Angioplasty Using 4-Hexylresorcinol-Incorporated Silk Vascular Patch in Rat Carotid Defect Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cellular Experiment
2.2. Blood Coagulation Test
2.3. Experimental Animals
2.4. Patch Materials and Scanning Electron Microscopy (SEM) Examination
2.4.1. Preparation of 4-HR-Incorporated Silk Patch
2.4.2. PTFE Patch
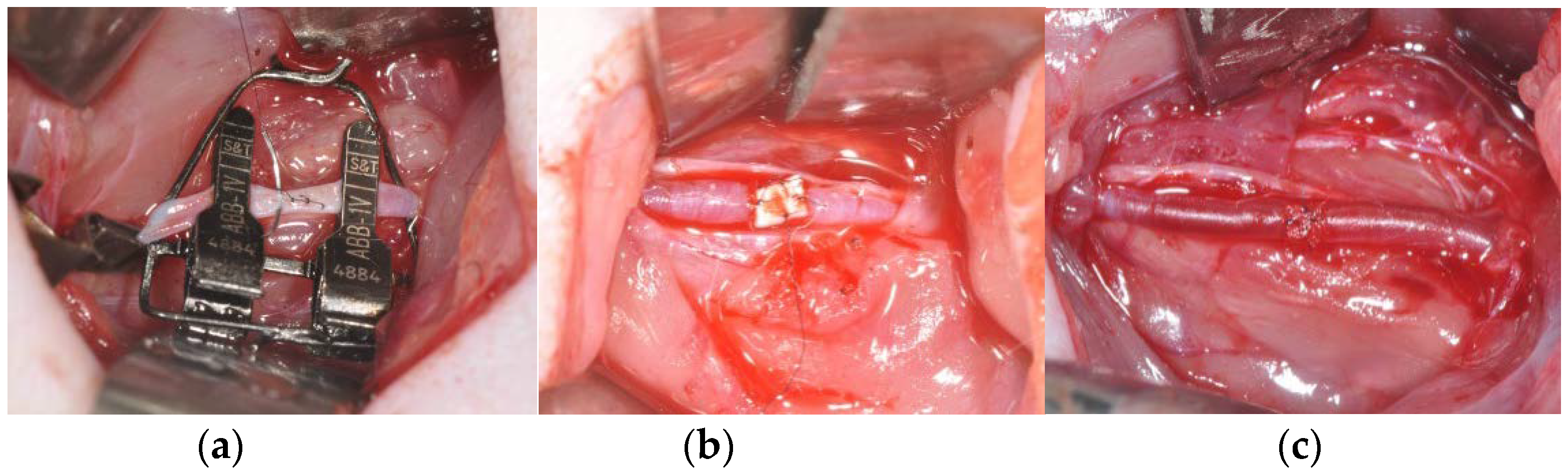
2.5. Study Design and Surgical Procedure
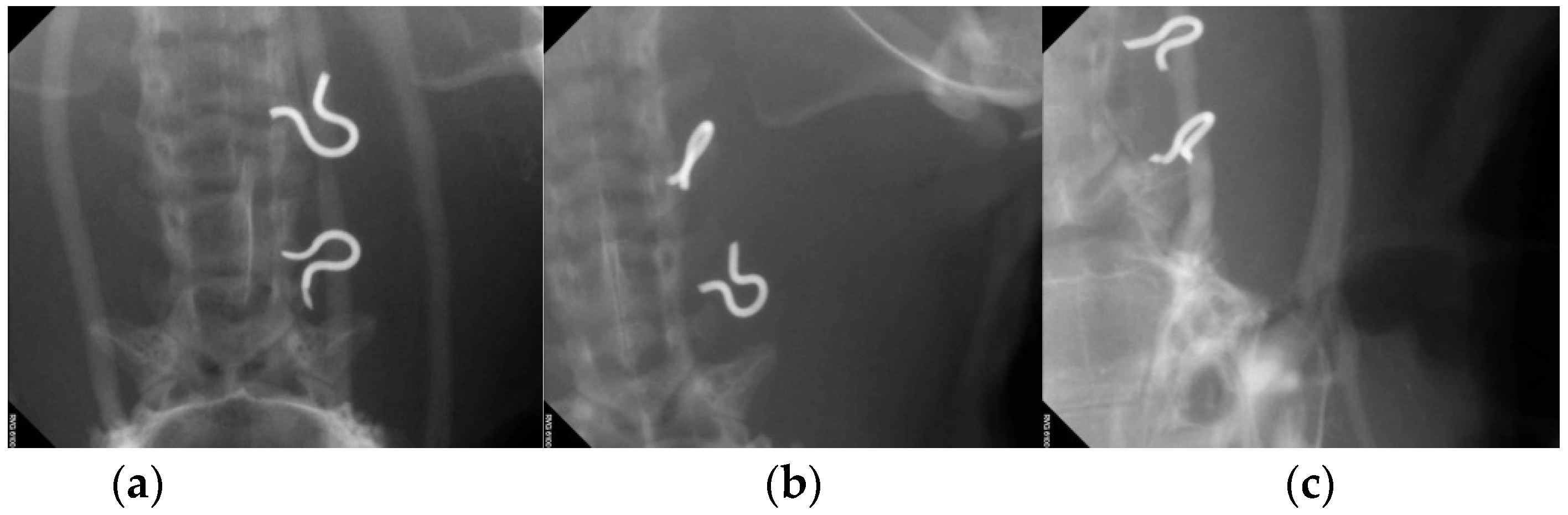
2.6. Ultrasonography and Angiography
2.7. Histological Analysis
2.8. Fluorescent and Immunohistochemical Analysis of Endothelial Cell Marker
2.9. Statistical Analysis
3. Results
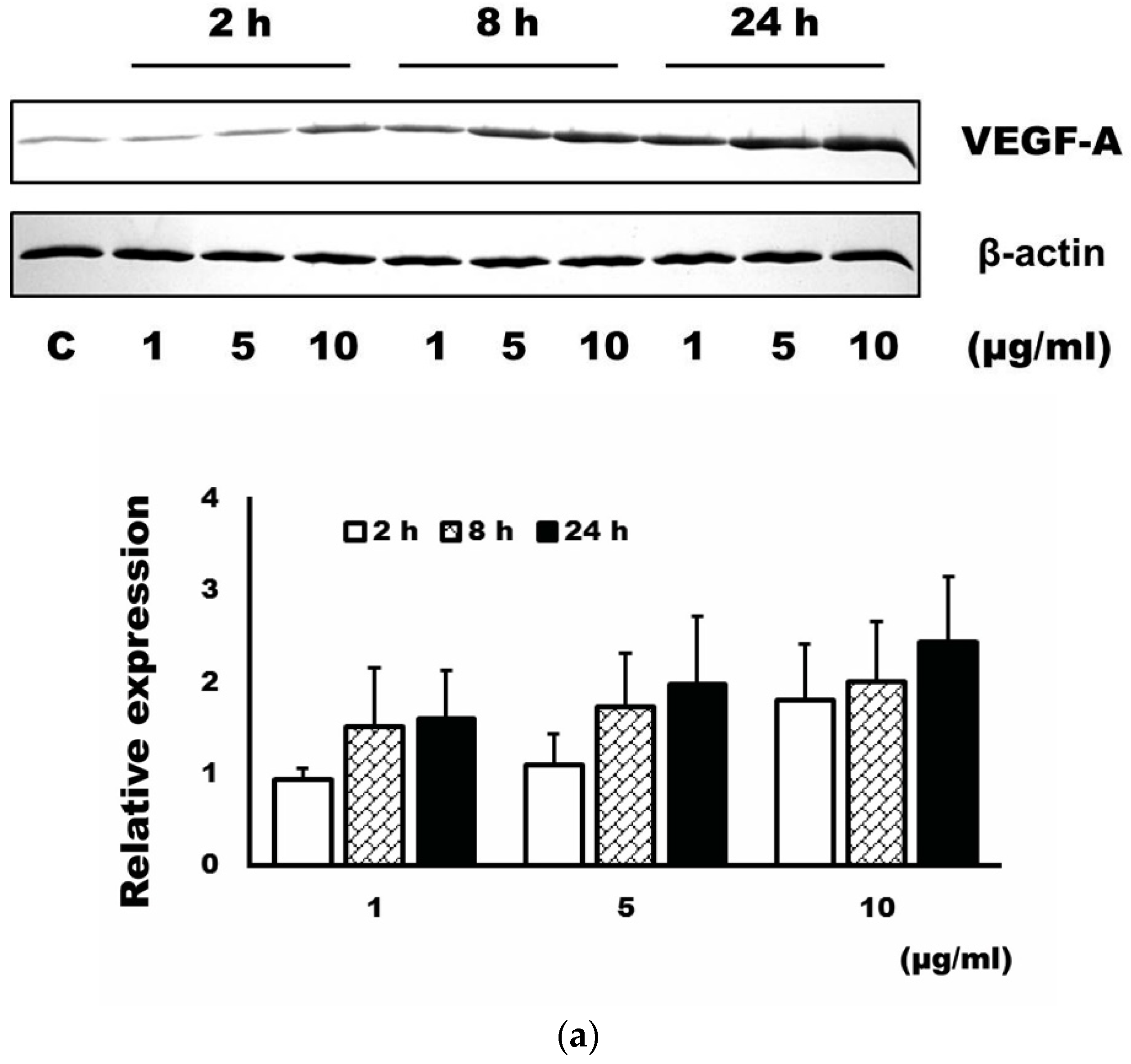
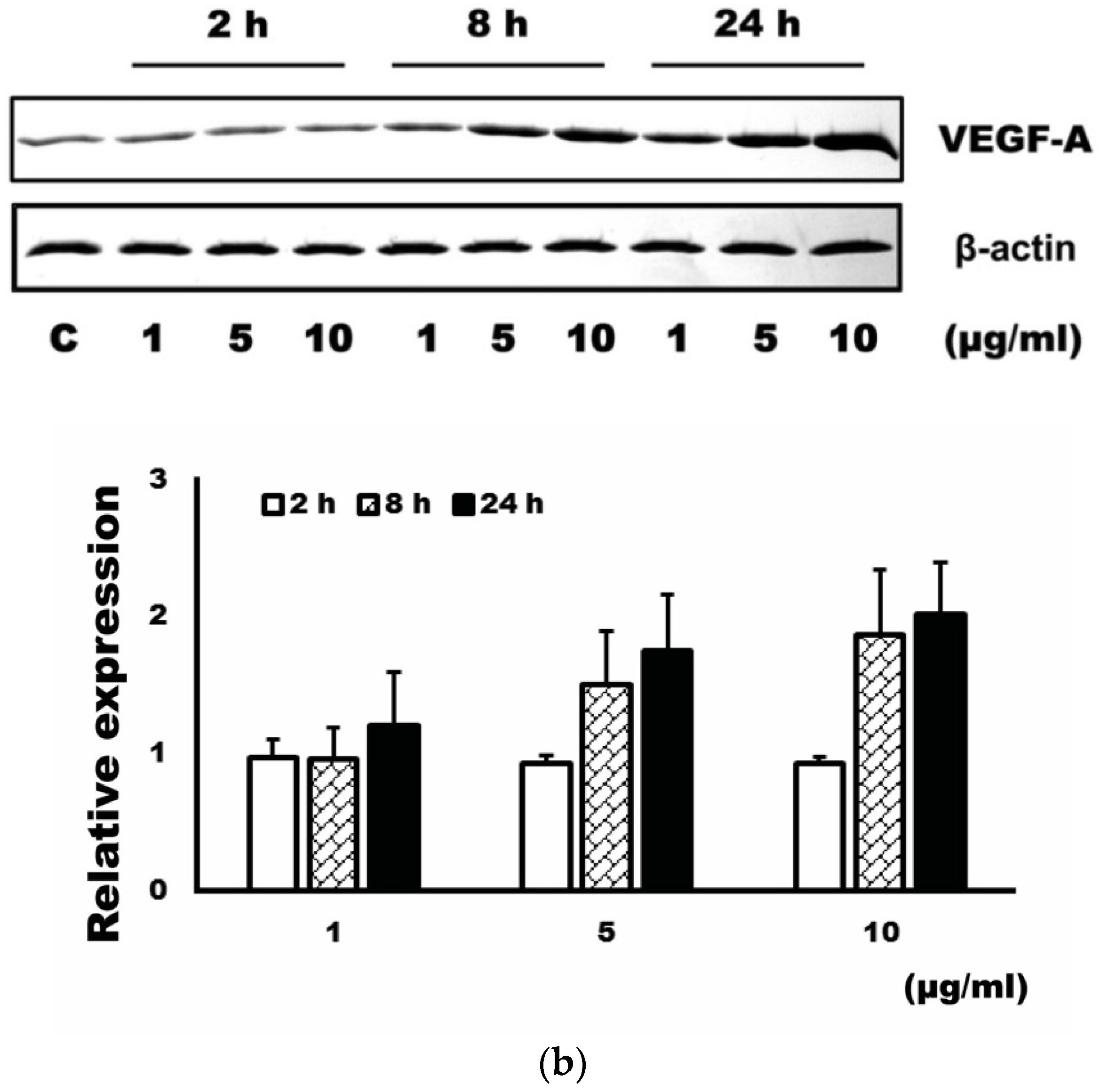
3.1. Expression Level of VEGF-A after Administration of 4-HR
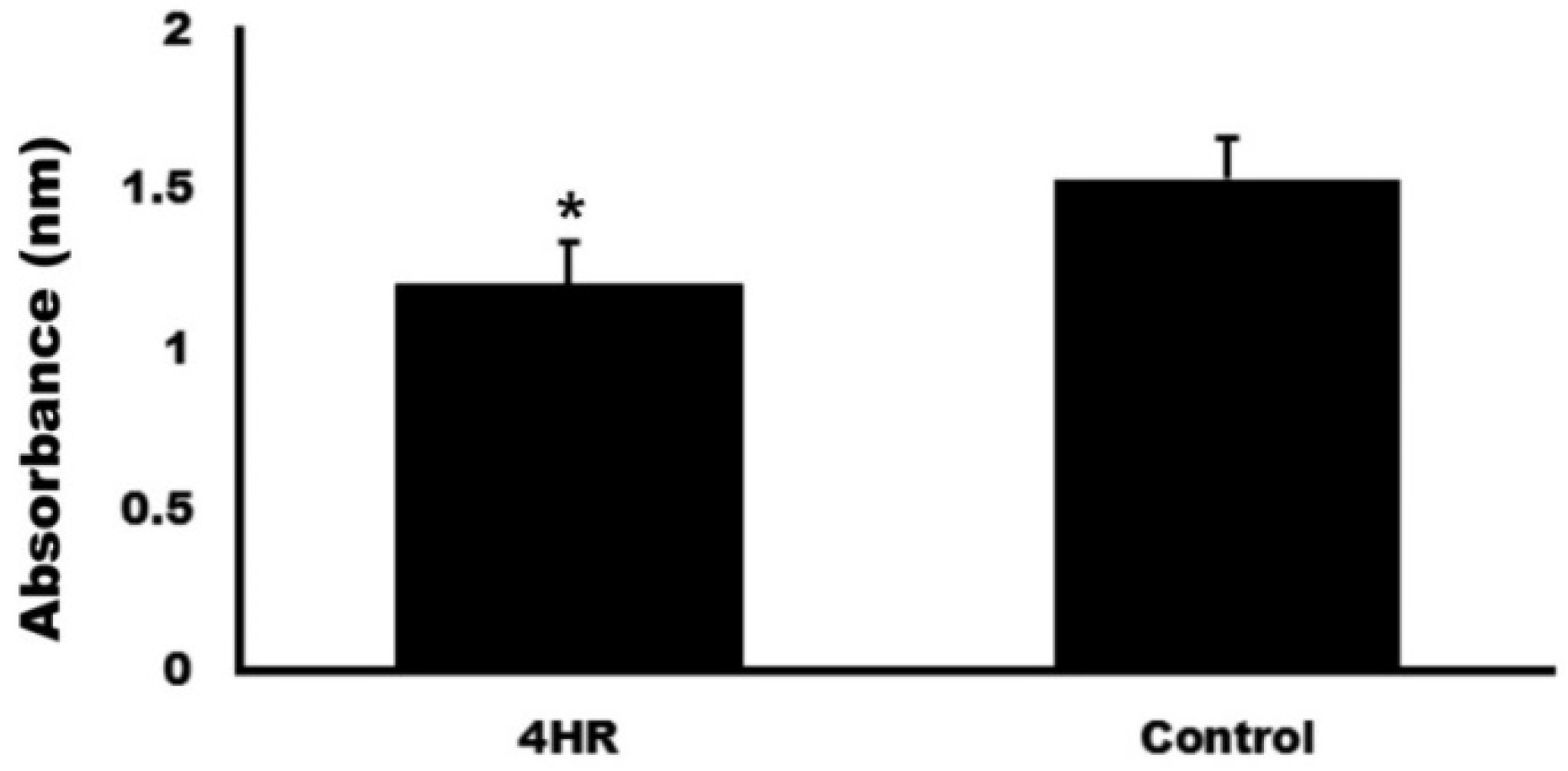
3.2. Blood Coagulation Test
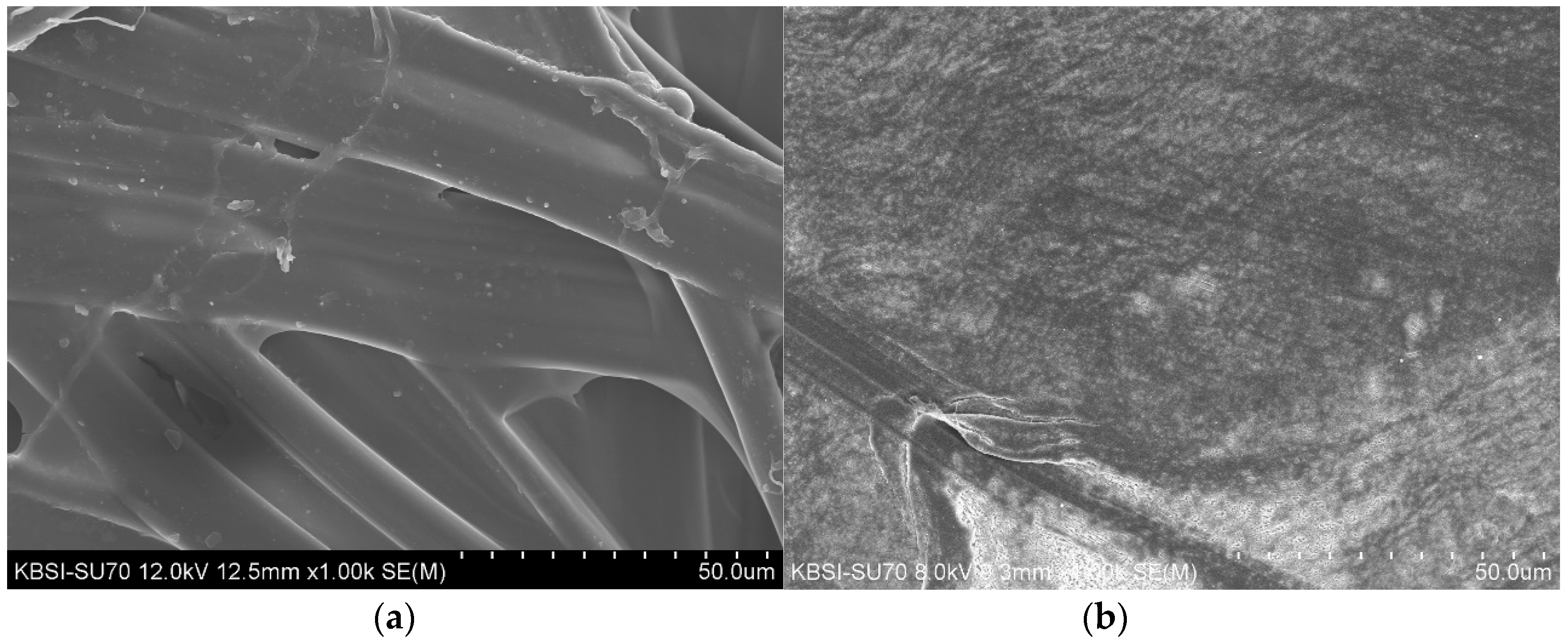
3.3. SEM Morphology Results for the 4-HR Silk and PTFE Patches
3.4. Artery Patency after Patch Angioplasty
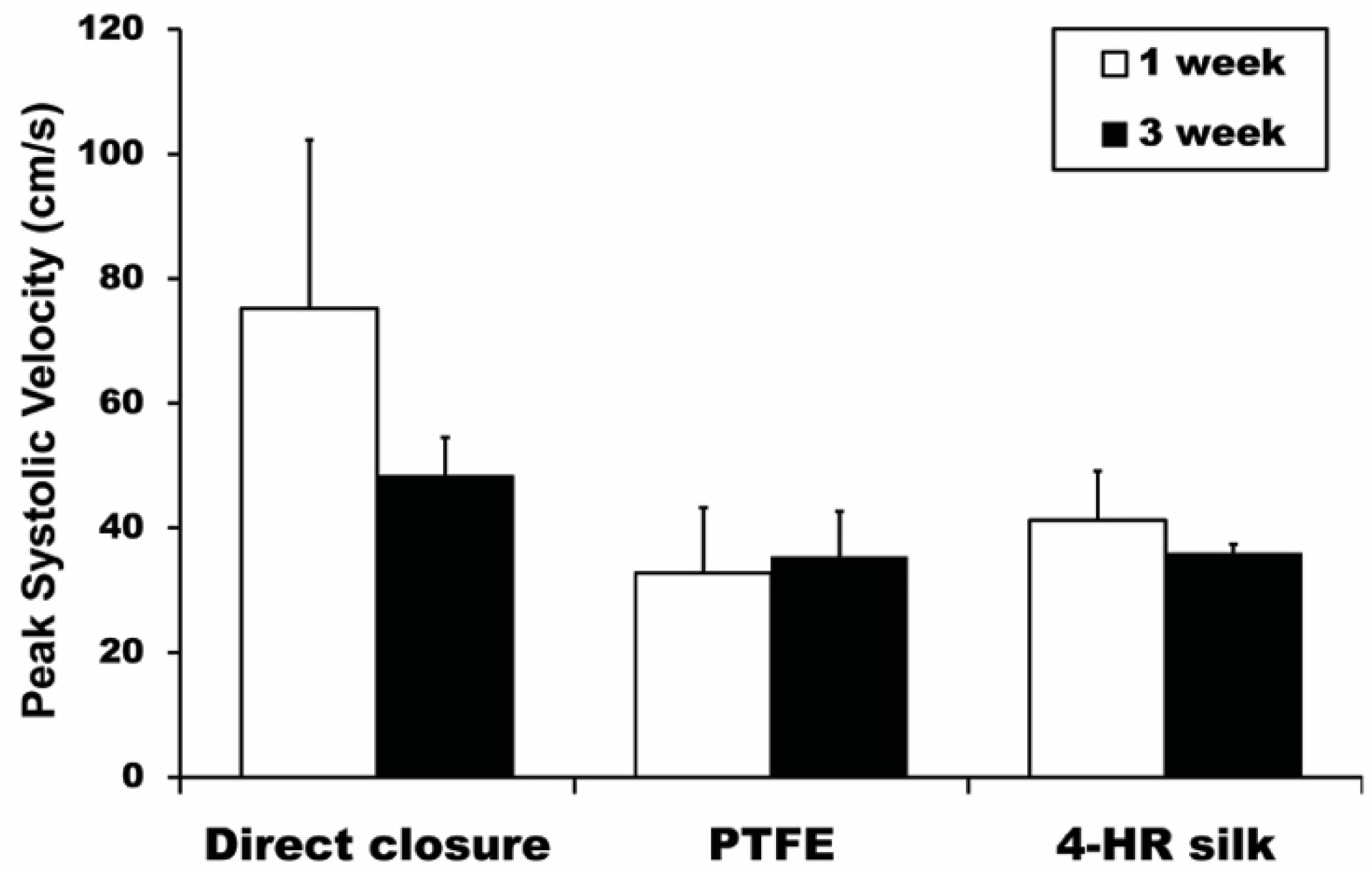
3.5. Ultrasonography PSV Results
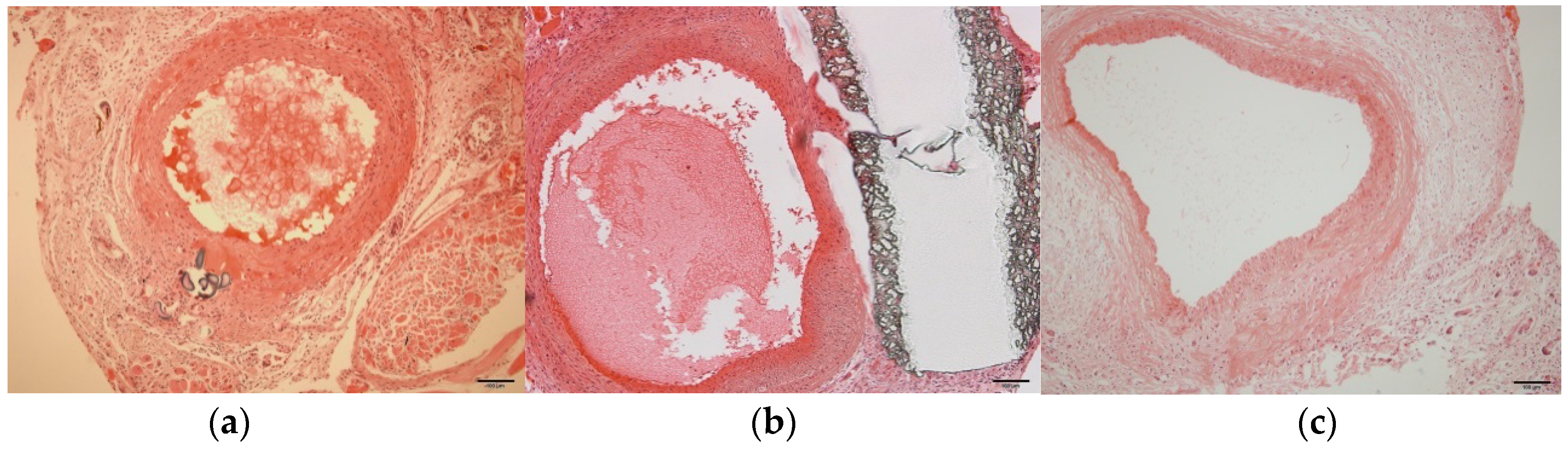
3.6. Histological Examination
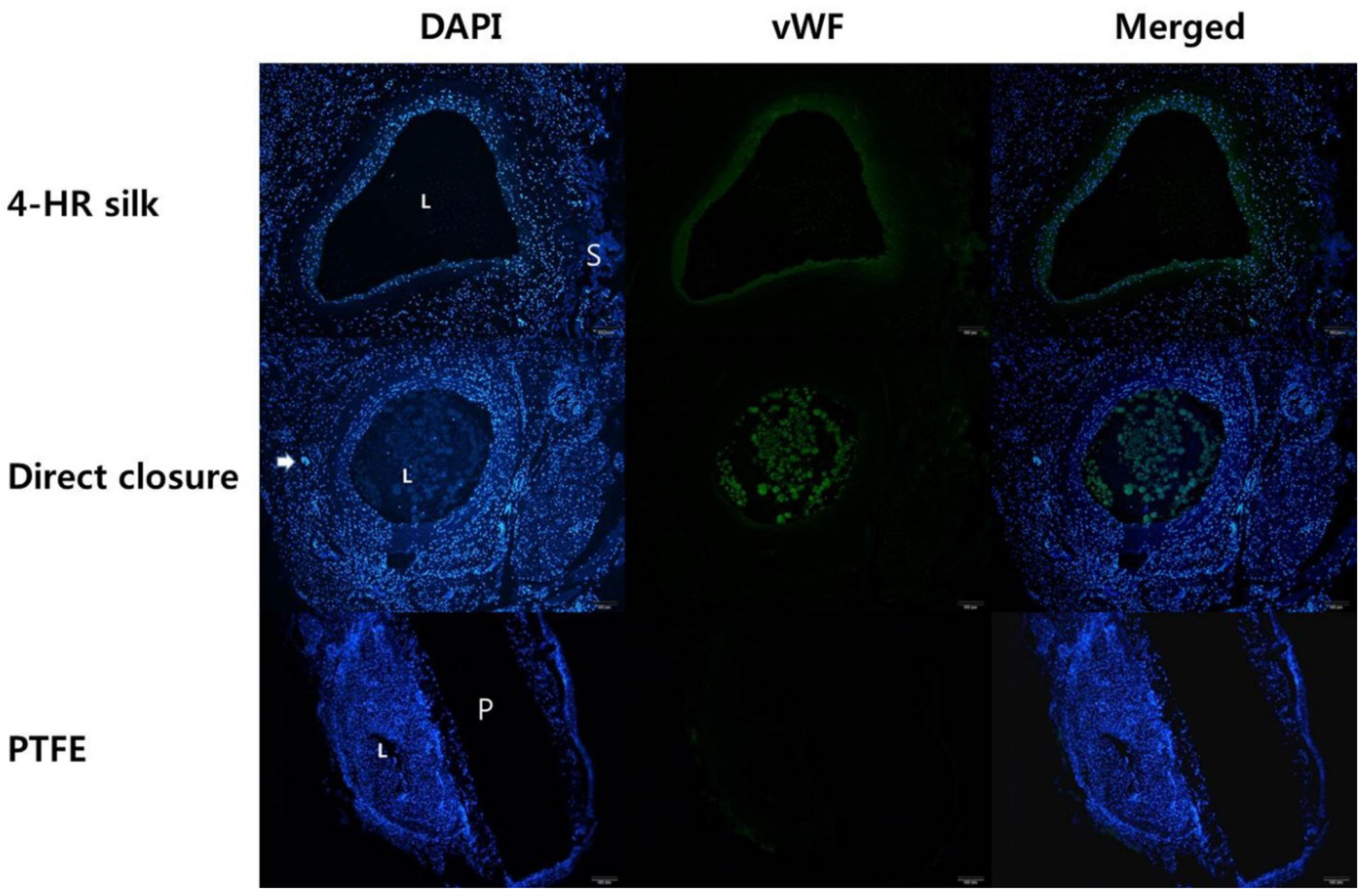
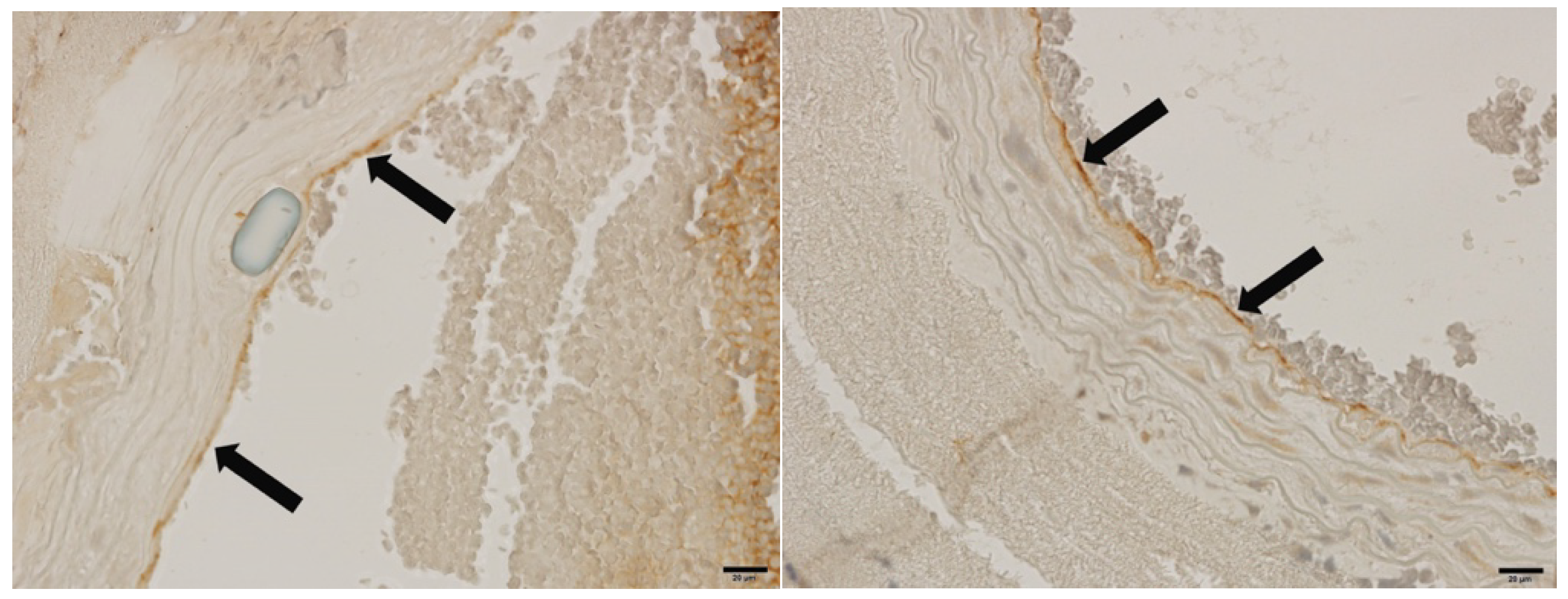
3.7. Fluorescent and Immunohistochemical Examination of vWF Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ketcham, A.S.; Hoye, R.C. Spontaneous carotid artery hemorrhage after head and neck surgery. Am. J. Surg. 1965, 110, 649–655. [Google Scholar] [CrossRef]
- Powitzky, R.; Vasan, N.; Krempl, G.; Medina, J. Carotid blowout in patients with head and neck cancer. Ann. Otol. Rhinol. Laryngol. 2010, 119, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, Y.P.; Kwon, T.W.; Kim, H.; Kim, G.E. Ten-year comparative analysis of bovine pericardium and autogenous vein for patch angioplasty in patients undergoing carotid endarterectomy. Ann. Vasc. Surg. 2012, 26, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, J.C.; Avgos, S.; Arnaoutoglou, E.; Nassis, C.; Peroulis, M.; Bali, C.; Papadopoulos, G.; Matsagkas, M.I. Use of the vascu-guard bovine pericardium patch for arteriotomy closure in carotid endarterectomy. Early and long-term results. Ann. Vasc. Surg. 2014, 28, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Marler, J.R.; Goldstein, M.; Grady, P.A.; Toole, J.F.; Baker, W.H.; Castaldo, J.E.; Chambless, L.E.; Moore, W.S.; Robertson, J.T. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995, 273, 1421–1428. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Robinson, P.A.; Saiedy, S.; Khan, J.H.; Boland, J.P. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: Long-term follow-up. J. Vasc. Surg. 1998, 27, 222–234. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Stone, P.; Deem, S.; Dean, L.S.; Keiffer, T.; Deem, E. Proposed duplex velocity criteria for carotid restenosis following carotid endarterectomy with patch closure. J. Vasc. Surg. 2009, 50, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Rerkasem, K.; Naylor, A.; Aburahma, A.; Rothwell, P. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J. Vasc. Surg. 2004, 40, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.A.; Srivastava, M.; Campbell, J.E.; Mousa, A.Y.; Hass, S.H.; Kazmi, H.; Dearing, D.D.; AbuRahma, A.F. A 10-year experience of infection following carotid endarterectomy with patch angioplasty. J. Vasc. Surg. 2011, 53, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, W.A.; Almerey, T.; Selim, M.; Farres, H.; Hakaim, A.G. Durability of carotid endarterectomy with bovine pericardial patch. Ann. Vasc. Surg. 2018, 50, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Kamenskiy, A.V.; MacTaggart, J.N.; Pipinos, I.I.; Gupta, P.K.; Dzenis, Y.A. Hemodynamically motivated choice of patch angioplasty for the performance of carotid endarterectomy. Ann. Biomed. Eng. 2013, 41, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.; Tulloch, A.; Cossman, D.V.; Cohen, J.L.; Rao, R.; Barmparas, G.; Mirocha, J.; Wagner, W. The influence of collagen impregnation of a knitted dacron patch used in carotid endarterectomy. Ann. Vasc. Surg. 2017, 39, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Awad, I.A.; Little, J.R. Patch angioplasty in carotid endarterectomy. Advantages, concerns, and controversies. Stroke 1989, 20, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Terzian, W.H.; Schadt, S.; Sheth, S.U. Right carotid-cutaneous fistula and right carotid pseudoaneurysm formation secondary to a chronically infected polyethylene terephthalate patch. Int. J. Crit. Illn. Inj. Sci. 2018, 8, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, A. Tissue engineering of vascular grafts. Matrix Biol. 2000, 19, 353–357. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, W.; Cui, L.; Cao, Y. Tissue engineering of blood vessel. J. Cell. Mol. Med. 2007, 11, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.B.; DeSimone, E.; Scheibel, T. Biomedical applications of recombinant silk-based materials. Adv. Mater. 2018, 30, 1704636. [Google Scholar] [CrossRef] [PubMed]
- Ghassemifar, R.; Redmond, S.; Zainuddin; Chirila, T.V. Advancing towards a tissue-engineered tympanic membrane: Silk fibroin as a substratum for growing human eardrum keratinocytes. J. Biomater. Appl. 2010, 24, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.Y.; Kim, S.G.; Kwon, K.J.; Kweon, H.; Chae, W.S.; Yang, W.G.; Lee, E.Y.; Seok, H. Silk fibroin-alginate-hydroxyapatite composite particles in bone tissue engineering applications in vivo. Int. J. Mol. Sci. 2017, 18, 858. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.T.; Kwon, K.J.; Park, Y.W.; Kim, S.G.; Kim, C.W.; Jo, Y.Y.; Kweon, H.Y.; Kang, S.W. The effect of silk fibroin/nano-hydroxyapatite/corn starch composite porous scaffold on bone regeneration in the rabbit calvarial defect model. Maxillofac. Plast. Reconstr. Surg. 2011, 33, 459–466. [Google Scholar]
- Khan, M.M.R.; Tsukada, M.; Gotoh, Y.; Morikawa, H.; Freddi, G.; Shiozaki, H. Physical properties and dyeability of silk fibers degummed with citric acid. Bioresour. Technol. 2010, 101, 8439–8445. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Cannizzaro, C.; Daheron, L.; Messmer, B.; Vunjak-Novakovic, G.; Kaplan, D.L. Silk fibroin microtubes for blood vessel engineering. Biomaterials 2007, 28, 5271–5279. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Jo, Y.Y.; Seok, H.; Kim, S.G.; Chae, W.S.; Sapru, S.; Kundu, S.C.; Kim, D.W.; Park, N.R.; Che, X. In vivo bone regeneration ability of different layers of natural silk cocoon processed using an eco-friendly method. Macromol. Res. 2017, 25, 806–816. [Google Scholar] [CrossRef]
- Yoo, C.K.; Jeon, J.Y.; Kim, Y.J.; Kim, S.G.; Hwang, K.G. Cell attachment and proliferation of osteoblast-like mg63 cells on silk fibroin membrane for guided bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, M.K.; Kweon, H.; Jo, Y.Y.; Lee, K.G.; Lee, J.K. Comparison of unprocessed silk cocoon and silk cocoon middle layer membranes for guided bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 11. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.Y.; Park, Y.W.; Kweon, H.; Jo, Y.Y.; Kim, S.G. Comparison of the physical properties and in vivo bioactivities of silkworm-cocoon-derived silk membrane, collagen membrane, and polytetrafluoroethylene membrane for guided bone regeneration. Macromol. Res. 2014, 22, 1018–1023. [Google Scholar] [CrossRef]
- Kozubek, A.; Tyman, J.H. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Martı, O.; López-Caballero, M.; Montero, P.; Gómez-Guillén, M.D.C. Spraying of 4-hexylresorcinol based formulations to prevent enzymatic browning in norway lobsters (nephrops norvegicus) during chilled storage. Food Chem. 2007, 100, 147–155. [Google Scholar]
- Rabbani, G.; Gilman, R.; Kabir, I.; Mondel, G. The treatment of fasciolopsis buski infection in children: A comparison of thiabendazole, mebendazole, levamisole, pyrantel pamoate, hexylresorcinol and tetrachloroethylene. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 513–515. [Google Scholar] [CrossRef]
- Kang, Y.J.; Noh, J.E.; Lee, M.J.; Chae, W.S.; Lee, S.Y.; Kim, S.G. The effect of 4-hexylresorcinol on xenograft degradation in a rat calvarial defect model. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 29. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.T.; Park, S.Y.; Kim, M.K.; Kim, S.G.; Park, Y.W.; Kwon, K.J. Effect of 4-hexylresorcinol on blood coagulation and healing of injured vessel in a rat model. Maxillofac. Plast. Reconstr. Surg. 2013, 35, 284–293. [Google Scholar] [CrossRef]
- Kim, S.G.; JeonG, J.H.; Park, Y.W.; SonG, J.Y.; Kim, A.S.; CHoI, J.Y.; Chae, W.S. 4-hexylresorcinol inhibits transglutaminase-2 activity and has synergistic effects along with cisplatin in kb cells. Oncol. Rep. 2011, 25, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Kim, S.G.; Choi, J.Y. Inhibition of foreign body giant cell formation by 4-hexylresorcinol through suppression of diacylglycerol kinase delta gene expression. Biomaterials 2014, 35, 8576–8584. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, K.; Koutsouris, M.; Panagiotopoulos, E.; Antonopoulos, D.; Panagiotopoulos, V.; Papalois, A.; Kepenekidis, A. The effect of nifedipine on the patency of microvascular anastomosis in rats. Acta. Chir. Plast. 2008, 50, 33–35. [Google Scholar] [PubMed]
- Lee, E.H.; Kim, J.Y.; Kweon, H.Y.; Jo, Y.Y.; Min, S.K.; Park, Y.W.; Choi, J.Y.; Kim, S.G. A combination graft of low-molecular-weight silk fibroin with choukroun platelet-rich fibrin for rabbit calvarial defect. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e33–e38. [Google Scholar] [CrossRef] [PubMed]
- Fukayama, T.; Takagi, K.; Tanaka, R.; Hatakeyama, Y.; Aytemiz, D.; Suzuki, Y.; Asakura, T. Biological reaction to small-diameter vascular grafts made of silk fibroin implanted in the abdominal aortae of rats. Ann. Vasc. Surg. 2015, 29, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Baguneid, M.; Seifalian, A.; Salacinski, H.; Murray, D.; Hamilton, G.; Walker, M. Tissue engineering of blood vessels. Br. J. Surg. 2006, 93, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Mi, H.Y.; Zhang, J.; Thomson, J.A.; Turng, L.S. Development of biomimetic thermoplastic polyurethane/fibroin small-diameter vascular grafts via a novel electrospinning approach. J. Biomed. Mater. Res. A 2018, 106, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dong, C.; Lu, G.; Lu, Q.; Li, Z.; Kaplan, D.L.; Zhu, H. Bilayered vascular grafts based on silk proteins. Acta Biomater. 2013, 9, 8991–9003. [Google Scholar] [CrossRef] [PubMed]
- Chantawong, P.; Tanaka, T.; Uemura, A.; Shimada, K.; Higuchi, A.; Tajiri, H.; Sakura, K.; Murakami, T.; Nakazawa, Y.; Tanaka, R. Silk fibroin-pellethane® cardiovascular patches: Effect of silk fibroin concentration on vascular remodeling in rat model. J. Mater. Sci. Mater. Med. 2017, 28, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, H.; Ju, R.; Chen, K.; Li, S.; Wang, W.; Yan, Y. In vivo biocompatibility and hemocompatibility of a polytetrafluoroethylene small diameter vascular graft modified with sulfonated silk fibroin. Am. J. Surg. 2017, 213, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Li, J.; Bowlin, G.L.; Li, D.; Rodriguez, I.A.; Wang, J.; Wu, T.; EI-Hamshary, H.A.; Al-Deyab, S.S.; Mo, X. Fabrication of cell penetration enhanced poly (l-lactic acid-co-ɛ-caprolactone)/silk vascular scaffolds utilizing air-impedance electrospinning. Colloids Surf. B Biointerfaces 2014, 120, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, K.; Mei, J.; Li, C.; Zhang, J.; Zheng, W.; An, D.; Xiao, N.; Zhao, Q.; Kong, D. Fabrication of highly interconnected porous silk fibroin scaffolds for potential use as vascular grafts. Acta Biomater. 2014, 10, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Higuchi, A.; Kubo, R.; Murakami, T.; Nakazawa, Y.; Tanaka, R. The effect of a silk fibroin/polyurethane blend patch on rat vessels. Organogenesis 2017, 13, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rigueiro, J.; Elices, M.; Llorca, J.; Viney, C. Tensile properties of silkworm silk obtained by forced silking. J. Appl. Polym. Sci. 2001, 82, 1928–1935. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.J.; How, T.V.; Poole, R.J.; Brennan, J.A.; Naik, J.B.; Vallabhaneni, S.R.; Fisher, R.K. Closure technique after carotid endarterectomy influences local hemodynamics. J. Vasc. Surg. 2014, 60, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.; Feustel, P.; Darling, R.C., III. In Primary closure, routine patching, and eversion endarterectomy: What is the current state of the literature supporting use of these techniques? Semin. Vasc. Surg. 2007, 20, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.A.; AbuRahma, A.F.; Mousa, A.Y.; Phang, D.; Hass, S.M.; Modak, A.; Dearing, D. Prospective randomized trial of acuseal versus vascu-guard patching in carotid endarterectomy. Ann. Vasc. Surg. 2014, 28, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Ostdiek, A.M.; Ivey, J.R.; Hansen, S.A.; Gopaldas, R.; Grant, S.A. Feasibility of a nanomaterial-tissue patch for vascular and cardiac reconstruction. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Maertens, V.; Maertens, H.; Kint, M.; Coucke, C.; Blomme, Y. Complication rate after carotid endarterectomy comparing patch angioplasty and primary closure. Ann. Vasc. Surg. 2016, 30, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Inflammatory response to implants. ASAIO Trans. 1988, 34, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Baker, P.; Coburn, R.; Fischman, S.; Genco, R. In vitro antiplaque effects of antiseptic phenols. J. Periodontol. 1977, 48, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, S.G.; Kim, M.K.; Kim, D.W.; Lee, J.H.; Seok, H.; Choi, J.Y. Topical delivery of 4-hexylresorcinol promotes wound healing via tumor necrosis factor-α suppression. Burns 2016, 42, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jo, Y.Y.; Kweon, H.Y.; Kim, D.W.; Kim, S.G. The effects of proteins released from silk mat layers on macrophages. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 10. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Matanzo, A.; Manferrari, G.; Marchetti, B.; Pluchino, S. Wnt3a promotes pro-angiogenic features in macrophages in vitro: Implications for stroke pathology. Exp. Biol. Med. 2018, 243, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Kawai, T.; Osugi, M.; Sugimura-Wakayama, Y.; Sakaguchi, K.; Kojima, T.; Kobayashi, T. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 8. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.H.; Nam, W.; Cha, I.H.; Kim, H.J. Revisiting radial forearm free flap for successful venous drainage. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 14. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.A.; Chung, C.U.; Bauder, A.R.; Wu, L.C. Prevention of thrombosis in hypercoagulable patients undergoing microsurgery: A novel anticoagulation protocol. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 307–312. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-W.; Kim, M.-K.; Kim, S.-G.; Park, Y.-W.; Park, Y.-T.; Kim, D.-W.; Seok, H. Angioplasty Using 4-Hexylresorcinol-Incorporated Silk Vascular Patch in Rat Carotid Defect Model. Appl. Sci. 2018, 8, 2388. https://doi.org/10.3390/app8122388
Kim C-W, Kim M-K, Kim S-G, Park Y-W, Park Y-T, Kim D-W, Seok H. Angioplasty Using 4-Hexylresorcinol-Incorporated Silk Vascular Patch in Rat Carotid Defect Model. Applied Sciences. 2018; 8(12):2388. https://doi.org/10.3390/app8122388
Chicago/Turabian StyleKim, Chan-Woo, Min-Keun Kim, Seong-Gon Kim, Young-Wook Park, Yong-Tae Park, Dae-Won Kim, and Hyun Seok. 2018. "Angioplasty Using 4-Hexylresorcinol-Incorporated Silk Vascular Patch in Rat Carotid Defect Model" Applied Sciences 8, no. 12: 2388. https://doi.org/10.3390/app8122388
APA StyleKim, C.-W., Kim, M.-K., Kim, S.-G., Park, Y.-W., Park, Y.-T., Kim, D.-W., & Seok, H. (2018). Angioplasty Using 4-Hexylresorcinol-Incorporated Silk Vascular Patch in Rat Carotid Defect Model. Applied Sciences, 8(12), 2388. https://doi.org/10.3390/app8122388





