshawaran, R.; Kalaiselvam, M. Effect of aggregation on thermal conductivity and heat transfer in hybrid nanocomposite phase change colloidal suspensions. Appl. Phys. Lett. 2013, 103, 193113. [Google Scholar] [CrossRef]
- Lv, Y.Z.; Zhang, S.N.; Du, Y.F.; Chen, T.M.; Li, C.R. Effect of oleic acid surface modification on dispersibility of TiO2 nanoparticles in transformer oils. J. Inorg. Mater. 2013, 28, 1–5. [Google Scholar] [CrossRef]
- Viali, W.R.; Alcantara, G.A.; Sartoratto, P.P.C.; Soler, M.A.G.; Mosiniewicz-Szablewska, E.; Andrzejewski, B.; Morais, P.C. Investigation of the molecular surface coating on the stability of insulating magnetic oils. J. Phys. Chem. C 2010, 114, 179–188. [Google Scholar] [CrossRef]
- Du, B.; Li, J.; Wang, B.M.; Zhang, Z.T. Preparation and breakdown strength of Fe3O4 nanofluid based on transformer oil. In Proceedings of the International Conference on High Voltage Engineering and Application (ICHVE), Shanghai, China, 17–20 November 2012; pp. 311–313. [Google Scholar]
- Miao, J.; Dong, M.; Shen, L.P. A modified electrical conductivity model for insulating oil-based nanofluids. In Proceedings of the IEEE International Conference on Condition Monitoring and Diagnosis, Bali, Indonesia, 23–27 September 2012; pp. 1126–1129. [Google Scholar]
- Chiesa, M.; Das, S.K. Experimental investigation of the dielectric and cooling performance of colloidal suspensions in insulating media. Colloids Surf. A 2009, 335, 88–97. [Google Scholar] [CrossRef]
- Pislaru-Danescu, L.; Morega, A.M.; Telipan, G.; Morega, M.; Dumitru, J.B.; Marinescu, V. Magnetic nanofluids applications in electrical engineering. IEEE Trans. Magn. 2013, 49, 5489–5497. [Google Scholar] [CrossRef]
- Andritsch, T.; Fabiani, D.; Vazquez, I.R. Nanodielectric—Examples of preparation and microstructure. IEEE Electr. Insul. Mag. 2013, 29, 21–28. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 235–271. ISBN 978-0-470-54194-4. [Google Scholar]
- Kayhani, M.H.; Soltanzadeh, H.; Heyhat, M.M.; Nazari, M.; Kowsary, F. Experimental study of convective heat transfer and pressure drop of TiO2/water nanofluids. Int. Commun. Heat Mass Transf. 2012, 39, 456–462. [Google Scholar] [CrossRef]
- Dombek, G.; Nadolny, Z.; Przybylek, P. Investigation of parameters which have influence on the ability of heat transport of electro-insulating liquids. Prz. Elektrotech. 2014, 10, 148–151. (In Polish) [Google Scholar]
- International Organization of Standardization (ISO). ISO 3104:1994: Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity; International Organization of Standardization (ISO): Geneva, Switzerland, 1994. [Google Scholar]
- International Organization of Standardization (ISO). ISO 3675:1998: Crude Petroleum and Liquid Petroleum Products—Laboratory Determination of Density—Hydrometer Method; International Organization of Standardization (ISO): Geneva, Switzerland, 1998. [Google Scholar]
- American Society for Testing and Materials (ASTM). ASTM D 1903-96: Standard Test Method for Coefficient of Thermal Expansion of Electrical Insulating Liquids of Petroleum Origin, and Askarels; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 1996. [Google Scholar]
- Poiana, M.A.; Alexa, E.; Munteanu, M.F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Gomez, N.A.; Abonia, R.; Cadavid, H.; Vargas, I.H. Chemical and spectroscopic characterization of a vegetable oil used as dielectric coolant in distribution transformers. J. Braz. Chem. Soc. 2011, 22, 2292–2303. [Google Scholar] [CrossRef]
- Valchos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573–574, 459–465. [Google Scholar] [CrossRef]
- Al-Eshaikh, M.A.; Qureshi, M.I. Evaluation of food grade corn oil for electrical applications. Int. J. Green Energy 2012, 9, 441–455. [Google Scholar] [CrossRef]
- Kragulj, M.; Trickovic, J.; Dalmacija, B.; Kukovecz, A.; Konya, Z.; Molnar, J.; Roncevic, S. Molecular interactions between organic compounds and functionally modified multiwalled carbon nanotubes. Chem. Eng. J. 2013, 225, 144–152. [Google Scholar] [CrossRef]
- Allen, K.; Hellman, F. Specific heat of endohedral and higher fullerene thin films. J. Chem. Phys. 1999, 111, 5291–5294. [Google Scholar] [CrossRef]
- Olson, J.R.; Topp, K.A.; Pohl, R.O. Specific-heat and thermal-conductivity of solid fullerenes. Science 1993, 259, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.P.; Silotia, P.; Bera, K. Effect of cubic and planar collective and localized modes on the specific heat of C60 fullerite for 0.2≤T≤300 K. Solid State Commun. 1998, 107, 129–133. [Google Scholar] [CrossRef]
- Saeedian, M.; Mahjour-Shafiei, M.; Shojaee, E.; Mohammadizedeh, M.R. Specific heat capacity of TiO2 nanoparticles. J. Comput. Theor. Nanosci. 2012, 9, 616–620. [Google Scholar] [CrossRef]
- Xin-Ming, W.; Lan, W.; Zhi-Cheng, T.; Guang-Hai, L.; Song-Sheng, Q. Preparation, characterization, and low-temperature heat capacities of nanocrystalline TiO2 ultrafine powder. J. Solid State Chem. 2001, 156, 220–224. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).