Effect of Hydrophobicity on the Self-Assembly Behavior of Urea Benzene Derivatives in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of UBD-1–3
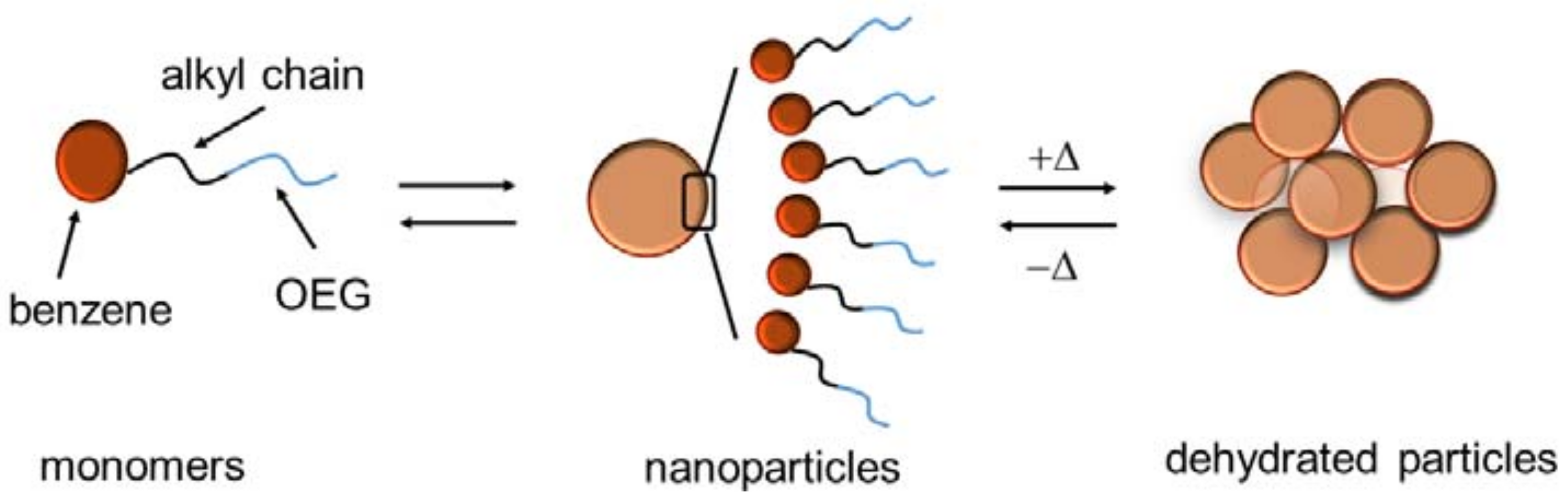
2.2. Self-Assembly Behavior in Aqueous Solution
2.2.1. 1H NMR spectra in D2O
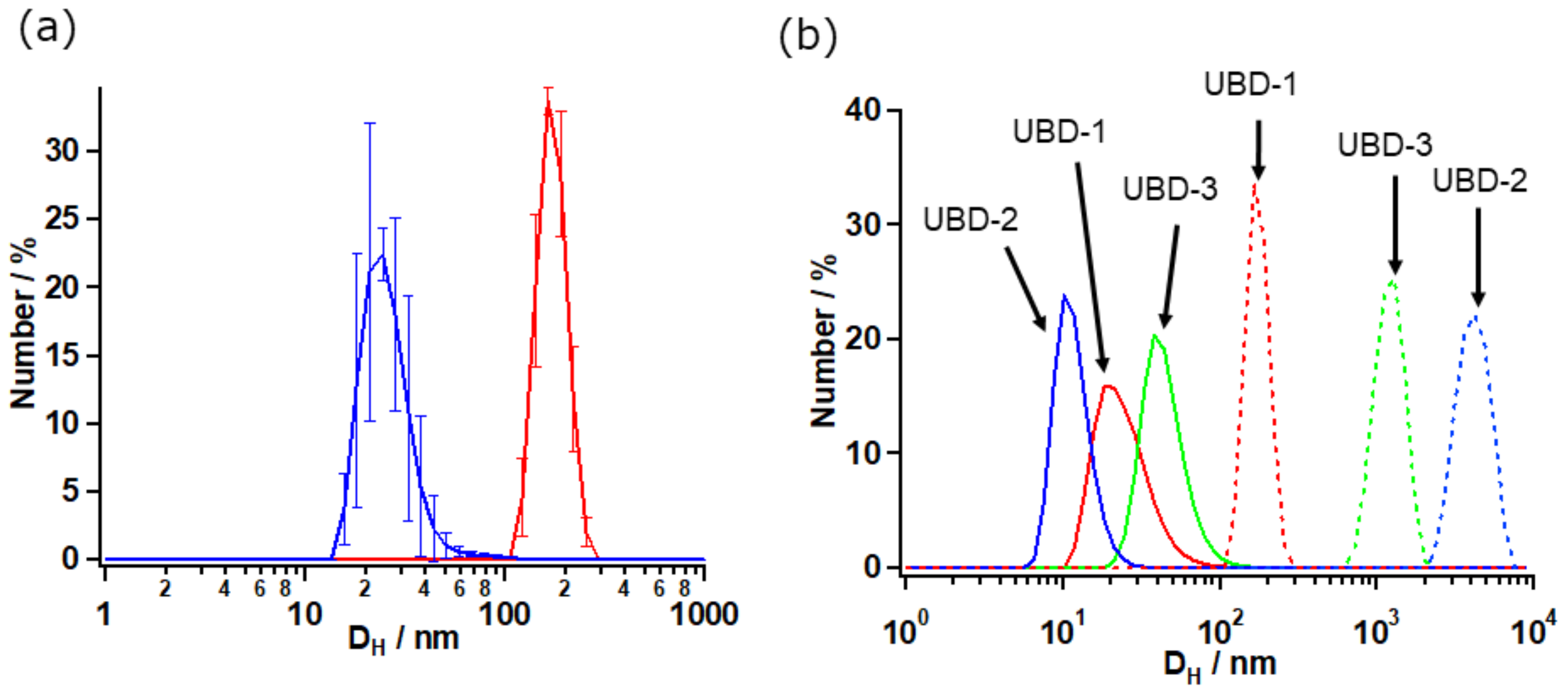
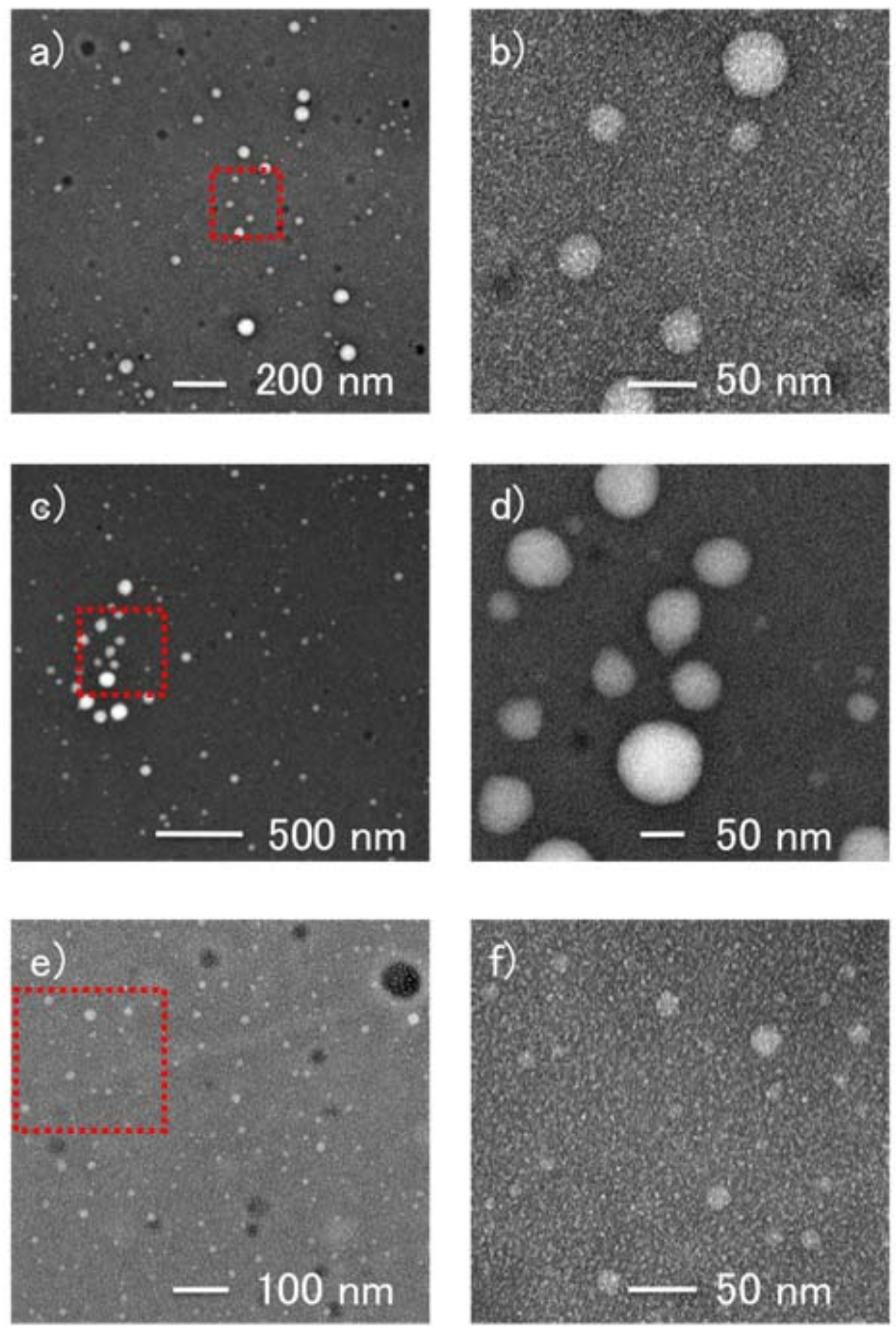
2.2.2. Size Distribution and Morphology of UBD-1–3 by Dynamic Light Scattering (DLS) and Transmission Electron Microscopy (TEM)
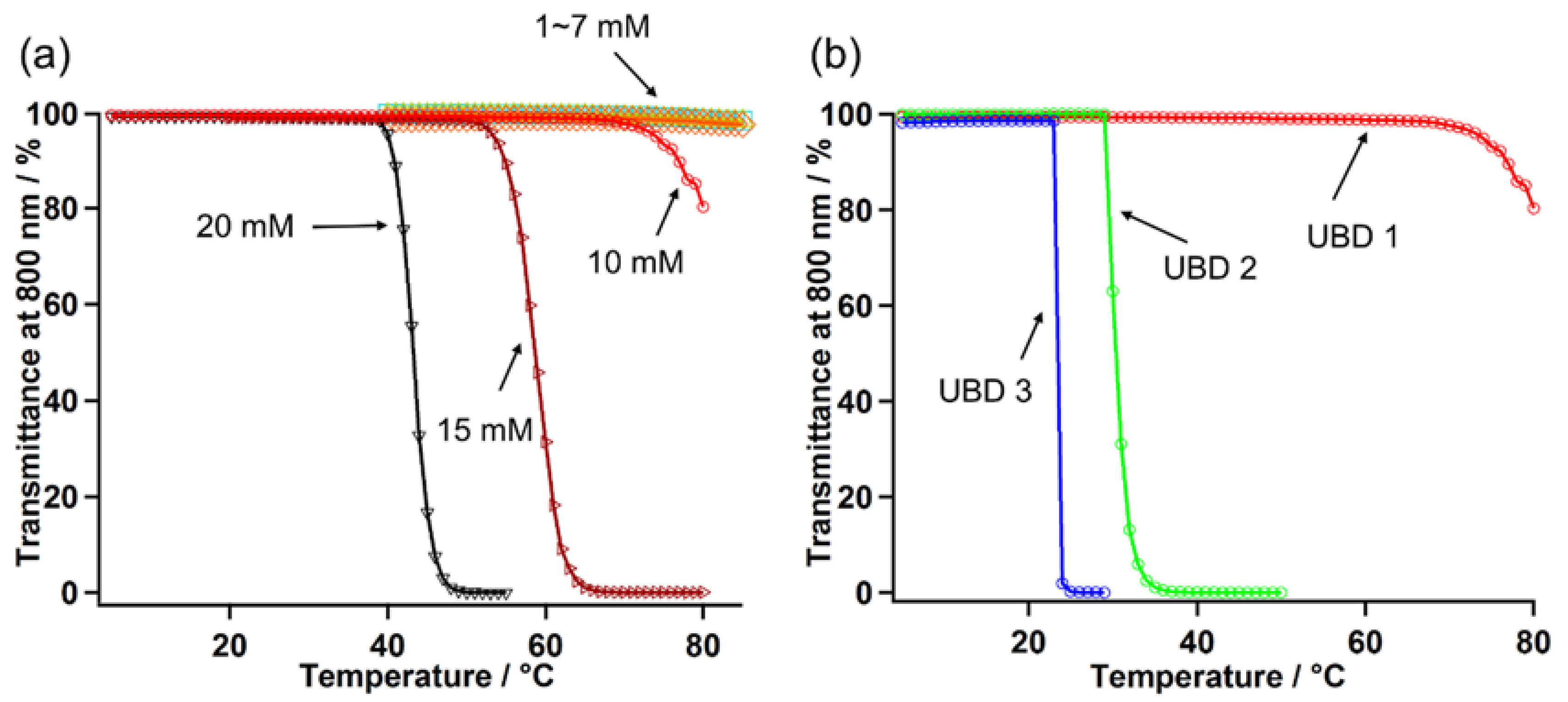
2.2.3. Additional Heat-Induced Self-Assembly
3. Materials and Methods
3.1. Experimental
3.2. Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moro, T.; Takatori, Y.; Ishihara, K.; Konno, T.; Takigawa, Y.; Matsushiba, T.; Chung, U.I.; Nakamura, K.; Kawaguchi, H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat. Mater. 2004, 3, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Aoki, D.; Ajiro, D. Design of Polyurethane Composed of Only Hard Main Chain with Oligo(ethylene glycol) Units as Side Chain Simultaneously Achieved High Biocompatible and Mechanical Properties. Macromolecules 2017, 50, 6529–6538. [Google Scholar] [CrossRef]
- Fujiyabu, T.; Li, X.; Shibayama, M.; Chung, U.; Sakai, T. Permeation of Water through Hydrogels with Controlled Network Structure. Macromolecules 2017, 50, 9411–9416. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, S.; Kang, E.; Kim, K.; Choi, K.; Kwon, I.C. New Generation of Multifunctional Nanoparticles for Cancer Imaging and Therapy. Adv. Funct. Mater. 2009, 19, 1553–1566. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987, 6, 559–593. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar] [PubMed]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and properties of chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Hayashi, H.; Ohkubo, K.; Karasawa, S.; Koga, N. Assemblies of Functional Small-Sized Molecules Having 4-Amio-2,2,6,6-tetramethylpiperidine-1-oxyl Responsive to Heat and pH in Water and Their Water Proton Relaxivities. Langmuir 2011, 27, 12709–12719. [Google Scholar] [CrossRef] [PubMed]
- Morishita, K.; Murayama, S.; Araki, T.; Aoki, I.; Karasawa, S. Thermal- and pH-Dependent Size Variable Radical Nanoparticles and Its Water Proton Relaxivity for Metal-Free MRI Functional Contrast Agents. J. Org. Chem. 2016, 81, 8351–8362. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Karasawa, S.; Koga, N. Crystal Structures and Emitting Properties of Trifluoromethylaminoquinoline Derivatives: Thermal Single-Crystal-to-Single-Crystal Transformation of Polymorphic Crystals That Emit Different Colors. Chem. Eur. J. 2012, 18, 15038–15048. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, S.; Todo, J.; Usui, K.; Harada, N.; Yoza, K.; Suemune, H.; Koga, N. Regioselective Photocyclizations of Di(quinolinyl)arylamines and Tri(quinolinyl)amine with Emission Color Changes and Photoreaction-induced Self-assemblies. Chem. Eur. J. 2016, 22, 7771–7781. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, S.; Hagihara, R.; Abe, Y.; Harada, N.; Todo, J.; Koga, N. Structures, Thermal Properties, and Emission Behaviors of N,N-R-Phenyl-7-amino-2,4-trifluoromethylquinoline Derivatives: Supercooled Liquid-to-Crystal Transformation Induced by Mechanical Stimuli. Cryst. Growth Des. 2014, 14, 2468–2478. [Google Scholar] [CrossRef]
- Harada, N.; Abe, Y.; Karasawa, S.; Koga, N. Polymorphic Equilibrium Responsive Thermal and Mechanical Stimuli in Light-emitting Crystals of N-Methylaminonaphthyridine. Org. Lett. 2012, 14, 6282–6285. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, R.; Usui, K.; Karasawa, S. Two-step transformation of p-anisolylaminoquinoline derivatives induced by conformation- and packing-dominated processes. Dyes Pigments 2017, 143, 401–408. [Google Scholar] [CrossRef]
- Araki, T.; Murayama, S.; Usui, K.; Shimada, T.; Aoki, I.; Karasawa, S. Self-Assembly Behavior of Emissive Urea Benzene Derivatives Enables Heat-Induced Accumulation in Tumor Tissue. Nano Lett. 2017, 17, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Allampally, N.A.; Florian, A.; Mayoral, M.A.; Rest, C.; Stepanenko, V.; Fernández, G. H-Aggregates of Oligophenyleneethynylene (OPE)-BODIPY Systems in Water: Guest Size-Dependent Encapsulation Mechanism and Co-aggregate Morphology. Chem. Eur. J. 2014, 20, 10669–10678. [Google Scholar] [CrossRef] [PubMed]
- Rödle, A.; Lambov, M.; Mück-Lichtenfeld, C.; Stepanenko, V.; Fernández, G. Cooperative nanoparticle H-type self-assembly of a bolaamphiphilic BODIPY derivative in aqueous medium. Polymer 2017, 128, 317–324. [Google Scholar] [CrossRef]
- Fielding, L.A.; Lane, J.A.; Derry, M.J.; Mykhaylyk, O.O.; Armes, S.P. Thermo-responsive Diblock Copolymer Worm Gels in Non-polar Solvents. J. Am. Chem. Soc. 2014, 136, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, J.J.; Xing, L.; Tang, N.; Cuccia, L.A. Design and synthesis of urea-linked aromatic oligomers—A route towards convoluted foldamers. Chem. Eur. J. 2009, 15, 10030–10038. [Google Scholar] [CrossRef] [PubMed]
- Aroua, S.; Gabrielle, E.; Tiu, V.; Ishikawa, T.; Yamakoshi, Y. Well-Defined Amphiphilic C60-PEG Conjugates: Water-Soluble and Thermoresponsive Materials. Helv. Chim. Acta 2016, 99, 805–813. [Google Scholar] [CrossRef]
- Matsuura, H.; Miyazawa, T. Vibrational Analysis of Molten Poly(ethylene glycol). J. Polym. Sci. 1969, 7, 1735–1769. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, S.; Fan, W. Zhao, Y. Ultrasensitive pH-Induced Water Solubility Switch Using UCST Polymers. Macromolecules 2016, 49, 1424–1433. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S. Hoogenboom, R. Thermoresponsive Polymers with Llower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Wyss, H.M.; Sijbesma, R.P. Effects of Surfactant and Urea on Dynamics and Viscoelastic Properties of Hydrophobically Assembled Supramolecular Hydrogel. Macromolecules 2018. [Google Scholar] [CrossRef]
- Besson, E.; Mehdi, A.; Reyé, C.; Gaveau, P.; Corriu, R.J.P. Self-assembly of layered organosilicas based on weak intermolecular interactions. Dalton Trans. 2010, 39, 7534–7539. [Google Scholar] [CrossRef] [PubMed]
- Morishita, K.; Okamoto, Y.; Murayama, S.; Usui, K.; Aoki, I.; Hirai, G.; Karasawa, S. Water-Proton Relaxivities of Radical Nanoparticles Self-Assembled via Hydration or Dehydration Processes. Langmuir 2017, 33, 7810–7817. [Google Scholar] [CrossRef] [PubMed]


| UBD-1 | UBD-2 | UBD-3 | |
|---|---|---|---|
| CAC | |||
| 16 mM | 9 mM | 2 mM | |
| Concentration/mM | LCST | ||
| 20 | 38 °C | 26 °C | - |
| 15 | 51 °C | - | 23 °C |
| 10 | 71 °C | 29 °C | - |
| 7 | >80 °C | - | 23 °C |
| 5 | >80 °C | 57 °C | - |
| 3 | - | >80 °C | 27 °C |
| 1 | >80 °C | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, Y.; Morishita, K.; Fuchi, Y.; Kobayashi, S.; Karasawa, S. Effect of Hydrophobicity on the Self-Assembly Behavior of Urea Benzene Derivatives in Aqueous Solution. Appl. Sci. 2018, 8, 1080. https://doi.org/10.3390/app8071080
Okamoto Y, Morishita K, Fuchi Y, Kobayashi S, Karasawa S. Effect of Hydrophobicity on the Self-Assembly Behavior of Urea Benzene Derivatives in Aqueous Solution. Applied Sciences. 2018; 8(7):1080. https://doi.org/10.3390/app8071080
Chicago/Turabian StyleOkamoto, Yuna, Kosuke Morishita, Yasufumi Fuchi, Shigeki Kobayashi, and Satoru Karasawa. 2018. "Effect of Hydrophobicity on the Self-Assembly Behavior of Urea Benzene Derivatives in Aqueous Solution" Applied Sciences 8, no. 7: 1080. https://doi.org/10.3390/app8071080
APA StyleOkamoto, Y., Morishita, K., Fuchi, Y., Kobayashi, S., & Karasawa, S. (2018). Effect of Hydrophobicity on the Self-Assembly Behavior of Urea Benzene Derivatives in Aqueous Solution. Applied Sciences, 8(7), 1080. https://doi.org/10.3390/app8071080





