Sustainable Cultivation of Galdieria phlegrea in an IoT-Integrated Twin-Layer Photobioreactor: System Design, Growth Dynamics, and Isotopic Perspective
Abstract
1. Introduction
Rationale Behind the Selection of Twin-Layer Cultivation Technology
2. Materials and Methods
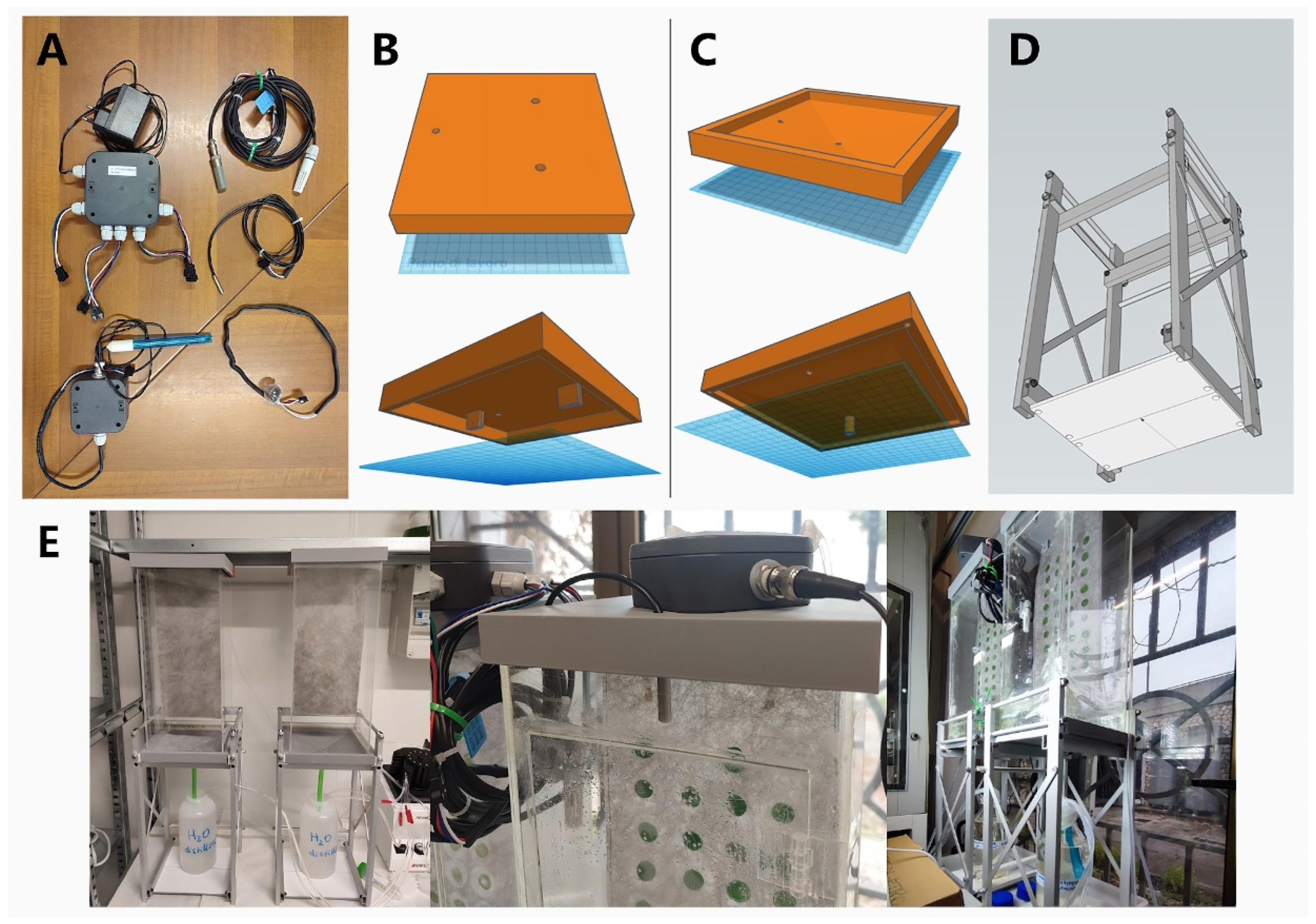
2.1. The TL-PBR Structure
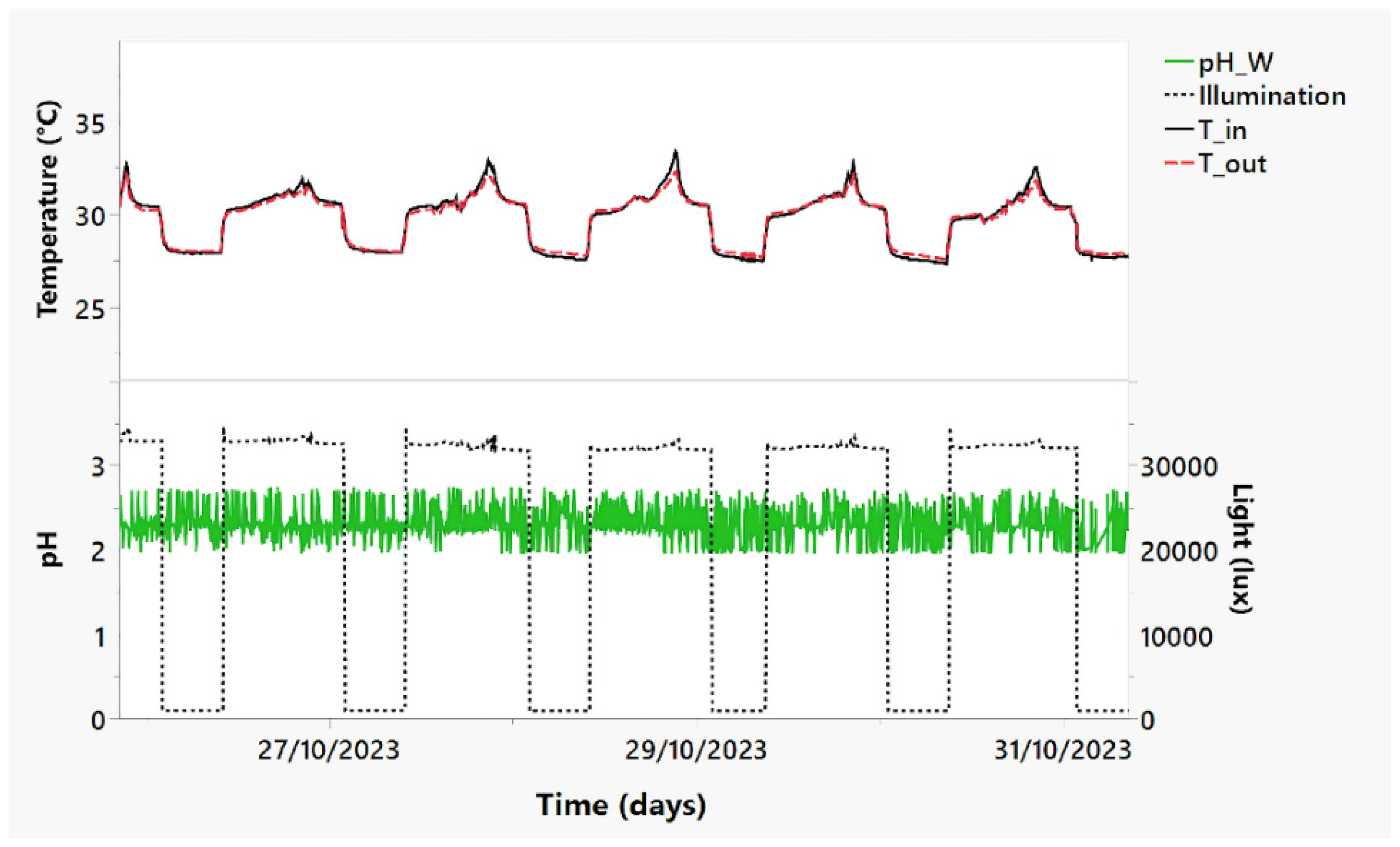
2.2. The Smart Monitoring System
2.3. Cultivation Tests
2.3.1. Experimental Setup
2.3.2. Culture Media Preparation
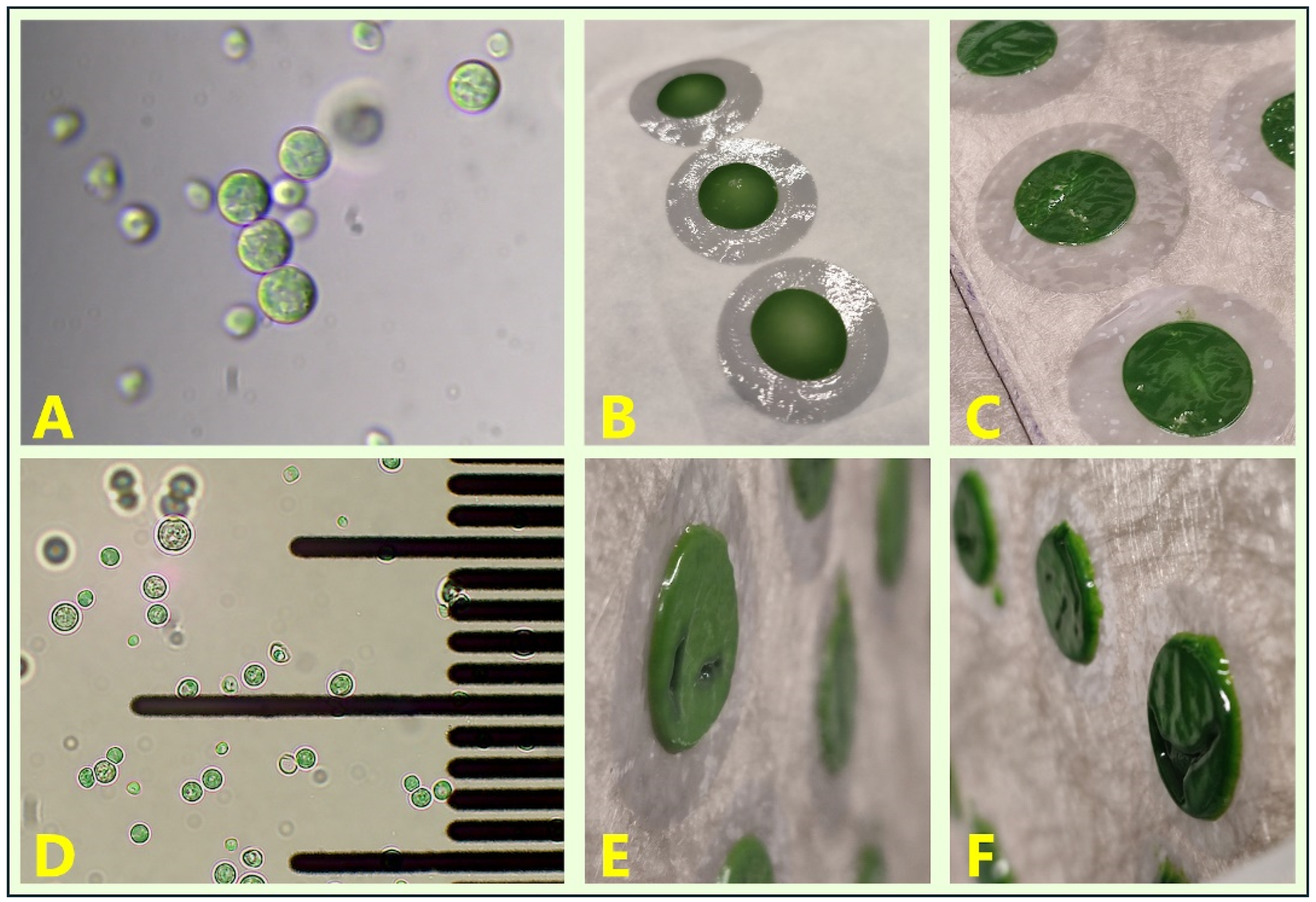
2.3.3. Initial Biomass Inoculum Preparation and Growth Monitoring
2.4. Biomass Characterisation
2.4.1. EA-IRMS Analysis
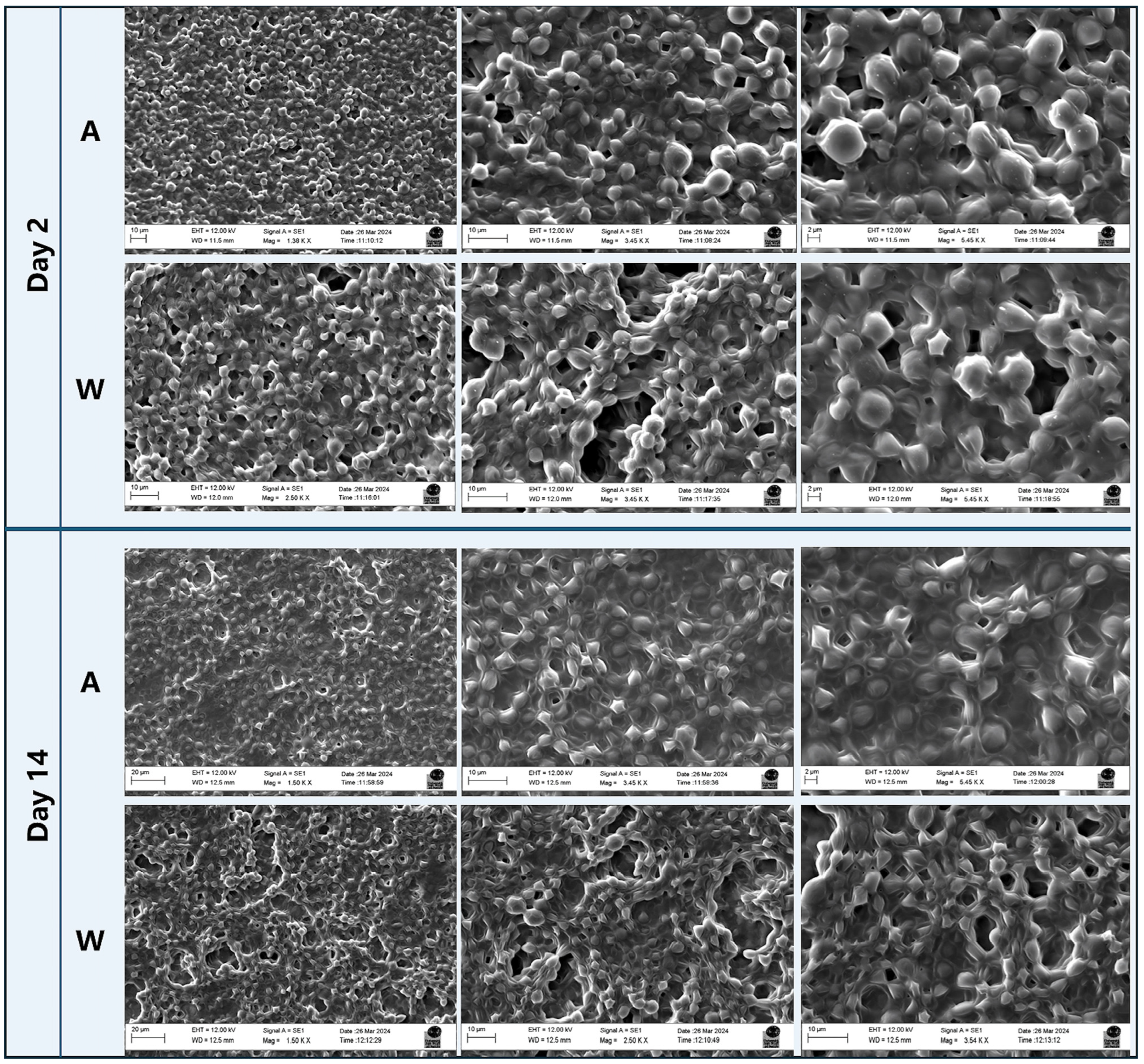
2.4.2. SEM-EDS Analysis
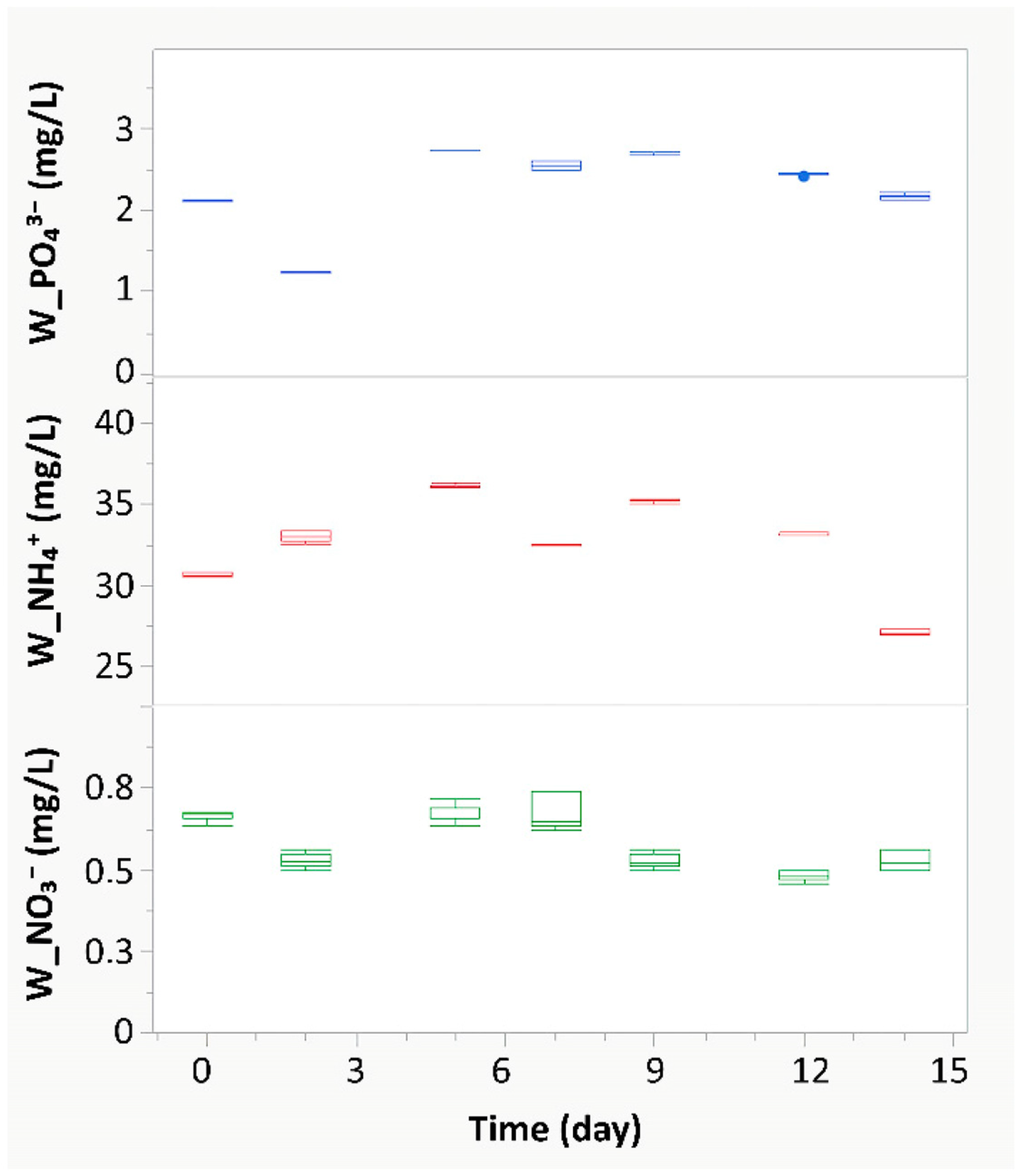
2.5. Analysis of NH4+, NO3− and PO43− Content in W e A
2.6. Data Processing and Statistical Analysis
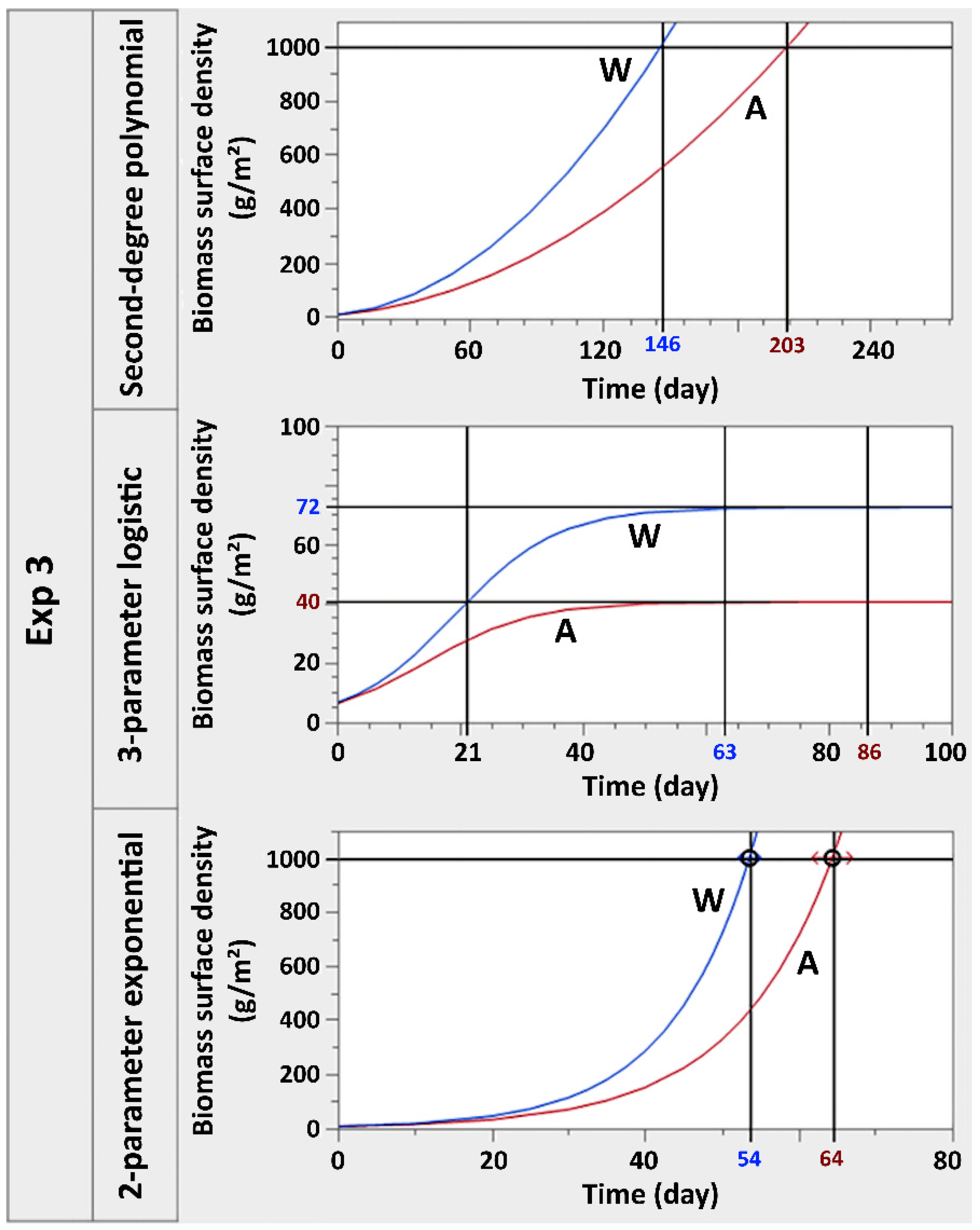
- Second-degree polynomial, . In this equation, a and b are the growth rate coefficients of the second and first-degree functions of x, respectively, while c represents the value of the surface density at time 0;
- Three-parameter logistic, . In this equation, a represents the growth rate, b is the value of x for which and c represents the asymptotic algal density value reached at saturation according to this model;
- Two-parameter exponential, . In this equation, a represents the surface algal density at time 0 and b represents the growth rate.
3. Results and Discussion
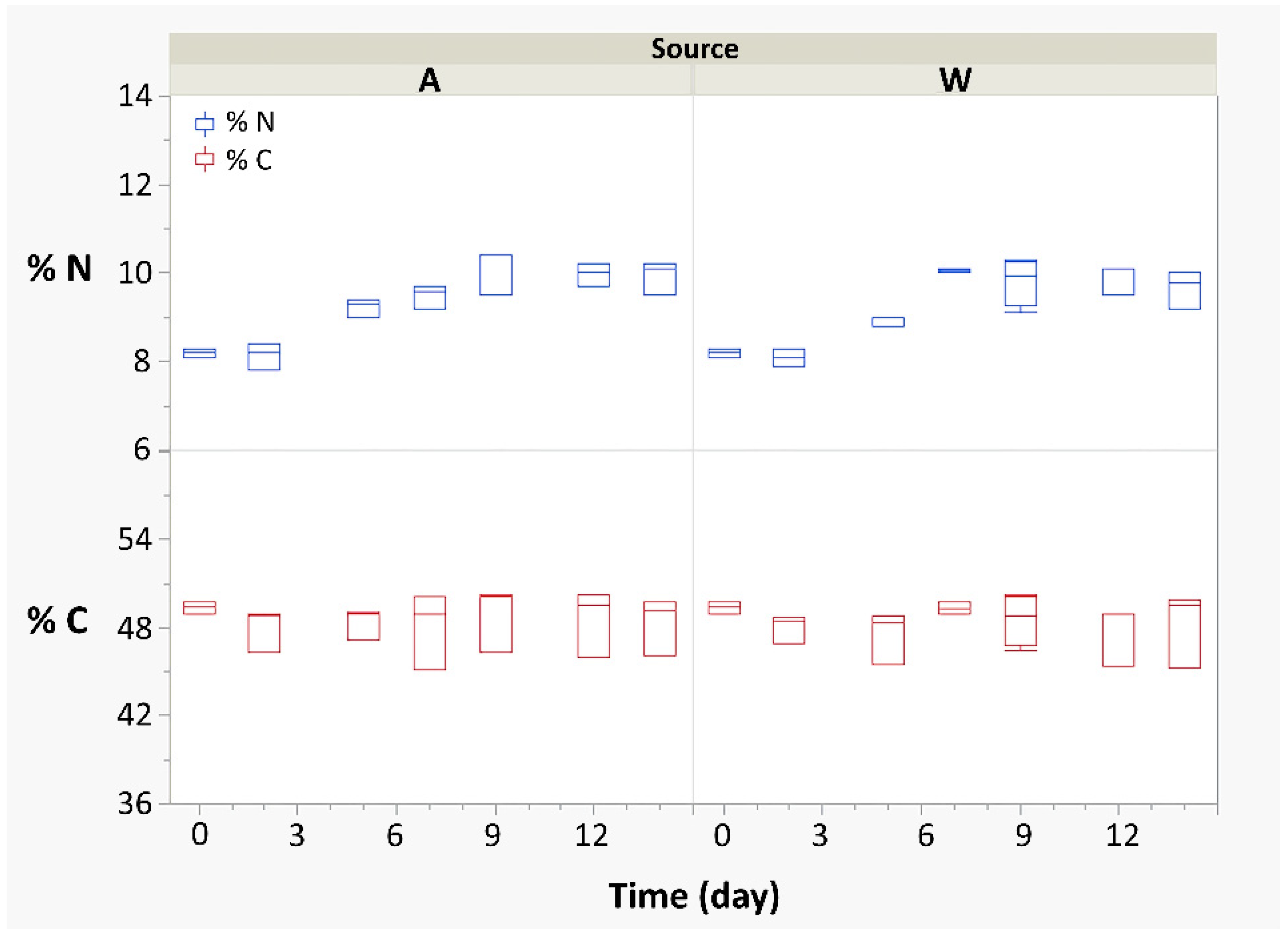
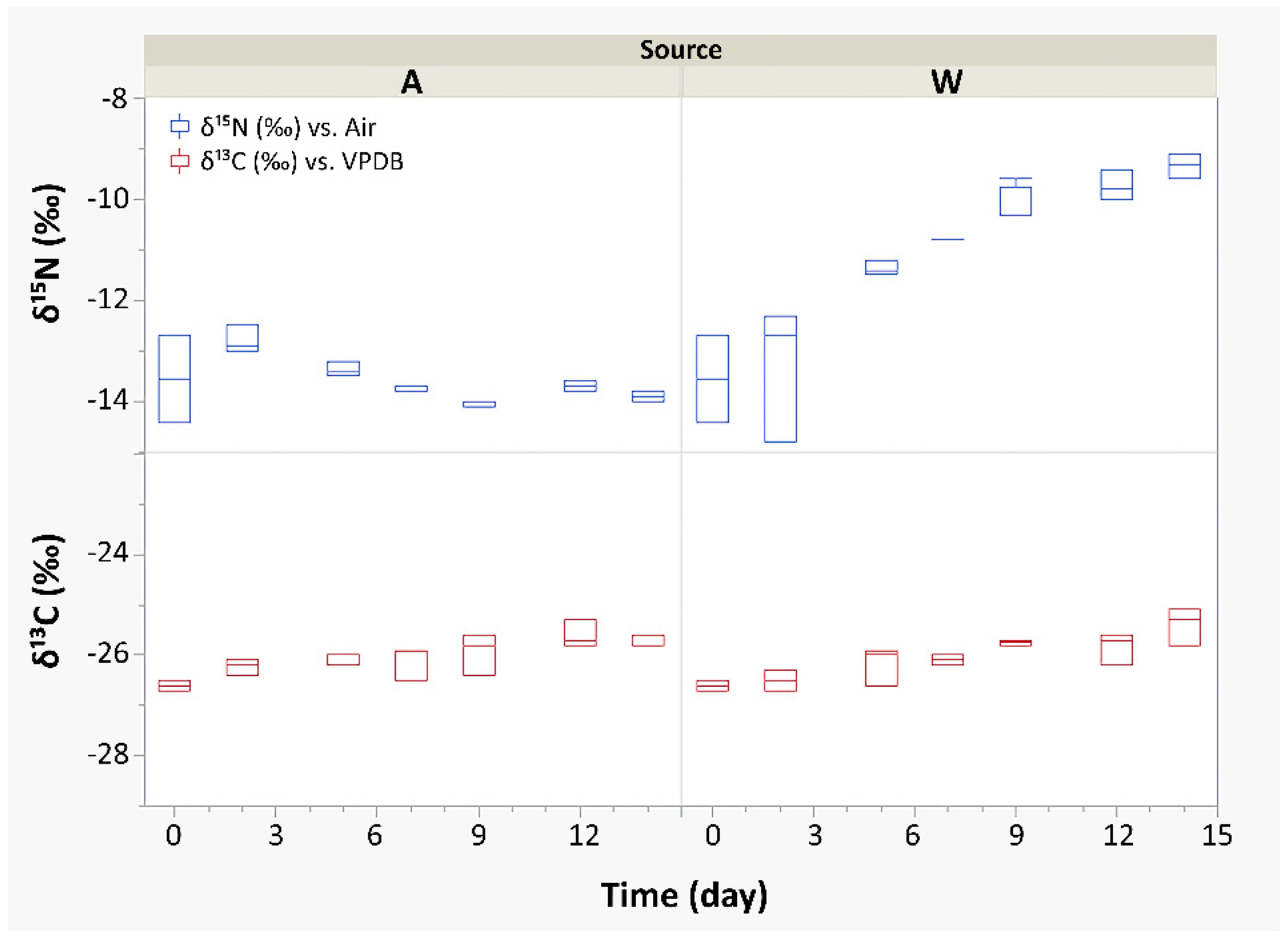
3.1. Metabolic Response and Connections with Isotopic, Spectrophotometric, and Monitoring Data
3.2. SEM Characterisation and Projections of Biomass Yields Obtainable from the System
3.3. Opportunities for Process Scale-Up and Use of Biomass Produced
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Allen medium |
| AICc | Akaike Information Criterion corrected |
| BIC | Bayesian Information Criterion |
| EA-IRMS | Elemental Analysis coupled with Isotopic Ratio Mass Spectrometry |
| IoT | Internet of Things |
| MSE | Mean Squared Error |
| PBR | Photobioreactor |
| RMSE | Root Mean Squared Error |
| SEM-EDS | Scanning Electron Microscopy coupled with Energy-Dispersive Spectroscopy |
| SSE | Sum of Squared Errors |
| TL | twin layers |
| TL-PBR | twin-layer type photobioreactor |
| W | wastewater medium |
References
- Canelli, G.; Abiusi, F.; Vidal Garcia, A.; Canziani, S.; Mathys, A. Amino acid profile and protein bioaccessibility of two Galdieria sulphuraria strains cultivated autotrophically and mixotrophically in pilot-scale photobioreactors. Innov. Food Sci. Emerg. Technol. 2023, 84, 103287. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Show, K.-Y.; Yan, Y.; Zong, C.; Guo, N.; Chang, J.-S.; Lee, D.-J. State of the art and challenges of biohydrogen from microalgae. Bioresour. Technol. 2019, 289, 121747. [Google Scholar] [CrossRef]
- González, I.; Herrero, N.; Siles, J.Á.; Chica, A.F.; Martín, M.; Izquierdo, C.G.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2020, 82, 1044–1061. [Google Scholar] [CrossRef] [PubMed]
- Iovinella, M.; Palmieri, M.; Papa, S.; Auciello, C.; Ventura, R.; Lombardo, F.; Race, M.; Lubritto, C.; di Cicco, M.R.; Davis, S.J.; et al. Biosorption of rare earth elements from luminophores by G. sulphuraria (Cyanidiophytina, Rhodophyta). Environ. Res. 2023, 239, 117281. [Google Scholar] [CrossRef] [PubMed]
- Čížková, M.; Mezricky, P.; Mezricky, D.; Rucki, M.; Zachleder, V.; Vítová, M. Bioaccumulation of Rare Earth Elements from Waste Luminophores in the Red Algae, Galdieria phlegrea. Waste Biomass Valorization 2020, 12, 3137–3146. [Google Scholar] [CrossRef]
- Yang, S.; Fan, Y.; Cao, Y.; Wang, Y.; Mou, H.; Sun, H. Technological readiness of commercial microalgae species for foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 7993–8017. [Google Scholar] [CrossRef]
- Gallego, I.; Medic, N.; Pedersen, J.S.; Ramasamy, P.K.; Robbens, J.; Vereecke, E.; Romeis, J. The microalgal sector in Europe: Towards a sustainable bioeconomy. New Biotechnol. 2025, 86, 1–13. [Google Scholar] [CrossRef]
- Malavasi, V.; Soru, S.; Cao, G. Extremophile Microalgae: The potential for biotechnological application. J. Phycol. 2020, 56, 559–573. [Google Scholar] [CrossRef]
- Palmieri, M.; Iovinella, M.; Davis, S.J.; di Cicco, M.R.; Lubritto, C.; Race, M.; Papa, S.; Fabbricino, M.; Ciniglia, C. Galdieria sulphuraria ACUF427 Freeze-Dried Biomass as Novel Biosorbent for Rare Earth Elements. Microorganisms 2022, 10, 2138. [Google Scholar] [CrossRef]
- Retta, B.; Iovinella, M.; Ciniglia, C. Significance and Applications of the Thermo-Acidophilic Microalga Galdieria sulphuraria (Cyanidiophytina, Rhodophyta). Plants 2024, 13, 1786. [Google Scholar] [CrossRef] [PubMed]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour. Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef] [PubMed]
- di Cicco, M.R.; Iovinella, M.; Palmieri, M.; Lubritto, C.; Ciniglia, C. Extremophilic Microalgae Galdieria Gen. for Urban Wastewater Treatment: Current State, the Case of “POWER” System, and Future Prospects. Plants 2021, 10, 2343. [Google Scholar] [CrossRef] [PubMed]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar] [CrossRef]
- di Cicco, M.R.; Palmieri, M.; Altieri, S.; Ciniglia, C.; Lubritto, C. Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields. Int. J. Environ. Res. Public Health 2021, 18, 2291. [Google Scholar] [CrossRef]
- Wan, M.; Wang, Z.; Zhang, Z.; Wang, J.; Li, S.; Yu, A.; Li, Y. A novel paradigm for the high-efficient production of phycocyanin from Galdieria sulphuraria. Bioresour. Technol. 2016, 218, 272–278. [Google Scholar] [CrossRef]
- Fasaei, F.; Bitter, J.; Slegers, P.; Van Boxtel, A. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M.; Barbosa, M.; Gouveia, L.; Sepúlveda, C.; Bazaes, J.; Arbib, Z. 20—Economics of microalgae production. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 485–503. [Google Scholar]
- Carbone, D.A.; Olivieri, G.; Pollio, A.; Melkonian, M. Biomass and phycobiliprotein production of Galdieria sulphuraria, immobilized on a twin-layer porous substrate photobioreactor. Appl. Microbiol. Biotechnol. 2020, 104, 3109–3119. [Google Scholar] [CrossRef]
- Naumann, T.; Çebi, Z.; Podola, B.; Melkonian, M. Growing microalgae as aquaculture feeds on twin-layers: A novel solid-state photobioreactor. J. Appl. Phycol. 2013, 25, 1413–1420. [Google Scholar] [CrossRef]
- Muñoz, M.; Guzmán, J.L.; Torres, M.; Acién, F.G. An IoT platform for data management in an industrial-scale microalgae cultivation plant. IEEE Access 2022, 10, 127128–127139. [Google Scholar] [CrossRef]
- Wang, K.; Khoo, K.S.; Leong, H.Y.; Nagarajan, D.; Chew, K.W.; Ting, H.Y.; Selvarajoo, A.; Chang, J.-S.; Show, P.L. How does the Internet of Things (IoT) help in microalgae biorefinery? Biotechnol. Adv. 2022, 54, 107819. [Google Scholar] [CrossRef] [PubMed]
- di Cicco, M.R.; Masiello, A.; Spagnuolo, A.; Vetromile, C.; Borea, L.; Giannella, G.; Iovinella, M.; Lubritto, C. Real-Time Monitoring and Static Data Analysis to Assess Energetic and Environmental Performances in the Wastewater Sector: A Case Study. Energies 2021, 14, 6948. [Google Scholar] [CrossRef]
- Aron, N.S.M.; Khoo, K.S.; Chew, K.W.; Veeramuthu, A.; Chang, J.-S.; Show, P.L. Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies. J. Water Process Eng. 2021, 39, 101701. [Google Scholar]
- Pinto, G.; Ciniglia, C.; Cascone, C.; Pollio, A. Species Composition of Cyanidiales Assemblages in Pisciarelli (Campi Flegrei, Italy) and Description of Galdieria phlegrea SP. NOV. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 487–502. [Google Scholar]
- Zuccaro, G.; Yousuf, A.; Pollio, A.; Steyer, J.-P. Chapter 2—Microalgae Cultivation Systems. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 11–29. [Google Scholar]
- Altieri, S.; Saiano, K.; Biondi, M.; Ricci, P.; Lubritto, C. Traceability of ‘Mozzarella di Bufala Campana’production chain by means of carbon, nitrogen and oxygen stable isotope ratios. J. Sci. Food Agric. 2020, 100, 995–1003. [Google Scholar] [CrossRef]
- Criss, R.E. Principles of Stable Isotope Distribution; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Germinario, C.; Cultrone, G.; Cavassa, L.; De Bonis, A.; Izzo, F.; Langella, A.; Mercurio, M.; Morra, V.; Munzi, P.; Grifa, C. Local production and imitations of Late Roman pottery from a well in the Roman necropolis of Cuma in Naples, Italy. Geoarchaeology 2019, 34, 62–79. [Google Scholar] [CrossRef]
- Yousuf, A. Fundamentals of microalgae cultivation. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–9. [Google Scholar]
- Varshney, P.; Sohoni, S.; Wangikar, P.P.; Beardall, J. Effect of high CO2 concentrations on the growth and macromolecular composition of a heat- and high-light-tolerant microalga. J. Appl. Phycol. 2016, 28, 2631–2640. [Google Scholar] [CrossRef]
- Abiusi, F.; Moñino Fernández, P.; Canziani, S.; Janssen, M.; Wijffels, R.H.; Barbosa, M. Mixotrophic cultivation of Galdieria sulphuraria for C-phycocyanin and protein production. Algal Res. 2022, 61, 102603. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Wan, M.; Wang, R.; Huang, J.; Zhang, K.; Guo, J.; Bai, W.; Li, Y. Comparative study on light attenuation models of Galdieria sulphuraria for efficient production of phycocyanin. J. Appl. Phycol. 2020, 32, 165–174. [Google Scholar] [CrossRef]
- Carbone, D.A.; Olivieri, G.; Pollio, A.; Melkonian, M. Comparison of Galdieria growth and photosynthetic activity in different culture systems. AMB Express 2020, 10, 170. [Google Scholar] [CrossRef]
- Sloth, J.K.; Wiebe, M.G.; Eriksen, N.T. Accumulation of phycocyanin in heterotrophic and mixotrophic cultures of the acidophilic red alga Galdieria sulphuraria. Enzym. Microb. Technol. 2006, 38, 168–175. [Google Scholar] [CrossRef]
- Buckeridge, E.; Caballero, C.C.; Smith, D.H.; Stott, M.B.; Carere, C.R. Substrate and nutrient manipulation during continuous cultivation of extremophilic algae, Galdieria spp. RTK 37.1, substantially impacts biomass productivity and composition. Biotechnol. Bioeng. 2024, 121, 3428–3439. [Google Scholar] [CrossRef]
- NOAA. NOAA Earth System Research Laboratories. Available online: https://www.esrl.noaa.gov/ (accessed on 11 June 2024).
- di Cicco, M.R.; Altieri, S.; Mantile, N.; Petitti, P.; Persiani, C.; Conti, A.M.; Allegrezza, L.; Cavazzuti, C.; Lubritto, C. Exploring Burial and Dietary Patterns at the Copper Age Necropolis of Selvicciola (Viterbo, Italy): New Perspectives from 14C and Stable Isotope Data. Heritage 2024, 7, 3291–3309. [Google Scholar] [CrossRef]
- Mohd Udaiyappan, A.F.; Hasan, H.A.; Takriff, M.S.; Abdullah, S.R.S.; Maeda, T.; Mustapha, N.A.; Mohd Yasin, N.H.; Nazashida Mohd Hakimi, N.I. Microalgae-bacteria interaction in palm oil mill effluent treatment. J. Water Process Eng. 2020, 35, 101203. [Google Scholar] [CrossRef]
- Iovinella, M.; Carbone, D.A.; Diana, C.; Seth, J.D.; Michele, I.; Esposito, S.; Ciniglia, C. Prevalent pH Controls the Capacity of Galdieria maxima to Use Ammonia and Nitrate as a Nitrogen Source. Plants 2020, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signatures. Isot. Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar]
- Azuara, M.P.; Aparicio, P.J. In Vivo Blue-Light Activation of Chlamydomonas reinhardii Nitrate Reductase 1. Plant Physiol. 1983, 71, 286–290. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Sloth, J.K.; Jensen, H.C.; Pleissner, D.; Eriksen, N.T. Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresour. Technol. 2017, 238, 296–305. [Google Scholar] [CrossRef]
- Bellido-Pedraza, C.M.; Calatrava, V.; Sanz-Luque, E.; Tejada-Jiménez, M.; Llamas, Á.; Plouviez, M.; Guieysse, B.; Fernández, E.; Galván, A. Chlamydomonas reinhardtii, an Algal Model in the Nitrogen Cycle. Plants 2020, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xi, J.; Yang, R.; Lu, L.; Qiu, W.; Yang, B. Structures and compositions of biofilms in moving bed biofilm reactors pretreated by four drying methods. Chem. Eng. J. 2023, 477, 147228. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, M.; Lu, T.; Ding, D.; Sun, Y.; Yuan, Y. Bio-removal of PtCl62− complex by Galdieria sulphuraria. Sci. Total Environ. 2021, 796, 149021. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Zhuang, L.-L.; Xu, X.-Q.; Deantes-Espinosa, V.M.; Wang, X.-X.; Hu, H.-Y. Microalgal attachment and attached systems for biomass production and wastewater treatment. Renew. Sustain. Energy Rev. 2018, 92, 331–342. [Google Scholar]
- Polizzi, B.; Bernard, O.; Ribot, M. A time-space model for the growth of microalgae biofilms for biofuel production. J. Theor. Biol. 2017, 432, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chu, R.; Chang, T.; Cheng, P.; Jiang, J.; Yao, T.; Zhou, C.; Liu, T.; Ruan, R. Modeling and improving arrayed microalgal biofilm attached culture system. Bioresour. Technol. 2021, 331, 124931. [Google Scholar]
- Li, L.; Wang, Y.; Gao, L.; Zhou, W.; Chen, L.; Zhang, Z.; Liu, T. Experiments and cellular automata simulation reveal light/carbon transportation and growth mechanism of Chlorella vulgaris biofilm in attached cultivation. Chem. Eng. J. 2023, 457, 141177. [Google Scholar] [CrossRef]
- de Assis, L.R.; Calijuri, M.L.; Assemany, P.P.; Berg, E.C.; Febroni, L.V.; Bartolomeu, T.A. Evaluation of the performance of different materials to support the attached growth of algal biomass. Algal Res. 2019, 39, 101440. [Google Scholar] [CrossRef]
- Murshid, S.; Antonysamy, A.; Dhakshinamoorthy, G.; Jayaseelan, A.; Pugazhendhi, A. A review on biofilm-based reactors for wastewater treatment: Recent advancements in biofilm carriers, kinetics, reactors, economics, and future perspectives. Sci. Total Environ. 2023, 892, 164796. [Google Scholar] [CrossRef]
- Yu, L.; Acosta, N.; Bautista, M.A.; McCalder, J.; Himann, J.; Pogosian, S.; Hubert, C.R.; Parkins, M.D.; Achari, G. Quantitative evaluation of municipal wastewater disinfection by 280 nm UVC LED. Water 2023, 15, 1257. [Google Scholar] [CrossRef]
- Havlik, I.; Lindner, P.; Scheper, T.; Reardon, K.F. On-line monitoring of large cultivations of microalgae and cyanobacteria. Trends Biotechnol. 2013, 31, 406–414. [Google Scholar] [CrossRef]
- Sandnes, J.M.; Ringstad, T.; Wenner, D.; Heyerdahl, P.H.; Källqvist, T.; Gislerød, H.R. Real-time monitoring and automatic density control of large-scale microalgal cultures using near infrared (NIR) optical density sensors. J. Biotechnol. 2006, 122, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Rioual, S.; Talbot, P.; Faÿ, F.; Hellio, C.; Lescop, B. A high sensitive microwave sensor to monitor bacterial and biofilm growth. Sens. Bio-Sens. Res. 2022, 36, 100493. [Google Scholar] [CrossRef]
- Porras Reyes, L.; Havlik, I.; Beutel, S. Software sensors in the monitoring of microalgae cultivations. Rev. Environ. Sci. Bio/Technol. 2024, 23, 67–92. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, Z.; Yu, H.; Zou, X. Prediction of microalgae harvesting efficiency and identification of important parameters for ballasted flotation using an optimized machine learning model. Algal Res. 2025, 87, 103985. [Google Scholar] [CrossRef]
- Syed, T.; Krujatz, F.; Ihadjadene, Y.; Mühlstädt, G.; Hamedi, H.; Mädler, J.; Urbas, L. A review on machine learning approaches for microalgae cultivation systems. Comput. Biol. Med. 2024, 172, 108248. [Google Scholar] [CrossRef]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Y.; Zhang, Q. A Review of Energy Consumption in the Acquisition of Bio-Feedstock for Microalgae Biofuel Production. Sustainability 2021, 13, 8873. [Google Scholar] [CrossRef]
- Quinn, J.C.; Davis, R. The potentials and challenges of algae based biofuels: A review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef]
- Davis, R.; Markham, J.; Kinchin, C.; Grundl, N.; Tan, E.C.; Humbird, D. Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing Through Dewatering for Downstream Conversion; National Renewable Energy Laboratory(NREL): Golden, CO, USA, 2016. [Google Scholar]
- Manninen, K.; Sonck, M.; Spilling, K. Environmental Impact Assessment of Algae Cultivation Under Finnish Conditions; CLEEN Ltd.: Helsinki, Finland, 2013. [Google Scholar]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.-O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Vázquez-Romero, B.; Perales, J.A.; Pereira, H.; Barbosa, M.; Ruiz, J. Techno-economic assessment of microalgae production, harvesting and drying for food, feed, cosmetics, and agriculture. Sci. Total Environ. 2022, 837, 155742. [Google Scholar] [CrossRef]
- Bhatt, A.; Khanchandani, M.; Rana, M.S.; Prajapati, S.K. Techno-economic analysis of microalgae cultivation for commercial sustainability: A state-of-the-art review. J. Clean. Prod. 2022, 370, 133456. [Google Scholar] [CrossRef]
- Ennaceri, H.; Ishika, T.; Mkpuma, V.O.; Moheimani, N.R. Microalgal biofilms: Towards a sustainable biomass production. Algal Res. 2023, 72, 103124. [Google Scholar] [CrossRef]
- Loke Show, P. Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Benavente-Valdés, J.R.; Rodríguez-Zúñiga, D.; Cepeda-Tovar, V.; Solís-Quiroz, O. Commercial Compounds from Algae. In Microbial Bioactive Compounds: Industrial and Agricultural Applications; Soni, R., Suyal, D.C., Morales-Oyervides, L., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 37–58. [Google Scholar]
- Hirooka, S.; Miyagishima, S.-Y. Cultivation of acidophilic algae Galdieria sulphuraria and Pseudochlorella sp. YKT1 in media derived from acidic hot springs. Front. Microbiol. 2016, 7, 2022. [Google Scholar] [CrossRef]
- Fan, Q.; Fu, P.; Song, C.; Fan, Y. Valorization of waste biomass through hydrothermal liquefaction: A review with focus on linking hydrothermal factors to products characteristics. Ind. Crops Prod. 2023, 191, 116017. [Google Scholar] [CrossRef]
- Iovinella, M.; Lombardo, F.; Ciniglia, C.; Palmieri, M.; di Cicco, M.R.; Trifuoggi, M.; Race, M.; Manfredi, C.; Lubritto, C.; Fabbricino, M.; et al. Bioremoval of Yttrium (III), Cerium (III), Europium (III), and Terbium (III) from Single and Quaternary Aqueous Solutions Using the Extremophile Galdieria sulphuraria (Galdieriaceae, Rhodophyta). Plants 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.M.; Mirkouei, A.; Reed, D.; Thompson, V. Current nature-based biological practices for rare earth elements extraction and recovery: Bioleaching and biosorption. Renew. Sustain. Energy Rev. 2023, 173, 113099. [Google Scholar] [CrossRef]
- Anuardo, R.G.; Espuny, M.; Costa, A.C.F.; Espuny, A.L.G.; Kazançoğlu, Y.; Kandsamy, J.; de Oliveira, O.J. Transforming E-Waste into Opportunities: Driving Organizational Actions to Achieve Sustainable Development Goals. Sustainability 2023, 15, 14150. [Google Scholar] [CrossRef]
- Shittu, O.S.; Williams, I.D.; Shaw, P.J. Global E-waste management: Can WEEE make a difference? A review of e-waste trends, legislation, contemporary issues and future challenges. Waste Manag. 2021, 120, 549–563. [Google Scholar] [CrossRef]

| Parameter | Type of Probe |
|---|---|
| pH of culture media | Analog pH sensor for acidic solutions Accuracy: ±0.1 at 25 °C |
| Environmental Light intensity | Photodiode Accuracy: ±0.054 lx |
| External Temperature and Relative Humidity | Thermo-hygrometer, CMOS-type sensor Temperature accuracy: ±0.2 °C Humidity accuracy: ±2% |
| Internal Temperature | Programmable Resolution 1-Wire Digital Thermometer Accuracy: ±0.5 °C |
| ID Experiment | Duration (Days) | pH | Temperature | Light–Dark Hours | Light Intensity (Lux) | Experimental Control | Test | Fresh Feeding Frequency of Culture medium |
|---|---|---|---|---|---|---|---|---|
| Exp 1 | 15 | 2.5 | 37 °C | 16:8 | ~1000 | Allen medium (1×) | Wastewater | Never (batch) |
| Exp 2 | 15 | 2.5 | 28–35 °C | 16:8 | ~1000 | Allen medium (1×) | Wastewater | Never (batch) |
| Exp 3 | 15 | 2.5 | 28–35 °C | 16:8 | ~32,000 | Allen medium (1×) | Wastewater | Never (batch) |
| Exp 4 | 15 | 2.5 | 28–35 °C | 16:8 | ~32,000 | Allen medium (1×) | Wastewater | Every 2–3 days (semi-batch) |
| Exp 5 | 15 | 2.5 | 28–35 °C | 16:8 | ~32,000 | Allen medium (1×) | Wastewater | Continuously |
| δ13C (‰) vs. VPDB | δ15N (‰) vs. Air | % C | % N | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Biomass grown in A | −26.0 ± 0.4 | −13.6 ± 0.5 | 48.5 ± 1.7 | 9.3 ± 0.8 |
| Biomass grown in W | −26.0 ± 0.5 | −11.0 ± 1.6 | 48.3 ± 1.6 | 9.2 ± 0.8 |
| Allen medium (dry residue) | - | −0.9 ± 0.2 | - | 21.5 ± 0.3 |
| Wastewater (dry residue) | −26.3 ± 1.8 | 5.1 ± 1.4 | 0.9 ± 0.1 | 1.3 ± 0.1 |
| Treatment | Model | AICc | BIC | SSE | MSE | RMSE | R2 |
|---|---|---|---|---|---|---|---|
| A | Second-degree polynomial | 678.35 | 736.57 | 142.71 | 0.47 | 0.69 | 0.96 |
| 3-parameter logistic | 773.64 | 831.85 | 193.12 | 0.64 | 0.80 | 0.95 | |
| 2-parameter exponential | 787.01 | 827.41 | 208.62 | 0.68 | 0.83 | 0.95 | |
| W | Second-degree polynomial | 716.85 | 775.07 | 161.26 | 0.54 | 0.73 | 0.97 |
| 3-parameter logistic | 718.05 | 776.26 | 161.87 | 0.54 | 0.73 | 0.97 | |
| 2-parameter exponential | 763.52 | 803.93 | 193.63 | 0.63 | 0.80 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Cicco, M.R.; Altieri, S.; Spagnuolo, A.; Ciniglia, C.; Germinario, C.; Bove, S.; Masiello, A.; Vetromile, C.; Galante, I.; Lubritto, C. Sustainable Cultivation of Galdieria phlegrea in an IoT-Integrated Twin-Layer Photobioreactor: System Design, Growth Dynamics, and Isotopic Perspective. Appl. Sci. 2025, 15, 5220. https://doi.org/10.3390/app15095220
di Cicco MR, Altieri S, Spagnuolo A, Ciniglia C, Germinario C, Bove S, Masiello A, Vetromile C, Galante I, Lubritto C. Sustainable Cultivation of Galdieria phlegrea in an IoT-Integrated Twin-Layer Photobioreactor: System Design, Growth Dynamics, and Isotopic Perspective. Applied Sciences. 2025; 15(9):5220. https://doi.org/10.3390/app15095220
Chicago/Turabian Styledi Cicco, Maria Rosa, Simona Altieri, Antonio Spagnuolo, Claudia Ciniglia, Chiara Germinario, Silvio Bove, Antonio Masiello, Carmela Vetromile, Iolanda Galante, and Carmine Lubritto. 2025. "Sustainable Cultivation of Galdieria phlegrea in an IoT-Integrated Twin-Layer Photobioreactor: System Design, Growth Dynamics, and Isotopic Perspective" Applied Sciences 15, no. 9: 5220. https://doi.org/10.3390/app15095220
APA Styledi Cicco, M. R., Altieri, S., Spagnuolo, A., Ciniglia, C., Germinario, C., Bove, S., Masiello, A., Vetromile, C., Galante, I., & Lubritto, C. (2025). Sustainable Cultivation of Galdieria phlegrea in an IoT-Integrated Twin-Layer Photobioreactor: System Design, Growth Dynamics, and Isotopic Perspective. Applied Sciences, 15(9), 5220. https://doi.org/10.3390/app15095220










