Aluminum-Loaded Bifunctional Resins for Efficient Fluoride Removal from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Loading Aluminum onto Resins
2.3. Analysis and Characterization
2.4. Batch Adsorption Experiments
2.5. Fixed-Bed Column Experiments
3. Results and Discussion
3.1. Material Characterization
3.2. Effect of Initial pH on Fluoride Adsorption
3.3. Effect of Coexisting Anions and Organics on Fluoride Adsorption
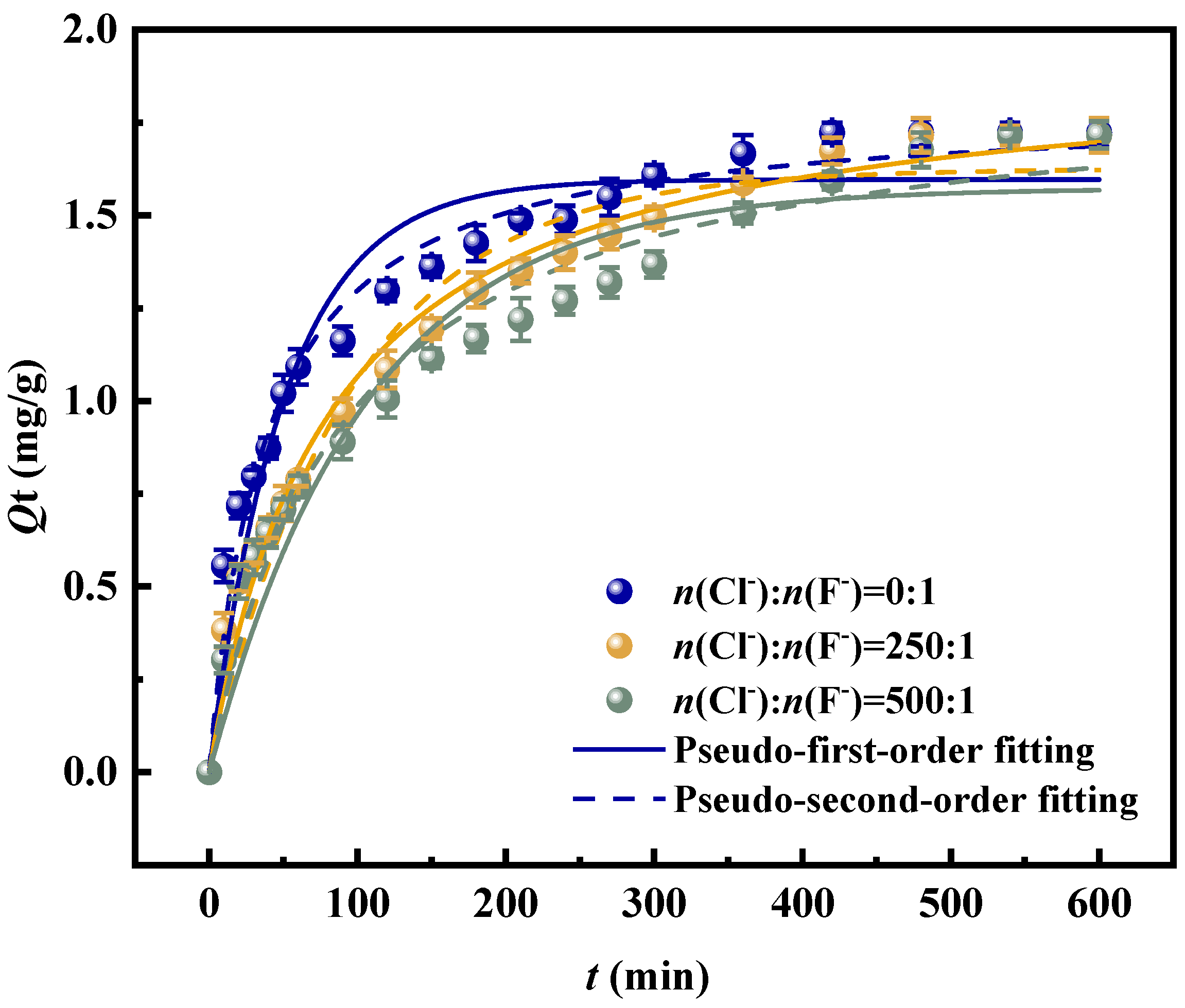
3.4. Adsorption Kinetics
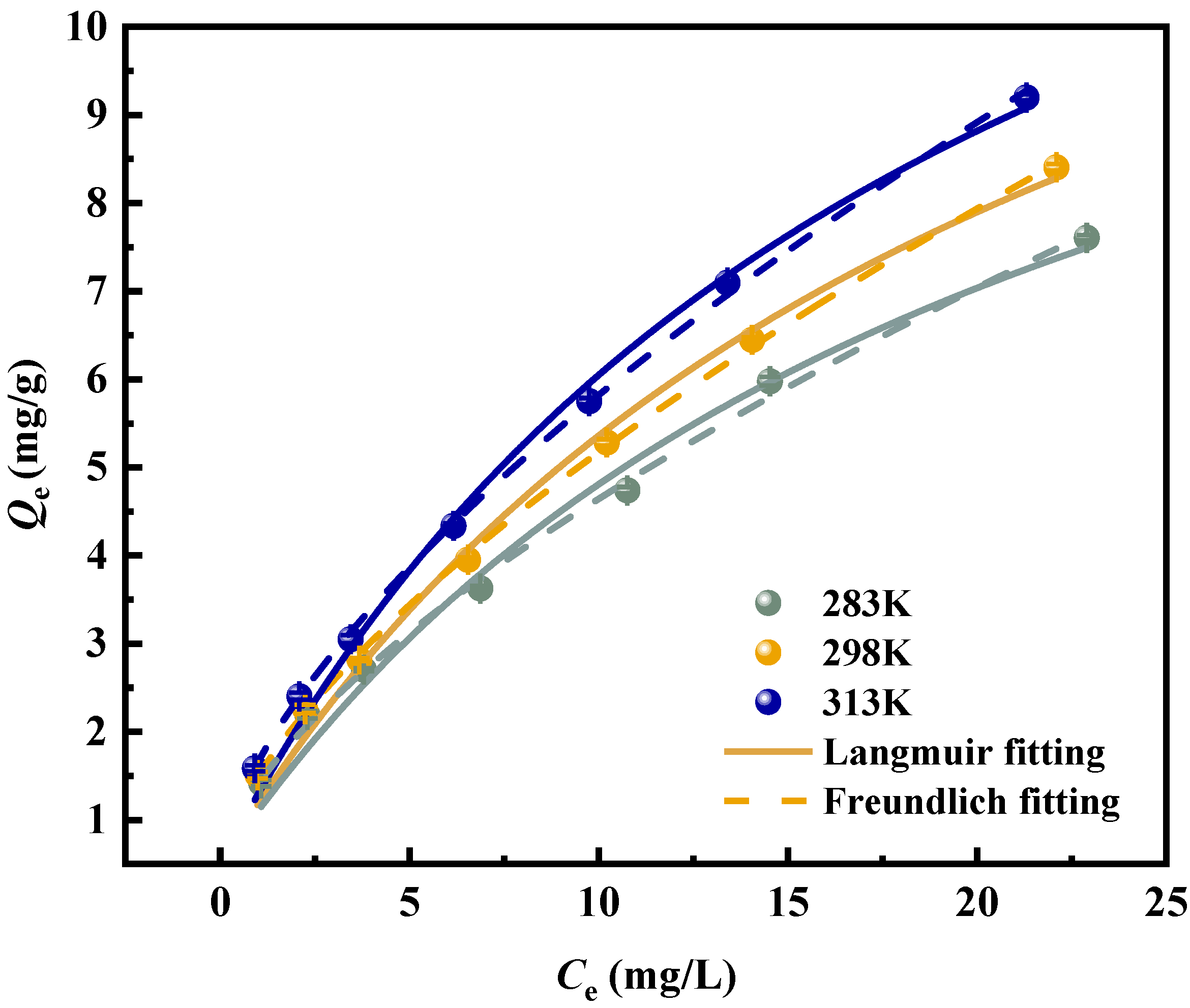
3.5. Adsorption Isotherms
3.6. Thermodynamic Study
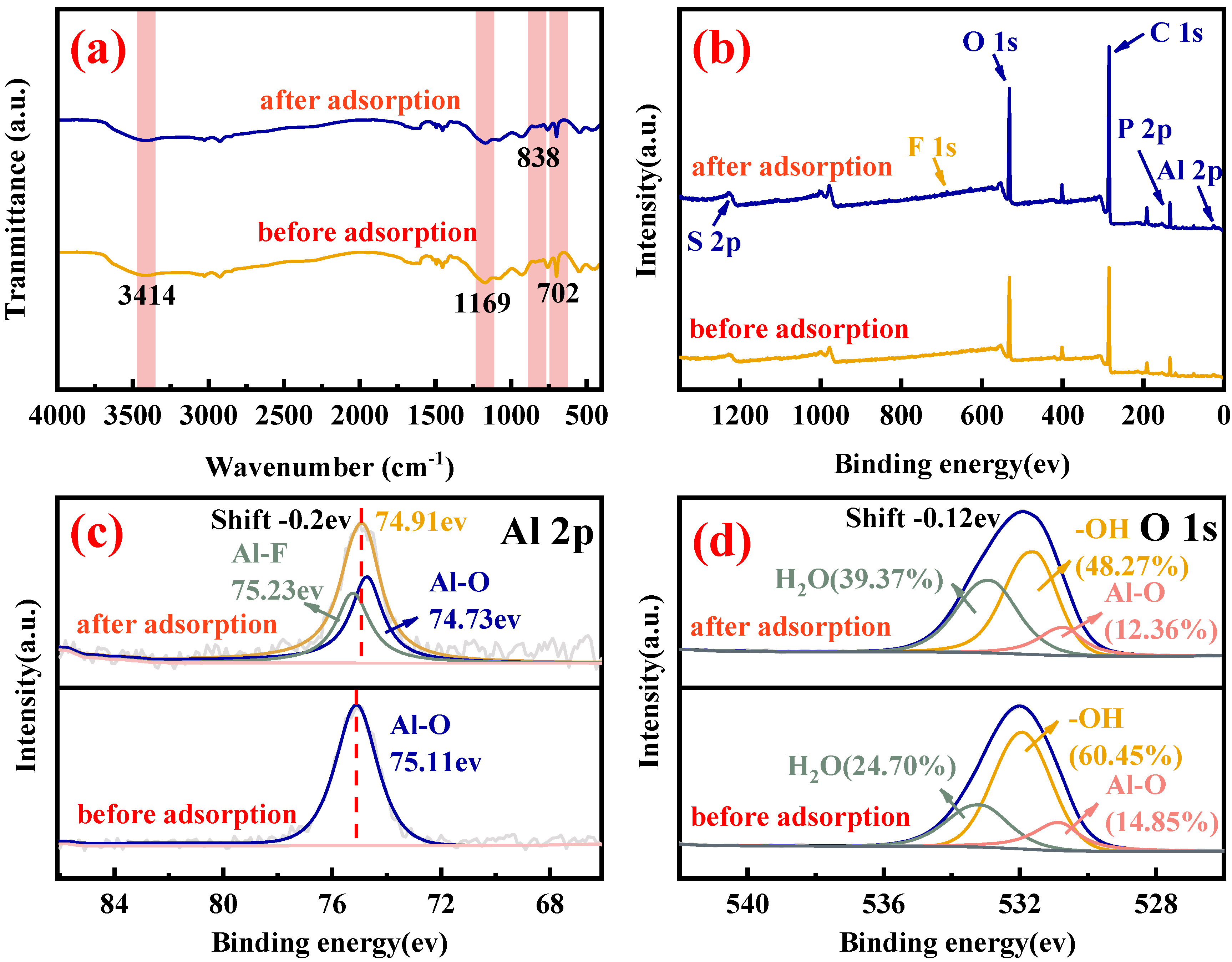
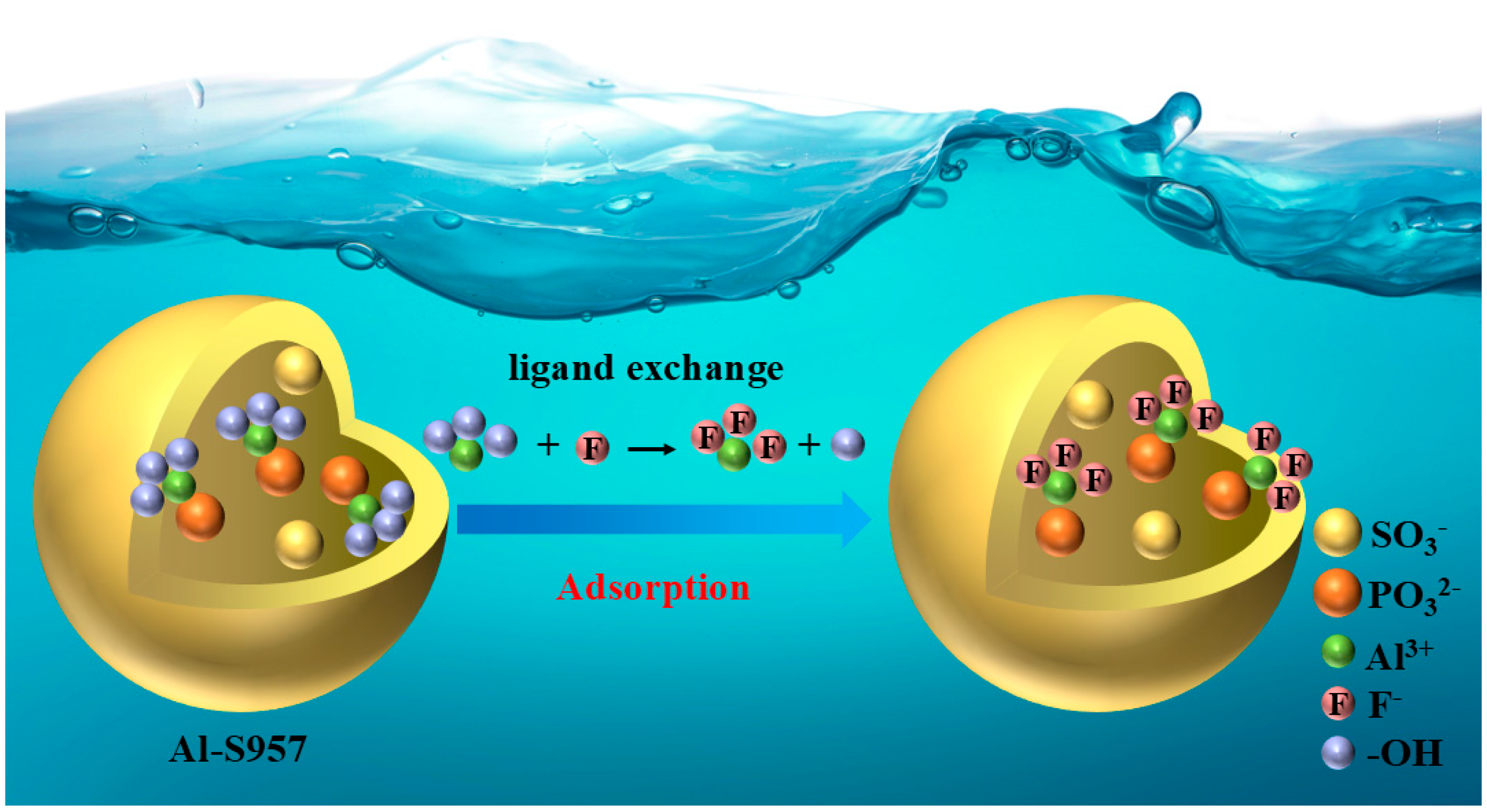
3.7. Adsorption Mechanism of Al-S957 for Fluoride
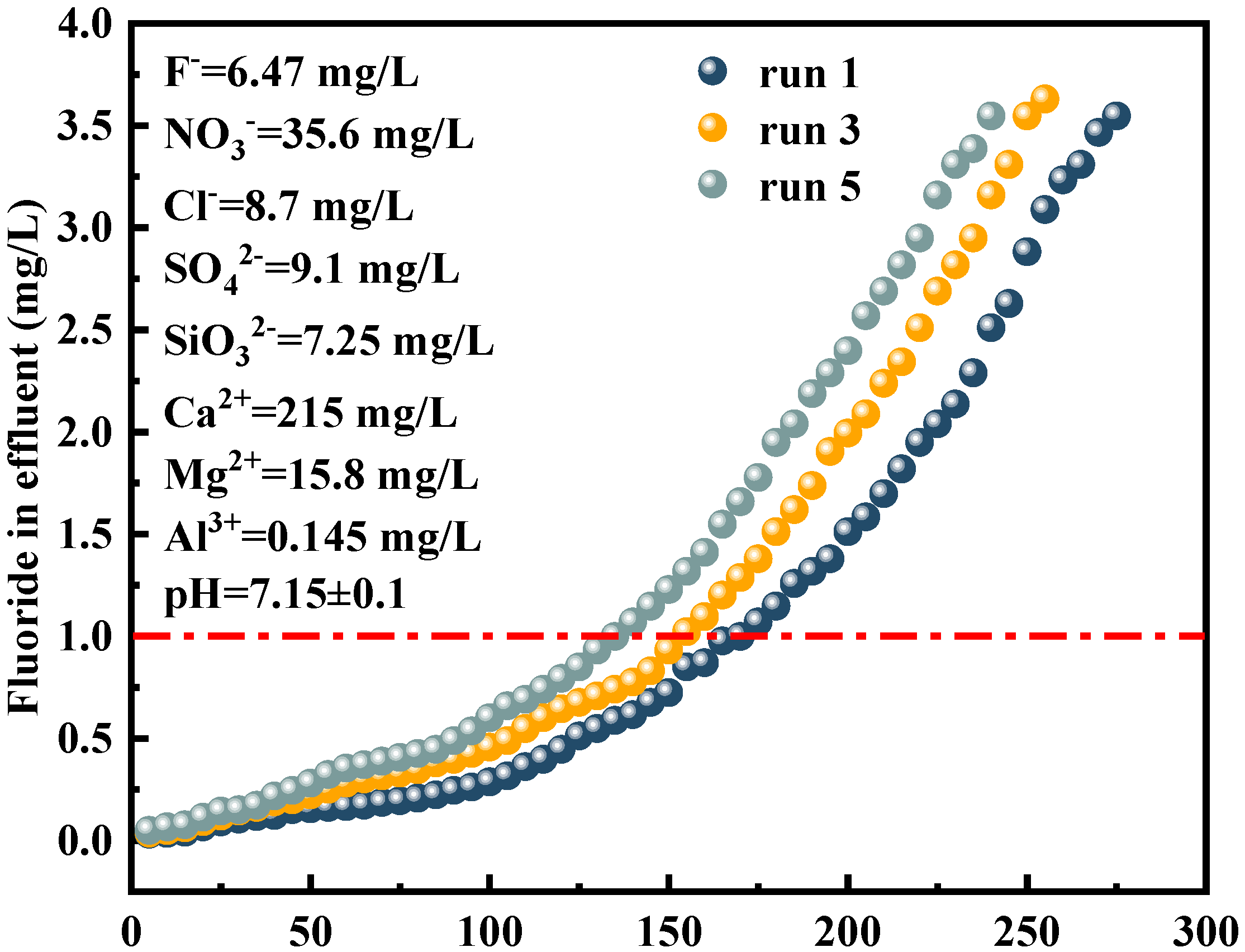
3.8. Application Performance and Scalability of Al-S957
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaji, E.; Sarath, K.V.; Santosh, M.; Krishnaprasad, P.K.; Arya, B.K.; Babu, M.S. Fluoride Contamination in Groundwater: A Global Review of the Status, Processes, Challenges, and Remedial Measures. Geosci. Front. 2024, 15, 101734. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, H.; Zhang, X.; Zeng, M.; Cao, B.; Liu, Y.; Qiu, L.; Zhang, T.; Lu, J. Carbonization-Derived Al@Carbon Composite for Efficient Fluoride Elimination: Structural Modification, Selective Adsorption, and Dual Ligand Exchange Mechanism. J. Colloid Interface Sci. 2025, 698, 138060. [Google Scholar] [CrossRef]
- Yin, C.; Huang, Q.; Zhu, G.; Liu, L.; Li, S.; Yang, X.; Wang, S. High-Performance Lanthanum-Based Metal–Organic Framework with Ligand Tuning of the Microstructures for Removal of Fluoride from Water. J. Colloid Interface Sci. 2022, 607, 1762–1775. [Google Scholar] [CrossRef]
- Yadav, A.K.; Bhattacharyya, S. A Review of the Emerging Ceramic Adsorbents for Defluoridation of Groundwater. J. Water Process Eng. 2020, 36, 101365. [Google Scholar] [CrossRef]
- Jayashree, D.E.; Kumar, P.S.; Ngueagni, P.T.; Vo, D.-V.; Chew, K.W. Effective Removal of Excessive Fluoride from Aqueous Environment Using Activated Pods of Bauhinia Variegata: Batch and Dynamic Analysis. Environ. Pollut. 2021, 272, 115969. [Google Scholar] [CrossRef] [PubMed]
- Lacson, C.F.Z.; Lu, M.-C.; Huang, Y.-H. Fluoride-Containing Water: A Global Perspective and a Pursuit to Sustainable Water Defluoridation Management -An Overview. J. Clean. Prod. 2021, 280, 124236. [Google Scholar] [CrossRef]
- Ni, C.; Liu, C.; Xie, Y.; Xie, W.; He, Z.; Zhong, H. A Critical Review on Adsorption and Recovery of Fluoride from Wastewater by Metal-Based Adsorbents. Environ. Sci. Pollut. Res. Int. 2022, 29, 82740–82761. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, L.; Yang, B.; Ji, G.; Zhao, Z.; Zhang, H.; Wang, F.; Xia, M.; Tao, Y. Adaptive Structural Modification of Zr-Based MOF-808 via Solvent and Ligand Engineering for Enhanced Fluoride Ion Adsorption Efficiency. Sep. Purif. Technol. 2024, 348, 127731. [Google Scholar] [CrossRef]
- Li, W.; Dai, R.; Al-shiaani, N.H.A.; Li, J.; Sun, C.; Wang, K.; Chen, K.; Guo, A.; Liu, H. High-Efficiency N-Doped Activated Carbon-Based Defluoridation Adsorbent Prepared from Itaconic Acid Fermentation Waste Liquid. New J. Chem. 2022, 46, 21092–21102. [Google Scholar] [CrossRef]
- Bera, B.; Saha Chowdhury, S.; Sonawane, V.R.; De, S. High Capacity Aluminium Substituted Hydroxyapatite Incorporated Granular Wood Charcoal (Al-HApC) for Fluoride Removal from Aqueous Medium: Batch and Column Study. Chem. Eng. J. 2023, 466, 143264. [Google Scholar] [CrossRef]
- Li, R.; Tian, X.; Ashraf, I.; Chen, B. Fluoride Removal Using a Chelating Resin Containing Phosphonic-Sulfonic Acid Bifunctional Group. J. Chromatogr. A 2020, 1613, 460697. [Google Scholar] [CrossRef]
- Guo, S.; Zheng, F.; Xu, J.; Jiang, J.; Cui, Z.; Wu, C.; Lin, Y.; Sun, Q.; Zheng, Y.; Sa, B. Enhanced Fluoride Removal from Drinking Water by Activated Carbon Supported Ce–Al Oxides: Performance and Mechanism. RSC Adv. 2025, 15, 14363–14374. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, Y.; He, P.; Zhao, B.; Zhang, X.; Xiao, H.; Diwu, M.; Liu, D.; Gu, Q.; Lu, C. Development of a Rare Earth Modified Residue of Leaching Aluminium from Gangue for Fluoride Ion Adsorption from Mine Water. Miner. Eng. 2025, 232, 109536. [Google Scholar] [CrossRef]
- Jiang, K.; Zeng, X. Desorption and Recovery of Fluoride Adsorbed by the Aluminum-Loaded Chelating Resin. Desalin. Water Treat. 2021, 224, 178–186. [Google Scholar] [CrossRef]
- Miao, Y.; Gu, J.; Zhu, D.; Ye, C.; Yang, Y.; Zhou, Q. Characterization and Performance of Novel Carbon-Doped Aluminum-Modified Bentonite for Cost-Effective Fluoride Removal. Mater. Today Commun. 2025, 42, 111156. [Google Scholar] [CrossRef]
- Kim, W.-T.; Lee, J.-W.; An, H.-E.; Cho, S.-H.; Jeong, S. Efficient Fluoride Wastewater Treatment Using Eco-Friendly Synthesized AlOOH. Nanomaterials 2023, 13, 2838. [Google Scholar] [CrossRef]
- Lange, N.A. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 2005; ISBN 978-0-07-143220-7. [Google Scholar]
- Uthappa, U.T.; Ajeya, K.V.; Sannasi, V.; Lee, S.G.; Sohn, E.-H.; Chang, B.-J.; Park, I.-J.; Kim, J.H.; Kurkuri, M.D.; Jung, H.-Y. Green Aluminum Metal-Organic Frameworks (Al-MOFs) Supported on Commercial Activated Carbon for Enhanced Removal Performances of Industrial Fluoride Pollutants. J. Water Process Eng. 2024, 63, 105450. [Google Scholar] [CrossRef]
- Bao, Y.; Qi, Y.; Li, Q.; Wang, L.; Cao, Z.; Li, J.; Wu, M.; Chen, J.; Zhang, H.; Guo, Q.; et al. Fluoride Removal from Coal Mining Water Using Novel Polymeric Aluminum Modified Activated Carbon Prepared through Mechanochemical Process. J. Environ. Sci. 2024, 146, 226–236. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S. Adsorption Characterization and Mechanism of Aluminum Based Chitosan/Zeolite Molecular Sieve Composite for Fluoride Removal. Desalin. Water Treat. 2022, 247, 108–120. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of Fluoride Removal from Water Environment by Adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, J.; Alcalá, L.; Megido, L.; Castrillón, L. Removal of Fluoride from Coke Wastewater by Aluminum Doped Chelating Ion-Exchange Resins: A Tertiary Treatment. Environ. Sci. Pollut. Res. 2022, 29, 8705–8715. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, M.; Wang, R.; Sun, Y. Broad-Spectrum Insight into Precise Removal of Immobilized Hard Acids for Fluoride: DFT Simulations, Experiments, and QICAR Analysis. Water Res. 2025, 284, 124003. [Google Scholar] [CrossRef] [PubMed]
- Araucz, K.; Aurich, A.; Kołodyńska, D. Novel Multifunctional Ion Exchangers for Metal Ions Removal in the Presence of Citric Acid. Chemosphere 2020, 251, 126331. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, W.; Xue, M.; Lv, R.; Fan, C.; Li, A. Adsorption Mechanism of Ca2+, Mg2+, Fe3+, and Al3+ Ions in Phosphoric Acid–Nitric Acid Solution on 001 × 7 and S957 Resins. RSC Adv. 2024, 14, 7234–7240. [Google Scholar] [CrossRef]
- He, C.; Sun, Y.; Gu, Y.; Ji, H. Enhanced Fluoride Removal Using Mg-Zr Binary Metal Oxide Nanoparticles Confined in a Strong-Base Anion Exchanger. Chemosphere 2024, 358, 141980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, Y.; Yao, Q.; Zhang, F.; Chen, W.; Liu, Y. Comparison of Fluorine Removal Performance and Mechanism of Spheroidal Magnesium Oxide before and after Lanthanum Modification. Environ. Sci. Pollut. Res. 2022, 29, 80477–80490. [Google Scholar] [CrossRef] [PubMed]
- Güngör, Ç.; Şakir Ece, M. Competitive Adsorption of VOCs (Benzene, Xylene and Ethylbenzene) with Fe3O4@SiO2-NH@BENZOPHENONE Magnetic Nanoadsorbents. Chem. Eng. J. 2023, 475, 146034. [Google Scholar] [CrossRef]
- Lai, Y.-T.; Huang, Y.-S.; Chen, C.-H.; Lin, Y.-C.; Jeng, H.-T.; Chang, M.-C.; Chen, L.-J.; Lee, C.-Y.; Hsu, P.-C.; Tai, N.-H. Green Treatment of Phosphate from Wastewater Using a Porous Bio-Templated Graphene Oxide/MgMn-Layered Double Hydroxide Composite. iScience 2020, 23, 101065. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, S.; Li, Y.; Hu, S.; Yang, P. Swelling Characterization of Ionic Responsive Superabsorbent Resin Containing Carboxylate Sodium Groups. React. Funct. Polym. 2022, 170, 105144. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, X.; Li, J.; Li, F.; Li, X.; Yu, J.; Guo, L.; Song, G.; Xiao, C.; Zhou, F.; et al. Efficient Removal of Phosphate and Fluoride from Phosphogypsum Leachate by Lanthanum-Modified Zeolite: Synchronous Adsorption Behavior and Mechanism. J. Environ. Chem. Eng. 2024, 12, 113294. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Zhang, S.; Bai, Y.; Zhang, X.; Jiang, W.; Yang, S. Shallow Groundwater Quality and Health Risk Assessment of Fluoride and Arsenic in Northwestern Jiangsu Province, China. Appl. Water Sci. 2024, 14, 119. [Google Scholar] [CrossRef]
- Iqbal, J.; Su, C.; Wang, M.; Abbas, H.; Baloch, M.Y.J.; Ghani, J.; Ullah, Z.; Huq, M.E. Groundwater Fluoride and Nitrate Contamination and Associated Human Health Risk Assessment in South Punjab, Pakistan. Environ. Sci. Pollut. Res. Int. 2023, 30, 61606–61625. [Google Scholar] [CrossRef]
- Su, R.; Wang, M.; Jiang, Y.; Zhang, S.; Tan, J. Citrate-Stabilized Amorphous Calcium Phosphate Nanoparticles as an Effective Adsorbent for Defluorination. Nanomaterials 2025, 15, 621. [Google Scholar] [CrossRef] [PubMed]
- Raghav, S.; Sapna; Kumar, D. Cubical-Shaped Rods of Pectin–Hydroxyapatite Composite for Adsorption Studies of Fluoride by Statistical Method and Adsorption Experiments. ACS Omega 2018, 3, 9675–9688. [Google Scholar] [CrossRef]
- Laxmi, V.; Singh, S.; Agarwal, S.; Bhardwaj, P.; Gautam, M.; Khan, S. Biopolymer Based Nanocomposite (Fe3O4/GONR/SA) for Targeted Fluoride Removal: Facile Synthesis, Adsorption Mechanism and Regeneration Potential. Mater. Res. Bull. 2025, 190, 113538. [Google Scholar] [CrossRef]
- Liang, S.; Wang, K.; Wang, K.; Wang, T.; Guo, C.; Wang, W.; Wang, J. Adsorption Behavior of Diclofenac on Polystyrene and Poly(Butylene Adipate-Co-Terephthalate) Microplastics: Influencing Factors and Adsorption Mechanism. Langmuir 2023, 39, 12216–12225. [Google Scholar] [CrossRef]
- Ghanbarian, M.; Ghanbarian, M.; Mahvi, A.H.; Tabatabaie, T. Enhanced Fluoride Removal over MgFe2O4–Chitosan–CaAl Nanohybrid: Response Surface Optimization, Kinetic and Isotherm Study. Int. J. Biol. Macromol. 2020, 148, 574–590. [Google Scholar] [CrossRef]
- Li, G.; Yang, M.; Ding, X.; Tan, W.; Li, G.; Fang, S.; Wang, H. Study on Sodium Functionalized Ultrasonic-Diatomite and Its Performance for Phosphorus Removal. DWT 2021, 237, 64–76. [Google Scholar] [CrossRef]
- Robshaw, T.; Tukra, S.; Hammond, D.B.; Leggett, G.J.; Ogden, M.D. Highly Efficient Fluoride Extraction from Simulant Leachate of Spent Potlining via La-Loaded Chelating Resin. An Equilibrium Study. J. Hazard. Mater. 2019, 361, 200–209. [Google Scholar] [CrossRef]
- Prathibha, C.; Biswas, A.; Chunduri, L.A.A.; Reddy, S.K.; Loganathan, P.; Kalaruban, M.; Venkatarmaniah, K. Zr(IV) Functionalized Graphene Oxide Anchored Sand as Potential and Economic Adsorbent for Fluoride Removal from Water. Diam. Relat. Mater. 2020, 109, 108081. [Google Scholar] [CrossRef]
- Li, W.; Xie, P.; Zhou, H.; Zhao, H.; Yang, B.; Xiong, J. Preparation of Lanthanum-Modified Tea Waste Biochar and Its Adsorption Performance on Fluoride in Water. Materials 2024, 17, 766. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zang, Z.; Zhang, S.; Ouyang, G.; Han, R. Enhanced Fluoride Adsorption from Aqueous Solution by Zirconium (IV)-Impregnated Magnetic Chitosan Graphene Oxide. Int. J. Biol. Macromol. 2021, 182, 1759–1768. [Google Scholar] [CrossRef]
- Zhou, Z.; Ali, A.; Su, J.; Wang, Z.; Huang, T.; Li, T. In-Situ Modified Biosynthetic Crystals with Lanthanum for Fluoride Removal Based on Microbially Induced Calcium Precipitation: Characterization, Kinetics, and Mechanism. Chemosphere 2023, 327, 138472. [Google Scholar] [CrossRef]
- Shin, E.; Dreisinger, D.B.; Burns, A.D. Removal of Fluoride from Sodium Sulfate Brine by Zirconium Pre-Loaded Chelating Resins with Amino-Methyl Phosphonic Acid Functionality. Desalination 2021, 505, 114985. [Google Scholar] [CrossRef]
- Preethi, J.; Karthikeyan, P.; Vigneshwaran, S.; Meenakshi, S. Facile Synthesis of Zr4+ Incorporated Chitosan/Gelatin Composite for the Sequestration of Chromium(VI) and Fluoride from Water. Chemosphere 2021, 262, 128317. [Google Scholar] [CrossRef] [PubMed]
- Turki, T.; Hamdouni, A.; Enesca, A. Fluoride Adsorption from Aqueous Solution by Modified Zeolite—Kinetic and Isotherm Studies. Molecules 2023, 28, 4076. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, F.; Ding, W.; Zhang, K.; Zhang, Q. Enhanced Removal of Fluoride Ions Using Lanthanum-Doped Coconut Shell Biochar in PVDF Ultrafiltration Membranes. J. Hazard. Mater. 2024, 480, 136393. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Miao, Y.; Zhu, D.; Li, J.; Yang, Y.; Ye, C. Optimizing Solvothermal Synthesis of Aluminum-Loaded Pomelo Peel for Enhanced Fluoride Adsorption Using Response Surface Methodology. Ind. Crops Prod. 2024, 222, 119932. [Google Scholar] [CrossRef]
- Sun, X.; Ke, C.; Lu, Q.; Liang, L.; Xie, G. Adsorption of Fluoride Ion on Al/La Modified Acidified Attapulgite Composite Materials. Sep. Purif. Technol. 2025, 354, 128791. [Google Scholar] [CrossRef]
- Wang, G.; Chen, D.; Yang, Z.; Liao, S.; Tamjidur, R.S.; Hu, S.; Wu, Q.; Zhang, W. Effectiveness of Capacitive Deionization for the Removal of Soluble Phosphorus and Fluoride with a Mg/Al Co-Doped Porous Biochar Electrode during the Process of Water-Washing of Phosphogypsum. Desalination 2025, 602, 118640. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, X.; Zhang, Y.; Zhang, X.; Ngo, H.H.; Liu, Y.; Jiang, W.; Tan, X.; Wang, X.; Zhang, J. Activated Nano-Al2O3 Loaded on Polyurethane Foam as a Potential Carrier for Fluorine Removal. J. Water Process Eng. 2021, 44, 102444. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, X.; Zhang, X.; Wang, X.; Zhang, J.; Liu, Y.; Tan, X.; Zhao, Y.; Kang, D.; Guo, W.; et al. New Easily Recycled Carrier Based Polyurethane Foam by Loading Al-MOF and Biochar for Selective Removal of Fluoride Ion from Aqueous Solutions. Sci. Total Environ. 2023, 901, 166312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, H.; Wang, D.; Wang, X.; Xu, H.; Wang, D. Mechanism of Fluoride Removal by AlCl3 and Al13: The Role of Aluminum Speciation. J. Hazard. Mater. 2020, 398, 12298. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, X.; Yu, J.; Li, S.; Guo, L.; Xiao, C.; Li, J.; Zhang, Y.; Chi, R. Enhanced Removal of Phosphate and Fluoride from Phosphogypsum Leachate by Cerium/Iron-Based Zeolite: Adsorption Behaviors and Competition Mechanisms. Sep. Purif. Technol. 2025, 376, 134185. [Google Scholar] [CrossRef]
- Zheng, F.; Guo, S.; Xu, J.; Jiang, J.; Wu, C.; Sun, Q.; Zheng, Y.; Lin, Y. Polyvinylpyrrolidone-Modified Ce[Sbnd]Al Bimetallic Oxide Loaded on Recyclable Activated Carbon for Selective Fluoride Ion Removal from Aqueous Solutions. J. Water Process Eng. 2025, 75, 107984. [Google Scholar] [CrossRef]
- Feng, J.; Li, X.; Yang, Y.; Zhou, Z. Preparation of Low-Cost Aluminum-Loaded Longan Shell Adsorbent for Fluoride Removal: Experimental and Modeling Studies. J. Environ. Chem. Eng. 2022, 10, 108917. [Google Scholar] [CrossRef]
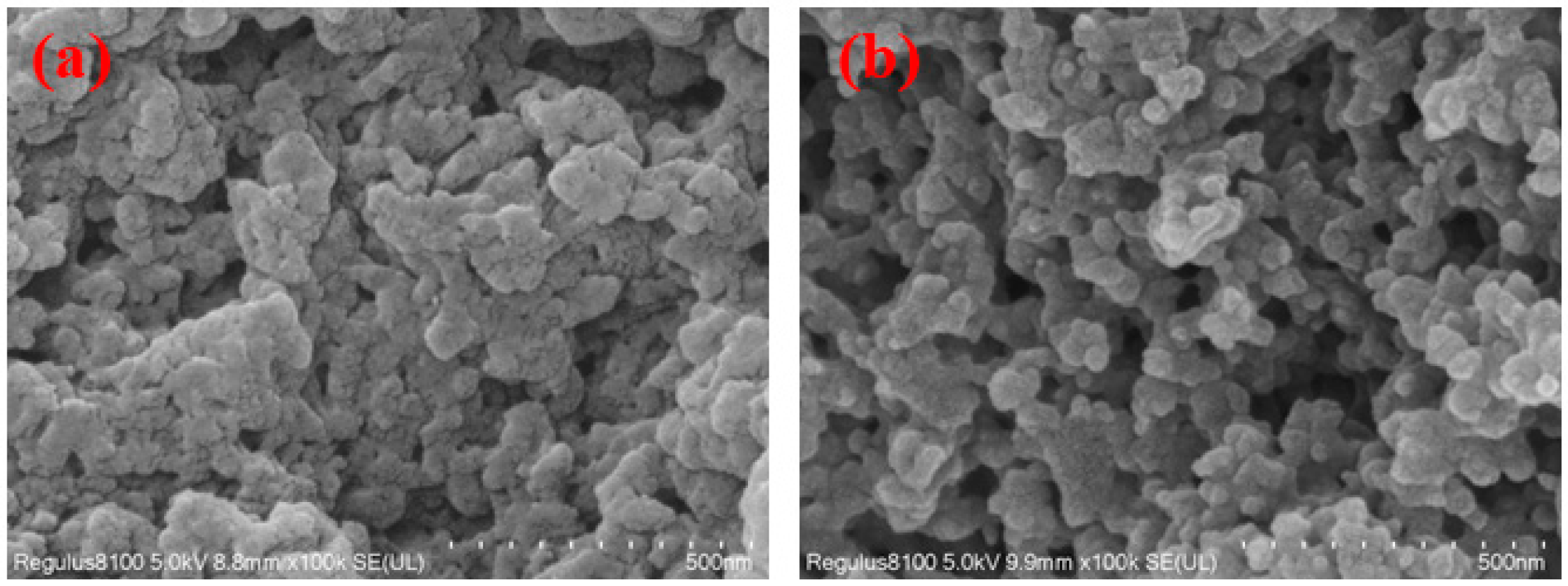
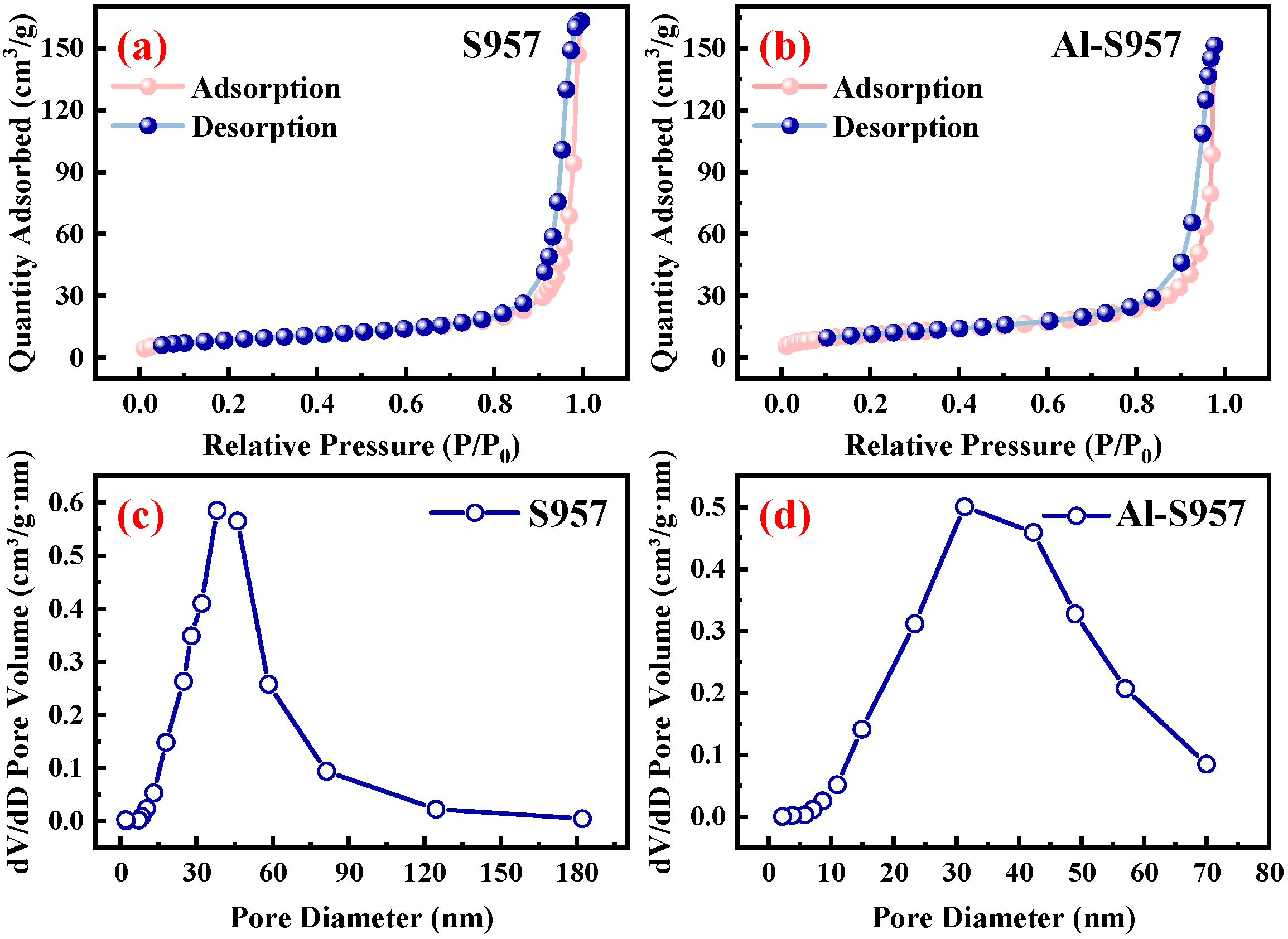


| Adsorbents | BET Surface Area (m2/g) | Average Pore Diameter (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| S957 | 31.38 | 32.66 | 0.25 |
| Al-S957 | 40.53 | 26.39 | 0.23 |
| Materials | Qmax (mg/g) | Reference |
|---|---|---|
| La-loaded chelating resin | 3.38 | Robshaw et al. [40] |
| Activated carbon fibers modified with zirconium | 6.12 | Prathibha et al. [41] |
| La-modified biochar (La-TBC) | 7.55 | Li et al. [42] |
| Zr-impregnated magnetic chitosan graphene oxide (Zr–MCGO) | 8.8 | Liu et al. [43] |
| La-modified biosynthetic crystal (BC-La) | 10.92 | Zhou et al. [44] |
| Al-loaded TP207 resin | 12.05 | Rodríguez-Iglesias et al. [22] |
| Zr-loaded TP260 resin | 12.1 | Shin et al. [45] |
| Zr impregnated highly functional binary biopolymeric composite | 12.13 | Preethi et al. [46] |
| Al-loaded S957 resin | 15.49 | In this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Gu, Y.; Wang, R.; Sun, Y. Aluminum-Loaded Bifunctional Resins for Efficient Fluoride Removal from Aqueous Solutions. Appl. Sci. 2025, 15, 7829. https://doi.org/10.3390/app15147829
Ma M, Gu Y, Wang R, Sun Y. Aluminum-Loaded Bifunctional Resins for Efficient Fluoride Removal from Aqueous Solutions. Applied Sciences. 2025; 15(14):7829. https://doi.org/10.3390/app15147829
Chicago/Turabian StyleMa, Mengfei, Yingpeng Gu, Ruijie Wang, and Yue Sun. 2025. "Aluminum-Loaded Bifunctional Resins for Efficient Fluoride Removal from Aqueous Solutions" Applied Sciences 15, no. 14: 7829. https://doi.org/10.3390/app15147829
APA StyleMa, M., Gu, Y., Wang, R., & Sun, Y. (2025). Aluminum-Loaded Bifunctional Resins for Efficient Fluoride Removal from Aqueous Solutions. Applied Sciences, 15(14), 7829. https://doi.org/10.3390/app15147829






