Effects of Ozone Oxidation Process on Residual Antibiotics and Antibiotic Resistance Genes in a Swine Wastewater Treatment Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analytical Methods for Water Quality Parameters
2.3. Examination of Antibiotic Concentrations
2.4. DNA Extraction and Quantification of ARGs
2.5. Statistical Analysis
3. Results
3.1. Changes in Physico-Chemical Properties During the Treatment Process
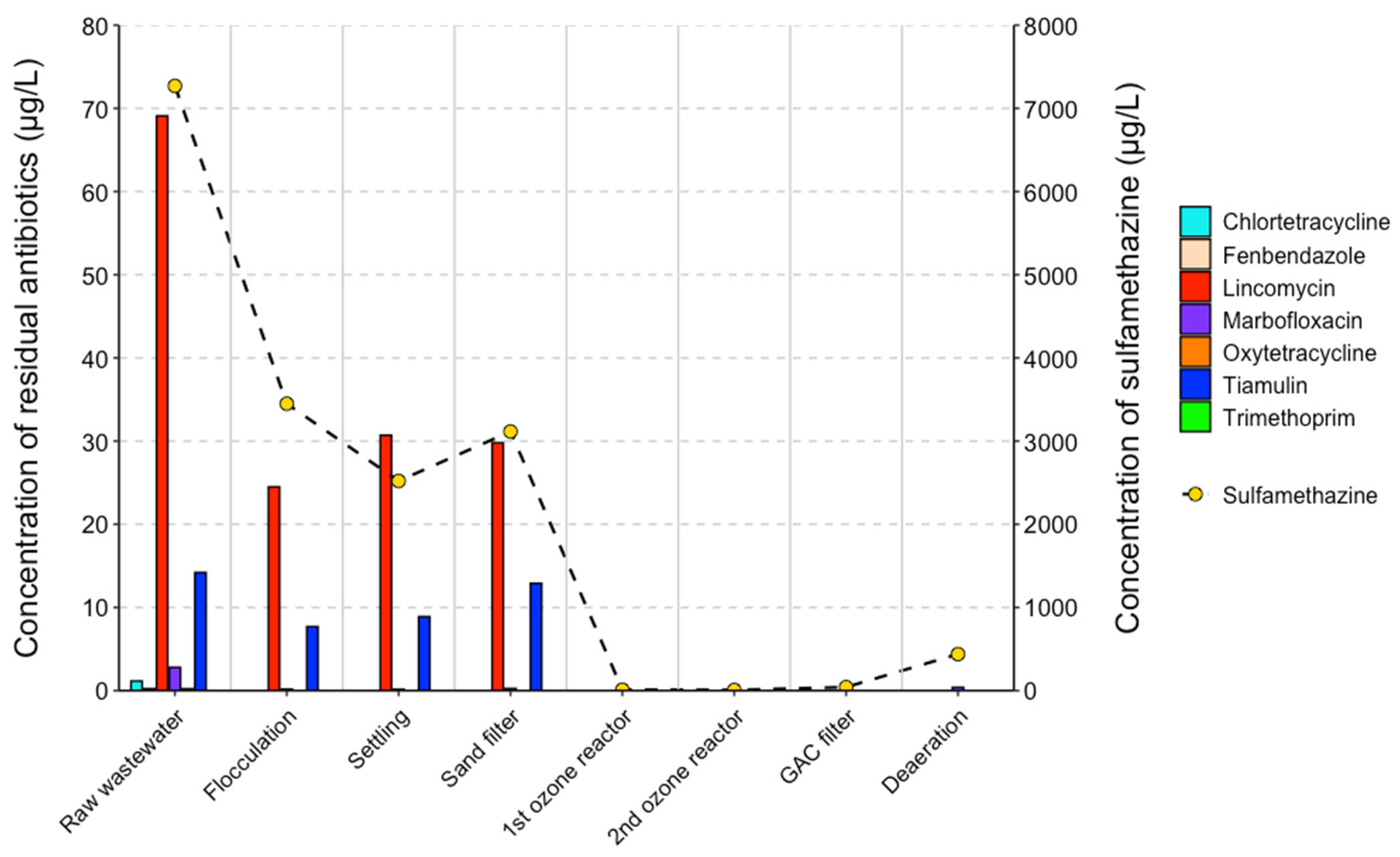
3.2. Occurrence and Removal of Residual Antibiotics During Wastewater Treatment
3.3. Prevalence of ARGs in Swine Wastewater Treatment Process
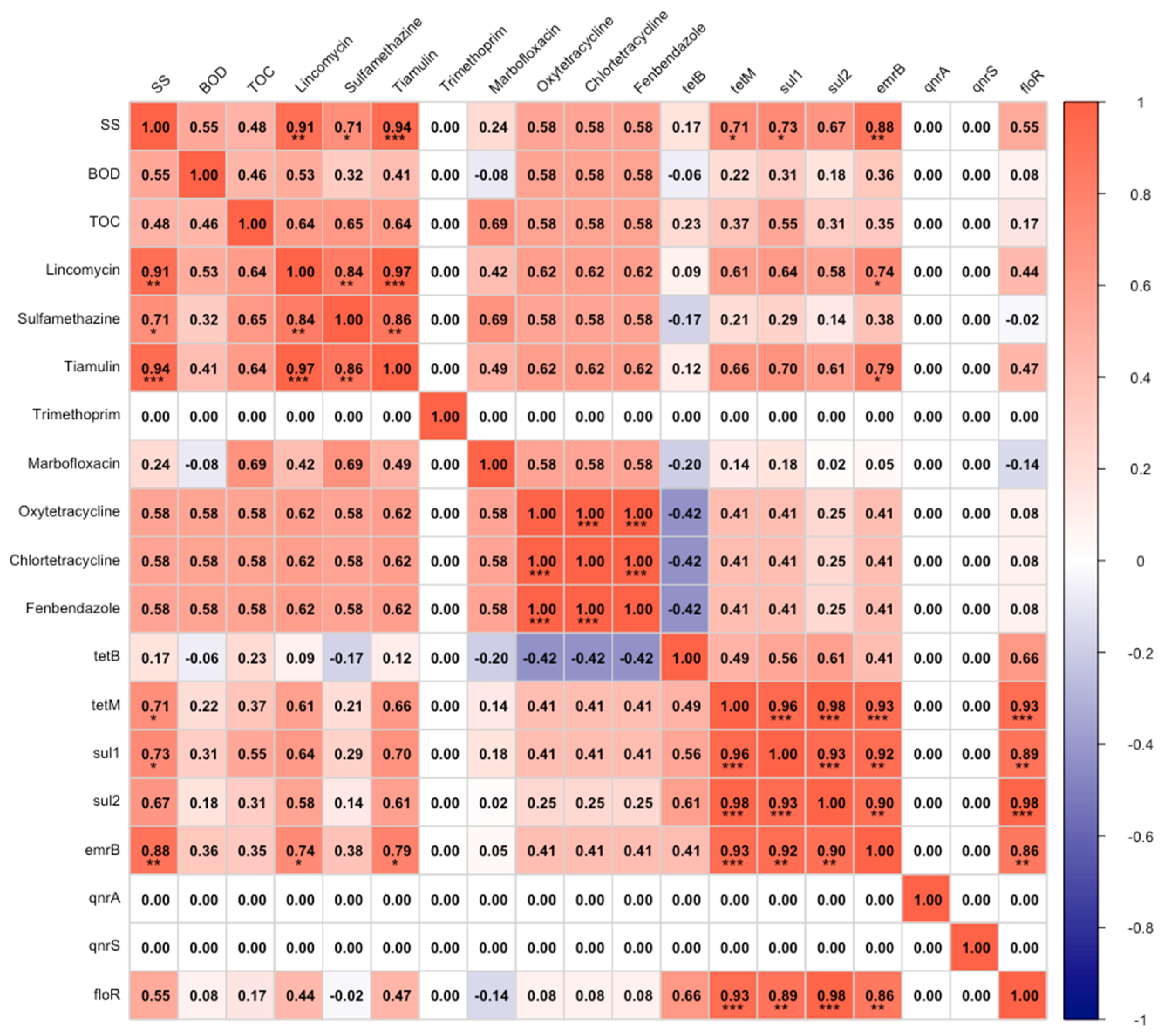
3.4. Relationship Between Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Yang, H.; Paruch, L.; Chen, X.; Van Eerde, A.; Skomedal, H.; Wang, Y.; Liu, D.; Liu Clarke, J. Antibiotic Application and Resistance in Swine Production in China: Current Situation and Future Perspectives. Front. Vet. Sci. 2019, 6, 136. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Residual Veterinary Pharmaceuticals in Animal Manures and Their Environmental Behaviors in Soils. In Applied Manure and Nutrient Chemistry for Sustainable Agriculture and Environment; Springer: Berlin/Heidelberg, Germany, 2014; pp. 23–52. [Google Scholar]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.-L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in Poultry Manure and Their Associated Health Issues: A Systematic Review. J. Soils Sediments 2020, 20, 486–497. [Google Scholar] [CrossRef]
- Pikkemaat, M.G.; Yassin, H.; Fels-Klerx, H.J.; Berendsen, B.J.A. Antibiotic Residues and Resistance in the Environment; RIKILT: Wageningen, The Netherlands, 2016. [Google Scholar]
- Ratasuk, N.; Boonsaner, M.; Hawker, D.W. Effect of Temperature, PH and Illumination on Abiotic Degradation of Oxytetracycline in Sterilized Swine Manure. J. Environ. Sci. Health Part. A 2012, 47, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Huang, H.; Chen, Y. Effects of Emerging Pollutants on the Occurrence and Transfer of Antibiotic Resistance Genes: A Review. J. Hazard. Mater. 2021, 420, 126602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Levy, K.; Trueba, G.; Cevallos, W.; Trostle, J.; Foxman, B.; Marrs, C.F.; Eisenberg, J.N.S. Effects of Selection Pressure and Genetic Association on the Relationship between Antibiotic Resistance and Virulence in Escherichia Coli. Antimicrob. Agents Chemother. 2015, 59, 6733–6740. [Google Scholar] [CrossRef]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Zhou, H.; Beltrán, J.F.; Brito, I.L. Functions Predict Horizontal Gene Transfer and the Emergence of Antibiotic Resistance. Sci. Adv. 2021, 7, eabj5056. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. Removal of Antibiotic Resistance Genes (ARGs) in Various Wastewater Treatment Processes: An Overview. Crit. Rev. Environ. Sci. Technol. 2022, 52, 571–630. [Google Scholar] [CrossRef]
- Ma, H.; Du, J.; Xu, T.; Yin, D.; Fang, X.; Guo, X. Distribution and Risk Assessment of Antibiotic Resistance Genes in Swine Farm Wastewater and Its Surrounding Environments: From Soil to Water. Environ. Sci. Process. Impacts 2025, 27, 741–751. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, X.; Du, Q.; Huo, J.; Wang, H.; Wang, Z.; Guo, W.; Ngo, H.H. Advancements in Swine Wastewater Treatment: Removal Mechanisms, Influential Factors, and Optimization Strategies. J. Water Process Eng. 2023, 54, 103986. [Google Scholar] [CrossRef]
- Wu, H.; Li, A.; Zhang, H.; Gao, S.; Li, S.; Cai, J.; Yan, R.; Xing, Z. The Potential and Sustainable Strategy for Swine Wastewater Treatment: Resource Recovery. Chemosphere 2023, 336, 139235. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.B. Bioprocessing for Elimination Antibiotics and Hormones from Swine Wastewater. Sci. Total Environ. 2018, 621, 1664–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.-S.; Zhao, J.-L.; Liu, W.-R.; Chen, J.; Zhang, Q.-Q.; He, L.-Y.; Ying, G.-G. Variations of Antibiotic Resistome in Swine Wastewater during Full-Scale Anaerobic Digestion Treatment. Environ. Int. 2021, 155, 106694. [Google Scholar] [CrossRef]
- Yang, F.; Han, B.; Gu, Y.; Zhang, K. Swine Liquid Manure: A Hotspot of Mobile Genetic Elements and Antibiotic Resistance Genes. Sci. Rep. 2020, 10, 15037. [Google Scholar] [CrossRef]
- Alvarino, T.; Suarez, S.; Lema, J.M.; Omil, F. Understanding the Removal Mechanisms of PPCPs and the Influence of Main Technological Parameters in Anaerobic UASB and Aerobic CAS Reactors. J. Hazard. Mater. 2014, 278, 506–513. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Nguyen, Q.A.; Zhang, J.; Liang, S. Improving Sulfonamide Antibiotics Removal from Swine Wastewater by Supplying a New Pomelo Peel Derived Biochar in an Anaerobic Membrane Bioreactor. Bioresour. Technol. 2021, 319, 124160. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Song, X.; Ding, X.; Xia, R.; Lin, X.; Li, G.; Nghiem, L.D.; Luo, W. Antibiotic Removal from Swine Farming Wastewater by Anaerobic Membrane Bioreactor: Role of Hydraulic Retention Time. J. Memb. Sci. 2023, 677, 121629. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Ma, Y.-L.; Zhang, J.-T.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. A Critical Review of Antibiotic Removal Strategies: Performance and Mechanisms. J. Water Process Eng. 2020, 38, 101681. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Li, F.; Hua, T.; Zhou, Q.; Ho, S.-H. Technologies towards Antibiotic Resistance Genes (ARGs) Removal from Aquatic Environment: A Critical Review. J. Hazard. Mater. 2021, 411, 125148. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, Y.; Yao, Q.; Addo-Bankas, O.; Ji, B.; Yuan, Y.; Wei, T.; Esteve-Núñez, A. A Review on Antibiotics Removal: Leveraging the Combination of Grey and Green Techniques. Sci. Total Environ. 2022, 838, 156427. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yuan, T.; Zhou, L.; Cheng, S.; Qu, X.; Lu, P.; Feng, Q. Impact Factors of the Accumulation, Migration and Spread of Antibiotic Resistance in the Environment. Environ. Geochem. Health 2021, 43, 1741–1758. [Google Scholar] [CrossRef]
- Yi, J.; Chen, Z.; Xu, D.; Wu, D.; Howard, A. Preparation of a Coagulant of Polysilicate Aluminum Ferric from Foundry Dust and Its Coagulation Performance in Treatment of Swine Wastewater. J. Clean. Prod. 2024, 434, 140400. [Google Scholar] [CrossRef]
- Yang, Z.; Jia, S.; Zhuo, N.; Yang, W.; Wang, Y. Flocculation of Copper(II) and Tetracycline from Water Using a Novel PH- and Temperature-Responsive Flocculants. Chemosphere 2015, 141, 112–119. [Google Scholar] [CrossRef]
- Liang, C.; Wei, D.; Zhang, S.; Ren, Q.; Shi, J.; Liu, L. Removal of Antibiotic Resistance Genes from Swine Wastewater by Membrane Filtration Treatment. Ecotoxicol. Environ. Saf. 2021, 210, 111885. [Google Scholar] [CrossRef]
- Košutić, K.; Dolar, D.; Ašperger, D.; Kunst, B. Removal of Antibiotics from a Model Wastewater by RO/NF Membranes. Sep. Purif. Technol. 2007, 53, 244–249. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Chen, X.; Han, Y.; Cao, G. Transformation Kinetics and Pathways of Sulfamonomethoxine by UV/H2O2 in Swine Wastewater. Chemosphere 2021, 265, 129125. [Google Scholar] [CrossRef]
- Sarrai, A.E.; Hanini, S.; Merzouk, N.K.; Tassalit, D.; Szabó, T.; Hernádi, K.; Nagy, L. Using Central Composite Experimental Design to Optimize the Degradation of Tylosin from Aqueous Solution by Photo-Fenton Reaction. Materials 2016, 9, 428. [Google Scholar] [CrossRef]
- Suzuki, H.; Araki, S.; Yamamoto, H. Evaluation of Advanced Oxidation Processes (AOP) Using O3, UV, and TiO2 for the Degradation of Phenol in Water. J. Water Process Eng. 2015, 7, 54–60. [Google Scholar] [CrossRef]
- Jiang, Q.; Feng, M.; Ye, C.; Yu, X. Effects and Relevant Mechanisms of Non-Antibiotic Factors on the Horizontal Transfer of Antibiotic Resistance Genes in Water Environments: A Review. Sci. Total Environ. 2022, 806, 150568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, M.; Zhang, K.; Wang, N.; Wang, K.; Wang, H.; Meng, S.; Mu, R. Effect of Ultrasound Irradiation Combined with Ozone Pretreatment on the Anaerobic Digestion for the Biosludge Exposed to Trace-Level Levofloxacin: Degradation, Microbial Community and ARGs Analysis. J. Environ. Manag. 2020, 262, 110356. [Google Scholar] [CrossRef]
- Oh, J.; Salcedo, D.E.; Medriano, C.A.; Kim, S. Comparison of Different Disinfection Processes in the Effective Removal of Antibiotic-Resistant Bacteria and Genes. J. Environ. Sci. 2014, 26, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Domingues, E.; Fernandes, E.; Gomes, J.; Martins, R.C. Advanced Oxidation Processes Perspective Regarding Swine Wastewater Treatment. Sci. Total Environ. 2021, 776, 145958. [Google Scholar] [CrossRef]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Effect of O3 Dose on the O3/UV Treatment Process for the Removal of Pharmaceuticals and Personal Care Products in Secondary Effluent. ChemEngineering 2019, 3, 53. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association American Water Works Association Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- National Institute of Food and Drug Safety Evaluation; Ministry of Agriculture, Food and Rural Affairs; Animal and Plant Quarantine Agency. Monitoring of Antimicrobial Use and Antimicrobial Resistance; Ministry of Agriculture, Food and Rural Affairs: Sejong, Republic of Korea, 2021; pp. 7–17.
- Mao, D.; Yu, S.; Rysz, M.; Luo, Y.; Yang, F.; Li, F.; Hou, J.; Mu, Q.; Alvarez, P.J.J. Prevalence and Proliferation of Antibiotic Resistance Genes in Two Municipal Wastewater Treatment Plants. Water Res. 2015, 85, 458–466. [Google Scholar] [CrossRef]
- Pei, R.; Kim, S.-C.; Carlson, K.H.; Pruden, A. Effect of River Landscape on the Sediment Concentrations of Antibiotics and Corresponding Antibiotic Resistance Genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [CrossRef]
- Khan, S.A.; Sung, K.; Nawaz, M.S. Detection of AacA-AphD, QacEδ1, MarA, FloR, and TetA Genes from Multidrug-Resistant Bacteria: Comparative Analysis of Real-Time Multiplex PCR Assays Using EvaGreen® and SYBR® Green I Dyes. Mol. Cell. Probes 2011, 25, 78–86. [Google Scholar] [CrossRef]
- Parmar, K.A.; Prajapati, S.; Dabhi, Y. Effective Use of Ferrous Sulfate and Alum as a Coagulant in Treatment of Dairy Industry Wastewater. ARPN J. Eng. Appl. Sci. 2011, 6, 42–45. [Google Scholar]
- Kang, C.; Zhao, Y.; Tang, C.; Addo-Bankas, O. Use of Aluminum-Based Water Treatment Sludge as Coagulant for Animal Farm Wastewater Treatment. J. Water Process Eng. 2022, 46, 102645. [Google Scholar] [CrossRef]
- Deng, L.; Zheng, D.; Zhang, J.; Yang, H.; Wang, L.; Wang, W.; He, T.; Zhang, Y. Treatment and Utilization of Swine Wastewater–A Review on Technologies in Full-Scale Application. Sci. Total Environ. 2023, 880, 163223. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.M.; Soja, U.B.; Mambo, A.D.; Kutty, S.R.M.; Jagaba, A.H.; Hayder, G.; Abubakar, S.; Umaru, I. Adsorption of Abattoir Wastewater Contaminants by Coconut Shell-Activated Carbon. In Sustainability Challenges and Delivering Practical Engineering Solutions: Resources, Materials, Energy, and Buildings; Springer: Berlin/Heidelberg, Germany, 2023; pp. 145–150. [Google Scholar]
- Folino, A.; Zema, D.A.; Calabrò, P.S. Organic Matter Removal and Ammonia Recovery by Optimised Treatments of Swine Wastewater. J. Environ. Manag. 2020, 270, 110692. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, H.; Liu, J.; Zuo, D.; Deng, L. Sequencing Batch Reactor (SBR) and Anoxic and Oxic Process (A/O) Display Opposite Performance for Pollutant Removal in Treating Digested Effluent of Swine Wastewater with Low and High COD/N Ratios. J. Clean. Prod. 2022, 372, 133643. [Google Scholar] [CrossRef]
- Montalvo, S.; Huiliñir, C.; Castillo, A.; Pagés-Díaz, J.; Guerrero, L. Carbon, Nitrogen and Phosphorus Recovery from Liquid Swine Wastes: A Review. J. Chem. Technol. Biotechnol. 2020, 95, 2335–2347. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Q.; Xiao, J.; Liu, Y.; Yan, C.; Liu, J.; Sand, W.; Chow, C.W.K. The Key Factors and Removal Mechanisms of Sulfadimethoxazole and Oxytetracycline by Coagulation. Environ. Sci. Pollut. Res. 2020, 27, 16167–16176. [Google Scholar] [CrossRef]
- Zhong, S.-F.; Yang, B.; Lei, H.-J.; Xiong, Q.; Zhang, Q.-Q.; Liu, F.; Ying, G.-G. Transformation Products of Tetracyclines in Three Typical Municipal Wastewater Treatment Plants. Sci. Total Environ. 2022, 830, 154647. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, W.; Xie, H.; Feng, X.; Wang, S.; Song, H.; Xiong, J.; Mailhot, G. Co-Adsorption and Interaction Mechanism of Cadmium and Sulfamethazine onto Activated Carbon Surface. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126540. [Google Scholar] [CrossRef]
- Lertpaitoonpan, W.; Ong, S.K.; Moorman, T.B. Effect of Organic Carbon and PH on Soil Sorption of Sulfamethazine. Chemosphere 2009, 76, 558–564. [Google Scholar] [CrossRef]
- Kalli, M.; Noutsopoulos, C.; Mamais, D. The Fate and Occurrence of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes during Advanced Wastewater Treatment and Disinfection: A Review. Water 2023, 15, 2084. [Google Scholar] [CrossRef]
- Czekalski, N.; Imminger, S.; Salhi, E.; Veljkovic, M.; Kleffel, K.; Drissner, D.; Hammes, F.; Burgmann, H.; Von Gunten, U. Inactivation of Antibiotic Resistant Bacteria and Resistance Genes by Ozone: From Laboratory Experiments to Full-Scale Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11862–11871. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous Ozonation of Urban Wastewater: Removal of Antibiotics, Antibiotic-Resistant Escherichia Coli and Antibiotic Resistance Genes and Phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, B.; Wang, Z.; Wei, J.; Zhao, Y.; Wang, S. Enhanced Antibiotics and Antibiotics Resistance Genes Removal from Aquaculture Wastewater by Microalgae-Based System Induced with Plant Hormones. Int. Biodeterior. Biodegrad. 2025, 200, 106045. [Google Scholar] [CrossRef]
- Chen, Y.; Su, J.-Q.; Zhang, J.; Li, P.; Chen, H.; Zhang, B.; Gin, K.Y.-H.; He, Y. High-Throughput Profiling of Antibiotic Resistance Gene Dynamic in a Drinking Water River-Reservoir System. Water Res. 2019, 149, 179–189. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Wang, G.; Liu, Y.; Chang, J.; Jiang, T.; Li, G.; Ma, R.; Yang, Y.; Yuan, J. The Enrichment of Antibiotic Resistance Genes in Swine Manure Compost Was Related to the Bulking Agent Types. Environ. Technol. Innov. 2024, 36, 103765. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, H.; Su, C.; Yan, S. Abundance and Persistence of Antibiotic Resistance Genes in Livestock Farms: A Comprehensive Investigation in Eastern China. Environ. Int. 2013, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ruan, J.; Huang, L.; Zhao, J.; Xu, Y. Comparison of the Elimination Effectiveness of Tetracycline and AmpC β-Lactamase Resistance Genes in a Municipal Wastewater Treatment Plant Using Four Parallel Processes. Ecotoxicology 2021, 30, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yuan, C.; Ruan, C.; Zheng, M.; Liu, L.; Wang, G.; Chen, G. Coagulation Promotes the Spread of Antibiotic Resistance Genes in Secondary Effluents. Environ. Pollut. 2024, 355, 124245. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wu, H.; Zhang, K.; Xu, X.; Wang, C.; Zhu, W.; Wu, W. Long-Term Low Dissolved Oxygen Accelerates the Removal of Antibiotics and Antibiotic Resistance Genes in Swine Wastewater Treatment. Chem. Eng. J. 2018, 334, 630–637. [Google Scholar] [CrossRef]
- Stefańska, I.; Kwiecień, E.; Kizerwetter-Świda, M.; Chrobak-Chmiel, D.; Rzewuska, M. Tetracycline, Macrolide and Lincosamide Resistance in Streptococcus Canis Strains from Companion Animals and Its Genetic Determinants. Antibiotics 2022, 11, 1034. [Google Scholar] [CrossRef]
- Sartini, I.; Vercelli, C.; Lebkowska-Wieruszewska, B.; Lisowski, A.; Fadel, C.; Poapolathep, A.; Dessì, F.; Giorgi, M. Pharmacokinetics and Antibacterial Activity of Tiamulin after Single and Multiple Oral Administrations in Geese. Vet. Anim. Sci. 2023, 22, 100317. [Google Scholar] [CrossRef]
- Shen, Q.; Tang, J.; Wang, X.; Li, Y.; Yao, X.; Sun, H.; Wu, Y. Fate of Antibiotic Resistance Genes and Metal Resistance Genes during the Thermophilic Fermentation of Solid and Liquid Swine Manures in an Ectopic Fermentation System. Ecotoxicol. Environ. Saf. 2021, 213, 111981. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Z.; Xing, S.; Liao, X. The Correlation between Antibiotic Resistance Gene Abundance and Microbial Community Resistance in Pig Farm Wastewater and Surrounding Rivers. Ecotoxicol. Environ. Saf. 2019, 182, 109452. [Google Scholar] [CrossRef] [PubMed]
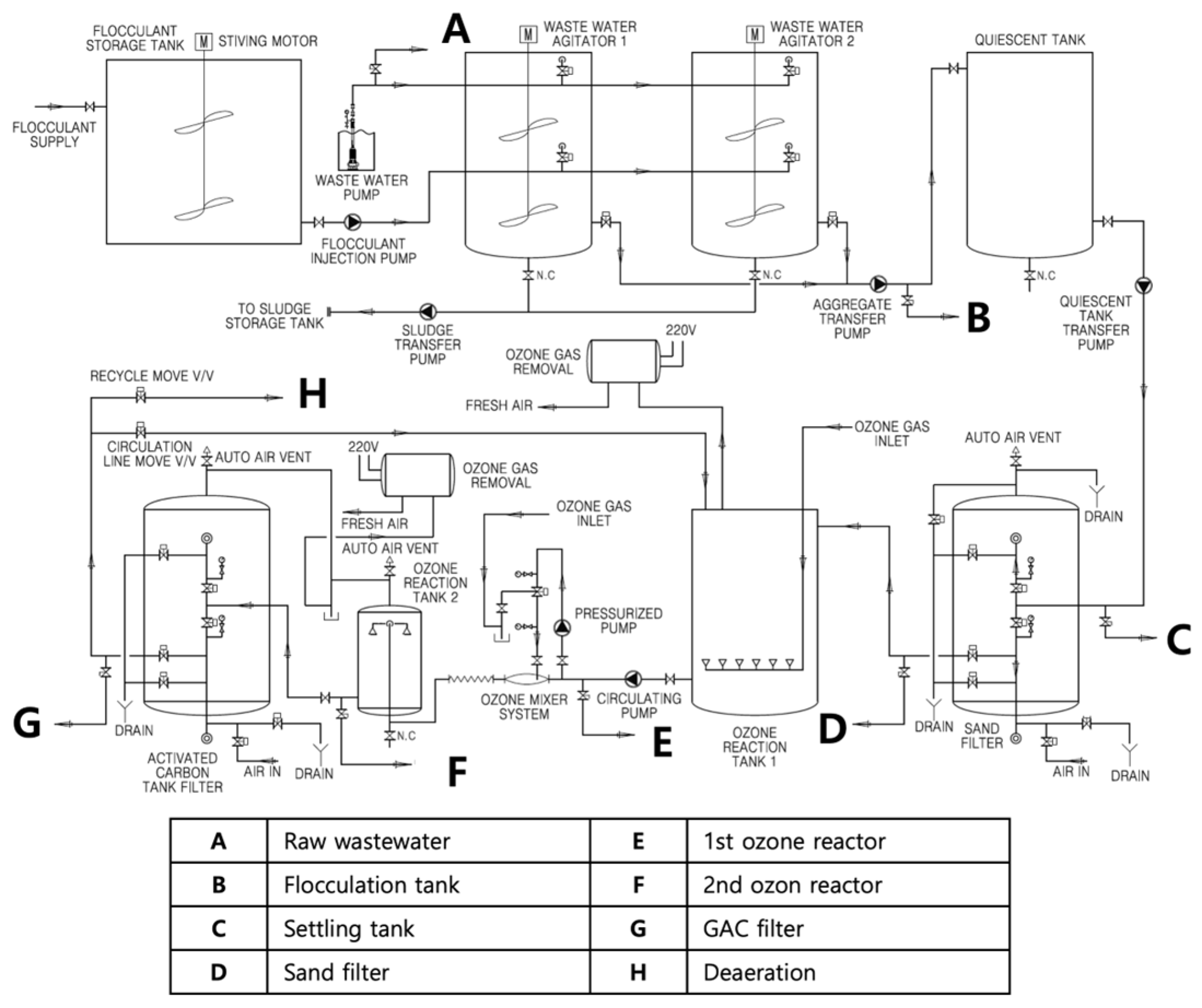


| Title 1 | SS | BOD | TOC | TN | TP |
| Removal Efficiency (%) | 92.2 | 33.6 | 69.8 | 3.1 | 96.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, T.; Kim, M.-S.; Hwang, Y.; Jeong, E.S.; Jo, H.; Cho, S.-K. Effects of Ozone Oxidation Process on Residual Antibiotics and Antibiotic Resistance Genes in a Swine Wastewater Treatment Plant. Appl. Sci. 2025, 15, 5158. https://doi.org/10.3390/app15095158
Cha T, Kim M-S, Hwang Y, Jeong ES, Jo H, Cho S-K. Effects of Ozone Oxidation Process on Residual Antibiotics and Antibiotic Resistance Genes in a Swine Wastewater Treatment Plant. Applied Sciences. 2025; 15(9):5158. https://doi.org/10.3390/app15095158
Chicago/Turabian StyleCha, Taeyoung, Min-Sang Kim, Yuhoon Hwang, Eun Sook Jeong, Hongmok Jo, and Si-Kyung Cho. 2025. "Effects of Ozone Oxidation Process on Residual Antibiotics and Antibiotic Resistance Genes in a Swine Wastewater Treatment Plant" Applied Sciences 15, no. 9: 5158. https://doi.org/10.3390/app15095158
APA StyleCha, T., Kim, M.-S., Hwang, Y., Jeong, E. S., Jo, H., & Cho, S.-K. (2025). Effects of Ozone Oxidation Process on Residual Antibiotics and Antibiotic Resistance Genes in a Swine Wastewater Treatment Plant. Applied Sciences, 15(9), 5158. https://doi.org/10.3390/app15095158








