Abstract
This study aimed to examine associations between functional capacity (FC), brain β-amyloid (Aβ) burden, and longitudinal cognitive performance. Data from 89 cognitively normal women (70.0 ± 2.7 years) in the Women’s Healthy Ageing Project cohort were analyzed. FC was assessed using the timed up and go (TUG) test and the Aβ burden was quantified via a F-18 Florbetaben PET scan with Standardized Uptake Value Ratio (SUVR). Cognition was evaluated longitudinally using the Preclinical Alzheimer Cognitive Composite (PACC) over 3.9 ± 2.6 years. Multiple linear regression, mediation analysis, and linear mixed-effects models were applied. Baseline Aβ burden and years of education were associated with cognitive performance two to six years later, while the TUG performance was associated with cognitive outcomes at two years. Aβ burden was found to mediate the relationship between FC and cognition over time. A significant three-way interaction (TUG × SUVR × time) was observed, indicating that declines in the TUG performance over time were exclusively associated with steeper cognitive decline among women with elevated Aβ burden (SUVR ≥ 1.42). These findings suggest that maintaining functional mobility may be particularly relevant for women with increased Aβ burden and support future research targeting early motor-cognitive markers.
1. Introduction
The life expectancy that developed countries have experienced in recent decades, together with medical advances, has led to an exponential increase in the number of people reaching an advanced age. For this reason, diseases that in the past were not present in many people have today become the leading causes of morbidity and mortality. Dementia is one of the costliest long-term illnesses to society, with 85% of costs related to family or social care [1], and is associated with difficulties in performing daily activities and a reduced quality of life [2]. The latest reports establish that around 55 million people have dementia worldwide [3], with Alzheimer’s disease (AD) being the most common form of dementia. Furthermore, it is worth mentioning that AD disproportionately affects women, both in prevalence and severity.
Alzheimer’s disease is a progressive, neurodegenerative disease that occurs when nerve cells in the brain die. The pathophysiological process of AD begins decades before clinical symptoms emerge and is characterized by early brain beta-amyloid (Aβ) deposition taking place in senile plaques and cerebrovascular deposits [4]. Evidence suggests that the accumulation of Aβ is one of the first biomarkers of AD and a hallmark of a downstream chain of events, which eventually causes neurodegeneration, cognitive impairment, and finally, dementia [5]. Currently, there are no effective pharmacological treatments that can stop or significantly slow the disease. Thus, determining the modifiable factors that may delay and even prevent disease is clinically important.
Physical activity (PA) is considered an essential modifiable factor related to cognitive function, being associated with a lower incidence of dementia, including AD [6]. A physically active lifestyle may prevent dementia through several pathways, such as improving cerebral vascularization, blood flow, and antioxidant and inflammatory processes in the brain, as well as decreasing Aβ production and increasing Aβ clearance [7,8]. In the same way, PA has indirect pathways through improvements in mood, sleep, and other cardiovascular risk factors. However, there are limitations when assessing PA using both subjective and objective methods, as they often do not accurately reflect personal physical performance. For this reason, evaluating physical functional capacity (FC) has been identified as an objective measure, a reflection of individual physical fitness positively associated with many health indices and as an early predictor of brain deterioration [9,10]. Nevertheless, the association between FC and AD biomarkers has not been consistently replicated, particularly regarding brain Aβ deposition. In a recent study published by our group [11], we reported that higher FC performance was associated with lower brain Aβ deposition. However, it is essential to note that much of the evidence to date comes from cross-sectional studies or short follow-ups likely to reverse causality [12].
The primary goals of the current study are (a) to assess the association between baseline FC and brain Aβ burden with longitudinal cognitive performance in cognitively healthy older women, and (b) to examine the association between changes in FC performance over time and baseline brain Aβ burden with longitudinal cognitive decline.
2. Materials and Methods
2.1. Participants
The study was approved by the University of Melbourne Human Research Ethics Committee and conducted in accordance with the National Health and Medical Research Council’s ethical guidelines (HREC 931149X, 1034765, 110525, 1339373, 010411, 1647448, 1750632) and the Declaration of Helsinki. Written informed consent was obtained from each participant.
Participants were drawn from the Women’s Healthy Ageing Project (WHAP), a longitudinal, prospective cohort study of Australian women, with the detailed methodology described previously [13]. WHAP is an extension of the Melbourne Women’s Midlife Health Project, a cross-sectional study that initially recruited 2001 women through random population sampling. Women were eligible for participation if they were aged 45–55 years, had a uterus and at least one ovary, were menstruating, and had not used hormone replacement therapy in the three months prior to recruitment. Of those eligible, 438 women consented to participate in the longitudinal follow-up study.
Since its inception in 1992, the WHAP has conducted multiple waves of data collection. In 2012, 125 participants consented to undergo cerebral magnetic resonance imaging (MRI) and positron emission tomography (PET) using F-18 Florbetaben (Bundoora, Australia), a radioligand that enables the in vivo quantification of amyloid-β (Aβ) pathology. Participants were subsequently reassessed in 2014 and 2019. Figure 1 shows the participant flow chart and Table 1 summarizes the variables collected across each wave, along with their corresponding time points of interest.
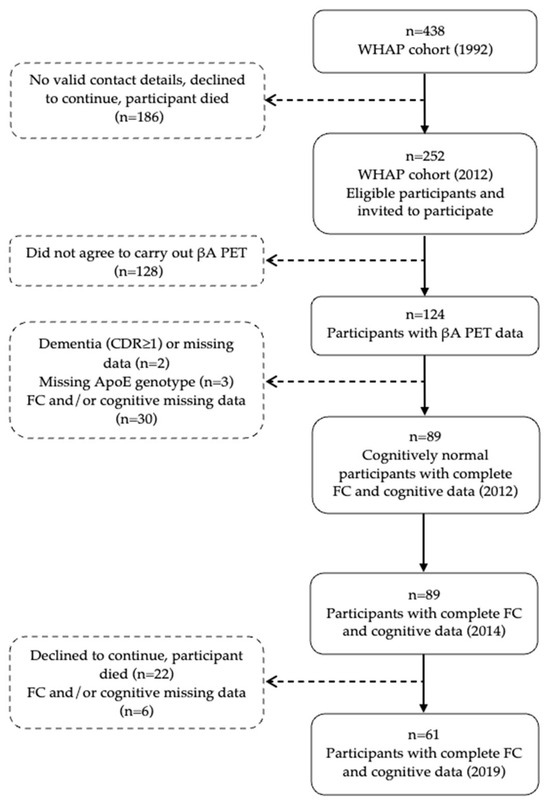
Figure 1.
Participant flow chart. Abbreviations: ApoE, apolipoprotein E; βA, beta-amyloid; CDR, clinical dementia rating; FC, functional capacity; PET, positron emission tomography; and WHAP, Women’s Healthy Ageing Project.

Table 1.
Summary of the different variables and the year of data collection.
For the present analysis, participants were included if they (i) were cognitively normal at baseline, (ii) had available PET-Aβ imaging data, and (iii) completed at least one follow-up assessment of FC and cognitive performance after 2012. A total of 89 women met these criteria (see Figure 1).
2.2. PET Imaging
The brain Aβ burden data used in the present study were obtained at baseline with participants receiving 250 MBq of F-18 Florbetaben intravenously, with a 20 min acquisition commencing 90 min post injection. The standardized uptake values (SUVs) were calculated for all the brain regions examined, and the SUV ratio (SUVR) was generated by normalizing the regional SUV using the cerebellar cortex. The neocortical SUVR, a global measure of Aβ burden, is expressed as the average SUVR of the area-weighted mean of the frontal, superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate regions [13]. This index was used to stratify participants as low (SUVR ≤ 1.10), intermediate (SUVR 1.11–1.41), and high (SUVR ≥ 1.42) neocortical βA load [14,15,16].
2.3. Evaluation of Physical Functional Capacity
FC was evaluated using the timed up and go (TUG) test [17]. The TUG measures the time (in seconds) taken to rise from a seated position, walk 3 m from the chair, walk back to the chair, and sit down again. A shorter time to complete this test reflects better physical performance.
To assess whether maintaining FC performance over time attenuates the negative association between baseline neocortical Aβ load and longitudinal PACC decline, participants were classified into two groups (maintained/improved FC performance vs. decline in FC performance) based on the change in TUG performance between baseline and the last follow-up. For this, we considered the minimum detectable change proposed by Donoghue et al., according to which TUG changes greater than 27% are considered significant [18]. In this regard, participants whose last follow-up TUG result was equal to or faster than the baseline (TUG change ≤ 27% or TUG change faster ≥ 27%) were classified as “Maintain/Improve TUG performance”; the rest of the participants were classified as “decline TUG” (TUG change slower > 27% than baseline).
2.4. Cognitive Measures
The Preclinical Alzheimer Cognitive Composite [19] (PACC) was the primary measure of cognitive change. Cognition was evaluated in three years (2012, 2014, and 2019). The PACC consists of the Mini Mental State Examination [20], Delayed Recall from the California Verbal Learning Test [21], Digit Symbol Coding [22], and Logical Memory Delayed Recall [23]. Raw scores were z-transformed based on the mean and SD from the baseline data and averaged together. Higher PACC scores indicate a better performance.
2.5. Covariates
Potential confounders were selected from the literature and included age, years of education, cardiovascular disease risk (assessed using the Framingham score for cardiovascular risk at baseline) [24], and ApoE4 genotype (carrier or noncarrier).
2.6. Statistical Analyses
Statistical analyses were conducted using the statistical package for the social sciences (IBM SPSS), version 29.0 (IBM Corp, Armonk, NY, USA). The normality of the variables was examined using the Kolmogorov–Smirnov test. As a result, it was found that the data had a normal distribution. Statistical significance was set at p < 0.05.
Pearson correlation coefficients adjusted based on age and education were used to examine the cross-sectional associations among the PACC, brain βA load, and TUG. Between-group differences in demographic and clinical characteristics according to TUG performance in 2012 were evaluated using an independent sample Student’s t-test for continuous variables and chi-square test for categorical variables. Stepwise multiple linear regressions were conducted to examine the associations between the baseline variables and participants’ PACC scores reflecting longitudinal cognitive decline. The predictor variables entered were baseline TUG, baseline brain βA load, age, years of education, ApoE4 genotype, and cardiovascular disease risk (FHS-CVD). The regression model’s results were corrected for multiple comparisons using the Benjamini–Hochberg procedure, implemented in SPSS, with a cut-off of q < 0.05.
We statistically assessed baseline βA load as a potential pathway linking FC with the risk of cognitive deterioration. For this, the baseline Aβ measure was tested as a potential mediator of the association between the baseline TUG and longitudinal PACC score. The potential mediator was tested in a simple mediation model, by calculating bias-corrected 95% confidence intervals using a bootstrapping procedure with 10,000 resamples, using the PROCESS macro for SPSS by Andrew Hayes [25]. The indirect effect of X on Y through a candidate mediator is significant if the confidence intervals do not include zero. The analyses were controlled by all covariates.
Finally, a linear mixed-effects (LME) model was performed to assess the association between changes in TUG performance over time and longitudinal cognitive decline. The outcome measure was the longitudinal PACC score. The full model included time (years between baseline and each follow-up), FC change groups (Maintain/Improve TUG performance or decline TUG performance), baseline βA load groups (low, intermediate, or high SUVR), and covariates, as well as all possible interaction terms with time. The random effects were time/subject, which modeled a subject-specific intercept and allowed the results to vary by subject.
3. Results
3.1. Baseline Characteristics
The cross-sectional analysis was run with baseline PET data from 2012. Of the 89 participants included from the WHAP cohort, 31 (34.1%) were ApoE4 carriers, with a mean (SD) age of 70.0 (2.7) years. Table 2 summarizes the demographic and clinical characteristics of the sample.

Table 2.
Demographic and clinical characteristics of the sample in 2012.
3.2. Association Between FC and Brain βA Load with Cognition
The association of PACC scores with βA load and TUG performance from 2012 was examined, observing a negative correlation in both cases after adjusting for age and education (r = −0.428; p< 0.001; r = −0.267; p < 0.05, respectively), such that higher Aβ deposition and lower performance in TUG were associated with lower PACC scores. Additionally, a positive correlation was observed between higher Aβ deposition and lower TUG performance (r = 0.243, p < 0.05).
3.3. Factors Associated with Cognitive Performance over Time
Table 3 presents the results of stepwise multiple linear regression analyses. Baseline βA load and years of education were associated with PACC score over time, such that a greater baseline βA load and fewer years of education were linked to a lower PACC score. Baseline TUG performance was associated with PACC scores at two years (p < 0.01). All associations remained significant after correction for multiple comparisons.

Table 3.
Stepwise multiple linear regressions for associations of cognitive function (PACC) over time with baseline brain amyloid burden (SUVR) and baseline functional capacity (TUG).
3.4. Mediation Analysis
To examine whether the association between baseline FC and longitudinal cognitive function was potentially mediated by the baseline Aβ burden, a mediation analysis was performed controlling for age, years of education, ApoE4 genotype, and FHS-CVD. The association between the TUG and PACC scores was statistically accounted for by baseline Aβ burden in 2012 (Figure 2, cognition 2012). However, for cognition in 2014, FC was partially mediated by the baseline Aβ burden (Figure 2, cognition 2014). In both cases (model 1 and model 2), TUG performance showed an indirect association with cognition, such that faster TUG results were associated with lower Aβ deposition.
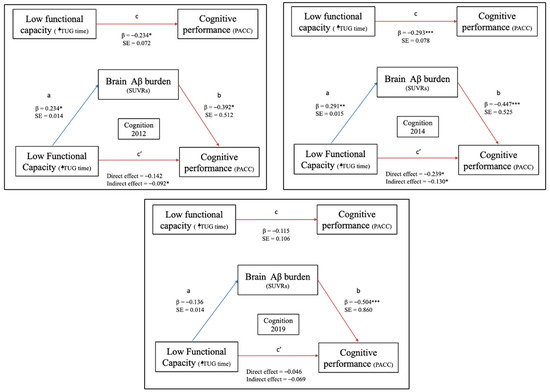
Figure 2.
Mediation of functional capacity (FC) and cognitive performance by brain Aβ burden for cognitively healthy women. Note. c is coefficient linking X and Y (total causal effect), c’ is the coefficient for the effect of X on Y adjusting for M (direct effect), b is the effect of M on Y adjusting for explanatory variable, “a” is the coefficient relating to the effect of X on M. Significance of the indirect effect is assessed via 95% confidence intervals. * p < 0.05; ** p < 0.01; *** p < 0.001. Cognition 2012: PACC score from 2012; Cognition 2014: PACC score from 2014; and Cognition 2019: PACC score from 2019. Abbreviations: PACC, Preclinical Alzheimer Cognitive Composite; SUVR, Standardized Uptake Value Ratio; and TUG, timed up and go test.
No significant associations were found between the baseline TUG and PACC scores in 2019 (Figure 2, cognition 2019).
3.5. Association of FC Deterioration with Longitudinal Cognitive Function
A total of 44.4% of the participants presented a worsening in TUG performance over time. As shown in Table 2, there were no differences between the TUG performance groups, except for the baseline TUG, where the women who exhibited a longitudinal decline in TUG were those who achieved the fastest baseline performance in this test.
As anticipated, the majority of participants (81%) had a low brain Aβ burden, with smaller proportions demonstrating intermediate (9%) or high (10%) Aβ levels. In the LME model examining PACC score over time, a significant interaction between the SUVR and TUG was observed, although only in women with high βA burden. However, the primary interest was whether FC moderated the association between Aβ load and prospective cognitive decline. When examining the PACC scores over time, there was a significant three-way interaction (TUG × SUVR × time), with time having a steeper slope on PACC for women with a high Aβ load (SUVR ≥ 1.42) who decreased TUG performance over time (β, −0.284; 95% CI, −0.54 to −0.03; p = 0.029) (Table 4; Figure 3).

Table 4.
Association of change in functional capacity over time and β-amyloid load with longitudinal cognitive decline.
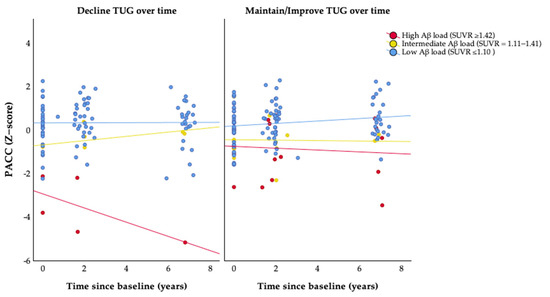
Figure 3.
Associations of change in functional capacity over time and β-amyloid load with cognitive decline. Abbreviations: PACC, Preclinical Alzheimer Cognitive Composite; SUVR, Standardized Uptake Value Ratio; and TUG, timed up and go test. Maintain/Improve TUG = last follow-up TUG result equal to (TUG changes ≤ 27%) or faster (TUG changes > 27%) than baseline. Decline TUG = last follow-up TUG result slower (TUG changes > 27%) than baseline.
4. Discussion
In this prospective study of cognitively healthy older women, we observed that (a) cognitive status was associated with brain Aβ load and years of education; (b) current FC performance was associated with sustained cognitive status for 2 years, although it required maintenance as the initial impact subsided over 2 years; and (c) maintaining TUG performance over time was associated with better cognitive outcomes in women with high Aβ loads (SUVR ≥ 1.42).
Lifestyle and its underlying mechanisms have become a priority research field due to their immense effects on health outcomes and promising savings in healthcare costs. Among the main modifiable components of lifestyle, PA is considered a protective component against cognitive impairment, neurodegeneration, and dementia through mechanisms related to cardiovascular health [12,26]; however, disentangling the specific contributions of PA to cognitive trajectories and brain pathologies remains a challenge.
Although the number of human studies investigating if greater amounts of daily PA mitigate brain Aβ deposition has been limited, the finding of lower Aβ burden in older women with greater FC converges with previous studies [27,28], but is inconsistent with other reports [29,30]. Participants with different characteristics and PA measures could explain this variation across studies. Concerning our work, the study sample was made up of exclusively women and, according to the results of a recently published study, both sexes experience a modified effect of PA on Aβ load [31]. Secondly, most studies have evaluated PA via questionnaires, and only a few have assessed PA via objective measures such as an accelerometer [32]. Although PA and FC are related, they are separate physiological and behavioral measures that can explain different aspects and predict various health outcomes. In this sense, our results are based on TUG performance as an objective measure of FC, which reflects individual fitness and is an early predictive factor of brain deterioration [10].
Consistent with the literature [30,31], our regression model found that greater Aβ deposition at baseline and fewer years of education were associated with increased risk of cognitive impairment at baseline and 2–6 years after the Aβ assessment. Furthermore, we reported that worse TUG performance at baseline was associated with a greater risk of cognitive decline 2 years later, suggesting that FC performance may be linked to better cognitive outcomes over a 2-year period. However, it requires maintenance, as baseline impact wanes over 2 years; hence, those maintaining TUG showed the best outcomes.
With an increasing emphasis on early diagnosis, to allow people with dementia and their families earlier access to cost-effective interventions to delay cognitive loss and improve quality of life, global policy has recommended that primary care play a most important role in pre-diagnosis evaluation [3]. For this reason, if a simple test, such as the TUG, which is easily applied in clinical consultations, helps to predict the possible cognitive deterioration an individual may experience two years in advance, it would be a great help.
In addition, through the mediation models, we provide statistical evidence suggesting that greater TUG performance at baseline may be associated with reduced risk of cognitive impairment through lower brain Aβ deposition, maintaining this process for up to 2 years—a longitudinal association that has never been shown before in the existing literature (to the Authors’ knowledge). Moreover, we also observed the direct effect of TUG at baseline on the risk of cognitive impairment at 2 years, such that better FC performance showed both direct and indirect associations with cognitive outcomes. These results are partially in line with previous studies, in which Aβ mediated the relationship between PA and cognitive impairment [28,31]. By contrast, a recent study analyzing the effect of lifestyle on Aβ burden and cognition by sex observed that elevated Aβ burden tends to increase cognitive impairment, although PA only showed a significantly beneficial effect in men, not women [31]. Although these results are contrary to ours, it should be noted that the questionnaires and accelerometers provide the amount of PA the subjects engage in at a given moment or period, whereas functional capacity is objective data on a person’s physical capacity. This fact may be one of the causes of the discrepancies in the said study.
Considering the existing literature, findings regarding the relationship between PA and Aβ burden are mixed. It may be because most of the findings were obtained through cross-sectional or longitudinal studies, in which some of the measures of interest were assessed only at baseline (for example, PA). In the present study, almost 50% of the samples significantly decreased FC performance over time. However, longitudinal analysis revealed that significant cognitive decline was observed only in women with amyloidosis (Aβ+ = SUVR ≥ 1.42) compared to those with low or intermediate Aβ burden. Maintaining or improving FC over time was associated with a reduced risk of cognitive impairment related to Aβ deposition in Aβ+ older women. This result agrees with the finding previously reported by Raffin et al. [33], in which reverse association between self-reported PA and cognitive decline tended to be more pronounced in a worsened Aβ profile. Rabin et al. [9] also reported that PA ameliorated Aβ-associated cognitive decline. Nevertheless, although PA may slow the progression of AD-related pathologies, previous studies observed no cross-sectional associations between PA and Aβ burden [9,33]. By contrast, we found a significant cross-sectional association between TUG and Aβ, as well as a longitudinal interaction between both. However, the latter was only in those women who were Aβ+ and had decreased FC performance.
Our study has several strengths. This study enriches the scarce literature about the interactive association of AD biomarkers and PA with cognition. In addition, instead of PA, we used the TUG test to evaluate FC as an objective measure of physical performance, offering, for the first time in the literature to our knowledge, a longitudinal analysis of the evolution of FC and its influence on the cognitive deterioration associated with Aβ. Lastly, the sample was made up entirely of women, this being a population group for which research is still lacking. Our study is not free of limitations. The relatively modest number of subjects included in the study is critical to interpreting and generalizing the findings. Furthermore, Aβ burden was assessed only at baseline, limiting the ability to track its progression over time and its evolving relationship with cognitive decline. Additionally, while the TUG test is an objective measure of functional capacity, it may not fully capture all the dimensions of physical activity that impact cognitive decline. Despite these limitations, the study offers important contributions to understanding the role of FC in cognitive decline and suggests directions for future research.
5. Conclusions
In this prospective observational study of cognitively unimpaired older women, both baseline Aβ burden and years of education were found to be associated with longitudinal cognitive performance. Functional capacity, as measured via the TUG test, was associated with cognitive outcomes at two years and appeared to be linked indirectly to cognitive performance through its relationship with Aβ levels. Furthermore, declines in functional mobility over time were associated with a steeper cognitive decline among women with an elevated Aβ burden. These findings suggest that maintaining functional mobility may be particularly relevant for women with increased Aβ burden and highlight the importance of investigating early motor-cognitive markers. Future studies are warranted to confirm these associations and to explore targeted interventions aimed at preserving mobility as a potential strategy to support cognitive health during aging.
Author Contributions
R.P.-C.: conceptualization, design, data analysis, and writing—review and editing. C.S.: funding acquisition, project administration, conceptualization, design, data analysis, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for the Healthy Aging Program (HAP) has been provided by the National Health and Medical Research Council [NHMRC grants 547600, 1032350, and 1062133], Ramaciotti Foundation, Australian Healthy Aging Organisation, the Brain Foundation, the Alzheimer’s Association [NIA320312], and Australian Menopausal Society, Bayer Healthcare, Shepherd Foundation, Scobie and Claire Mackinnon Foundation, Collier Trust Fund, J.O. and J.R. Wicking Trust, Mason Foundation, the Alzheimer’s Association of Australia, and Royal Australian College of Physicians. Inaugural funding was provided by VicHealth and the NHMRC. The Principal Investigator of HAP (C.S.) is supported by the National Health and Medical Research Council. R.P.C. was supported under a Universidad Politécnica de Madrid grant as part of the national research programme “Recualificación del Sistema Universitario Español” financed by the Recovery and Resilience Package—NextGenerationEU (European Commission).
Institutional Review Board Statement
The studies involving human participants were reviewed and approved by the University of Melbourne Human Research Ethics Committee, in 4 July 2011, fully compliant with the guidelines of the National Health and Medical Research Council ethical standards (HREC 931149X, 1034765, 110525, 1339373, 010411, 1647448, and 1750632), and carried out by the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Deidentified data for ongoing analysis is available via the BioGrid Platform. BioGrid has ethics approval for its data linkage platform at all participating sites where data are connected. Biogrid uses state-of-the-art techniques to encrypt and transmit data when accessed for research, and has implemented systems and processes to ensure the privacy and confidentiality of clinician and participant data within our research platform. BioGrid’s privacy charter ensures ethical data use, confidentiality, and secure transactions, emphasizing joint intellectual property ownership and consent for publications (https://www.biogrid.org.au/privacy-charter).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient confidentiality and participant privacy reasons. Requests to access the deidentified datasets can be found in online repository searching by title or data custodian. The name of the repository is Health Populations—Data Custodian C Szoeke in BioGrid and applications for data access can be made through the following URL: https://www.biogrid.org.au/data-directory.
Acknowledgments
We would like to acknowledge the contributions of the participants and their supporters for their time and commitment over the past 30 years to the University. We thank BioGrid for providing data linkage and Melbourne Health Pathology for their services, including blood biomarker storage and analysis. We thank the research assistants who assisted in data collection. A comprehensive list of all researchers contributing to the project, as well as the membership of our Scientific Advisory Board, is available online.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D.E. Global and Regional Projections of the Economic Burden of Alzheimer’s Disease and Related Dementias from 2019 to 2050: A Value of Statistical Life Approach. EClinicalMedicine 2022, 51, 101580. [Google Scholar] [CrossRef] [PubMed]
- Skaria, A.P. The Economic and Societal Burden of Alzheimer Disease: Managed Care Considerations. Am. J. Manag. Care 2022, 28, S188–S196. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report: Journey Through the Diagnosis of Dementia. Alzheimer’s Dis. Int. 2021, 30, 19. [Google Scholar]
- Thal, D.R.; Capetillo-Zarate, E.; Del Tredici, K.; Braak, H. The Development of Amyloid Beta Protein Deposits in the Aged Brain. Sci. Aging Knowl. Environ. 2006, 2006, re1. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking Pathophysiological Processes in Alzheimer’s Disease: An Updated Hypothetical Model of Dynamic Biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Peters, R.; Booth, A.; Rockwood, K.; Peters, J.; D’Este, C.; Anstey, K.J. Combining Modifiable Risk Factors and Risk of Dementia: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e022846. [Google Scholar] [CrossRef]
- Ebrahimi, K.; Majdi, A.; Baghaiee, B.; Hosseini, S.H.; Sadigh-Eteghad, S. Physical Activity and Beta-Amyloid Pathology in Alzheimer’s Disease: A Sound Mind in a Sound Body. EXCLI J. 2017, 16, 959–972. [Google Scholar]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical Exercise in the Prevention and Treatment of Alzheimer’s Disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Rabin, J.S.; Klein, H.; Kirn, D.R.; Schultz, A.P.; Yang, H.S.; Hampton, O.; Jiang, S.; Buckley, R.F.; Viswanathan, A.; Hedden, T.; et al. Associations of Physical Activity and β-Amyloid with Longitudinal Cognition and Neurodegeneration in Clinically Normal Older Adults. JAMA Neurol. 2019, 76, 1203–1210. [Google Scholar] [CrossRef]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical Activity, Fitness, and Gray Matter Volume. Neurobiol. Aging 2014, 35, S20–S28. [Google Scholar] [CrossRef]
- Pedrero-Chamizo, R.; Szoeke, C.; Dennerstein, L.; Campbell, S. Influence of Physical Activity Levels and Functional Capacity on Brain β-Amyloid Deposition in Older Women. Front. Aging Neurosci. 2021, 13, 697528. [Google Scholar] [CrossRef] [PubMed]
- Iso-Markku, P.; Kujala, U.M.; Knittle, K.; Polet, J.; Vuoksimaa, E.; Waller, K. Physical Activity as a Protective Factor for Dementia and Alzheimer’s Disease: Systematic Review, Meta-Analysis and Quality Assessment of Cohort and Case-Control Studies. Br. J. Sports Med. 2022, 56, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Szoeke, C.; Coulson, M.; Campbell, S.; Dennerstein, L. Cohort Profile: Women’s Healthy Ageing Project (WHAP)—A Longitudinal Prospective Study of Australian Women since 1990. Womens Midlife Health 2016, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Duara, R.; Loewenstein, D.A.; Lizarraga, G.; Adjouadi, M.; Barker, W.W.; Greig-Custo, M.T.; Rosselli, M.; Penate, A.; Shea, Y.F.; Behar, R.; et al. Effect of Age, Ethnicity, Sex, Cognitive Status and APOE Genotype on Amyloid Load and the Threshold for Amyloid Positivity. Neuroimage Clin. 2019, 22, 101800. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Ong, K.; Mulligan, R.S.; Holl, G.; Pejoska, S.; Jones, G.; O’Keefe, G.; Ackerman, U.; Tochon-Danguy, H.; Chan, J.G.; et al. Amyloid Imaging with 18F-Florbetaben in Alzheimer Disease and Other Dementias. J. Nucl. Med. 2011, 52, 1210–1217. [Google Scholar] [CrossRef]
- Bullich, S.; Roé-Vellvé, N.; Marquié, M.; Landau, S.M.; Barthel, H.; Villemagne, V.L.; Sanabria, Á.; Tartari, J.P.; Sotolongo-Grau, O.; Doré, V.; et al. Early Detection of Amyloid Load Using 18F-Florbetaben PET. Alzheimers Res. Ther. 2021, 13, 67. [Google Scholar] [CrossRef]
- Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Donoghue, O.A.; Savva, G.M.; Börsch-Supan, A.; Kenny, R.A. Reliability, Measurement Error and Minimum Detectable Change in Mobility Measures: A Cohort Study of Community-Dwelling Adults Aged 50 Years and over in Ireland. BMJ Open 2019, 9, e030475. [Google Scholar] [CrossRef]
- Insel, P.S.; Weiner, M.; Scott MacKin, R.; Mormino, E.; Lim, Y.Y.; Stomrud, E.; Palmqvist, S.; Masters, C.L.; Maruff, P.T.; Hansson, O.; et al. Determining Clinically Meaningful Decline in Preclinical Alzheimer Disease. Neurology 2019, 93, E322–E333. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E. Mini-Mental State. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiat. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Delis, D.; Kramer, J.; Kaplan, E.; Ober, B.A. CVLT-II Adult Version; Harcourt-Brace: San Antonio, CA, USA, 2000. [Google Scholar]
- Wechsler, D.A. Wechsler Adult Intelligence Scale-Third Edition; Psychological Corporation: New York, NY, USA, 1997. [Google Scholar]
- Wechsler, D.A. Wechsler Memory Scale-Revised (WMS-R); Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. PROCESS: A Versatile Computational Tool for Observed Variable Mediation, Moderation, and Conditional Process Modeling [White Paper]. 2012. Available online: http://www.afhayes.com/public/process2012.pdf (accessed on 26 April 2025).
- Buchman, A.S.; Boyle, P.A.; Yu, L.; Shah, R.C.; Wilson, R.S.; Bennett, D.A. Total Daily Physical Activity and the Risk of AD and Cognitive Decline in Older Adults. Neurology 2012, 78, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Peiffer, J.J.; Taddei, K.; Lui, J.K.; Laws, S.M.; Gupta, V.B.; Taddei, T.; Ward, V.K.; Rodrigues, M.A.; Burnham, S.; et al. Physical Activity and Amyloid-β Plasma and Brain Levels: Results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol. Psychiatry 2013, 18, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Lopez, O.L.; Becker, J.T.; Kuller, L.H.; Mehta, P.D.; Tracy, R.P.; Erickson, K.I. Physical Activity Predicts Reduced Plasma β Amyloid in the Cardiovascular Health Study. Ann. Clin. Transl. Neurol. 2017, 4, 284–291. [Google Scholar] [CrossRef]
- De Souto Barreto, P.; Andrieu, S.; Payoux, P.; Demougeot, L.; Rolland, Y.; Vellas, B. Physical Activity and Amyloid-β Brain Levels in Elderly Adults with Intact Cognition and Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2015, 63, 1634–1639. [Google Scholar] [CrossRef]
- Vemuri, P.; Lesnick, T.G.; Przybelski, S.A.; Knopman, D.S.; Roberts, R.O.; Lowe, V.J.; Kantarci, K.; Senjem, M.L.; Gunter, J.L.; Boeve, B.F.; et al. Effect of Lifestyle Activities on Alzheimer Disease Biomarkers and Cognition. Ann. Neurol. 2012, 72, 730–738. [Google Scholar] [CrossRef]
- Bachmann, D.; Roman, Z.J.; Buchmann, A.; Zuber, I.; Studer, S.; Saake, A.; Rauen, K.; Gruber, E.; Nitsch, R.M.; Hock, C.; et al. Lifestyle Affects Amyloid Burden and Cognition Differently in Men and Women. Ann. Neurol. 2022, 92, 451–463. [Google Scholar] [CrossRef]
- Brown, B.M.; Peiffer, J.J.; Rainey-Smith, S.R. Exploring the Relationship between Physical Activity, Beta-Amyloid and Tau: A Narrative Review. Ageing Res. Rev. 2019, 50, 9–18. [Google Scholar] [CrossRef]
- Raffin, J.; Rolland, Y.; Aggarwal, G.; Nguyen, A.D.; Morley, J.E.; Li, Y.; Bateman, R.J.; Vellas, B.; Barreto, P.D.S. Associations between Physical Activity, Blood-Based Biomarkers of Neurodegeneration, and Cognition in Healthy Older Adults: The MAPT Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 1382–1390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).