Marine Gelatine from Rest Raw Materials
Abstract
1. Introduction
Opportunities for by-Catch Utilization
2. Properties and Applications of Marine-Derived Gelatine
- Gelatine is not chemically modified and has no, possibly harmful, by-products of chemical modification
- It does not contain and is not made of any genetically modified organisms
- Is not a food additive and therefore does not require an E-number
- It is considered Generally Recognised As Safe (GRAS)
- It does not cause any known allergies
- It has been consumed for more than 2000 years and is known for generations [23]
2.1. Legislative and Safety Considerations of Marine Gelatine Production
2.2. Comparison of Fish and Mammalian Gelatine
3. Production Strategies for Gelatine
3.1. Pre-Treatment and Extraction Strategies
3.1.1. Chemical Pre-Treatment
3.1.2. Enzymatic Pre-Treatment
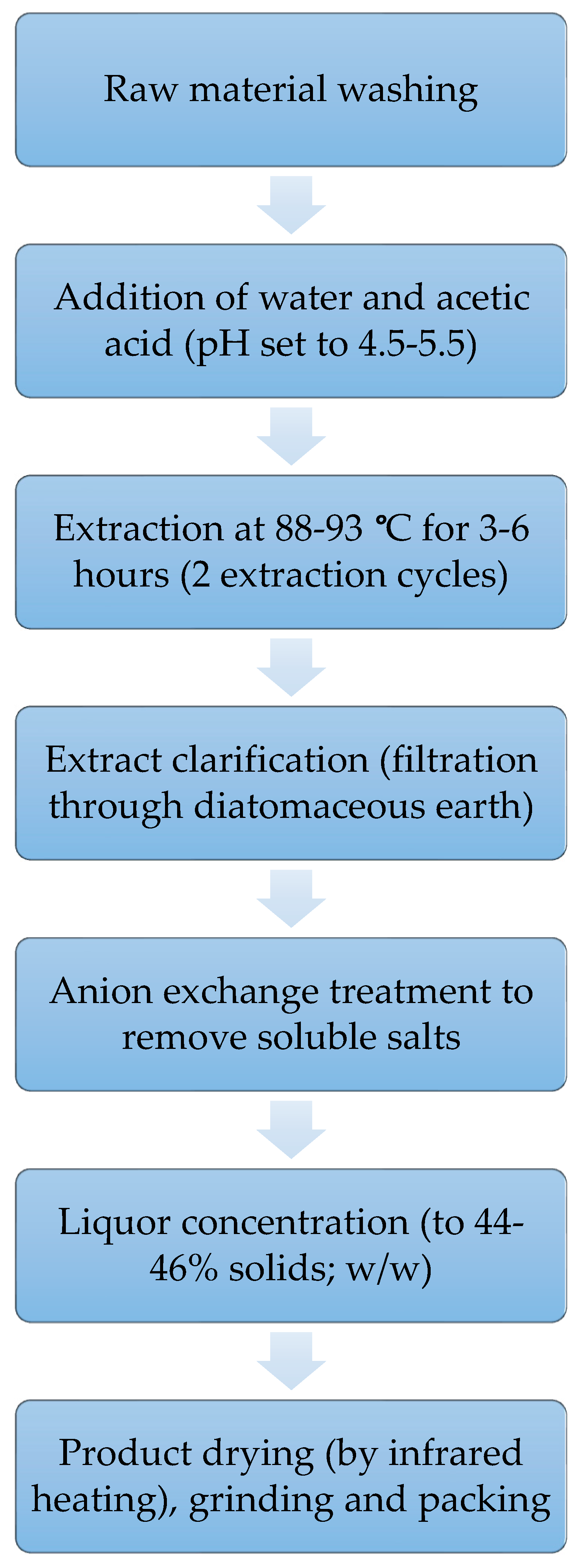
3.1.3. Extraction of Gelatine
3.2. Improving the Properties of Fish Gelatine
4. Opportunities for Novel Applications of Fish Gelatine and Collagen
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Valorisation of fish by-products against waste management treatments–Comparison of environmental impacts. Waste Manag. 2015, 46, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2016 (SOFIA): Contributing to Food Security and Nutrition for All; Food and Agriculture Organization: Rome, Italy, 2016. [Google Scholar]
- Hayatudin, H. More Effort Needed to Produce Halal Medicinal Products. Halal J. Online J. Glob. Halal Mark. 2005. Available online: www.halaljournal.com (accessed on 21 November 2018).
- Jaswir, I.; Mirghani, M.E.S.; Hassan, T.; Yaakob, C.M. Extraction and characterization of gelatin from different marine fish species in Malaysia. Int. Food Res. J. 2009, 16, 381–389. [Google Scholar]
- The Common Fisheries Policy (CFP). Available online: https://ec.europa.eu/fisheries/cfp_en (accessed on 8 November 2018).
- Hayes, M.; McKeon, K. Advances in the Processing of Marine Discard and By-products. In Seafood Processing By-Products; Springer: New York, NY, USA, 2014; pp. 125–143. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.A.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- FAO. Torry advisory note No. 81. In Handling and Processing Blue Whiting; Food and Agriculture Organization: Rome, Italy, 2001. [Google Scholar]
- Capros Aper. Available online: https://en.wikipedia.org/wiki/Capros_aper (accessed on 6 September 2018).
- O’Donnell, C.; Farrell, E.; Saunders, R.; Campbell, A. The Abundance of Boarfish (Capros aper) along the Western Shelf Estimated Using Hydro-Acoustics; Marine Institute: Oranmore, Ireland, 2012. [Google Scholar]
- Blanchard, F.; Vandermeirsch, F. Warming and exponential abundance increase of the subtropical fish Capros aper in the Bay of Biscay (1973–2002). C. R. Biol. 2005, 328, 505–509. [Google Scholar] [CrossRef] [PubMed]
- KFO. Available online: http://www.kfo.ie/assets/kfo_december-16update-(2).pdf (accessed on 6 September 2018).
- White, E.; Minto, C.; Nolan, C.P.; King, E.; Mullins, E.; Clarke, M. First estimates of age, growth, and maturity of boarfish (Capros aper): A species newly exploited in the Northeast Atlantic. ICES J. Mar. Sci. 2010, 68, 61–66. [Google Scholar] [CrossRef]
- Lucy Towers. An Overview on Alternative Species of Fish Available in the Markets. Available online: http://dev.thefishsite.com/articles/an-overview-on-alternative-species-of-fish-available-in-the-markets (accessed on 21 November 2018).
- Hayes, M.; Mora, L.; Hussey, K.; Aluko, R.E. Boarfish protein recovery using the pH-shift process and generation of protein hydrolysates with ACE-I and antihypertensive bioactivities in spontaneously hypertensive rats. Innov. Food Sci. Emerg. Technol. 2016, 37, 253–260. [Google Scholar] [CrossRef]
- Blanco, M.; Sotelo, C.G.; Pérez-Martín, R.I. Hydrolysis as a Valorization Strategy for Unused Marine Food Biomass: Boarfish and Small-Spotted Catshark Discards and By-Products. J. Food Biochem. 2015, 39, 368–376. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Norziah, M.H.; Al-Hassan, A.; Khairulnizam, A.B.; Mordi, M.N.; Norita, M. Characterization of fish gelatin from surimi processing wastes: Thermal analysis and effect of transglutaminase on gel properties. Food Hydrocoll. 2009, 23, 1610–1616. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocoll. 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Zotos, A. Fish processing by-products as a potential source of gelatin: A review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- GMIA. Gelatin Handbook; Gelatin Manufacturers Institute of America: New York, NY, USA, 2012. [Google Scholar]
- GME. Available online: https://www.gelatine.org/gelatine/safety.html (accessed on 8 November 2018).
- Food Safety: Overview. Available online: https://ec.europa.eu/food (accessed on 9 November 2018).
- GRAS Substances (SCOGS) Database. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ (accessed on 8 November 2018).
- Kuehn, A.; Swoboda, I.; Arumugam, K.; Hilger, C.; Hentges, F. Fish allergens at a glance: Variable allergenicity of parvalbumins, the major fish allergens. Front. Immunol. 2014, 5, 179. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, A.; Hilger, C.; Lehners-Weber, C.; Codreanu-Morel, F.; Morisset, M.; Metz-Favre, C.; Pauli, G.; De Blay, F.; Revets, D.; Muller, C.P. Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: Component resolved diagnosis using parvalbumin and the new allergens. Clin. Exp. Allergy 2013, 43, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Akiyama, H.; Huge, J.; Kubota, H.; Chikazawa, S.; Satoh, T.; Miyake, T.; Uhara, H.; Okuyama, R.; Nakagawara, R. Fish collagen is an important panallergen in the Japanese population. Allergy 2016, 71, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Dıaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Olsen, C.W.; Olson, D.A.; Chiou, B.; Yee, E.; Bechtel, P.J.; McHugh, T.H. Water vapor permeability of mammalian and fish gelatin films. J. Food Sci. 2006, 71, E202–E207. [Google Scholar] [CrossRef]
- Atma, Y. Amino acid and proximate composition of fish bone gelatin from different warm-water species: A comparative study. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 58, p. 012008. [Google Scholar]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Zhou, P.; Regenstein, J.M. Comparison of water gel desserts from fish skin and pork gelatins using instrumental measurements. J. Food Sci. 2007, 72, C196–C201. [Google Scholar] [CrossRef] [PubMed]
- Norland, R.E. Fish gelatin. In Advances in Fisheries Technology and Biotechnology for Increased Profitability; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1990; pp. 325–333. [Google Scholar]
- Shyni, K.; Hema, G.S.; Ninan, G.; Mathew, S.; Joshy, C.G.; Lakshmanan, P.T. Isolation and characterization of gelatin from the skins of skipjack tuna (Katsuwonus pelamis), dog shark (Scoliodon sorrakowah), and rohu (Labeo rohita). Food Hydrocoll. 2014, 39, 68–76. [Google Scholar] [CrossRef]
- Sripriya, R.; Kumar, R. A Novel Enzymatic Method for Preparation and Characterization of Collagen Film from Swim Bladder of Fish Rohu (Labeo rohita). Food Nutr. Sci. 2015, 6, 1468. [Google Scholar]
- Al-Mazeedi, H.M.; Regenstein, J.M.; Riaz, M.N. The issue of undeclared ingredients in halal and kosher food production: A focus on processing aids. Compr. Rev. Food Sci. Food Saf. 2013, 12, 228–233. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to a notification from DSM on fish gelatine for use as a formulation aid (carrier) in vitamin and carotenoid preparations pursuant to Article 6, paragraph 11 of Directive 2000/13/EC—For permanent exemption from labelling. EFSA J. 2007, 5, 568. [Google Scholar]
- Fish Gelatin—A Short Market Survey. Available online: http://fiskeviden.dk/wp-content/uploads/2016/03/T2C_Delrapport_2_6.pdf (accessed on 21 November 2018).
- A Fish Gelatine Plant in Hanstholm—A First Outline of Economics. Available online: http://fiskeviden.dk/wp-content/uploads/2016/03/T2C_Delrapport_2_5.pdf (accessed on 21 November 2018).
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Prodpran, T.; Tanaka, M. Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocoll. 2006, 20, 492–501. [Google Scholar] [CrossRef]
- Alfaro, A.T.; Biluca, F.C.; Marquetti, C.; Tonial, I.B.; de Souza, N.E. African catfish (Clarias gariepinus) skin gelatin: Extraction optimization and physical–chemical properties. Food Res. Int. 2014, 65, 416–422. [Google Scholar] [CrossRef]
- Chandra, M.V.; Shamasundar, B.A. Rheological properties of gelatin prepared from the swim bladders of freshwater fish Catla catla. Food Hydrocoll. 2015, 48, 47–54. [Google Scholar] [CrossRef]
- Giménez, B.; Gómez-Guillén, M.C.; Montero, P. The role of salt washing of fish skins in chemical and rheological properties of gelatin extracted. Food Hydrocoll. 2005, 19, 951–957. [Google Scholar] [CrossRef]
- Haddar, A.; Bougatef, A.; Balti, R.; Souissi, N.; Koched, W.; Nasri, M. Physicochemical and functional properties of gelatin from tuna (Thunnus thynnus) head bones. J. Food Nutr. Res. 2011, 50, 150–159. [Google Scholar]
- Khiari, Z.; Rico, D.; Martin-Diana, A.B.; Barry-Ryan, C. Comparison between gelatines extracted from mackerel and blue whiting bones after different pre-treatments. Food Chem. 2013, 139, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Sinthusamran, S.; Kishimura, H. Gelatin from clown featherback skin: Extraction conditions. LWT-Food Sci. Technol. 2016, 66, 186–192. [Google Scholar] [CrossRef]
- Kołodziejska, I.; Kaczorowski, K.; Piotrowska, B.; Sadowska, M. Modification of the properties of gelatin from skins of Baltic cod (Gadus morhua) with transglutaminase. Food Chem. 2004, 86, 203–209. [Google Scholar] [CrossRef]
- Niu, L.; Zhou, X.; Yuan, C.; Bai, Y.; Lai, K.; Yang, F.; Huang, Y. Characterization of tilapia (Oreochromis niloticus) skin gelatin extracted with alkaline and different acid pretreatments. Food Hydrocoll. 2013, 33, 336–341. [Google Scholar] [CrossRef]
- Norziah, M.H.; Kee, H.Y.; Norita, M. Response surface optimization of bromelain-assisted gelatin extraction from surimi processing wastes. Food Biosci. 2014, 5, 9–18. [Google Scholar] [CrossRef]
- Shakila, R.J.; Jeevithan, E.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Functional characterization of gelatin extracted from bones of red snapper and grouper in comparison with mammalian gelatin. LWT-Food Sci. Technol. 2012, 48, 30–36. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Regenstein, J.M. Effects of alkaline and acid pretreatments on Alaska pollock skin gelatin extraction. J. Food Sci. 2005, 70, c392–c396. [Google Scholar] [CrossRef]
- Giménez, B.; Turnay, J.; Lizarbe, M.A.; Montero, P.; Gómez-Guillén, M.C. Use of lactic acid for extraction of fish skin gelatin. Food Hydrocoll. 2005, 19, 941–950. [Google Scholar] [CrossRef]
- Gómez-Guilløen, M.C.; Montero, P. Extraction of gelatin from megrim (Lepidorhombus boscii) skins with several organic acids. J. Food Sci. 2001, 66, 213–216. [Google Scholar] [CrossRef]
- Grossman, S.; Bergman, M. Process for the Production of Gelatin from Fish Skins. U.S. Patent 5,093,474, 3 March 1992. [Google Scholar]
- Herpandi, N.H.; Adzitey, F. Fish bone and scale as a potential source of halal gelatin. J. Fish. Aquat. Sci. 2011, 6, 379–389. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; Montero, P. Extraction of gelatin from fish skins by high pressure treatment. Food Hydrocoll. 2005, 19, 923–928. [Google Scholar] [CrossRef]
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem. 2008, 108, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Akagündüz, Y.; Mosquera, M.; Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Sea bream bones and scales as a source of gelatin and ACE inhibitory peptides. LWT-Food Sci. Technol. 2014, 55, 579–585. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, S.; Wang, Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod. Process. 2011, 89, 185–193. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Improvement of gelatin extraction from bigeye snapper skin using pepsin-aided process in combination with protease inhibitor. Food Hydrocoll. 2008, 22, 615–622. [Google Scholar] [CrossRef]
- Hou, P.Z.; Regenstein, J.M. Optimization of extraction conditions for pollock skin gelatin. J. Food Sci. 2004, 69, C393–C398. [Google Scholar] [CrossRef]
- Cho, S.M.; Gu, Y.S.; Kim, S.B. Extracting optimization and physical properties of yellowfin tuna (Thunnus albacares) skin gelatin compared to mammalian gelatins. Food Hydrocoll. 2005, 19, 221–229. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Al-Saidi, G.S.; Guizani, N. Thermal characterisation of gelatin extracted from yellowfin tuna skin and commercial mammalian gelatin. Food Chem. 2008, 108, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Karim, A.A. Ultraviolet irradiation improves gel strength of fish gelatin. Food Chem. 2009, 113, 1160–1164. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Towards producing novel fish gelatin films by combination treatments of ultraviolet radiation and sugars (ribose and lactose) as cross-linking agents. J. Food Sci. Technol. 2014, 51, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Avena-Bustillos, R.J.; Chiou, B.-S.; Bilbao-Sainz, C.; Bechtel, P.J.; McHugh, T.H. Ultraviolet-B Radiation Induced Cross-linking Improves Physical Properties of Cold-and Warm-Water Fish Gelatin Gels and Films. J. Food Sci. 2012, 77, E215–E223. [Google Scholar] [CrossRef] [PubMed]
- Deiber, J.A.; Ottone, M.L.; Piaggio, M.V.; Peirotti, M.B. Characterization of cross-linked polyampholytic gelatin hydrogels through the rubber elasticity and thermodynamic swelling theories. Polymer 2009, 50, 6065–6075. [Google Scholar] [CrossRef]
- Farris, S.; Schaich, K.M.; Liu, L.; Piergiovanni, L.; Yam, K.L. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: A review. Trends Food Sci. Technol. 2009, 20, 316–332. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Pourjavadi, A.; Salimi, H.; Kurdtabar, M. Protein-and homo poly (amino acid)-based hydrogels with super-swelling properties. Polym. Adv. Technol. 2009, 20, 655–671. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, A.I.; Gómez-Guillén, M.C.; Montero, P. The effect of added salts on the viscoelastic properties of fish skin gelatin. Food Chem. 2000, 70, 71–76. [Google Scholar] [CrossRef]
- Koli, J.M.; Basu, S.; Nayak, B.B.; Kannuchamy, N.; Gudipati, V. Improvement of gel strength and melting point of fish gelatin by addition of coenhancers using response surface methodology. J. Food Sci. 2011, 76, E503–E509. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Moslehi, Z.; Mohammadi Nafchi, A.; Mostahsan, A.; Salamat, N.; Daraei Garmakhany, A. Cold water fish gelatin modification by a natural phenolic cross-linker (ferulic acid and caffeic acid). Food Sci. Nutr. 2015, 3, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Benjakul, S. Physico-chemical properties and fishy odour of gelatin from seabass (Lates calcarifer) skin stored in ice. Food Biosci. 2015, 10, 59–68. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S.; O’Brien, N.M. Effects of defatting and tannic acid incorporation during extraction on properties and fishy odour of gelatin from seabass skin. LWT-Food Sci. Technol. 2016, 65, 661–667. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Hatta, K.; Nakanishi, T. 3D-culture system for heart regeneration and cardiac medicine. BioMed Res. Int. 2013, 2013, 895967. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. BioMed Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Nakayama, Y.; Zhou, Y.-M.; Takamizawa, K.; Mori, K.; Munekata, M. Development of salmon collagen vascular graft: Mechanical and biological properties and preliminary implantation study. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, R.; Iohara, K.; Murakami, M.; Fukuta, O.; Nakashima, M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 2012, 33, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals: Beyond the Diet before the Drugs. Available online: https://www.ingentaconnect.com/content/ben/cbc/2014/00000010/00000001/art00001 (accessed on 8 November 2018).
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Brit. J. Pharm. 2018, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Choonpicharn, S.; Jaturasitha, S.; Rakariyatham, N.; Suree, N.; Niamsup, H. Antioxidant and antihypertensive activity of gelatin hydrolysate from Nile tilapia skin. J. Food Sci. Technol. 2015, 52, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Cryoprotective effect of gelatin hydrolysate from blacktip shark skin on surimi subjected to different freeze-thaw cycles. LWT-Food Sci. Technol. 2012, 47, 437–442. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.; Yang, N.; Xu, B.; Jin, Z.; Xu, X. Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J. Funct. Foods 2014, 7, 609–620. [Google Scholar] [CrossRef]
- Hong, P.K.; Gottardi, D.; Ndagijimana, M.; Betti, M. Glycation and transglutaminase mediated glycosylation of fish gelatin peptides with glucosamine enhance bioactivity. Food Chem. 2014, 142, 285–293. [Google Scholar] [CrossRef] [PubMed]

| Type of Gelatine | Typical Usage |
|---|---|
| Food grade | Confectionary, gelatine desserts, gelatine in meats, clarification of beverages and juices, special dietary uses |
| Pharmaceutical | Gelatine capsules (hard and soft type), tablets and tablet coating, suppositories, gelatine emulsions, microencapsulation, absorbable gelatine sponge and films, plasma substitute, pastilles and troches, bacterial growth media |
| Photographic | Photographic emulsions |
| Other (technical) | Coating and sizing, paper manufacture, printing processes, colloidal applications, matches, coated abrasives, adhesives, films and light filters, cosmetics, microencapsulation |
| Authors/Year | Material | Pre-Treatment | Extraction |
|---|---|---|---|
| [42] | African catfish (Clarias gariepinus) skin | NaOH at various concentration and time range (0.15–0.35% (w/v) and 40–120 min); Sulphuric acid at various concentration and time range (0.08–0.35% (w/v) and 40–120 min); Citric acid at various concentration and time range (0.6–1.4% (w/v) and 40–120 min) | Water at various temperature and time range (33–67 °C and 4–14 h) |
| [43] | Swim bladders of catla (Catla catla) | 0.15% NaOH (w/v) for 40 min; sulphuric acid (0.15%, v/v) and citric acid (0.5%, v/v) for 40 min (×2) | Water, 45–50 °C for 17 h |
| [44] | Dover sole (Solea vulgaris) skin |
| Water, 45 °C overnight |
| [45] | Tuna (Thunnus thynnus) head bones | Alkaline protease from Bacillus mojavensis, 50 °C for 4 h; 0.4 M HCl for 7.5 h; 0.9% Ca(OH)2 (w/v) for 144 h | Water, 75 °C for 4 h |
| [4] | Skins of several marine species (kerapu (Epinephelus sexfasciatus), jenahak (Lutjianus argentimaculatus), kembung (Rastrelliger kanagurta), kerisi (Pristipomodes typus) | 0.2% NaOH (w/v) for 40 min; sulphuric acid (0.2%, v/v) and citric acid (1%, v/v) for 40 min (×2) | Water, 45 °C for 18 h |
| [41] | Brownstripe red snapper (Lutjanus vitta) and bigeye snapper (Priacanthus macracanthus) skin | 0.2 M NaOH (3 × 30 min); 0.05 M acetic acid for 3 h | Water, 45 °C for 12 h |
| [46] | Mackerel (Scomber scombrus) and blue whiting (Micromesistius poutassou) bones |
| Water, 45 °C for 18 h |
| [47] | Clown featherback (Chitala ornata) skin | 0.1 M NaOH for 2 h; 0.05 M acetic acid for 30 min | Water at various temperature and time range (45, 65, 85 °C and 6 h and 12 h) |
| [48] | Baltic cod (Gadus morhua) skin | No pre-treatment (only manual cleaning of material) | Water at various temperature and time range (30–60 °C and 15–120 min) |
| [32] | Nile perch (Lates niloticus) skin and bone | Skin: 0.01 M sulphuric acid (pH of 2.5–3.0) for 16 h Bones: 3% HCl for 9–12 days | Three sequential extractions for 5 h, at 50, 60 and 70 °C; followed by boiling for 5 h |
| [20] | Splendid squid (Loligo formosana) skin | 0.05 M NaOH for 6 h; 0.05 M phosphoric acid for 24 h | Water, with different temperatures (50, 60, 70 and 80 °C) |
| [49] | Tilapia (Oreochromis niloticus) skin | 0.3 M NaOH for 1 h; HCl, citric and acetic acid at various concentrations (0.01–0.20 M) | Water, 50 °C for 3 h |
| [19] | Herring species (Tenualosa ilisha) skin | 0.2 M Ca(OH)2 for 1 h; 0.1 M citric acid for 3 h | Water, 50 °C for 3 h |
| [50] | Ribbon fish (Lepturacanthus savel) surimi processing waste | 0.2 M Ca(OH)2 for 1 h; 0.1 M citric acid containing bromelain in various concentrations for varying times | Water, at different combinations of temperatures and durations |
| [51] | Red snapper (Lutjanus campechanus) and grouper (Epinephelus chlorostigma) bones | 0.2% NaOH (w/v) for 45 min; sulphuric acid (0.2%, v/v) and citric acid (1%, v/v) for 45 min (×2) | Water, 45 °C for 24 h |
| [35] | Skins of dog shark (Scoliodon sorrakowah), skipjack tuna (Katsuwonus pelamis) and rohu (Labeo rohita) | 0.1 M NaOH for 2 h; 0.05 M acetic acid for 24 h | Water, 45 °C for 12 h |
| [52] | Seabass (Lates calcarifer) skin | 0.1 M NaOH for 3 h; 0.05 M acetic acid for 2 h | Water at various temperature and time range (45, 55 °C and 3, 6 and 12 h) |
| [53] | Alaska Pollock skin | NaOH/Ca(OH)2 at various concentrations for 60 min; acetic, citric and sulfuric acid at various concentrations for 60 min | Water, 50 °C for 3 h |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanovic, I.; Hayes, M. Marine Gelatine from Rest Raw Materials. Appl. Sci. 2018, 8, 2407. https://doi.org/10.3390/app8122407
Milovanovic I, Hayes M. Marine Gelatine from Rest Raw Materials. Applied Sciences. 2018; 8(12):2407. https://doi.org/10.3390/app8122407
Chicago/Turabian StyleMilovanovic, Ivan, and Maria Hayes. 2018. "Marine Gelatine from Rest Raw Materials" Applied Sciences 8, no. 12: 2407. https://doi.org/10.3390/app8122407
APA StyleMilovanovic, I., & Hayes, M. (2018). Marine Gelatine from Rest Raw Materials. Applied Sciences, 8(12), 2407. https://doi.org/10.3390/app8122407





