Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented S. maxima Fermentation
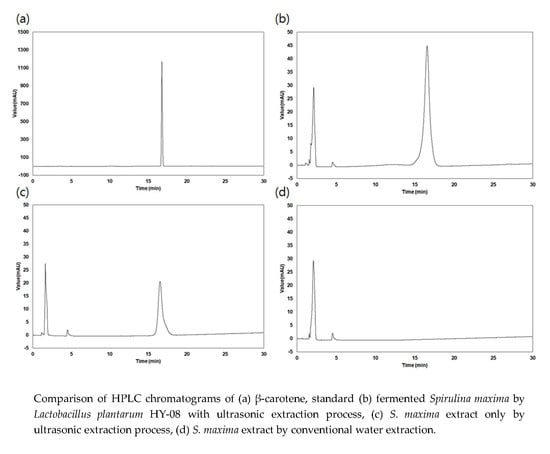
2.2. Determination of β-Carotene Content in the Extracts
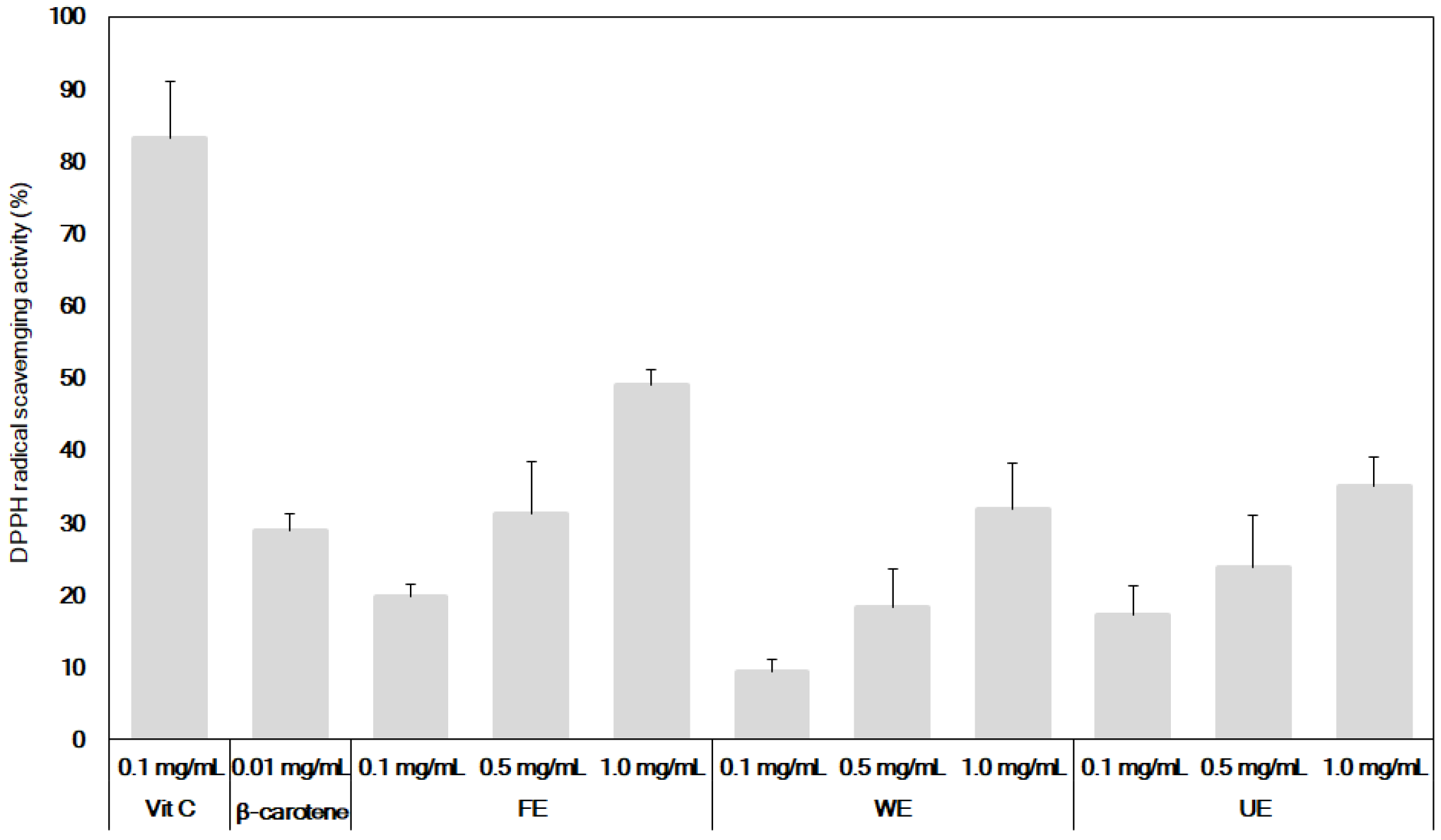
2.3. Measurement of the DPPH Free Radical-Scavenging Activity of the Extracts
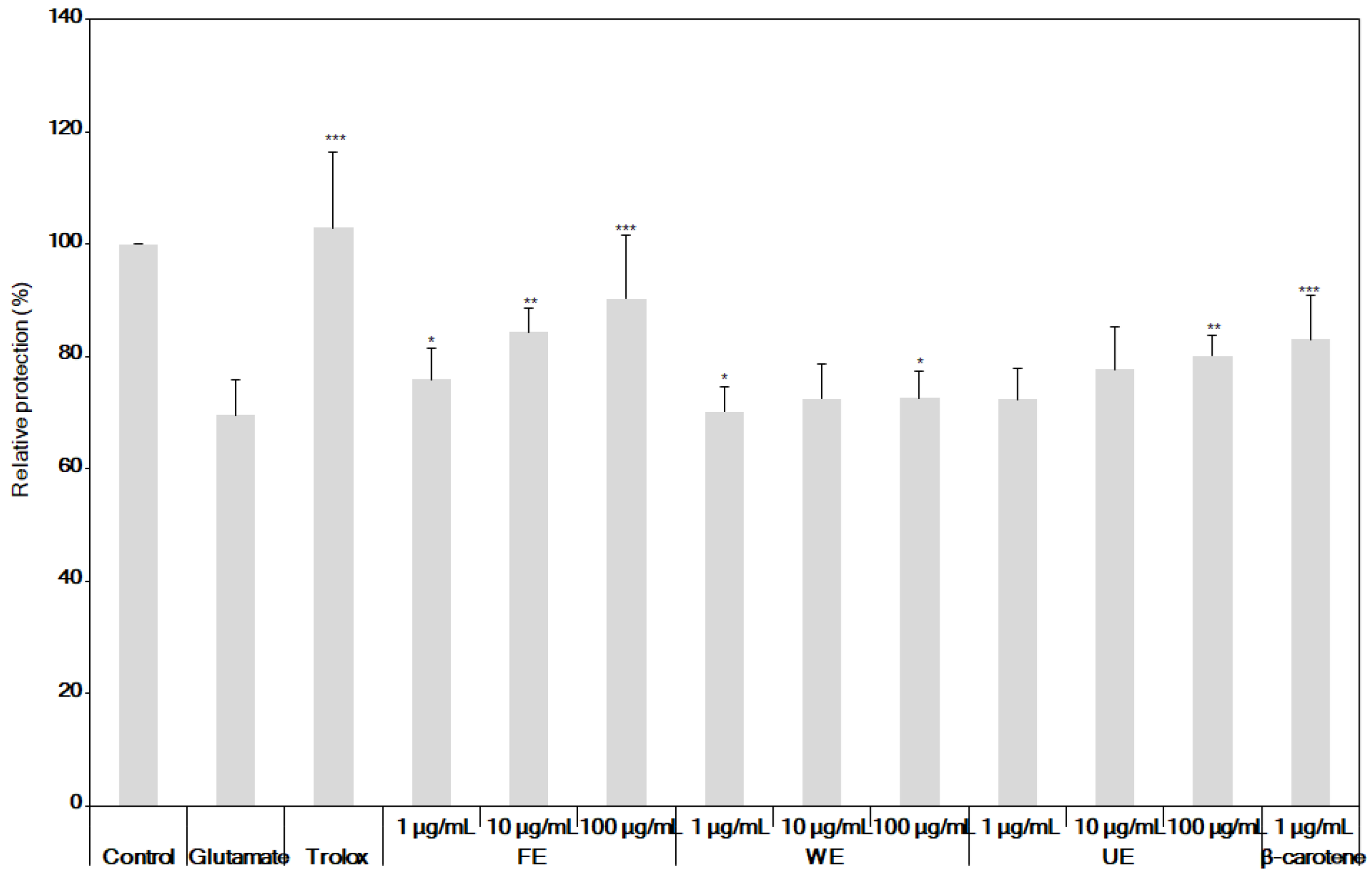
2.4. Measurement of the Neuroprotective Activity of the Extracts
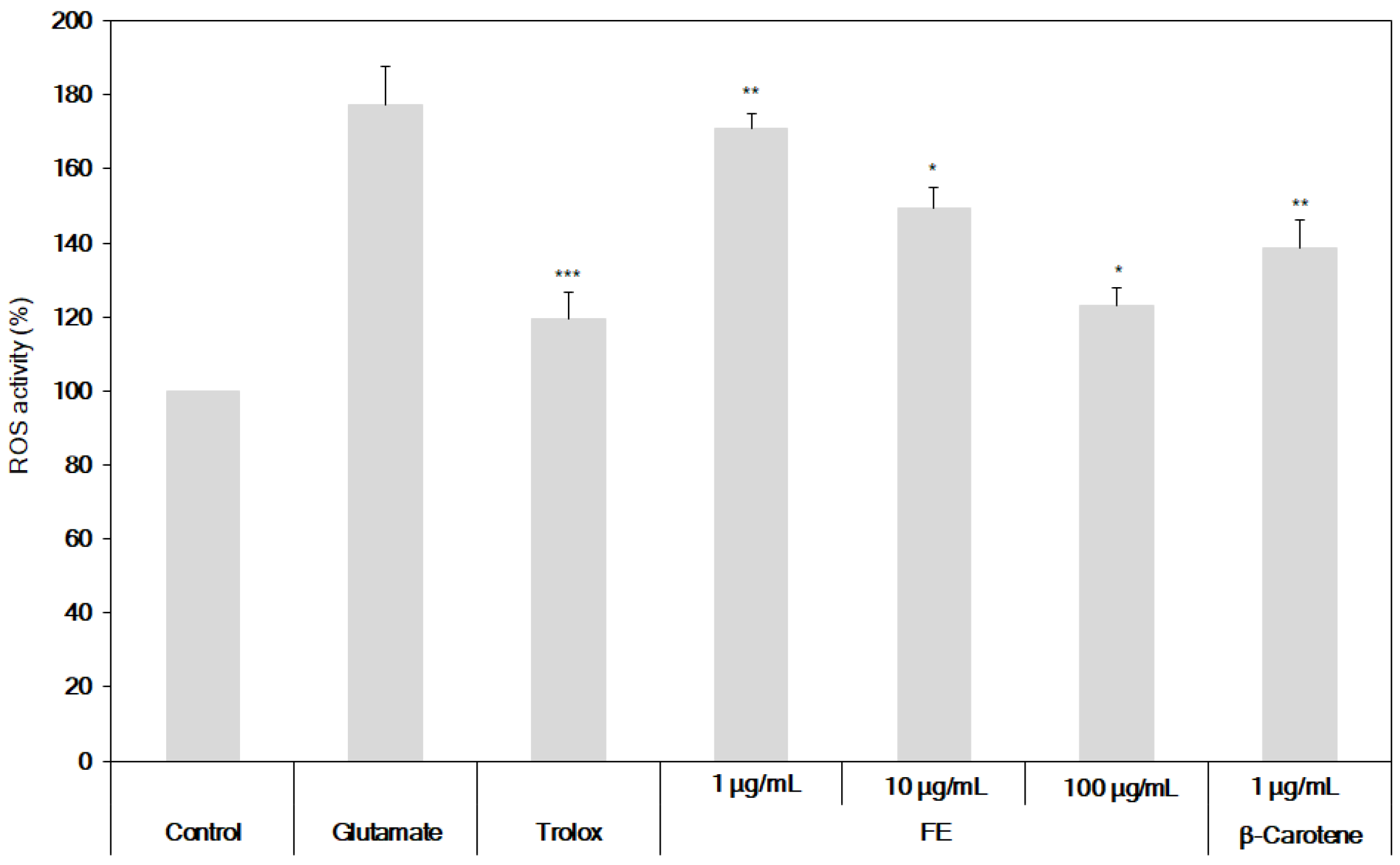
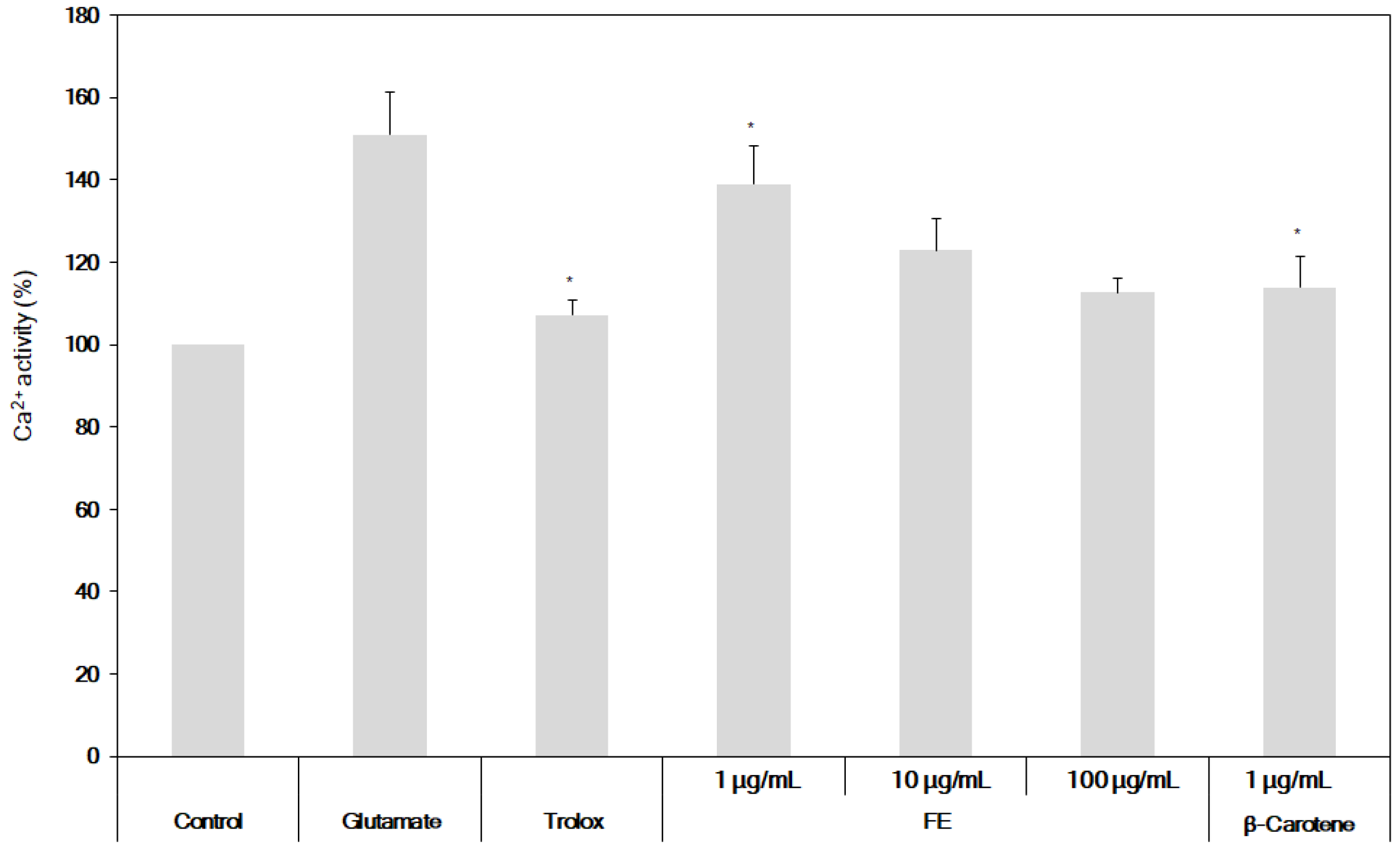
2.5. Determination of the Inhibition of ROS and Ca2+ Production from HT22 Cells
2.6. Measurement of Glutathione Peroxidase and Glutathione Reductase Activities via Treatment of the Extracts
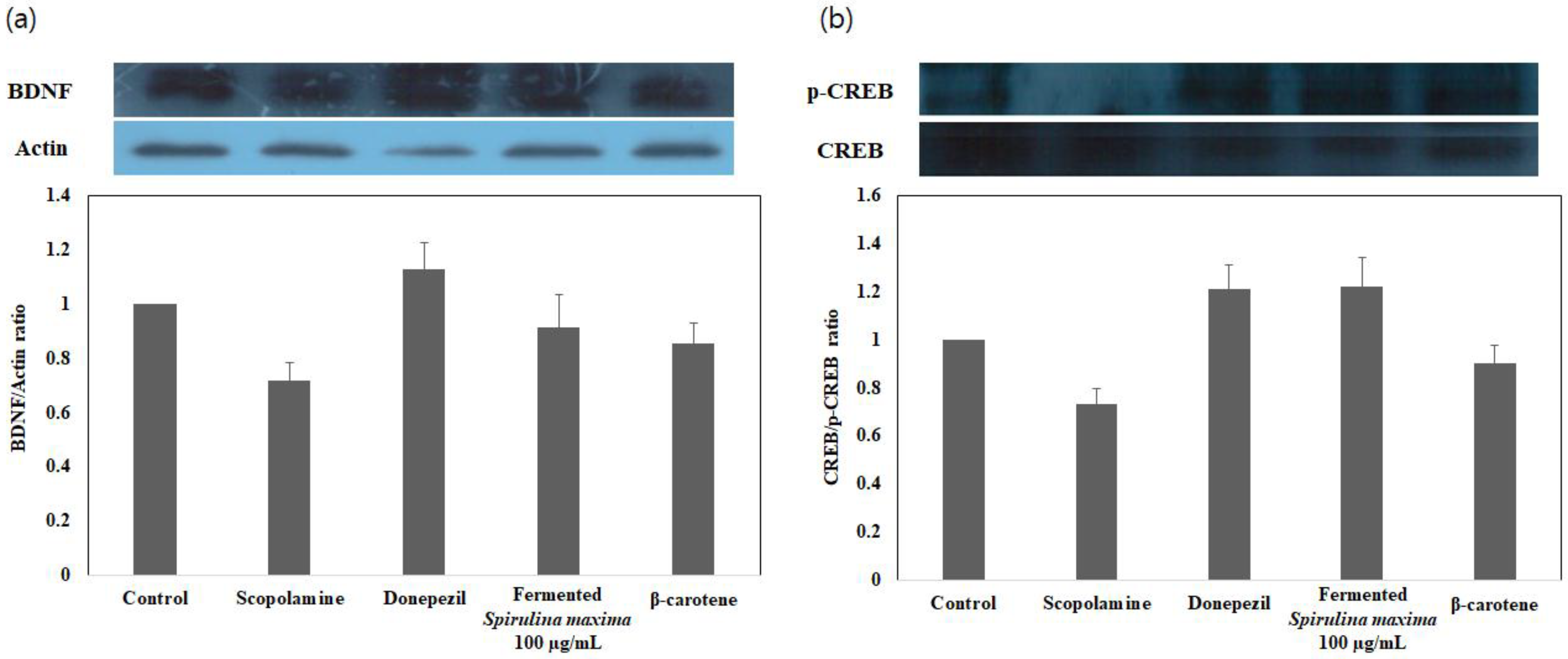
2.7. Western Blot Analysis of BDNF (Brain-Derived Neurotrophic Factor) and p-CREB (cAMP-Responsive Element-Binding Protein) Gene Expression Induced by the Extract
2.8. Statistical Analysis
3. Results
3.1. Extraction Yield and β-Carotene Content of the Extracts
3.2. Comparison of Antioxidant and Neuroprotective Activities of β-Carotene and the Fermented S. maxima Extract
3.3. Neuroprotective Effects of the Fermented S. maxima Extract against Brain Oxidative Stress
3.4. Enhancement of BDNF/p-CREB Signaling Pathways in Glutamate-Induced, Oxidatively Stressed HT22 Cells with the Fermented S. maxima Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Citron, M. Alzheimer’s disease: Treatments in discovery and development. Nat. Neurosci. 2002, 5, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.R.; Iversen, S.D. The effects of novel cholinesterase inhibitors and selective muscarinic receptor agonists in tests of reference and working memory. Behav. Brain Res. 1993, 57, 143–153. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the β-amyloid peptide. J. Alzheimers Dis. 2010, 19, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Hannula, M.J.; Myöhänen, T.T.; Tenorio-Laranga, J.; Männistö, P.T.; Garcia-Horsman, J.A. Prolyl oligopeptidase colocalizes with α-synuclein, β-amyloid, tau protein and astroglia in the post-mortem brain samples with Parkinson’s and Alzheimer’s diseases. Neuroscience 2013, 242, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Toide, K.; Shinoda, M.; Fujiwara, T.; Iwamoto, Y. Effect of a novel prolyl endopeptidase inhibitor, JTP-4819, on spatial memory and central cholinergic neurons in aged rats. Pharmacol. Biochem. Behav. 1997, 56, 427–434. [Google Scholar] [CrossRef]
- Fukunari, A.; Kato, A.; Sakai, Y.; Yoshimoto, T.; Ishiura, S.; Suzuki, K.; Nakajima, T. Colocalization of prolyl endopeptidase and amyloid beta-peptide in brains of senescence-accelerated mouse. Neurosci. Lett. 1994, 176, 201–204. [Google Scholar] [CrossRef]
- Takeda, A.; Loveman, E.; Clegg, A.; Kirby, J.; Picot, J.; Payne, E.; Green, C. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Rodda, J.; Morgan, S.; Walker, Z. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer’s disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int. Psychogeriatr. 2009, 21, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Kim, D.H.; Moon, Y.S.; Jung, J.S.; Ahn, E.M.; Baek, N.I.; Song, D.K. Protection against β-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 25–30. [Google Scholar] [CrossRef]
- Oken, B.S.; Storzbach, D.M.; Kaye, J.A. The Efficacy of Ginkgo biloba on cognitive function in Alzheimer Disease. Arch. Neurol. 1998, 55, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.B.; Lee, B.; Yun, B.R.; Lee, J.; Lee, H.Y.; Park, D.S.; Chung, H.C.; Chung, J.Y.; Ma, C.J. Memory enhancing effect of Codonopsis lanceolata by high hydrostatic pressure process and fermentation. Korean J. Pharmacogn. 2013, 44, 41–46. [Google Scholar]
- Hosseini, S.M.; Shahbazizadeh, S.; Khosravi-Darani, K.; Mozafari, M.R. Spirulina paltensis: Food and Function. Curr. Nutr. Food Sci. 2013, 9, 189–193. [Google Scholar] [CrossRef]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell. Int. 2013, 13, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.A. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–783. [Google Scholar] [CrossRef] [PubMed]
- Son, M.H.; Park, K.H.; Choi, A.R.; Yoo, G.; In, M.J.; Kim, D.H.; Chae, H.J. Investigation of biological activities of enzymatic hydrolysate of Spirulina. J. Korean Soc. Food Sci. Nutr. 2009, 38, 136–141. [Google Scholar] [CrossRef]
- Yang, H.N.; Lee, E.H.; Kim, H.M. Spirulina platensis inhibits anaphylactic reaction. Life Sci. 1997, 61, 1237–1244. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.H.; Kim, J.S.; Han, J.A.; Seo, H.J.; Lim, H.J.; Choi, S.Y. Studies on simultaneous determination of chlorophyll a and b, pheophorbide a, and β-carotene in Chlorella and Spirulina products. J. Food Hyg. Saf. 2005, 20, 141–146. [Google Scholar]
- Yamada, T.; Sakaguchi, K. Comparative studies on Chlorella cell walls: Induction of protoplast formation. Arch. Microbiol. 1982, 132, 10–13. [Google Scholar] [CrossRef]
- Oh, S.H.; Ahn, J.H.; Kang, D.H.; Lee, H.Y. The effect of ultrasonificated extracts of Spirulina maxima on the anticancer activity. Mar. Biotechnol. 2011, 13, 205–214. [Google Scholar] [CrossRef]
- Eom, S.H.; Kang, Y.M.; Park, J.H.; Yu, D.U.; Jeong, E.T.; Lee, M.S.; Kim, Y.M. Enhancement of polyphenol content and antioxidant activity of brown alga Eisenia bicyclis extract by microbial fermentation. Fish. Aquat. Sci. 2011, 14, 192–197. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chan, C.F.; Huang, W.Y.; Lin, J.S.; Chan, P.; Liu, H.Y.; Lin, Y.S. Applications of Lactobacillus rhamnosus spent culture supernatant in cosmetic antioxidation, whitening and moisture retention applications. Molecules 2013, 18, 14161–14171. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.Y.; Lee, H.Y. Enhancement of Chlorophyll a Production from Marine Spirulina maxima by an Optimized Ultrasonic Extraction Process. Appl. Sci. 2018, 8, 26. [Google Scholar] [CrossRef]
- Dietz, B.M.; Kang, Y.H.; Liu, G.; Eggler, A.L.; Yao, P.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; Mesecar, A.D.; Breemen, R.B.; et al. Xanthohumol isolated from humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem. Res. Toxicol. 2005, 18, 1296–1305. [Google Scholar] [CrossRef]
- Lee, J.; Weon, J.B.; Ma, C.J. Neuroprotective activity of phytosterols isolated from Artemisia apiacea. Korean J. Pharmacogn. 2014, 45, 214–219. [Google Scholar]
- Dar, M.A.; Khan, A.M.; Raina, R.; Beigh, S.A.; Sultana, M. Effect of repeated oral administration of bifenthrin antioxidant status and acetylcholinesterase activity in brain of rats. Toxicol. Environ. Chem. 2015, 97, 961–967. [Google Scholar] [CrossRef]
- Lee, M.R.; Moon, S.H.; Choi, A.R.; Lee, S.C.; Ahn, K.H.; Park, H.R. Neuroprotective effects of extracts from Diospyros kaki L. Peel. Korean J. Food Cookery Sci. 2011, 27, 67–73. [Google Scholar] [CrossRef]
- Kim, N.R.; Kim, H.Y.; Kim, M.H.; Kim, H.M.; Jeong, H.J. Improvement of depressive behavior by Sweetme Sweet Pumpkin™ and its active compound, β-carotene. Life Sci. 2016, 147, 39–45. [Google Scholar] [CrossRef]
- Ying, S.W.; Futter, M.; Rosenblum, K.; Webber, M.J.; Hunt, S.P.; Bliss, T.V.; Bramham, C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002, 22, 1532–1540. [Google Scholar] [CrossRef]
- Li, F.J.; Shen, L.; Ji, H.F. Dietary intakes of vitamin E, vitamin C, and β-carotene and risk of Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2012, 31, 253–258. [Google Scholar] [CrossRef]
- Craft, N.E.; Soares, J.H. Relative solubility, stability, and absorptivity of lutein and beta-carotene in organic solvents. J. Agric. Food Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Juan, M.Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Sergio, A.R.P.; Robert, M.R. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Tsuchihashi, H.; Kigoshi, M.; Iwatsuki, M.; Niki, E. Action of β-Carotene as an Antioxidant against Lipid Peroxidation. Arch. Biochem. Biophys. 1995, 323, 137–147. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Paiva, M.; Heaton, M.B. Vitamin E and β-carotene protect against ethanol combined with ischemia in an embryonic rat hippocampal culture model of fetal alcohol syndrome. Neurosci. Lett. 1999, 263, 189–192. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Paiva, M.; Heaton, M.B. The Antioxidants Vitamin E and β-Carotene Protect Against Ethanol-Induced Neurotoxicity in Embryonic Rat Hippocampal Cultures. Alcohol 1999, 17, 163–168. [Google Scholar] [CrossRef]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- Chen, D.Y.; Bambah-Mukku, D.; Pollonini, G.; Alberini, C.M. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat. Neurosci. 2012, 15, 1707–1714. [Google Scholar] [CrossRef]

| Extraction Process | β-Carotene Content (mg/g) | Extraction Yield (%) |
|---|---|---|
| FE 1 | 1.62 ± 0.22 | 18.26 ± 1.93 |
| WE 2 | 0.81 ± 0.13 | 10.60 ± 1.44 |
| UE 3 | 1.03 ± 0.34 | 12.77 ± 1.68 |
| Sample | Treated Concentration (µg/mL) | Glutathione (% of Control) | Glutathione Reductase (% of Control) | Glutathione Peroxidase (% of Control) |
|---|---|---|---|---|
| Control | - | 100.00 | 100.00 | 100.00 |
| Trolox | 50 μM | 91.88 ± 8.90 | 93.32 ± 7.21 | 91.26 ± 1.67 |
| Fermented Spirulina maxima extracts | 1 | 51.02 ± 3.31 | 61.42 ± 8.23 | 67.94 ± 5.50 |
| 10 | 63.76 ± 5.18 | 70.23 ± 4.49 | 75.07 ± 7.36 | |
| 100 | 80.38 ± 9.30 | 81.27 ± 5.11 | 80.33 ± 7.18 | |
| β-carotene | 1 | 47.73 ± 2.85 | 48.08 ± 1.07 | 43.75 ± 6.35 |
| Extraction Process | p-CREB/BDNF Expression Ratio |
|---|---|
| FE 1 | 1.338 |
| β-carotene | 1.055 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.Y.; Kang, D.H.; Heo, S.-J.; Lee, H.Y. Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction. Appl. Sci. 2018, 8, 2469. https://doi.org/10.3390/app8122469
Choi WY, Kang DH, Heo S-J, Lee HY. Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction. Applied Sciences. 2018; 8(12):2469. https://doi.org/10.3390/app8122469
Chicago/Turabian StyleChoi, Woon Yong, Do Hyung Kang, Soo-Jin Heo, and Hyeon Yong Lee. 2018. "Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction" Applied Sciences 8, no. 12: 2469. https://doi.org/10.3390/app8122469
APA StyleChoi, W. Y., Kang, D. H., Heo, S.-J., & Lee, H. Y. (2018). Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction. Applied Sciences, 8(12), 2469. https://doi.org/10.3390/app8122469