The Influence of Effective Microorganisms on Microbes and Nutrients in Kiwifruit Planting Soil
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Area
2.2. Strains and EMs Preparation
2.3. Fertilization
2.4. Collection of Soil Samples
2.5. Determination of Soil Microbe Counts
2.6. Determination of Soil Nutrients
3. Results
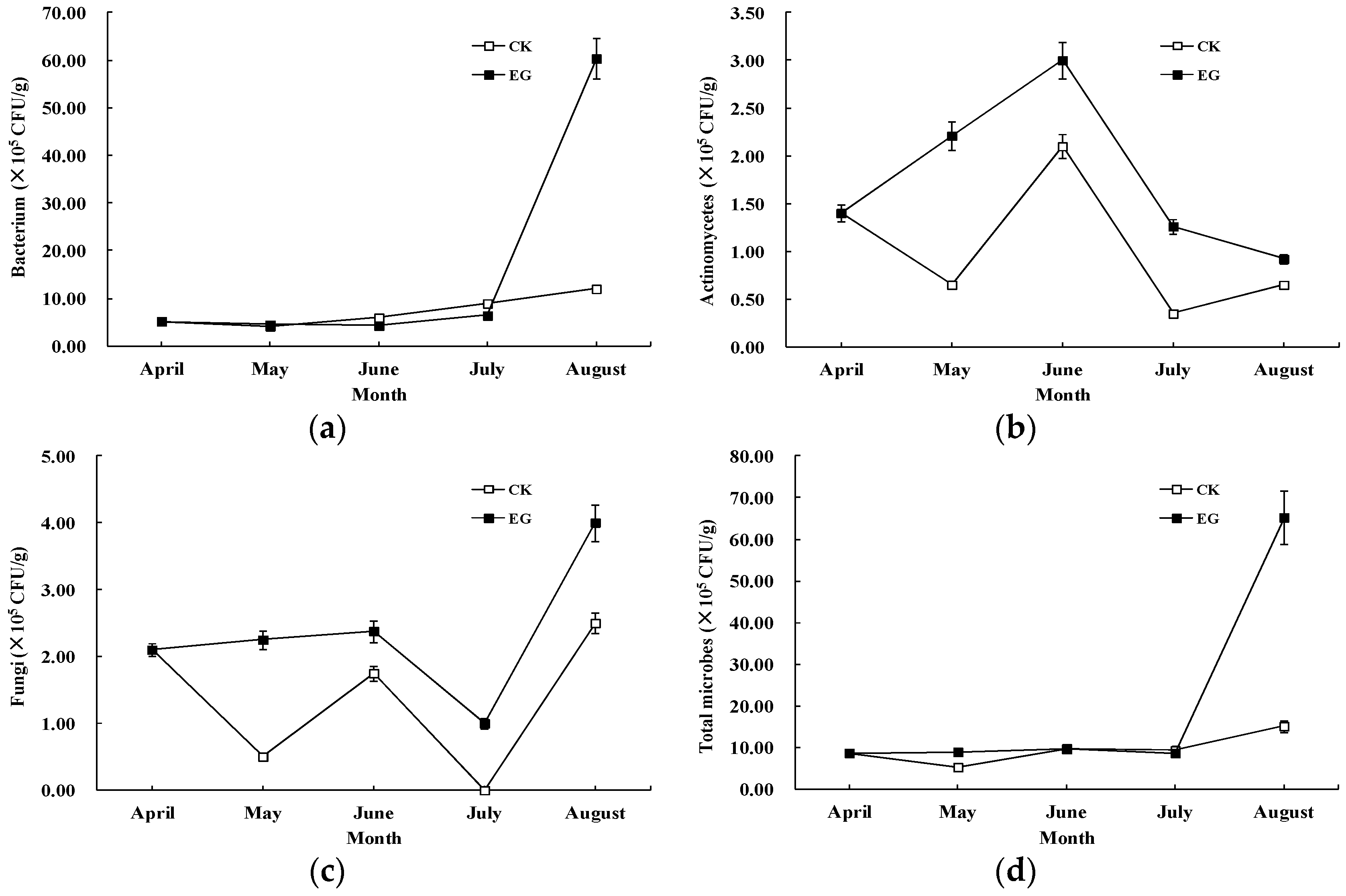
3.1. Effects of EMs on Microbe Counts
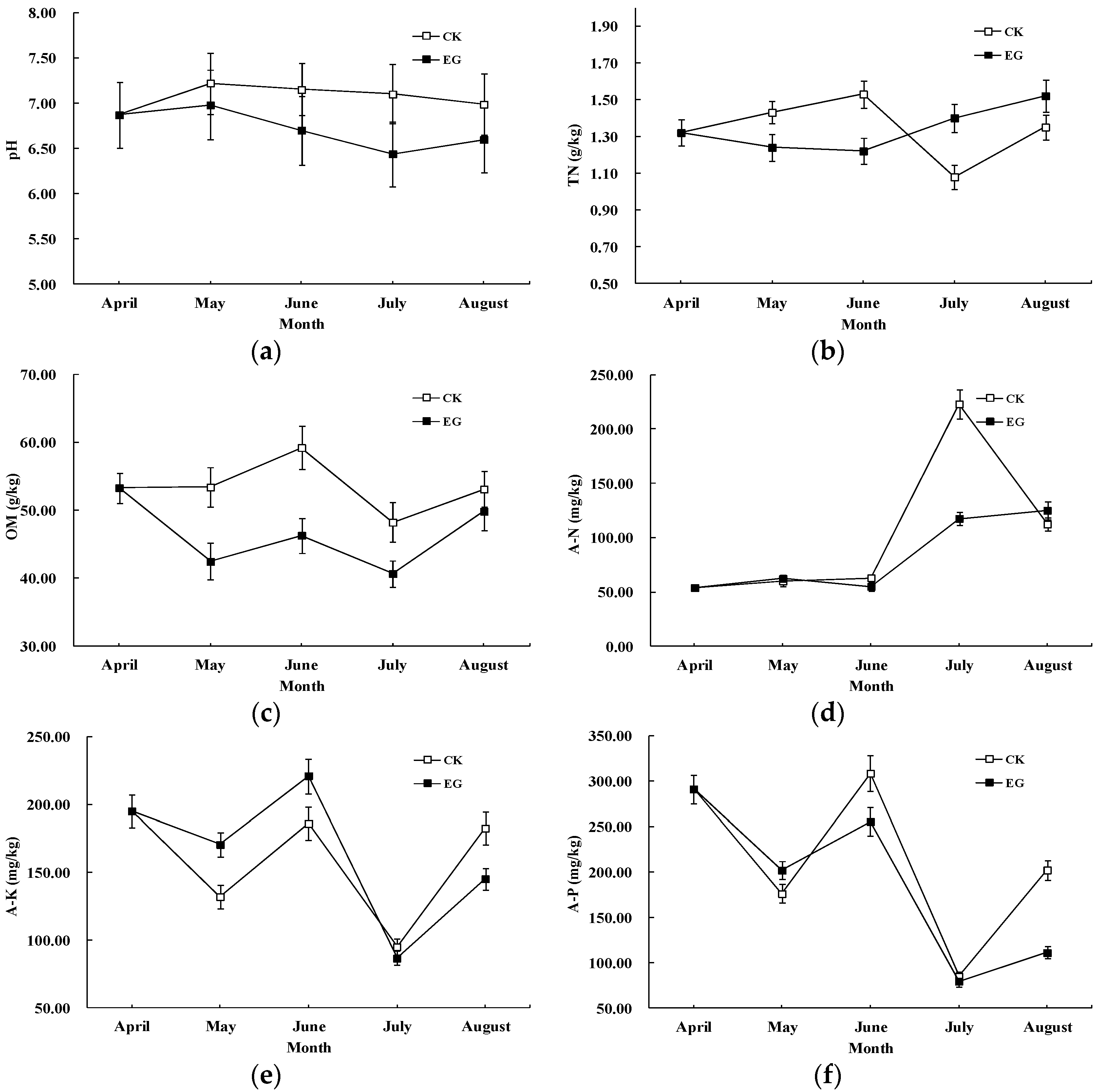
3.2. Effects of EMs on pH and Soil Nutrients
3.3. Association between Microbes and Soil Nutrients
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gammon, C.S.; Kruger, R.; Minihane, A.M.; Conlon, C.A.; von Hurst, P.R.; Stonehouse, W. Kiwifruitfruit consumption favourably affects plasma lipids in a randomised controlled trial in hypercholesterolaemic men. Br. J. Nutr. 2013, 109, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, Y.C.; Han, K.S.; Singh, H.; Yoon, M.; Park, J.H.; Cho, C.W.; Cho, S. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013, 136, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Imeh, U.; Khokhar, S. Distribution of conjugated and free phenols in fruits: Antioxidant activity and cultivar variations. J. Agric. Food Chem. 2002, 50, 6301–6306. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Sârbu, C.; Naşcu-Briciu, R.D.; Kot-Wasik, A.; Gorinstein, S.; Wasik, A.; Namieśnik, J. Classification and fingerprinting of kiwifruit and pomelo fruits by multivariate analysis of chromatographic and spectroscopic data. Food Chem. 2012, 130, 994–1002. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Im, M.H.; Choi, J.H.; Yim, S.H.; Leontowicz, H.; Leontowicz, M.; Suhaj, M.; Gorinstein, S. The effects of ethylene treatment on the bioactivity of conventional and organic growing ‘Hayward’ kiwifruit fruit. Sci. Hortic-Amst. 2013, 164, 589–595. [Google Scholar] [CrossRef]
- Zhou, D.M.; Hao, X.Z.; Wang, Y.J.; Dong, Y.H.; Cang, L. Copper and Zn uptake by radish and pakchoi as affected by application of livestock and poultry manures. Chemosphere 2005, 59, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, S.; Liu, L.L. Studies on phosphorus solubilizing activity of a strain of phosphobacteria isolated from chestnut type soil in China. Bioresour. Technol. 2008, 99, 6702–6707. [Google Scholar] [CrossRef] [PubMed]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Donmez, M.F.; Turan, M.; Gunes, A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci. Hortic-Amst. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Yu, X.; An, M.; Wang, H.; Shen, G.; Tian, S.; Sun, X.; Wang, L. Influence of Bacillus subtilis Bs-15 on the microbial population and functional diversity of microbial communities in the chestnut soil. Ecol. Environ. Sci. 2014, 23, 598–602. [Google Scholar]
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free living plant growth-promoting rhizobacteria. Antonie Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Cakmakci, R.; Dönmez, M.F.; Erdoğan, Ü. The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk. J. Agric. For. 2007, 31, 189–199. [Google Scholar]
- Ryu, C.; Murphy, J.F.; Reddy, M.; Kloepper, J.W. A two-strain mixture of rhizobacteria elicits induction of systemic resistance against Pseudomonas syringae and Cucumber mosaic virus coupled to promotion of plant growth on Arabidopsis thaliana. J. Microbiol. Biotechnol. 2007, 17, 280–286. [Google Scholar]
- Das, K.; Dang, R.; Shivananda, T.N. Effect of biofertilizers on the nutrient availability in soil in relation to growth, yield and yield attributes of Stevia rebaudiana. Arch. Agron. Soil Sci. 2009, 55, 359–366. [Google Scholar] [CrossRef]
- Da Silva Oliveira, W.; Stamford, N.P.; Vila Nova da Silva, E.; de Rosalia e Silva Santos, C.E.; Santiago de Freitas, A.D.; Stamford Arnaud, T.M.; Sarmento, B.F. Biofertilizer produced by interactive microbial processes affects melon yield and nutrients availability in a Brazilian semiarid soil. Aust. J. Crop. Sci. 2014, 8, 1124–1130. [Google Scholar]
- Zhang, D.; Xu, N.; Nu, M.; Luo, Y.; Li, J.; Liu, T. Technical Specification of Balanced Fertilization by Soil Testing; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006.
- Zou, L.; Li, L.; Pan, X.; Tian, G.; Luo, Y.; Wu, Q.; Li, B.; Cheng, L.; Xiao, J.; Hu, S.; et al. Molecular characterization of β-lactam-resistant Escherichia coli isolated from Fu River, China. World J. Microb. Biot. 2012, 28, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Soil Science Society of China. Methods of Soil and Agricultural Chemistry Analysis; China Agriculture Scientech Press: Beijing, China, 1999; pp. 147–194. [Google Scholar]
- Luo, H.; Liu, X.; Anderson, B.C.; Zhang, K.; Li, X.; Huang, B.; Li, M.; Mo, Y.; Fan, L.; Shen, Q.; et al. Carbon sequestration potential of green roofs using mixed-sewage-sludge substrate in Chengdu World Modern Garden City. Ecol. Indic. 2015, 49, 247–259. [Google Scholar] [CrossRef]
- Huang, S.W.; Jin, J.Y.; Yang, L.P.; Bai, Y.L. Spatial variability of soil nutrients and influencing factors in a vegetable production area of Hebei Province in China. Nutr. Cycl. Agroecosyst. 2006, 75, 201–212. [Google Scholar] [CrossRef]
- Sand, W. Microbial life in geothermal waters. Geothermics 2003, 32, 655–667. [Google Scholar] [CrossRef]
- Pang, X.; Ning, W.; Qing, L.; Bao, W. The relation among soil microorganism, enzyme activity and soil nutrients under subalpine coniferous forest in Western Sichuan. Acta Ecol. Sin. 2009, 29, 286–292. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, G.Y.; Tian, Y.Y.; Liu, L.Q.; Liu, C.F.; Yang, Q.; Zhou, J.C. Differential analysis of soil bacteria diversity in different mixed forests of Dalbergia odorifera. Acta Ecol. Sin. 2015, 35, 8117–8127. [Google Scholar]
- Gaind, S.; Gaur, A.C. Effects of pH on phosphate solubilization by microbes. Curr. Sci. India 1989, 58, 1208–1211. [Google Scholar]
- Turan, M.; Ataoglu, N.; Sezen, Y. Effects of phosphorus solubilizing bacteria (Bacillus megaterium) on yield and phosphorus contents of tomato plant (Lycopersicon esculentum L.). In Proceedings of the Third National Fertilizer Congress (Farming–Industry–Environment), Tokat, Turkey, 11–13 October 2004; pp. 939–945.
- Yan, H.; Guo, S.; Liu, W. Effects of Bacillus subtilis on rhizosphere enzyme activities of cucumber under salt-stress. Acta Agric. Boreali-Sin. 2010, 25, 209–212. [Google Scholar]
- Yin, H. Studies on Improving the Capacity of Salt-Tolerance of Cucumber by Bacillus subtilis. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2006. [Google Scholar]
- Walia, A.; Mehta, P.; Chauhan, A.; Shirkot, C.K. Effect of Bacillus subtilis strain CKT1 as inoculum on growth of tomato seedlings under net house conditions. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 145–155. [Google Scholar] [CrossRef]
- Shweta, G.; Rajesh, K.; Kirti, K.; Anjali, C.; Singh, S.R. Efficacy of indigenous plant growth promoting rhizobacteria on capsicum yield and soil health. Res. Crop. 2015, 16, 123–132. [Google Scholar]
- Li, Y.; Yan, J.; Hou, Q.; Xie, Y. Effects of bacterial manure on soil base dissolving N under different conditions of soil moisture content. J. Changzhi Univ. 2006, 23, 5–7. [Google Scholar]
- Ma, Y.; Luo, Y.; Teng, Y.; Li, Z. Plant growth promoting rhizobacteria and their role in phytoremediation of heavy metal contaminated soils. Acta Pedol. Sin. 2013, 50, 1021–1031. [Google Scholar]
- Mantelin, S.; Touraine, B. Plant growth-promoting bacteria and nitrate availability: Impacts on root development and nitrate uptake. J. Exp. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hameeda, B.; Harini, G.; Rupela, O.P.; Wani, S.P.; Reddy, G. Growth promotion of maize by phosphate solubilizing bacteria isolated from compost and microfauna. Microbiol. Res. 2008, 163, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol. 2014, 3, 121–128. [Google Scholar] [CrossRef]
- Idriss, E.E.; Makarewicz, O.; Farouk, A.; Rosner, K.; Greiner, R.; Bochow, H.; Richter, T.; Borriss, R. Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effecta. Microbiology 2002, 148, 2097–2109. [Google Scholar] [PubMed]
- Liu, P. Impact of Bacillus amyloliquefaciens and Phosphorus in Rhizosphere on the Growth and P uptake of maize. Master’s Thesis, Jilin University, Jilin, China, 2013. [Google Scholar]
| Microbe (y) | Soil Nutrients (x) | Correlation Equation | r | p |
|---|---|---|---|---|
| Bacteria | TN | y = 0.004x + 1.272 | 0.835 | 0.078 |
| A-N | y = 0.988x + 66.93 | 0.690 | 0.197 | |
| OM | y = 0.073x + 45.33 | 0.349 | 0.565 | |
| A-K | y = −0.477x + 171.3 | −0.230 | 0.710 | |
| A-P | y = −1.812x + 217.1 | −0.494 | 0.398 | |
| Actinomycetes | TN | y = −0.130x + 1.568 | −0.887 * | 0.045 |
| A-N | y = −30.41x + 136.3 | −0.724 | 0.166 | |
| OM | y = −1.570x + 49.27 | −0.253 | 0.682 | |
| A-K | y = 41.18x + 91.25 | 0.676 | 0.210 | |
| A-P | y = 60.62x + 81.30 | 0.562 | 0.325 | |
| Fungi | TN | y = 0.049x + 1.224 | 0.429 | 0.471 |
| A-N | y = 6.794x + 66.93 | 0.206 | 0.740 | |
| OM | y = 2.612x + 40.38 | 0.542 | 0.346 | |
| A-K | y = 14.42x + 129.8 | 0.302 | 0.622 | |
| A-P | y = −1.234x + 190.7 | −0.015 | 0.981 |
| Month | Bacteria (%) | Fungi (%) | Actinomycetes (%) |
|---|---|---|---|
| April | 59.76 | 24.17 | 16.07 |
| May (CK) | 78.24 | 9.46 | 12.30 |
| May (EG) | 50.00 | 25.00 | 25.00 |
| June (CK) | 61.00 | 18.00 | 21.00 |
| June (EG) | 44.10 | 24.70 | 31.20 |
| July (CK) | 96.24 | 0.00 | 3.76 |
| July (EG) | 73.95 | 11.53 | 14.52 |
| August (CK) | 79.21 | 16.50 | 4.29 |
| August (EG) | 92.46 | 6.13 | 1.41 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Zhou, X.; Li, Y.; Ji, L.; Wu, G.; Li, B.; Cheng, L.; Long, M.; Deng, W.; Zou, L. The Influence of Effective Microorganisms on Microbes and Nutrients in Kiwifruit Planting Soil. Appl. Sci. 2016, 6, 168. https://doi.org/10.3390/app6060168
Fan L, Zhou X, Li Y, Ji L, Wu G, Li B, Cheng L, Long M, Deng W, Zou L. The Influence of Effective Microorganisms on Microbes and Nutrients in Kiwifruit Planting Soil. Applied Sciences. 2016; 6(6):168. https://doi.org/10.3390/app6060168
Chicago/Turabian StyleFan, Liangqian, Xi Zhou, Yongsheng Li, Lin Ji, Guoyan Wu, Bei Li, Lin Cheng, Mei Long, Wenwen Deng, and Likou Zou. 2016. "The Influence of Effective Microorganisms on Microbes and Nutrients in Kiwifruit Planting Soil" Applied Sciences 6, no. 6: 168. https://doi.org/10.3390/app6060168





