Current Natural Degradation and Artificial Intervention Removal Techniques for Antibiotics in the Aquatic Environment: A Review
Abstract
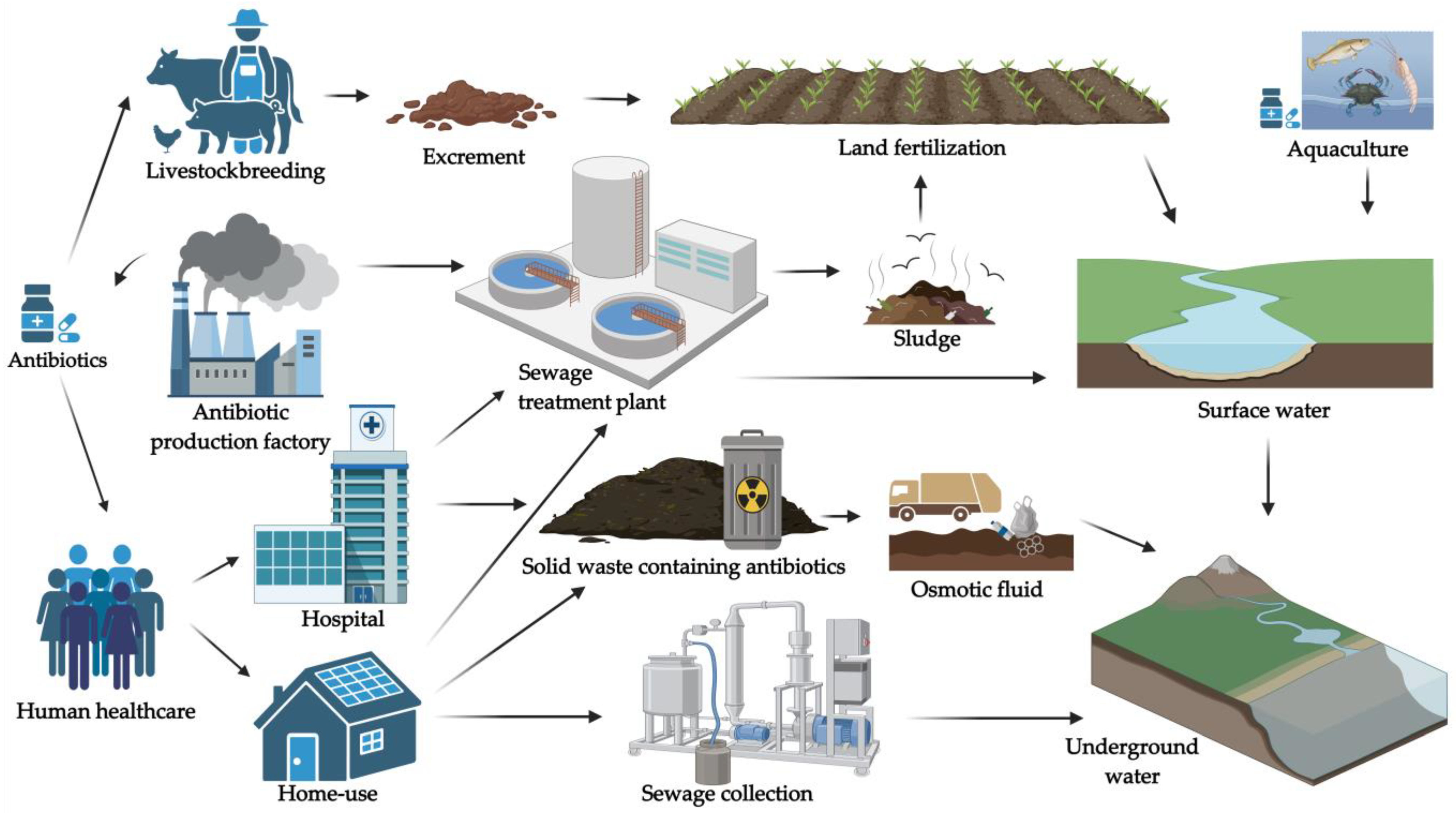
1. Introduction
2. The Degradation of Antibiotics in the Natural Environment
2.1. Photolysis and Hydrolysis
2.2. Biodegradation
3. Artificial Intervention in the Degradation of Antibiotics
3.1. Advanced Oxidation Processes (AOPs)
3.2. Supercritical Water Oxidation
3.3. Physical Chemistry Methods
3.3.1. Adsorption Method
3.3.2. Ultrafiltration Membrane Technology
3.4. Ionizing Radiation Degradation
3.5. New Type of Catalyst
3.5.1. Nano Photocatalyst
3.5.2. Metal–Organic Frameworks (MOFs)
3.5.3. Carbon-Based Single-Atom Photocatalysts
4. Hybrid Technology
4.1. Microbial Electrochemical Systems
4.2. Microalgae Technology
4.3. Constructed Wetland Technology
5. Conclusions and Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Cunha, R.D.; Fonseca, L.P.; Calado, C.R. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef]
- Huang, S.; Yu, J.; Li, C.; Zhu, Q.; Zhang, Y.; Lichtfouse, E.; Marmier, N. The Effect Review of Various Biological, Physical and Chemical Methods on the Removal of Antibiotics. Water 2022, 14, 3138. [Google Scholar] [CrossRef]
- Lienert, J.; Guedel, K.; Escher, B.I. Screening method for ecotoxicological hazard assessment of 42 pharmaceuticals considering human metabolism and excretory routes. Environ. Sci. Technol. 2007, 41, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current research trends on emerging contaminants pharmaceutical and personal care products (PPCPs): A comprehensive review. Sci. Total Environ. 2023, 859, 16003. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Wu, Y.; Li, S.; Hu, J.; Sun, W.; Ni, J. Profiles, drivers, and prioritization of antibiotics in China’s major rivers. J. Hazard. Mater. 2024, 477, 135399. [Google Scholar] [CrossRef]
- Booth, A.; Aga, D.S.; Wester, A.L. Retrospective analysis of the global antibiotic residues that exceed the predicted no effect concentration for antimicrobial resistance in various environmental matrices. Environ. Int. 2020, 141, 105796. [Google Scholar] [CrossRef]
- Le, T.X.; Munekage, Y. Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam. Mar. Pollut. Bull. 2005, 49, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Yin, D.; Zhang, H.; Yu, Z. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 2011, 82, 822–828. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef]
- Brandt, K.K.; Amezquita, A.; Backhaus, T.; Boxall, A.; Coors, A.; Heberer, T.; Lawrence, J.R.; Lazorchak, J.; Schoenfeld, J.; Snape, J.R. Ecotoxicological assessment of antibiotics: A call for improved consideration of microorganisms. Environ. Int. 2015, 85, 189–205. [Google Scholar] [CrossRef]
- Vlitalo, P.; Kruglova, A.; Mikola, A.; Vahala, R. Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review. Int. J. Hyg. Environ. Health 2017, 220, 558–569. [Google Scholar] [CrossRef]
- Cabello, F.C. Antibióticos y acuicultura en Chile: Consecuencias para la salud humana y animal. Rev. Médica Chile 2004, 132, 1001–1006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baran, W.; Adamek, E.; Ziemianska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, Y.; Huang, H.; Zhong, C. Flexibility induced high-performance MOF-based adsorbent for nitroimidazole antibiotics capture. Chem. Eng. J. 2018, 333, 678–685. [Google Scholar] [CrossRef]
- Martínez, J.L. Antibiotics and Antibiotic Resistance Genes in Natural Environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, H.; Zhang, K.; Xu, X.; Wang, C.; Zhu, W.; Wu, W. Long-term low dissolved oxygen accelerates the removal of antibiotics and antibiotic resistance genes in swine wastewater treatment. Chem. Eng. J. 2018, 334, 630–637. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. In Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2014; Available online: https://amr-review.org/ (accessed on 19 April 2025).
- Kusari, S.; Prabhakaran, D.; Lamshöft, M.; Spiteller, M. In vitro residual anti-bacterial activity of difloxacin, sarafloxacin and their photoproducts after photolysis in water. Environ. Pollut. 2009, 157, 2722–2730. [Google Scholar] [CrossRef]
- Asih, A.K.; Yetti, R.D.; Chandra, B. Photodegradation of Antibiotic Using TiO2 as a Catalyst: A Review. Int. J. Pharm. Sci. Med. 2021, 6, 37–43. [Google Scholar] [CrossRef]
- Lin, Y.C.; Hsiao, K.W.; Lin, Y.C. Photolytic degradation of ciprofloxacin in solid and aqueous environments: Kinetics, phototransformation pathways, and byproducts. Environ. Sci. Pollut. Res. 2017, 25, 2303–2312. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Aust, M.O. Effects of Pig Slurry on the Sorption of Sulfonamide Antibiotics in Soil. Arch. Environ. Contam. Toxicol. 2004, 47, 31–39. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Jian, H.; Wang, C.; Xing, B.; Sun, H.; Hao, Y. Effect of biochar-derived dissolved organic matter on adsorption of sulfamethoxazole and chloramphenicol. J. Hazard. Mater. 2020, 396, 122598. [Google Scholar] [CrossRef] [PubMed]
- Wegst-Uhrich, S.R.; Navarro, D.A.G.; Zimmerman, L.; Aga, D.S. Assessing antibiotic sorption in soil: A literature review and new case studies on sulfonamides and macrolides. Chem. Cent. J. 2014, 8, 5. [Google Scholar] [CrossRef]
- Mccabe, A.J.; Arnold, W.A. Multiple linear regression models to predict the formation efficiency of triplet excited states of dissolved organic matter in temperate wetlands. Limnol. Oceanogr. 2018, 63, 1992–2014. [Google Scholar] [CrossRef]
- Canonica, S.; Schönenberger, U. Inhibitory Effect of Dissolved Organic Matter on the Transformation of Selected Anilines and Sulfonamide Antibiotics Induced by the Sulfate Radical. Environ. Sci. Technol. 2019, 53, 11783–11791. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Feng, L.; Li, R.; Li, J.; Du, Z.; Zhang, L.; Liu, Y. Inhibitory effects of antioxidant moieties in humic substances on phototransformation of chlortetracycline mediated by the algae extracellular organic matter. Sci. Total. Environ. 2021, 798, 149001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xie, Q.; Wang, J.; Chen, X.; Chen, J. Effects of dissolved organic matter derived from freshwater and seawater on photodegradation of three antiviral drugs. Environ. Pollut. 2019, 258, 113700. [Google Scholar] [CrossRef]
- Chen, J.; Qiao, X.; Zhang, Y.N.; Uddin, M.; Guo, Z. Disparate effects of DOM extracted from coastal seawaters and freshwaters on photodegradation of 2,4-Dihydroxybenzophenone. Water Res. 2019, 151, 280–287. [Google Scholar]
- Niu, J.; Li, Y.; Wang, W. Light-source-dependent role of nitrate and humic acid in tetracycline photolysis: Kinetics and mechanism. Chemosphere 2013, 92, 1423–1429. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Qu, J.; Yang, M. Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J. Photochem. Photobiol. A Chem. 2008, 197, 81–87. [Google Scholar] [CrossRef]
- Jiang, R.; Wei, Y.; Sun, J.; Wang, J.; Zhao, Z.; Liu, Y.; Li, X.; Cao, J. Degradation of cefradine in alga-containing water environment: A mechanism and kinetic study. Environ. Sci. Pollut. Res. 2019, 26, 9184–9192. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. pH and temperature effects on the hydrolysis of three ??-lactam antibiotics: Ampicillin, cefalotin and cefoxitin. Sci. Total Environ. 2013, 466–467C, 547–555. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, S.; An, Y.; Wang, Y.; Lei, Y.; Song, L. Antibiotics and antibiotic resistance genes in landfills: A review. Sci. Total Environ. 2022, 806, 150647. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X. Detection of AmpC β-lactamases in Acinetobacter baumannii in the Xuzhou region and analysis of drug resistance. Exp. Ther. Med. 2015, 10, 933–936. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sekawi, Z.; Monjezi, A.; Maleki, M.H.; Soroush, S.; Sadeghifard, N.; Pakzad, I.; Azizi-Jalilian, F.; Emaneini, M.; Asadollahi, K. Emergence of SCCmec type III with variable antimicrobial resistance profiles and spa types among methicillin-resistant Staphylococcus aureus isolated from healthcare- and community-acquired infections in the west of Iran. Int. J. Infect. Dis. 2014, 25, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y. Mechanisms of bacterial resistance to macrolide antibiotics. J. Infect. Chemother. 1999, 5, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.V.; Day, P.; Lewendon, A.; Murray, I.A. Tinkering with antibiotic resistance: Chloramphenicol acetyltransferase and its substrates. Biochm. Soc. Trans. 1988, 16, 939–942. [Google Scholar] [CrossRef]
- Erickson, B.D.; Elkins, C.A.; Mullis, L.B.; Heinze, T.M.; Wagner, R.D.; Cerniglia, C.E. A metallo-β-lactamase is responsible for the degradation of ceftiofur by the bovine intestinal bacterium Bacillus cereus P41. Vet. Microbiol. 2014, 172, 499–504. [Google Scholar] [CrossRef]
- Maki, T.; Hasegawa, H.; Kitami, H.; Fumoto, K.; Munekage, Y.; Ueda, K. Bacterial degradation of antibiotic residues in marine fish farm sediments of Uranouchi Bay and phylogenetic analysis of antibiotic-degrading bacteria using 16S rDNA sequences. Fish. Sci. 2010, 72, 811–820. [Google Scholar] [CrossRef]
- Migliore, L.; Fiori, M.; Spadoni, A.; Galli, E. Biodegradation of oxytetracycline by Pleurotus ostreatus mycelium: A mycoremediation technique. J. Hazard. Mater. 2012, 215–216, 227–232. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Interaction between tetracycline and microorganisms during wastewater treatment: A review. Sci. Total Environ. 2021, 757, 143981. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Chen, C.; Zhang, C.; Su, G.; Ren, N.; Ho, S.-H. Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae. Water Res. 2020, 172, 115475. [Google Scholar] [CrossRef]
- Zhao, K.; Si, T.; Liu, S.; Liu, G.; Li, D.; Li, F. Co-metabolism of microorganisms: A study revealing the mechanism of antibiotic removal, progress of biodegradation transformation pathways. Sci. Total Environ. 2024, 954, 176561. [Google Scholar] [CrossRef]
- Kim, D.W.; Heinze, T.M.; Kim, B.S.; Schnackenberg, L.K.; Woodling, K.A.; Sutherland, J.B. Modification of Norfloxacin by a Microbacterium sp. Strain Isolated from a Wastewater Treatment Plant. Appl. Environ. Microbiol. 2011, 77, 6100–6108. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, H.; Li, Z.; Feng, Y.; Cheng, D.; Xue, J. Biodegradation of gentamicin by bacterial consortia AMQD4 in synthetic medium and raw gentamicin sewage. Sci. Rep. 2017, 7, 11004. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Khanal, S.K.; Zhang, H.; Chen, G.-H.; Lu, H. Sulfamethoxazole degradation in anaerobic sulfate-reducing bacteria sludge system. Water Res. 2017, 119, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2021, 20, 257–269. [Google Scholar] [CrossRef]
- Xu, B.; Mao, D.; Luo, Y.; Xu, L. Sulfamethoxazole biodegradation and biotransformation in the water–sediment system of a natural river. Bioresour. Technol. 2011, 102, 7069–7076. [Google Scholar] [CrossRef]
- Manaia, C.M. Assessing the Risk of Antibiotic Resistance Transmission from the Environment to Humans: Non-Direct Proportionality between Abundance and Risk. Trends Microbiol. 2017, 25, 173–181. [Google Scholar] [CrossRef]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Grad, Y.H.; Lipsitch, M.; Feldgarden, M.; Arachchi, H.M.; Cerqueira, G.C.; FitzGerald, M.; Godfrey, P.; Haas, B.J.; Murphy, C.I.; Russ, C.; et al. Genomic epidemiology of the Escherichia coli O104:H4 outbreaks in Europe, 2011. In Proceedings of the National Academy of Sciences of the United States of America, Washington, DC, USA, 17 January 2012. [Google Scholar]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 46, 127083. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Huebner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Anjali, R.; Shanthakumar, S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J. Environ. Manag. 2019, 246, 51–62. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Affam, A.C.; Chaudhuri, M. Optimization of Fenton treatment of amoxicillin and cloxacillin antibiotic aqueous solution. Desalination Water Treat. 2014, 52, 1878–1884. [Google Scholar] [CrossRef]
- Elmolla, E.; Chaudhuri, M. Optimization of Fenton process for treatment of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution-ScienceDirect. J. Hazard. Mater. 2009, 170, 666–672. [Google Scholar] [CrossRef]
- Mohsen, D.S.; Hadi, I.M.; Roya, O.; Mahmood, H. Erratum to “The Comparative Study of the Effectiveness of Cimetidine, Ranitidine, Famotidine, and Omeprazole in Treatment of Children with Dyspepsia”. Isrn Pediatr. 2013, 2013, 206546. [Google Scholar]
- Verma, M.; Haritash, A.K. Degradation of amoxicillin by Fenton and Fenton-integrated hybrid oxidation processes. J. Environ. Chem. Eng. 2019, 7, 102886. [Google Scholar] [CrossRef]
- Gou, Y.; Chen, P.; Yang, L.; Li, S.; Peng, L.; Song, S.; Xu, Y. Degradation of fluoroquinolones in homogeneous and heterogeneous photo-Fenton processes: A review. Chemosphere 2021, 270, 129481. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Vogler, B.; Lei, Y.; Wu, T. Metallic ion leaching from heterogeneous catalysts: An overlooked effect in the study of catalytic ozonation processes. Environ. Sci. Water Res. Technol. 2017, 3, 1143–1151. [Google Scholar] [CrossRef]
- Hassani, A.; Karaca, M.; Karaca, S.; Khataee, A.; Açışlı, Ö.; Yılmaz, B. Preparation of magnetite nanoparticles by high-energy planetary ball mill and its application for ciprofloxacin degradation through heterogeneous Fenton process. J. Environ. Manag. 2018, 211, 53–62. [Google Scholar] [CrossRef]
- Sang, F.; Yin, Z.; Wang, W.; Almatrafi, E.; Wang, Y.; Zhao, B.; Gong, J.; Zhou, C.; Zhang, C.; Zeng, G.; et al. Degradation of ciprofloxacin using heterogeneous Fenton catalysts derived from natural pyrite and rice straw biochar. J. Clean. Prod. 2022, 378, 134459. [Google Scholar] [CrossRef]
- Brillas, E. Progress of homogeneous and heterogeneous electro-Fenton treatments of antibiotics in synthetic and real wastewaters. A critical review on the period 2017–2021. Sci. Total Environ. 2022, 819, 153102. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Olvera-Vargas, H.; Oturan, N.; Oturan, M.A. Heterogeneous Electro-Fenton Process: Principles and Applications. In Electro-Fenton Process: New Trends and Scale-Up; Zhou, M., Oturan, M.A., Sirés, I., Eds.; Springer: Singapore, 2018; pp. 85–110. [Google Scholar]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.-S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef]
- Qi, H.; Sun, X.; Sun, Z. Cu-doped Fe2O3 nanoparticles/etched graphite felt as bifunctional cathode for efficient degradation of sulfamethoxazole in the heterogeneous electro-Fenton process. Chem. Eng. J. 2022, 427, 131695. [Google Scholar] [CrossRef]
- Jiang, Y.; Ran, J.; Mao, K.; Yang, X.; Zhong, L.; Yang, C.; Feng, X.; Zhang, H. Recent progress in Fenton/Fenton-like reactions for the removal of antibiotics in aqueous environments. Ecotoxicol. Environ. Saf. 2022, 236, 113464. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.; Yi, X.-H.; Wang, C.-C.; Fu, H.; Wang, P.; Zhao, Y. Heterogeneous photo-Fenton degradation toward sulfonamide matrix over magnetic Fe3S4 derived from MIL-100(Fe). J. Hazard. Mater. 2022, 424, 127415. [Google Scholar] [CrossRef]
- Du, Z.; Liu, F.; Xiao, C.; Dan, Y.; Jiang, L. Fabrication of poly (vinyl alcohol)/sodium alginate hydrogel beads and its application in photo-Fenton degradation of tetracycline. J. Mater. Sci. 2021, 56, 913–926. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Feng, W.; Wang, Y.; Liu, S.; Dong, Z.; Li, X. A critical review on metal complexes removal from water using methods based on Fenton-like reactions: Analysis and comparison of methods and mechanisms. J. Hazard. Mater. 2021, 414, 125517. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhu, Z.; Shang, Q.; Tang, Y.; Zhang, D.; Du, Y.; Liu, M.; Yin, K.; Liu, C. Engineering. Highly Efficient Continuous-Flow Electro-Fenton Treatment of Antibiotic Wastewater Using a Double-Cathode System. ACS Sustain. Chem. Eng. 2021, 9, 9. [Google Scholar] [CrossRef]
- Bugueño-Carrasco, S.; Monteil, H.; Toledo-Neira, C.; Sandoval, M.Á.; Thiam, A.; Salazar, R. Elimination of pharmaceutical pollutants by solar photoelectro-Fenton process in a pilot plant. Environ. Sci. Pollut. Res. 2021, 28, 23753–23766. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Shi, S.; Chen, D.; Liu, J.; Huang, L.; Zhang, J.; Feng, Y. Study on the mechanism of tetracycline removal in electrocoagulation coupled with electro-fenton reaction system with Fe anode and carbon nanotube cathode. Chem. Eng. J. 2022, 428, 131045. [Google Scholar] [CrossRef]
- Zhu, L.; Santiago-Schübel, B.; Xiao, H.; Hollert, H.; Kueppers, S. Electrochemical oxidation of fluoroquinolone antibiotics: Mechanism, residual antibacterial activity and toxicity change. Water Res. 2016, 102, 52–62. [Google Scholar] [CrossRef]
- Ke, M.K.; Huang, G.X.; Mei, S.C.; Wang, Z.H.; Zhang, Y.J.; Hua, T.W.; Zheng, L.R.; Yu, H.Q. Interface-Promoted Direct Oxidation of p -Arsanilic Acid and Removal of Total Arsenic by the Coupling of Peroxymonosulfate and Mn-Fe-Mixed Oxide. Environ. Sci. Technol. 2021, 55, 7063–7071. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Fang, X.; Miao, W.; Chen, X.; Sun, J.; Ni, B.-J.; Mao, S. Heterogeneous Electro-Fenton catalysis with HKUST-1-derived Cu@C decorated in 3D graphene network-ScienceDirect. Chemosphere 2020, 243, 125423. [Google Scholar] [CrossRef]
- Nieto-Juarez, J.I.; Pierzchla, R.; Sienkiewicz, R.; Kohn, R. Inactivation of MS2 coliphage in Fenton and Fenton-like systems: Role of transition metals, hydrogen peroxide and sunlight. Environ. Sci. Technol. 2010, 44, 3351–3356. [Google Scholar] [CrossRef]
- Dai, L.; Li, B.; Xu, H.; Wang, W.S.; Zhang, S.; Xu, Y.; Qi, S.; He, X.; Jin, L. Magnetic nanoreactor Fe3O4@HNTs as heterogeneous Fenton-like catalyst for acid fuchsin degradation: Efficiency, kinetics and mechanism. J. Phys. Chem. Solids 2023, 180, 111445. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Zhang, G.; Pang, T.; Zhang, X.; Cai, D.; Wu, Z.J.R. In situ degradation of antibiotic residues in medical intravenous infusion bottles using high energy electron beam irradiation. Sci. Rep. 2017, 7, 39928. [Google Scholar] [CrossRef]
- Wang, N.; Jin, L.; Li, C.; Liang, Y.; Wang, P. Preparation of coal fly ash-based Fenton-like catalyst and its application for the treatment of organic wastewater under microwave assistance. J. Clean. Prod. 2022, 342, 130926. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Han, H.; Zhang, M.; Wang, H.; Song, H.; Chen, Y. Effective removal of organic dyes using the ultrasonic-assisted hydrothermal synthesis of NaP zeolite doping Cu or Fe in Fenton-like oxidation systems. Sep. Purif. Technol. 2022, 299, 121767. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Liu, S.; Peng, Y.; Ki, S.Y. Ultrasound-assisted electrochemical treatment for phenolic wastewater. Ultrason. Sonochemistry 2020, 65, 105058. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, M.D.; Cocero, M.J. Supercritical water oxidation: A technical review. AIChE J. 2006, 52, 3933–3951. [Google Scholar] [CrossRef]
- Gong, M.; Wang, M.; Wang, L.; Feng, A.; Hu, J. Degradation of tetracycline hydrochloride in sub- and supercritical water with and without oxidation. Process Saf. Environ. Prot. 2022, 162, 373–383. [Google Scholar] [CrossRef]
- Stavbar, S.; Hrnčič, M.K.; Premzl, K.; Kolar, M.; Turk, S.Š. Sub- and super-critical water oxidation of wastewater containing amoxicillin and ciprofloxacin. J. Supercrit. Fluids 2017, 128, 73–78. [Google Scholar] [CrossRef]
- Dias, I.M.; Mourão, L.C.; Andrade, L.A.; Souza, G.B.M.; Viana, J.C.V.; Oliveira, S.B.; Alonso, C.G. Degradation of antibiotic amoxicillin from pharmaceutical industry wastewater into a continuous flow reactor using supercritical water gasification. Water Res. 2023, 234, 119826. [Google Scholar] [CrossRef]
- Chen, J.; Bai, Y.; Meng, T.; Wang, Q.; Wang, C.; Jiaqiang, E. Detailed mechanisms of amoxicillin decomposition in supercritical water by ReaxFF reactive molecular dynamics simulation. Chem. Eng. J. 2023, 451, 138644. [Google Scholar] [CrossRef]
- Top, S.; Akgün, M.; Kıpçak, E.; Bilgili, M.S. Treatment of hospital wastewater by supercritical water oxidation process. Water Res. 2020, 185, 116279. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.-H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef]
- Chen, C.X.; Aris, A.; Yong, E.L.; Noor, Z.Z. A review of antibiotic removal from domestic wastewater using the activated sludge process: Removal routes, kinetics and operational parameters. Environ. Sci. Pollut. Res. 2022, 29, 4787–4802. [Google Scholar] [CrossRef]
- He, P.; Yang, K.; Wang, W.; Dong, F.; Du, L.; Deng, Y. Reduced graphene oxide-CoFe2O4 composites for supercapacitor electrode. Russ. J. Electrochem. 2013, 49, 359–364. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ji, L.; Liu, J.; Yang, H.; Dong, T.; Liu, T. Zeolite-like algal biochar nanoparticles for enhanced antibiotics removal: Sorption mechanisms and theoretical calculations. Colloids Surf. B Biointerfaces 2025, 248, 114475. [Google Scholar] [CrossRef] [PubMed]
- Saljoughi, E.; Sadrzadeh, M.; Mohammadi, T. Effect of preparation variables on morphology and pure water permeation flux through asymmetric cellulose acetate membranes. J. Membr. Sci. 2009, 326, 627–634. [Google Scholar] [CrossRef]
- Sun, W.; Li, L.; Chen, C.; Li, J. Effects of operation conditions, solvent and gelation bath on morphology and performance of PPESK asymmetric ultrafiltration membrane. J. Appl. Polym. Sci. 2010, 108, 3662–3669. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook; Journal of Yangling Vocational & Technical College; Taylor & Francis Group: Boca Raton, FL, USA, 1998. [Google Scholar]
- Wenten, I.G.; Khoiruddin, K.; Wardani, A.K.; Aryanti, P.T.P.; Astuti, D.I.; Komaladewi, A. Preparation of antifouling polypropylene/ZnO composite hollow fiber membrane by dip-coating method for peat water treatment. J. Water Process Eng. 2020, 34, 101158. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L.; Fontananova, E. Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewaters by membrane technology: Limitations, successes, and future improvements. Sci. Total Environ. 2022, 838, 156010. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Preparation of a Novel Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane Modified with Reduced Graphene Oxide/Titanium Dioxide (TiO2) Nanocomposite with Enhanced Hydrophilicity and Antifouling Properties. Ind. Eng. Chem. Res. 2014, 53, 140819070522009. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Yu, S.; Hu, J.; Wang, J. Gamma radiation-induced degradation of p-nitrophenol (PNP) in the presence of hydrogen peroxide (H2O2) in aqueous solution. J. Hazard. Mater. 2011, 177, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J. Degradation of sulfamethazine by gamma irradiation in the presence of hydrogen peroxide. J. Hazard. Mater. 2013, 250–251, 99–105. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Radiation induced degradation of dyes—An overview. J. Hazard. Mater. 2009, 166, 6–16. [Google Scholar] [CrossRef]
- Alsager, O.A.; Alnajrani, M.N.; Alhazzaa, O. Decomposition of antibiotics by gamma irradiation: Kinetics, antimicrobial activity, and real application in food matrices. Chem. Eng. J. 2018, 338, 548–556. [Google Scholar] [CrossRef]
- Liu, F.; Guo, Z.; Zhao, Y.; Cao, H.; Zhu, S. Radiolytic decomposition of ciprofloxacin using gamma irradiation in aqueous solution. Environ. Sci. Pollut. Res. Int. 2015, 22, 15772–15780. [Google Scholar]
- Guo, Z.; Zhou, F.; Zhao, Y.; Zhang, C.; Liu, F.; Bao, C.; Lin, M. Gamma irradiation-induced sulfadiazine degradation and its removal mechanisms. Chem. Eng. J. 2012, 191, 256–262. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2018, 646, 1385–1397. [Google Scholar] [CrossRef]
- Rivas-Ortiz, I.B.; Cruz-González, G.; Lastre-Acosta, A.M.; Manduca-Artiles, M.; Rapado-Paneque, M.; Chávez-Ardanza, A.; Teixeira, A.C.S.C.; Jáuregui-Haza, U.J. Optimization of radiolytic degradation of sulfadiazine by combining Fenton and gamma irradiation processes. J. Radioanal. Nucl. Chem. 2017, 314, 2597–2607. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, R.; Zou, Z.; Zhang, L.; Ma, H. Optimizing physico-chemical properties of hierarchical ZnO/TiO2 nano-film by the novel heating method for photocatalytic degradation of antibiotics and dye. Chemosphere 2024, 346, 140392. [Google Scholar] [CrossRef]
- Wang, Y.; Xiu, J.; Gan, T.; Zou, H.; Li, F. Photocatalytic degradation of tetracycline hydrochloride by lanthanum doped TiO2@g-C3N4 activated persulfate under visible light irradiation. RSC Adv. 2023, 13, 8383–8393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Wang, Z.; Hu, C.; Ma, D.; Chen, W.; Ao, T. Effective and structure-controlled adsorption of tetracycline hydrochloride from aqueous solution by using Fe-based metal-organic frameworks—ScienceDirect. Appl. Surf. Sci. 2021, 542, 148662. [Google Scholar] [CrossRef]
- Liang, C.; Tang, Y.; Zhang, X.; Chai, H.; Huang, Y.; Feng, P. ZIF-mediated N-doped hollow porous carbon as a high performance adsorbent for tetracycline removal from water with wide pH range. Environ. Res. 2020, 182, 109059. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption Behaviors of Organic Micropollutants on Zirconium Metal-Organic Framework UiO-66: Analysis of Surface Interactions. Acs Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Chen, X.; Wang, L.; Cai, W. Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway. Chem. Eng. J. 2019, 369, 13. [Google Scholar] [CrossRef]
- Wang, A.J.; Cui, D.; Cheng, H.Y.; Guo, Y.Q.; Kong, F.Y.; Ren, N.Q.; Wu, W.M. A membrane-free, continuously feeding, single chamber up-flow biocatalyzed electrolysis reactor for nitrobenzene reduction. J. Hazard. Mater. 2012, 199, 401. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Sun, W.; Lei, Y.; Wang, Q.; Li, A.; Chen, W.; Zhou, G.; Zhang, Z.; Wang, Y.; et al. Engineering the Atomic Interface with Single Platinum Atoms for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2019, 59, 1295–1301. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Shen, S. Single Metal Atom Photocatalysis. Small Methods 2019, 3, 1800447. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, W.; Zhao, Z.; Liu, W.; Ye, J.; Tong, M.; Li, Y. Different degradation mechanisms of carbamazepine and diclofenac by single-atom Barium embedded g-C3N4: The role of photosensitation-like mechanism. J. Hazard. Mater. 2021, 416, 125936. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Cao, D.; Wang, Y.; Zhu, Y. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Appl. Catal. B Environ. 2017, 211, 79–88. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, G.; Huang, D.; Zhang, C.; He, D.; Zhou, C.; Wang, W.; Xiong, W.; Song, B.; Yi, H.; et al. In Situ Grown Single-Atom Cobalt on Polymeric Carbon Nitride with Bidentate Ligand for Efficient Photocatalytic Degradation of Refractory Antibiotics. Small 2020, 16, 2001634. [Google Scholar] [CrossRef]
- Kumar, A.; Hsu, L.H.-H.; Kavanagh, P.; Barrière, F.; Lens, P.N.L.; Lapinsonnière, L.; Lienhard V, J.H.; Schröder, U.; Jiang, X.; Leech, D. The ins and outs of microorganism–electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1, 0024. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Cheng, H.-Y.; Kong, D.-Y.; Gao, S.-H.; Sun, F.; Cui, D.; Kong, F.-Y.; Zhou, A.-J.; Liu, W.-Z.; Ren, N.-Q.; et al. Accelerated Reduction of Chlorinated Nitroaromatic Antibiotic Chloramphenicol by Biocathode. Environ. Sci. Technol. 2013, 47, 5353–5361. [Google Scholar] [CrossRef]
- Wang, L.; You, L.; Zhang, J.; Yang, T.; Zhang, W.; Zhang, Z.; Liu, P.; Wu, S.; Zhao, F.; Ma, J. Biodegradation of sulfadiazine in microbial fuel cells: Reaction mechanism, biotoxicity removal and the correlation with reactor microbes. J. Hazard. Mater. 2018, 360, 402–411. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Li, J.; Ly, Q.V.; Nguyen, T.A.H.; Tran, V.S. Applying a new pomelo peel derived biochar in microbial fell cell for enhancing sulfonamide antibiotics removal in swine wastewater. Bioresour. Technol. 2020, 318, 123886. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Liang, B.; Ma, J.; Cai, W.; Li, Z.; Liu, W.; Qi, M.; Zhao, Y.; Ma, X.; Deng, Y.; Wang, A.; et al. Response of chloramphenicol-reducing biocathode resistome to continuous electrical stimulation. Water Res. 2019, 148, 398–406. [Google Scholar] [CrossRef]
- Zhang, S.; Song, H.-L.; Cao, X.; Li, H.; Guo, J.; Yang, X.-L.; Singh, R.P.; Liu, S. Inhibition of methanogens decreased sulfadiazine removal and increased antibiotic resistance gene development in microbial fuel cells. Bioresour. Technol. 2019, 281, 188–194. [Google Scholar] [CrossRef]
- Cheng, S.; Kiely, P.; Logan, B.E. Pre-acclimation of a wastewater inoculum to cellulose in an aqueous–cathode MEC improves power generation in air–cathode MFCs. Bioresour. Technol. 2011, 102, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xiao, Y.; Yan, W.; Ding, R.; Wang, S.; Zhao, F. The effect of bioelectrochemical systems on antibiotics removal and antibiotic resistance genes: A review. Chem. Eng. J. 2019, 358, 17. [Google Scholar] [CrossRef]
- Dominguez-Benetton, X.; Varia, J.C.; Pozo, G.; Modin, O.; Ter Heijne, A.; Fransaer, J.; Rabaey, K. Metal recovery by microbial electro-metallurgy. Prog. Mater. Sci. 2018, 94, 435–461. [Google Scholar] [CrossRef]
- Xue, W.; Zhou, Q.; Li, F. Bacterial community changes and antibiotic resistance gene quantification in microbial electrolysis cells during long-term sulfamethoxazole treatment. Bioresour. Technol. 2019, 294, 122170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, L.; Crittenden, J.C. An electrochemical process that uses an Fe0/TiO2 cathode to degrade typical dyes and antibiotics and a bio-anode that produces electricity. Front. Environ. Sci. Eng. 2016, 10, 15. [Google Scholar] [CrossRef]
- Dai, M.; Zhang, Y.; Wu, Y.; Sun, R.; Zong, W.; Kong, Q. Mechanism involved in the treatment of sulfamethoxazole in wastewater using a constructed wetland microbial fuel cell system. J. Environ. Chem. Eng. 2021, 9, 10619. [Google Scholar] [CrossRef]
- Soltani, F.; Navidjouy, N.; Khorsandi, H.; Rahimnejad, M.; Alizadeh, S. A novel bio-electro-Fenton system with dual application for the catalytic degradation of tetracycline antibiotic in wastewater and bioelectricity generation. RSC Adv. 2021, 11, 27160–27173. [Google Scholar] [CrossRef]
- Sun, J.; Li, N.; Yang, P.; Zhang, Y.; Yuan, Y.; Lu, X.; Zhang, H. Simultaneous antibiotic degradation, nitrogen removal and power generation in a microalgae-bacteria powered biofuel cell designed for aquaculture wastewater treatment and energy recovery. Int. J. Hydrogen Energy 2020, 45, 10871–10881. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chang, J.S. Microalgal biosorption of heavy metals: A comprehensive bibliometric review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Hu, L.; Liu, Y.; Zhao, J.; He, L.; Ying, G. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems. Environ. Int. 2021, 155, 106594. [Google Scholar] [CrossRef]
- Norvill, Z.N.; Shilton, A.; Guieysse, B. Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J. Hazard. Mater. 2016, 313, 291–309. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants—Opportunities and challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lin, H.; Guo, W.; Zhao, F.; Li, J. Bioaccumulation and biodegradation of sulfamethazine in Chlorella pyrenoidosa. J. Ocean. Univ. China 2017, 16, 1167–1174. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem. Eng. J. 2016, 313, 1251–1257. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can Microalgae Remove Pharmaceutical Contaminants from Water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Johnson, N.W.; Liu, Y.; Miao, Y.; Mahendra, S. Biodegradation mechanisms of sulfonamides by Phanerochaete chrysosporium—Luffa fiber system revealed at the transcriptome level. Chemosphere 2021, 266, 129194. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, Y.-S.; Hu, L.-X.; Shi, Z.-Q.; Cai, W.-W.; He, L.-Y.; Ying, G.-G. Co-metabolism of sulfamethoxazole by a freshwater microalga Chlorella pyrenoidosa. Water Res. 2020, 175, 115656. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, J. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem. Eng. J. 2015, 260, 550–556. [Google Scholar] [CrossRef]
- Huang, K.-X.; Vadiveloo, A.; Zhong, H.; Mao, B.-D.; Qiu, J.; Gao, F. Enhancing the removal of sulfamethoxazole and microalgal lipid production through microalgae-biochar hybrids. Bioresour. Technol. 2024, 413, 131510. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef]
- Mishra, A.; Medhi, K.; Malaviya, P.; Thakur, I.S. Omics approaches for microalgal applications: Prospects and challenges. Bioresour. Technol. 2019, 291, 121890. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Chang, H.; Zhong, N.; Ren, N.; Ho, S.-H. Comprehensive insights into antibiotic resistance gene migration in microalgal-bacterial consortia: Mechanisms, factors, and perspectives. Sci. Total Environ. 2023, 901, 166029. [Google Scholar] [CrossRef]
- Ge, L.; Deng, H. Degradation of two fluoroquinolone antibiotics photoinduced by Fe (III)-microalgae suspension in an aqueous solution. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2015, 14, 693–699. [Google Scholar] [CrossRef]
- Wu, Y.; Li, T.; Yang, L. Mechanisms of removing pollutants from aqueous solutions by microorganisms and their aggregates: A review. Bioresour. Technol. 2012, 107, 10–18. [Google Scholar] [CrossRef]
- Xian, Q.; Hu, L.; Chen, H.; Chang, Z.; Zou, H. Removal of nutrients and veterinary antibiotics from swine wastewater by a constructed macrophyte floating bed system. J. Environ. Manag. 2010, 91, 2657–2661. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Fink, G.; Schluesener, M.P.; Sidrach-Cardona, R.; Martin-Villacorta, J.; Ternes, T.; Becares, E. Removal of antibiotics from urban wastewater by constructed wetland optimization. Chemosphere 2011, 83, 713–719. [Google Scholar] [CrossRef]
- Wang, Q.S.; Li, J.G.; Wang, C.J.; Cai, X.P.; Sun, H.; Tao, M. Treatment of Mariculture Wastewater Using Constructed Wetlands under Antibiotic Interference. Appl. Mech. Mater. 2014, 522–524, 849–853. [Google Scholar] [CrossRef]
| Antibiotic Class | Antibiotic Examples | Primary Target |
|---|---|---|
| Protein Synthesis Inhibitors | ||
| Aminoglycosides | Tobramycin, Gentamicin, Kanamycin | 30S ribosomal subunit (causes tRNA misreading) |
| Tetracyclines | Oxytetracycline, Doxycycline, Tetracycline | 30S ribosomal subunit (blocks aminoacyl-tRNA binding) |
| Macrolides | Erythromycin, Spiramycin, Azithromycin | 50S ribosomal subunit (inhibits elongation step) |
| Phenicols | Chloramphenicol, Thiamphenicol, Florfenicol | 50S ribosomal subunit (inhibits elongation step) |
| Cell Wall Synthesis Inhibitors | ||
| β-Lactams | Penicillin, Cephalosporins | Penicillin-binding proteins (disrupts cell wall synthesis) |
| Glycopeptides | Ampicillin, Vancomycin | Peptidoglycan subunits (inhibits cell wall biosynthesis) |
| DNA Synthesis Inhibitors | ||
| Quinolones | Ciprofloxacin, Levofloxacin | Topoisomerase II (DNA gyrase), Topoisomerase IV |
| Sulfonamides | Sulfamethoxazole, Sulfadiazine | Competitive inhibitor of DHPS (dihydropteroate synthase) in folate synthesis |
| RNA Synthesis Inhibitors | ||
| Rifamycins | Rifampin, Rifaximin | DNA-dependent RNA polymerase |
| Cell Membrane Disruptors | ||
| Polymyxins | Polymyxin B, Polymyxin E (Colistin) | Bacterial lipopolysaccharide layer (increases membrane permeability) |
| Comparison Item | Homogeneous Catalyst | Heterogeneous Catalyst |
|---|---|---|
| Advantages | 1. High activity: direct contact with reactants enables fast reaction rates (Fe2+ completely degrades amoxicillin in 12 min at pH 3 [62]). 2. Mild reaction conditions: efficient reactions at room temperature and pressure [63]. | 1. Broad pH tolerance: some catalysts (e.g., Fe3O4/montmorillonite) function at neutral pH [64]. 2. Recyclability: magnetic materials (e.g., Fe3O4) can be separated via magnetic fields [65]. 3. High stability: e.g., FeS2 retains activity after repeated cycles [66]. |
| Disadvantages | 1. pH limitation: requires acidic conditions (pH 2–3), forms iron sludge in neutral environment [67]. 2. Difficult recovery: catalyst separation is challenging, leading to secondary pollution [68]. 3. Toxic by-products: may generate harmful intermediates [69]. | 1. Complex synthesis: requires carrier modification (e.g., graphene), increasing costs [70]. 2. Metal leaching risk: long-term use may release Fe ions [64]. |
| Catalyst Type | Representative Materials | Degradation Mechanism | Advantages | Limitations |
|---|---|---|---|---|
| Nanophotocatalysts | TiO2, ZnO, g-C3N4 | Efficient separation of photogenerated electron–hole pairs and increased surface active sites. Heterojunctions (e.g., ZnO/TiO2) enhance charge separation; doping (N, C) broadens light absorption range and improves charge separation efficiency. | High catalytic activity; low cost. | Prone to e−/h+ recombination; some materials only respond to UV light; difficult to recover nanoparticles. |
| MOFs | MIL101(Fe), ZIF-8, UiO-66 | Adsorption (electrostatic, π-π, hydrophobic, acid–base interactions); photocatalytic generation of radicals (e.g., O2− and •OH) for pollutant degradation. | Ultra-high specific surface area (strong adsorption capacity); adjustable pore structure; multifunctional active sites. | Poor water stability (some MOFs are prone to hydrolysis); high synthesis cost; Low separation efficiency of photogenerated carriers. |
| Carbon-based Single-Atom Catalyst | Ba/g-C3N4, Ag/mpg-C3N4 | Single-atom metals (e.g., Ba and Co) optimize carrier band structure (e.g., g-C3N4) to enhance light absorption. | Atomic-level utilization (100%); significantly improved reaction kinetics. | Complex preparation process (requires precise control of single-atom dispersion); high cost. |
| Technology | Applicable Environmental Systems | Cost | Limitations |
|---|---|---|---|
| Natural Degradation Methods (Photolysis, Hydrolysis, and Biodegradation) | Surface water, soil | Low, relies on natural conditions, no additional equipment required | Slow degradation rate, limited efficiency, highly influenced by environmental conditions (e.g., light, pH, and temperature). Unable to handle high-concentration or complex pollutants and may generate secondary pollution such as antibiotic resistance genes (ARGs). |
| Microbial Degradation | Wastewater treatment plants (activated sludge process) | Moderate, requires optimization of reaction conditions for non-active substances | Limited degradation capacity for specific antibiotics; may require acclimation. High risk of ARGs dissemination, requiring subsequent monitoring. |
| Advanced Oxidation Processes (AOPs) | Industrial wastewater treatment | High, requires oxidants (e.g., H2O2, ozone), catalysts (e.g., TiO2), and energy (UV/electricity) | Low oxidant utilization, potential generation of toxic intermediates, requiring post-treatment. Harsh reaction conditions (e.g., acidic pH), complex equipment. |
| Supercritical Water Oxidation (SCWO) | High-concentration organic wastewater (pharmaceutical plants) | High operational cost, including equipment, energy, and maintenance costs | Acidic intermediates can severely corrode reactors, necessitating expensive materials. Requires continuous heating to maintain supercritical state, energy consumption higher than conventional biological treatment or AOPs. |
| Membrane Filtration Technology | Drinking water treatment | High, membrane materials (e.g., graphene and carbon nanotubes) are costly and require regular replacement | Severe membrane fouling issues, frequent cleaning or replacement increases maintenance costs. Concentrate disposal may cause secondary pollution. |
| Adsorption Method | Emergency or advanced treatment | Relatively high adsorbent cost (e.g., biochar and nano-modified materials) | Adsorbents require regeneration or disposal post-use, regeneration costs are high. Sensitive to dissolved organic matter (DOM) or ionic strength, efficiency easily affected by water quality. |
| Ionizing Radiation | Medical wastewater, high-risk pollutants | High, equipment costs (e.g., cobalt-60 radiation sources, accelerators) and regular maintenance | High initial investment unaffordable for small-scale plants. DOM and inorganic salts reduce efficiency. Scaling challenges: electron beam suitable for small–medium flow, gamma irradiation is mobile, suitable for fixed installations. |
| Bioelectrochemical Systems (BESs) | Laboratory or small-scale pilot projects | High, electrode materials (e.g., graphene) and system construction costs | Low electron transfer efficiency, difficult to scale up. Requires continuous power supply (MEC), high energy consumption. |
| Microalgae Technology | Ecological remediation | Moderate, requires light and nutrients for microalgae cultivation | Antibiotics may inhibit microalgae growth, requiring optimized ecosystems. High costs for harvesting and processing microalgae biomass. |
| Constructed Wetland Technology | Rural/decentralized wastewater treatment | Moderate, low construction cost but large land footprint | Treatment efficiency affected by seasons and climate, poor performance in winter. Long-term operation may accumulate pollutants, requiring regular maintenance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Li, H.; Liu, S. Current Natural Degradation and Artificial Intervention Removal Techniques for Antibiotics in the Aquatic Environment: A Review. Appl. Sci. 2025, 15, 5182. https://doi.org/10.3390/app15095182
Ji J, Li H, Liu S. Current Natural Degradation and Artificial Intervention Removal Techniques for Antibiotics in the Aquatic Environment: A Review. Applied Sciences. 2025; 15(9):5182. https://doi.org/10.3390/app15095182
Chicago/Turabian StyleJi, Jing, Haoqing Li, and Shejiang Liu. 2025. "Current Natural Degradation and Artificial Intervention Removal Techniques for Antibiotics in the Aquatic Environment: A Review" Applied Sciences 15, no. 9: 5182. https://doi.org/10.3390/app15095182
APA StyleJi, J., Li, H., & Liu, S. (2025). Current Natural Degradation and Artificial Intervention Removal Techniques for Antibiotics in the Aquatic Environment: A Review. Applied Sciences, 15(9), 5182. https://doi.org/10.3390/app15095182






