Antimicrobial Potential of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze Essential Oils for Food Applications: A Review of Their Chemical Compositions and Antimicrobial Efficacy
Abstract
1. Introduction
2. Methodology of the Literature Review
3. Chemical Composition of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze EOs
| Method of Extraction | Part of the Plant Used for Extraction | Stage of the Plant at Harvest | Mass of the Plant Material Used for Extraction | Yield | Origin of the Plant | Reference |
|---|---|---|---|---|---|---|
| Steam distillation | NM | NM | NM | NM | Bulgaria | [24] 1 |
| Steam distillation | Leaves | NM | 100 g | NM | Spain | [25] |
| Steam distillation | NM | Flowering | NM | 0.21% (v/w) | Romania | [26] |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | NM | 100 g | NM | Serbia | [8] |
| Hydrodistillation using a Deryng apparatus | Aerial parts | Flowering | 40 g | EO1: 0.7% EO2: 0.5% | Poland | [7] 2 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | NM | 200 g | 0.6% (w/w) | Serbia | [14] 3 |
| NM | Aerial parts | NM | NM | NM | Italy | [27] |
| NM | Aerial parts | NM | 10 kg | NM | Egypt | [28] |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts (stems, leaves, and flowers) | Flowering | 25 g | EO1: 0.70% EO2: 0.28% EO3: 0.40% | Italy | [29] 4 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | NM | 50 g | EO1: 0.42–0.56 (v/w) EO2: 0.24–0.56 (v/w) EO3: 0.60–0.90 (v/w) EO4: 1.70–2.00 (v/w) EO5: 0.80–1.06 (v/w) | Kosovo | [20] 5 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | NM | 50 g | NM | Iran | [30] |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Flowering | NM | 1.1% (w/w) | Iran | [31] |
| Hydrodistillation with a Clevenger-type apparatus | NM | NM | 160 g | NM | Iran | [32] |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Flowering | NM | EO1: 0.40% (v/w) EO2: 0.54% (v/w) EO3: 0.65% (v/w) EO4: 0.79% (v/w) EO5: 0.48% (v/w) | Montenegro | [23] 6 |
| Hydrodistillation with a Clevenger-type apparatus | Inflorescence parts | Flowering | 200 g | EO1: 0.40 ± 0.09% EO2: 0.45 ± 0.11% | Iran | [33] 7 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Flowering | NM | 1.1% (w/w) | Italy | [34] 8 |
| Hydrodistillation with an Aura Distillateur installation | Aerial parts (shoots and inflorescences) | Flowering | 10 kg | 0.27% (v/w) | Romania | [35] 9 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Vegetative | 100 g | 0.17% (v/v) | Serbia | [36] |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Vegetative (June) Flowering (September) Flowering (November) | 150 g | J: EO1: 1.3% (w/w) EO2: 1.2% (w/w) EO3: 1.4% (w/w) S: EO1: 0.5% (w/w) EO2: 0.4% (w/w) EO3: 0.4% (w/w) N: EO1: 0.1% (w/w) EO2: 0.1% (w/w) EO3: 0.1% (w/w) | Serbia | [21] 10 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Flowering | 10 g | 2015: EO1: 1.20% (v/w) EO2: 1.30% (v/w) EO3: 1.00% (v/w) 2016: EO1: 0.83% (v/w) EO2: 0.79% (v/w) EO3: 0.83% (v/w) | Poland | [37] 11 |
| Hydrodistillation with a Clevenger-type apparatus | Leaves and flowers | Flowering | 500 g | NM | Turkey | [38] |
| Steam distillation | NM | NM | NM | NM | Balkan | [39] 12 |
| Method of Extraction | Part of the Plant Used | Stage of the Plant at Harvest | Mass of the Plant Material Used for Extraction | Yield | Origin of the Plant | Reference |
|---|---|---|---|---|---|---|
| Steam distillation using a copper distillation apparatus | Aerial parts (flower and leaves) | NM | 500 g | 0.37% | Bulgaria | [19] |
| Hydrodistillation with a Clevenger-type apparatus | Stem, leaves, and flowers | Flowering | 50 g | 1.86 ± 0.64% (v/w) | Romania | [16] 1 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Flowering | 25 g | NM | Iran | [40] |
| Hydrodistilled using a Likens–Nickerson apparatus with continuous extraction with dichloromethane | Aerial parts | Flowering | EO1: 24.78 g EO2: 28.22 g EO3: 13.77 g | EO1: 1.48% EO2: 2.08% EO3: 2.30% | Alabama | [41] 2 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Flowering | NM | 0.83% | Finland | [42] |
| Hydrodistillation using a Neo-Clevenger type apparatus | Aerial parts | Flowering | 100 g | EO1: 1.74 ± 0.10 mL/100 g EO2: 1.76 ± 0.11 mL/100 g | Romania | [17] 3 |
| Hydrodistillation | EO1: Aerial parts EO2: Leaves EO3: Flowers | NM | 2000 g | EO1: 0.62 ± 0.020 g/100 g EO2: 0.75 ± 0.008 g/100 g EO3: 1.22 ± 0.011 g/100 g | Romania | [43] 4 |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Flowering | 40 g | EO1: 1.32% (w/w) EO2: 2.78% (w/w) | [22] 5 | |
| Hydrodistillation with a Clevenger-type apparatus | Aerial parts | Flowering | 50 g | Irana | [44] |
3.1. Chemical Composition of Hyssopus officinalis L. EO
3.2. Chemical Composition of Agastache foeniculum (Pursh) Kuntze EO
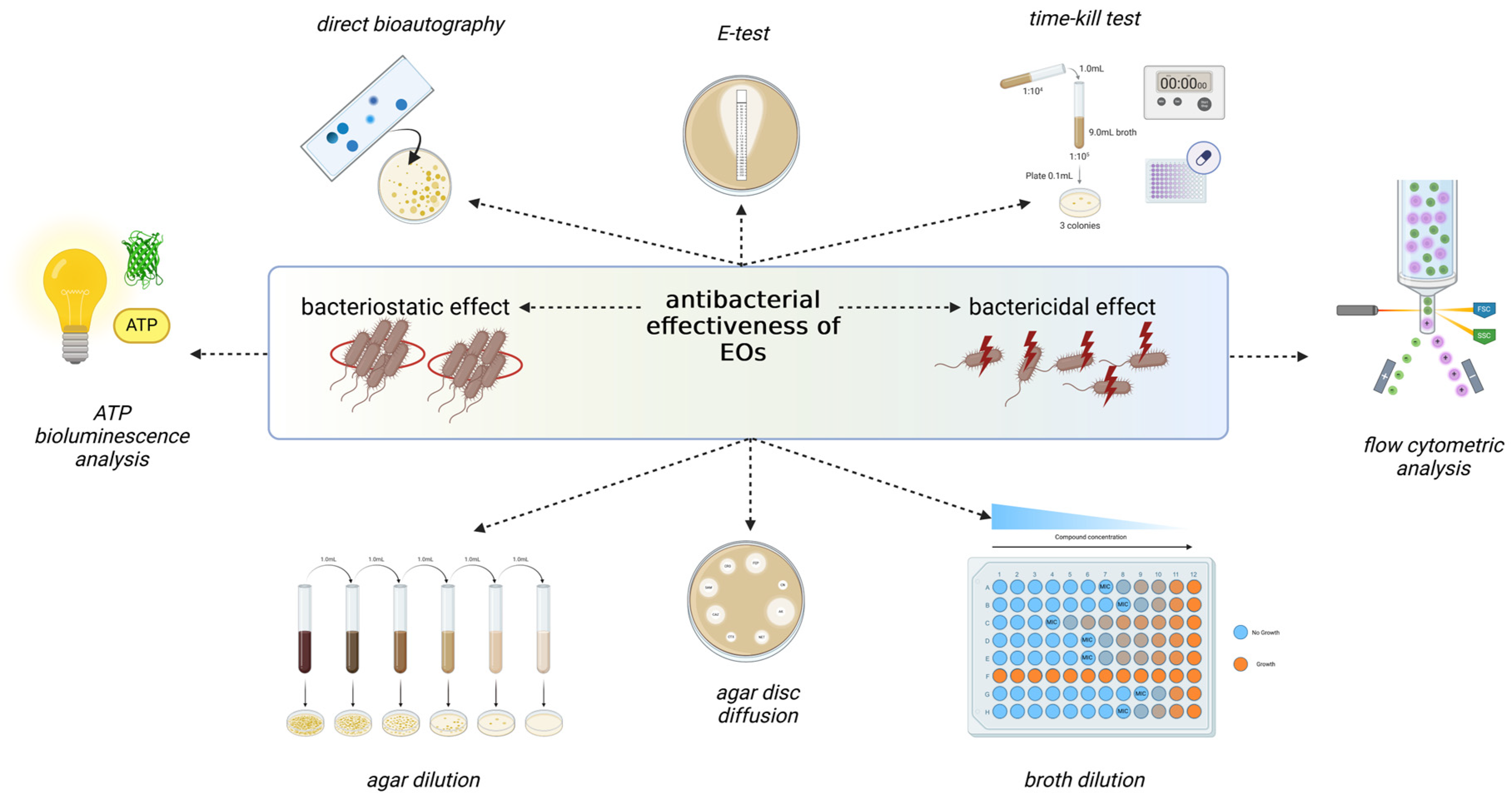
4. Antimicrobial Activities of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze EOs
4.1. Antimicrobial Activity of H. officinalis EO
4.1.1. Antifungal Activity
4.1.2. Antibacterial Activity
4.2. Antimicrobial Activity of Agastache foeniculum (Pursh) Kuntze
4.2.1. Antifungal Activity
4.2.2. Antibacterial Activity
5. Toxicity of Some Compounds of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze EOs
6. Food-Related Applications of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze EOs
7. Future Perspectives of the Food Utilization of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze EOs
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, J.; Chen, B.; Mcclements, D.J. Improving the Efficacy of Essential Oils as Antimicrobials in Foods: Mechanisms of Action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.d.A.; de Oliveira, M.S. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Baj, T.; Korona-Głowniak, I.; Kowalski, R.; Malm, A. Chemical composition and microbiological evaluation of essential oil from H. officinalis L. with white and pink flowers. Open Chem. 2018, 16, 317–323. [Google Scholar] [CrossRef]
- Aćimović, M.; Todosijević, M.; Varga, A.; Kiprovski, B.; Tešević, V.; Čabarkapa, I.; Sikora, V. Bioactivity of essential oils from cultivated winter savory, sage and H. officinalis. Lek. Sirovine 2019, 39, 11–17. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Nutraceutics of Lamiaceae. Stud. Nat. Prod. Chem. 2019, 62, 125–178. [Google Scholar] [CrossRef]
- Ortiz-Mendoza, N.; Martínez-Gordillo, M.J.; Martínez-Ambriz, E.; Basurto-Peña, F.A.; González-Trujano, M.E.; Aguirre-Hernández, E. Ethnobotanical, Phytochemical, and Pharmacological Properties of the Subfamily Nepetoideae (Lamiaceae) in Inflammatory Diseases. Plants 2023, 12, 3752. [Google Scholar] [CrossRef]
- Charles, D.J. Sources of Natural Antioxidants and Their Activities. In Antioxidant Properties of Spices, Herbs and Other Sources; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. H. officinalisus Essential Oil: An Update of Its Phytochemistry, Biological Activities, and Safety Profile. In Oxidative Medicine and Cellular Longevity; Wiley: Hoboken, NJ, USA, 2022; 8442734; 10p, Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1155/2022/8442734 (accessed on 15 December 2024).
- Judžentienė, A. Hyssop (H. officinalis L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; EditorPreedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 471–479. [Google Scholar] [CrossRef]
- Džamić, A.M.; Soković, M.D.; Novaković, M.; Jadranin, M.; Ristić, M.S.; Tešević, V.; Marin, P.D. Composition, antifungal and antioxidant properties of H. officinalis L. subsp. pilifer (Pant.) Murb. essential oil and deodorized extracts. Ind. Crops Prod. 2013, 51, 401–407. [Google Scholar] [CrossRef]
- de Elguea-Culebras, G.O.; Sánchez-Vioque, R.; Santana-Méridas, O.; Herraiz-Peñalver, D.; Carmona, M.; Berruga, M.I. In vitro antifungal activity of residues from essential oil industry against Penicillium verrucosum, a common contaminant of ripening cheeses. LWT 2016, 73, 226–232. [Google Scholar] [CrossRef]
- Bălănescu, F.; Botezatu, A.V.; Marques, F.; Busuioc, A.; Marincaş, O.; Vînătoru, C.; Cârâc, G.; Furdui, B.; Dinica, R.M. Bridging the Chemical Profile and Biological Activities of a New Variety of Agastache foeniculum (Pursh) Kuntze Extracts and Essential Oil. Int. J. Mol. Sci. 2023, 24, 828. [Google Scholar] [CrossRef]
- Nechita, M.A.; Pralea, I.E.; Țigu, A.B.; Iuga, C.A.; Pop, C.R.; Gál, E.; Vârban, R.; Nechita, V.I.; Oniga, O.; Toiu, A.; et al. Agastache Species (Lamiaceae) as a Valuable Source of Volatile Compounds: GC–MS Profiling and Investigation of In Vitro Antibacterial and Cytotoxic Activities. Int. J. Mol. Sci. 2024, 25, 5366. [Google Scholar] [CrossRef]
- Ivanov, I.G.; Vrancheva, R.Z.; Petkova, N.T.; Tumbarski, Y.; Dincheva, I.N.; Badjakov, I.K. Phytochemical compounds of anise hyssop (Agastache foeniculum) and antibacterial, antioxidant, and acetylcholinesterase inhibitory properties of its essential oil. J. Appl. Pharm. Sci. 2019, 9, 72–78. [Google Scholar] [CrossRef]
- Mollova, S.; Stanev, S.; Bojilov, D.; Manolov, S.; Kostova, I.; Damianova, S.; Fidan, H.; Stoyanova, A.; Ercisli, S.; Assouguem, A.; et al. Chemical composition and biological activity of essential oil from anise hyssop. Biotechnol. Biotechnol. Equip. 2024, 38, 2358995. [Google Scholar] [CrossRef]
- Hajdari, A.; Giorgi, A.; Beretta, G.; Gelmini, F.; Buratti, S.; Benedetti, S.; Merkouri, A.; Mala, X.; Kabashi, S.; Pentimalli, D.; et al. Phytochemical and sensorial characterization of H. officinalis subsp. aristatus (godr.) Nyman (Lamiaceae) by GC–MS, HPLC–UV–DAD, spectrophotometric assays and e-nose with aid of chemometric techniques. Eur. Food Res. Technol. 2018, 244, 1313–1327. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Dimitrijević, M.V.; Miladinović, L.C.; Stamenković, J.G.; Mihajilov-Krstev, T.M. Study of hyssop Essential Oil from Southeastern Serbia. Chem. Biodivers. 2024, 22, e202401954. [Google Scholar] [CrossRef]
- Talebi, S.F.; Saharkhiz, M.J.; Kermani, M.J.; Sharafi, Y.; Fard, F.R. Effect of Ploidy Level on the Nuclear Genome Content and Essential Oil Composition of Anise hyssop (Agastache foeniculum [Pursh.] Kuntze). Anal. Chem. Lett. 2016, 6, 678–687. [Google Scholar] [CrossRef]
- Mićović, T.; Topalović, D.; Živković, L.; Spremo-Potparević, B.; Jakovljević, V.; Matić, S.; Popović, S.; Baskić, D.; Stešević, D.; Samardžić, S.; et al. Antioxidant, antigenotoxic and cytotoxic activity of essential oils and methanol extracts of H. officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae). Plants 2021, 10, 711. [Google Scholar] [CrossRef]
- Hristova, Y.; Wanner, J.; Jirovetz, L.; Stappen, I.; Iliev, I.; Gochev, V. Chemical composition and antifungal activity of essential oil of H. officinalis L. from Bulgaria against clinical isolates of Candida species. Biotechnol. Biotechnol. Equip. 2015, 29, 592–601. [Google Scholar] [CrossRef]
- Muñoz-Tebar, N.; González-Navarro, E.J.; López-Díaz, T.M.; Santos, J.A.; de Elguea-Culebras, G.O.; García-Martínez, M.M.; Molina, A.; Carmona, M.; Berruga, M.I. Biological activity of extracts from aromatic plants as control agents against spoilage molds isolated from sheep cheese. Foods 2021, 10, 1576. [Google Scholar] [CrossRef] [PubMed]
- Jianu, C.; Golet, I.; Misca, C.; Jianu, A. Antimicrobial Properties and Chemical Composition of Essential Oils Isolated from Six Medicinal Plants Grown in Romania Against Foodborne Pathogens. Rev. Chim. Buchar.-Orig. Ed. 2016, 67, 1056–1061. Available online: https://www.researchgate.net/publication/306479909 (accessed on 15 December 2024).
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef]
- Eldeghedy, H.I.; El-Gendy, A.E.-N.G.; Nassrallah, A.A.; Aboul-Enein, A.M.; Omer, E.A. Essential oil composition and biological activities of H. officinalis and Perilla frutescens. Int. J. Health Sci. 2022, 6, 9963–9982. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Guevara, M.P.E.; Paganetto, G.; Grandini, A.; Maresca, I.; Menghini, L.; Martino, L.; Di Marengo, A.; Tacchini, M. Wild italian H. officinalis subsp. aristatus (Godr.) Nyman: From morphological and phytochemical evidences to biological activities. Plants 2021, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Karbin, S. Antifungal Activities of the Essential Oils on Post-Harvest Disease Agent Aspergilus flavus. Artic. Adv. Environ. Biol. 2009, 3, 219–225. Available online: https://www.researchgate.net/publication/228631568 (accessed on 15 December 2024).
- Mahboubi, M.; Haghi, G.; Kazempour, N. Antimicrobial Activity and Chemical Composition of H. officinalis L. Essential oil. J. Biol. Act. Prod. Nat. 2011, 1, 132–137. [Google Scholar] [CrossRef]
- Mehraie, A.; Khanzadi, S.; Hashemi, M.; Azizzadeh, M. New coating containing chitosan and H. officinalis essential oil (emulsion and nanoemulsion) to protect shrimp (Litopenaeus vannamei) against chemical, microbial and sensory changes. Food Chem. X 2023, 19, 100801. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Mohamadpoor, H.; Bajalan, I.; Malekpoor, F. Chemical Compositions and Antioxidant Activity of Essential Oils from Inflorescences of Two Landraces of Hyssop [H. officinalis L. subsp. angustifolius (Bieb.)] Cultivated in Southwestern, Iran. J. Essent. Oil-Bear. Plants 2019, 22, 1074–1081. [Google Scholar] [CrossRef]
- Rosato, A.; Maggi, F.; Cianfaglione, K.; Conti, F.; Ciaschetti, G.; Rakotosaona, R.; Fracchiolla, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Chemical composition and antibacterial activity of seven uncommon essential oils. J. Essent. Oil Res. 2018, 30, 233–243. [Google Scholar] [CrossRef]
- Stan, C.; Muscalu, A.; Burnichi, F.; Popescu, C.; Gatea, F.; Sicuia, O.-A.; Vlăduț, N.V.; Israel-Roming, F. Evaluation of essential oil and hydrolate from a new hyssop variety (H. officinalis L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12639. [Google Scholar] [CrossRef]
- Stanković, N.; Mihajilov-Krstev, T.; Zlatković, B.; Matejić, J.; Stankov Jovanović, V.; Kocić, B.; Čomić, L. Comparative Study of Composition, Antioxidant, and Antimicrobial Activities of Essential Oils of Selected Aromatic Plants from Balkan Peninsula. Planta Medica 2016, 82, 650–661. [Google Scholar] [CrossRef]
- Wesolowska, A.; Jadczak, D. Comparison of the Chemical Composition of Essential Oils Isolated from Hyssop(H. officinalis L.) with Blue, Pink and White Flowers. J. Essent. Oil-Bear. Plants 2018, 21, 938–949. [Google Scholar] [CrossRef]
- Metin, S.; Kara, N.; Didinen, B.I.; Kubilay, A. Antibacterial activity of essential oils and extracts of some medicinal plants against bacterial fish pathogens. Isr. J. Aquac.-Bamidgeh 2021, 73, 1–14. [Google Scholar] [CrossRef]
- Tančinová, D.; Barboráková, Z.; Mašková, Z.; Mrvová, M.; Medo, J.; Golian, M.; Štefániková, J.; Árvay, J. In vitro antifungal activity of essential oils (family Lamiaceae) against Cladosporium sp. Strains—Postharvest pathogens of fruits. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e9921. [Google Scholar] [CrossRef]
- Hashemi, M.; Ehsani, A.; Hassani, A.; Afshari, A.; Aminzare, M.; Sahranavard, T.; Azimzadeh, Z. Phytochemical, Antibacterial, Antifungal and Antioxidant Properties of Agastache foeniculum Essential Oil. J. Chem. Health Risks 2017, 7. [Google Scholar] [CrossRef]
- Lawson, S.K.; Satyal, P.; Setzer, W.N. The volatile phytochemistry of seven native american aromatic medicinal plants. Plants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Myadelets, M.A.; Vorobyeva, T.A.; Domrachev, D.V. Composition of the Essential Oils of Some Species Belonging to Genus Agastache clayton ex Gronov (Lamiaceae) Cultivated under the Conditions of the Middle Ural. Chem. Sustain. Dev. 2013, 21, 397–401. [Google Scholar]
- Stefan, D.-S.; Popescu, M.; Luntraru, C.-M.; Suciu, A.; Belcu, M.; Ionescu, L.-E.; Popescu, M.; Iancu, P.; Stefan, M. Comparative Study of Useful Compounds Extracted from Lophanthus anisatus by Green Extraction. Molecules 2022, 27, 7737. [Google Scholar] [CrossRef]
- Ebadollahi, A. Chemical constituents and toxicity of Agastache foeniculum (Pursh) Kuntze essential oil against two stored-product insect pests. Chil. J. Agric. Res. 2011, 71, 212–217. [Google Scholar] [CrossRef]
- Michalczyk, M.; Macura, R.; Tesarowicz, I.; Banaś, J. Effect of adding essential oils of coriander (Coriandrum sativum L.) and hyssop (H. officinalis L.) on the shelf life of ground beef. Meat Sci. 2012, 90, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.; Librán, C.M.; Berruga, M.I.; Zalacain, A.; Carmona, M. Mycotoxicogenic fungal inhibition by innovative cheese cover with aromatic plants. J. Sci. Food Agric. 2013, 93, 1112–1118. [Google Scholar] [CrossRef]
- Mićović, T.; Ušjak, D.; Milenković, M.; Samardžić, S.; Maksimović, Z. Antimicrobial activity of H. officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae) essential oils from Montenegro and Serbia. Lek. Sirovine 2023, 43, e173. [Google Scholar] [CrossRef]
- Harčárová, M.; Čonková, E.; Proškovcová, M.; Váczi, P.; Marcinčáková, D.; Bujňák, L. Comparison of antifungal activity of selected essential oils against fusarium graminearum in vitro. Ann. Agric. Environ. Med. 2021, 28, 414–418. [Google Scholar] [CrossRef]
- Ownagh, A.; Hasani, A.; Mardani, K.; Ebrahimzadeh, S.; Ghaffar Ownagh, A. Antifungal Effects of Thyme, Agastache and Satureja Essential Oils on Aspergillus fumigatus, Aspergillus flavus and Fusarium solani. Vet. Res. Forum 2010, 1, 99–105. [Google Scholar]
- Es, I.; Khaneghah, A.M.; Akbariirad, H. Global Regulation of Essential Oils. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Wiley: Hoboken, NJ, USA, 2017; pp. 327–338. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/9781119149392.ch11 (accessed on 23 January 2025). [CrossRef]
- Inanoglu, S.; Goksen, G.; Nayik, G.A.; Nejad, A.S.M. Essential oils from Lamiaceae family (rosemary, thyme, mint, basil). In Essential Oils Extraction, Characterization and Applications; Nayik, G.A., Ansari, M.J., Eds.; Academic Press: Kolkata, India, 2023; pp. 85–101. [Google Scholar] [CrossRef]
- Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC, Consolidated Version on 19.02.2025. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02008R1334-20250219 (accessed on 25 February 2025).
- Ebadollahi, A.; Roya, K.; Jalal, J.S.; Parisa, H.; Rahim, M.A. Toxicity and Physiological Effects of Essential Oil from Agastache Foeniculum (Pursh) Kuntze Against Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae) Larvae. Annu. Res. Rev. Biol. 2013, 3, 649–658. Available online: https://journalarrb.com/index.php/ARRB/article/view/674 (accessed on 15 December 2024).
- Ebadollahi, A.; Nouri-Ganbalani, G.; Hoseini, S.A.; Sadeghi, G.R. Insecticidal activity of essential oils of five aromatic plants against callosobruchus maculatus f. (coleoptera: Bruchidae) under laboratory conditions. J. Essent. Oil-Bear. Plants 2012, 15, 256–262. [Google Scholar] [CrossRef]
- Medeleanu, M.L.; Fărcaș, A.C.; Coman, C.; Leopold, L.; Diaconeasa, Z.; Sonia Socaci, S.A. Citrus essential oils—Based nano-emulsions: Functional properties and potential applications. Food Chem. X 2023, 20, 100960. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2018, 59, 2467–2480. [Google Scholar] [CrossRef]
- Medeleanu, M.L.; Fărcaș, A.C.; Coman, C.; Leopold, L.; Diaconeasa, Z.; Sendra, E.; Carbonell Pedro, A.A.; Sonia Socaci, S.A. Citrus Essential Oils’ Nano-emulsions: Formulation and Characterization, Bulletin Of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Food Sci. Technol. 2024, 81, 95–113. [Google Scholar] [CrossRef]
- Nikolić, I.; Čabarkapa, I.; Pavlić, B.; Kravić, S.; Đilas, M.; Iličić, M.; Bulut, S.; Kocić-Tanackov, S. Antibacterial and antibiofilm effect of essential oils on staphylococci isolated from cheese—Application of the oil mixture in a cheese model. Int. J. Food Microbiol. 2024, 425, 110873. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Gutiérrez-Praena, D.; Jos, A.; Cameán, A.M. In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: A review. Food Chem. Toxicol. 2015, 81, 9–27. [Google Scholar] [CrossRef]

| Total Number of Identified Compounds | Total Identified Compounds (%) | Main Classes of Compounds (%) | Main Compounds (%) | Reference |
|---|---|---|---|---|
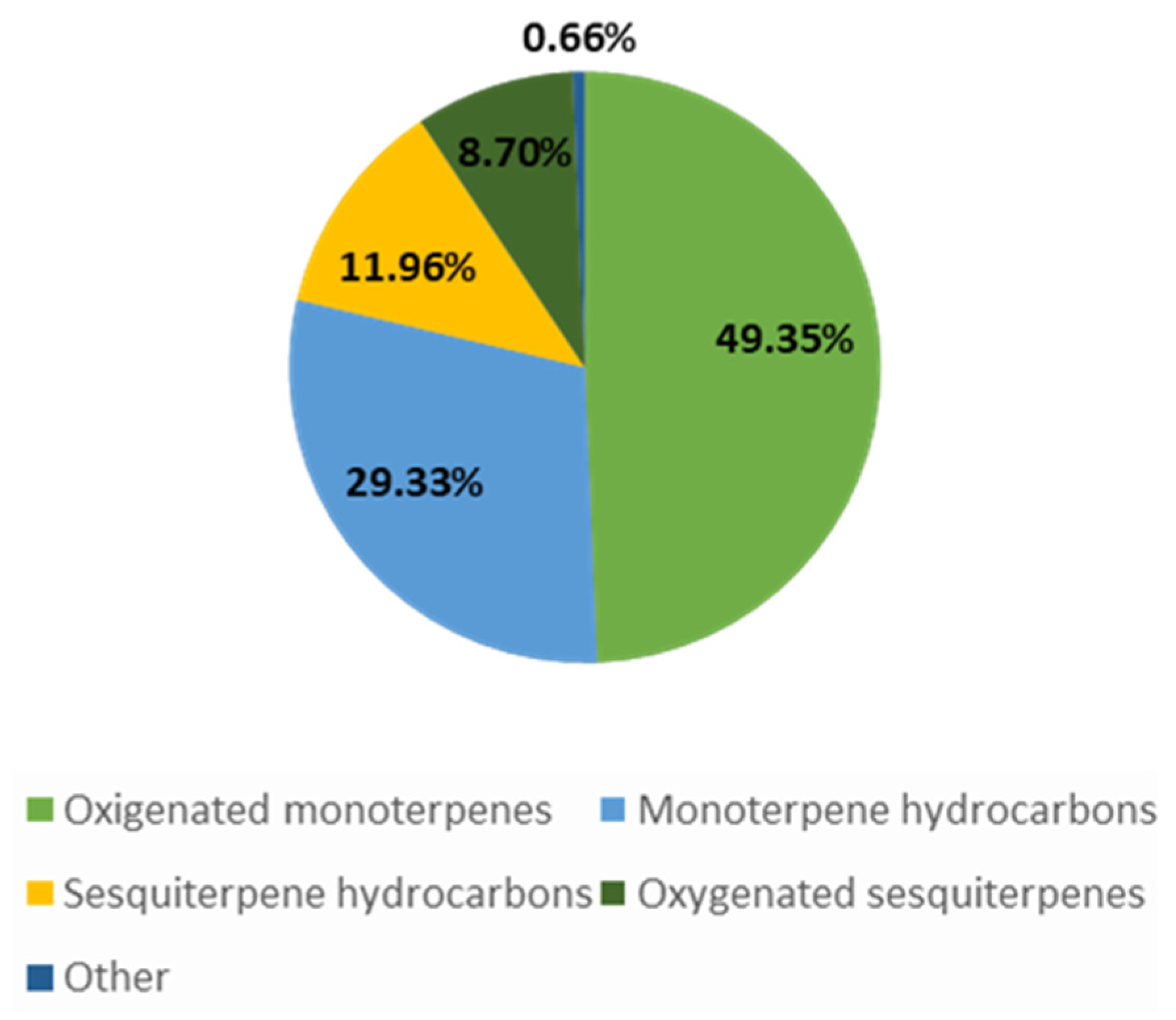
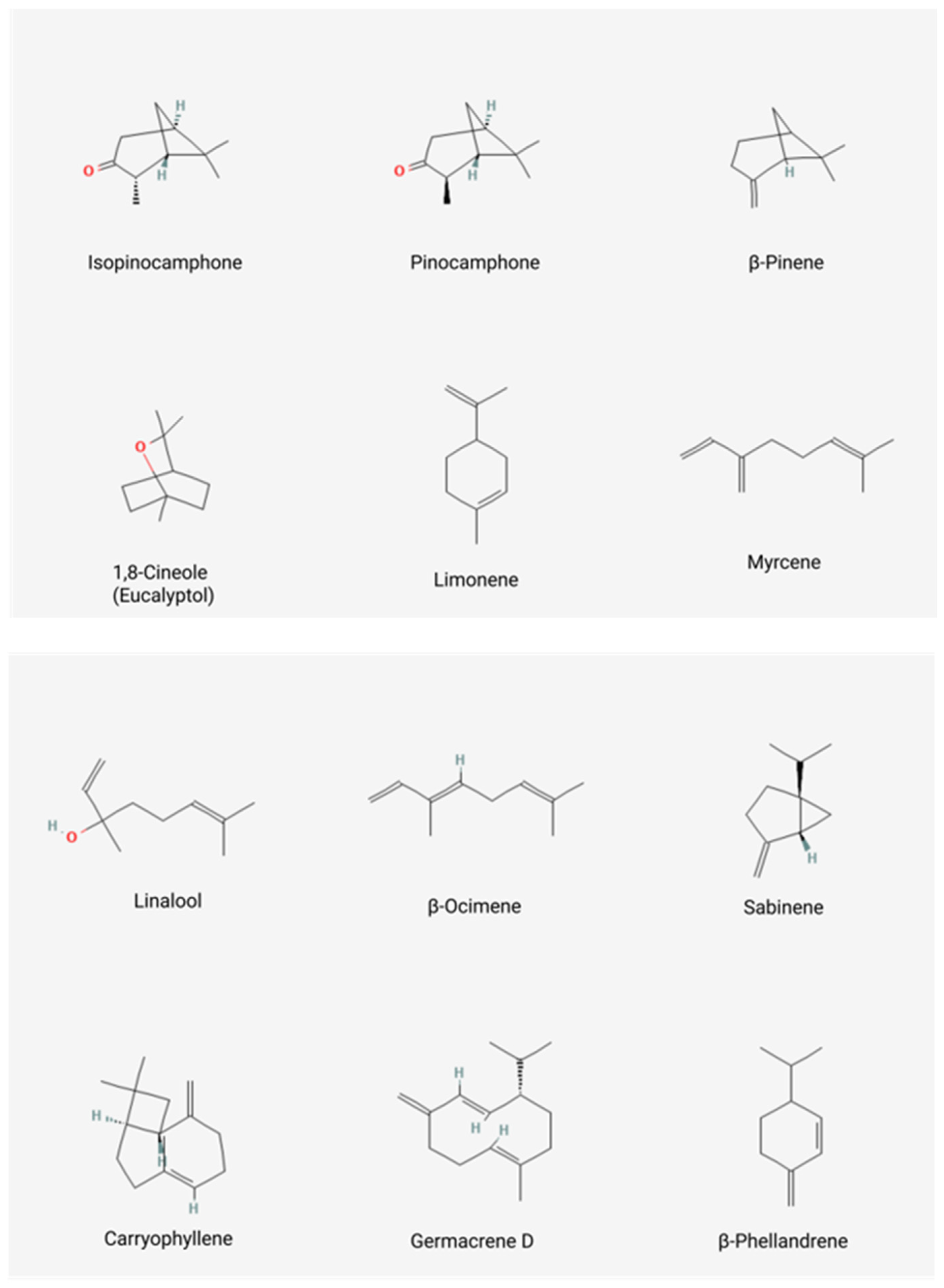
| 59 | 99.4 | Oxygenated monoterpenes: 67.8 Monoterpene hydrocarbons: 20.9 Sesquiterpene hydrocarbons: 6.4 Oxygenated sesquiterpenes: 2.4 Other: 0.1 | cis-Pinocamphone: 41.1; trans-Pinocamphone: 20.5; β-Pinene: 12; Myrcene: 1.5 | [8] |
| EO1: 44 EO2: 49 | 99.6 99.0 | Oxygenated monoterpenes: EO1: 70.6, EO2: 59.3 Monoterpene hydrocarbons: EO1: 23.2, EO2: 17.4 Sesquiterpene hydrocarbons: EO1: 4.9, EO2: 10.2 Oxygenated sesquiterpenes: EO1: 0.6, EO2:10.9 Other compounds: EO1:0.3, EO1:1.2 | β-Pinene: EO1: 12.4, EO2: 9.8; 1,8-Cineole: EO1: 7.6, EO2: 1.9; Pinocamphone: EO1: 51.0, EO2: 28.8; Isopinocamphone: EO1: 1.9, EO2: 21.9; Myrcene: EO1: 3.9, EO2: 1.7 | [7] |
| EO1: 66 EO2: 55 | 97.2 98 | Oxygenated monoterpenes: 61.69 Monoterpene hydrocarbons: 20.77 Sesquiterpene hydrocarbons: 15.19 Oxygenated sesquiterpenes: 1.39 Other compounds: 0.97 | β-Pinene: EO1: 13.38, EO2: 13.54; Myrcene: EO1: 1.90, EO2: 1.94; cis-Pinocamphone: EO1: 48.98, EO2: 50.77; trans-Pinocamphone: EO1: 5.78, EO2: 5.94; β-Phellandrene: EO1: 4.44, EO2: 5.17; Myrtenol: EO1: 1.62, EO2: 1.39; Germacrene D: EO1: 1.92, EO2: 1.87; Bicyclogermacrene: EO1: 1.53, EO2: 2.08; Sabinene: EO1: 1.54, EO2: 1.70; Limonene: EO1: 1.48, EO2: 1.50 | [24] 1 |
| 31 | 98.73 | Oxygenated monoterpenes: 59.78 Monoterpene hydrocarbons: 33.51 Sesquiterpene hydrocarbons: 4.54 Oxygenated sesquiterpenes: 0.87 Other compounds: 0.03 | 1,8-Cineole: 36.43; β-Pinene: 19.55; iso-Pinocamphone: 15.32; Pinocamphone: 6.39; Myrcene: 1.95; Sabinene: 2.90; Germacrene D: 1.65 | [14] |
| 57 | 96.81 | Oxygenated monoterpenes: 54.08 Monoterpene hydrocarbons: 28.39 Sesquiterpene hydrocarbons: 13.64 Oxygenated sesquiterpenes: 0.35 Other compounds: 0.35 | β-Pinene: 18.20; iso-Pinocamphone: 29.10; trans-Pinocamphone: 11.12; Lavandulol: 4.43; Myrcene: 1.84; β-Phellandrene: 1.85; Sabinene: 1.43; Limonene: 1.27; Myrtenol: 1.26 | [27] |
| 36 | 99.83 | Oxygenated compounds: 58.77 Nonoxygenated compounds: 41.03 Monoterpene compounds: 88.76 Sesquiterpene compounds: 11.07 | Isopinocamphone: 34; Pinocamphone: 21.27; β-Pinene: 13.19; β-Phellandrene: 13.10; Sabinene: 1.84; trans-Caryophyllene: 1.77; Germacrene-D: 1.28; bicyclo-Germacrene: 3.47 | [28] |
| EOEO1: 13 EOEO2: 33 EOEO3: 12 | 99.3 96.1 98.9 | NM | 1,8-Cineole: EO1: 15.5, EO2: 4.4, EO3: 39.7; Limonene: EO1: 5.8, EO2: 0.2, EO3: 7.6; trans-Pinocamphone: EO2: 11.0; iso-Pinocamphone; EO2: 43.2; Methyleugenol: EO2: 15.8, EO3: 41.5; (-)-Limonen-10-yl-acetate: EO1: 67.9; β-Pinene: EO1: 1.7, EO2: 3.1, EO3: 4.3; trans-Sabinol: EO2: 1.9; Spathulenol: EO1: 0.4, EO2: 2.1, EO3: 0.9; Caryophyllene oxide: EO2: 2.7, EO3:1.5; Limonen-10-ol: EO1: 2.8; Methyl ether: EO1: 1.9; | [29] |
| EO1: 57 EO2: 52 EO3: 44 EO4: 65 EO5: 66 | NM | NM | β-Pinene: EO1:3.3, EO2: 3.41, EO3:23.31, EO4: 13.66, EO5: 11.23; Limonene, EO1: 3.36, EO3: 14.47, EO4: 7.75, EO5: 3.92; Eucalyptol (cineol): EO1: 45.27, EO2: 7.15, EO3: 0.95, EO4: 12.29, EO5: 16.67; iso-Pinocamphone: EO1: 0.33, EO2: 14.67, EO3: 1.01, EO4: 0.61, EO5: 1.46; Pinocamphone: EO2: 57.73, EO3: 33.81, EO4: 37.86, EO5:30.44; E-Caryophyllene: EO1: 2.15, EO3: 13.73, EO4: 9.04, EO5: 4.98; Myrtenol: EO1: 1.55, EO2:2.36, EO3: 1.46; Sabinene: EO1: 1.33, EO3: 1.22, EO4: 1.10, EO5: 1.54; Z-β-Ocimene: EO3: 1.93, EO4: 4.43, EO5: 9.04; Borneol: EO3: 1.17, EO4: 3.81, EO5: 4.11; α-Pinene: EO1: 1.82, EO5: 1.12; Myrcene: EO1: 1.39, EO3: 1.03, EO5: 1.02; E-Anethenol: EO2: 4.83; Bicyclogermacrene: EO1: 2.16, EO4: 1.13; | [20] |
| 24 | 98.12 | NM | β-Pinene: 10.39; iso-Pinocamphone: 55.49; Pinocamphone: 1.75; Elemol: 3.74; D-Germacrene: 4.55; Myrtenol: 1.67; Myrtenyl acetate: 2.26; β-Bourbonene: 2.27; Caryophyllene: 3.30; Alloaromadendrene: 1.76; γ-Elemene: 2.70 | [26] |
| 18 | 93.9 | NM | Sabinene: 5.2; iso-Pinocamphone: 44.7; Pinocamphone: 14.1; Elemol: 5.6; Germacrene D-11-ol: 5.7; β-Phellandrene: 2.4; Myrtenol: 2.8; Germacrene D: 1.6; Spathulenol: 2.8; Caryophyllene oxide: 1.6; β-Caryophyllene: 1.4; cis-α-Bergamotene: 1.4 | [30] |
| 43 | 93.7 | NM | Isopinocamphone: 22.1; Isopinocamphopinone: 39.3; 2-Hydroxypinocamphone: 5.4; β-pinene: 2.9; β-Bourbonene: 1.7; Myrtenal: 2.0; Cymene: 1.2; Spathulenol: 2.8; Elemol: 1.7; cis-Pinonsaeure: 1.8; Caryophyllene oxide: 1.2 | [31] |
| 33 | 99.75 | NM | Pinocamphone: 11.81; Isopinocamphone: 35.45; β-Pinene: 10.12; Elemol: 5.11; Germacrene D: 3.68; Bicyclogermacrene: 4.04; β-Phellandrene: 5.65; β-Myrcene: 1.21; Linalool: 1.14; β-Bourbonone: 1.73; E-Caryophyllen: 3.25; γ-Eudesmol: 1.47 | [32] |
| NM | NM | NM | cis-Pinocamphone: 47.83; trans-Pinocamphone: 14.65; β-Pinene: 15.21; Myrtenol: 2.26; Sabinene: 1.53; Spathulenol: 1.52; β-Phellandrene: 1.47; Limonene: 1.33; β-Bourbonene: 1.06 | [45] |
| EO1: 16 EO2: 13 EO3: 13 EO4: 12 EO5: 14 | EO1: 86.84 EO2: 97.59 EO3: 99.21 EO4: 99.43 EO5: 98.47 | Oxygenated monoterpenes: EO1:57.94, EO2: 41.7, EO3: 27.35, EO4: 58.14, EO5: 57.9 Monoterpene hydrocarbons: EO1:28.9, EO2: 36.65, EO3: 43.53, EO4: 37.77, EO5: 26.2 Sesquiterpene hydrocarbons: EO5: 0.67 Phenylpropanoids: EO2: 19.24 EO3: 28.33, EO4: 3.52, EO5: 13.70 | 1,8-Cineole: EO1: 42.07, EO2: 9.77, EO3: 1.42, EO4: 38.19, EO5: 56.08; cis-Pinocamphone: EO1: 5.61, EO2: 22.75, EO3: 14.72, EO4: 14.54; β-Pinene: EO1: 9.13, EO2: 16.33, EO3: 15.79, EO4: 9.69, EO5: 5.48; Limonene: EO1: 7.99, EO2: 16.11, EO3: 23.81, EO4: 21.77, EO5: 15.43; trans-Pinocamphone: EO1:1.84, EO2: 3.34, EO3: 8.34, EO4: 4.72; Methyl eugenol: EO2: 19.24, EO3: 28.33, EO4: 3.52, EO5: 13.70; Sabinene: EO1: 1.24; Z-β-Ocimene: EO1: 2.94, EO2: 2.06, EO3: 1.88, EO4: 3.11, EO5: 3.06; Myrtenal: EO1: 3.71, EO2: 1.02 | [23] 2 |
| EO1: 26 EO2: 22 | WF: 97.75 PF: 97.38 | Oxygenated monoterpenes: WF: 74.22, PF: 63.82 Monoterpene hydrocarbons: WF: 17.93, PF: 28.96 Sesquiterpene hydrocarbons: WF: 4.59, PF: 4.60 Oxygenated sesquiterpenes: WF: 1.01, PF: 0.0 | cis-Pinocamphone: EO1: 30.11, EO2: 55.14; Camphor: EO1: 31.85; β-Pinene: EO1: 12.26, EO2: 17.06; Trans-pinocamphone: EO1: 6.09, EO2: 3.37; Sabinene: EO1: 1.61, EO2: 3.17; δ-3-Carene: EO1: 1.34, EO2: 2.57; Myrtenol: EO1: 2.62, EO2: 3.50; Germacrene D: EO1: 1.61, EO2: 1.69; Bicyclogermacrene: EO1: 1.33, EO2: 1.47; α-Pinene: WF: 0.EO1, EO2: 1.14; Myrcene: EO2: 1.60 | [33] |
| 44 | 99.6 | Oxygenated monoterpenes: 51.8 Monoterpene hydrocarbons: 22.7 Sesquiterpene hydrocarbons: 10.4 Oxygenated sesquiterpenes: 4.9 Phenylpropanoids: 9.9% | Linalool: 47.7; (Z)-β-Ocimene: 6.2; (E)-β-Ocimene: 5.4; Germacrene D: 5.8; Methyl eugenol: 9.9; β-Pinene: 1.7; Myrcene: 1.5; Limonene: 4.6; Palustrol: 2.4; Ledol: 2.2 | [34] |
| 28 | 96.15 | Oxygenated monoterpenes: 55.33 Monoterpene hydrocarbons: 24.51 Sesquiterpene hydrocarbons: 17.62 Oxygenated sesquiterpenes: 2.19 | cis-Pinocamphone: 36.63; trans-Pinocamphone: 11.72; β-Pinene: 10.46; Germacrene D: 7.27; Terpiene: 7.19; β-Elemene: 6.20; α-Thujene: 2.05; Myrcene: 1.78; Carrenol: 1.86; Pinenol: 1.74, β-Caryophyllen: 2.63; Longifolene: 2.02 | [35] |
| 43 | 98.6 | NM | 1,8-Cineole: 49.09; (Z)-β-Ocimene: 2.47; Isopinocamphone: 22.69; Limonene: 1.46; Sabinene: 1.33; α-Pinene: 1.13; β-Pinene: 11.26; trans-Pinocamphone: 1.24 | [36] |
| NM | NM | NM | Campholenone: 38.39; trans-3-Pinanone: 25.05; β-Pinene: 11.07; Myrtenyl methyl ether: 2.87; Caryophyllene: 2.16; β-Phellandrene: 1.71; (-)-β-Bourbonene: 1.66; (-)-Myrtenol: 1.63; Terpinene-4-ol: 1.22; Linalool: 1.14; D-Limonene: 1.10; Germacrene D: 1.08 | [39] |
| 38 | 93.47 | - | β-Pinene + Mircene: 16.36; 1,8-Cineole: 48.23; Pinocamphone: 3.42; Isopinocamphone: 4.38; Limonene: 6.02; 1-Octen-3-ol: 3.32; Pinocarvone: 3.16; α-Pinene: 2.67; β-e-Ocimene: 1.97; α-Terpineol: 1.87 | [25] |
| J: EO1: 25 EO2: 26 EO3: 23 S: EO1: 41 EO2: 51 EO3: 51 N: EO1: 53 EO2: 54 EO3: 52 | J: EO1: 97.86 EO2: 99.06 EO3: 99.67 S: EO1: 99.73 EO2: 99.01 EO3: 97.87 N: EO1: 98.05 EO2: 99.06 EO3: 98.82 | Oxygenated monoterpenes J: EO1: 61.43, EO2: 48.9, EO3: 27.32 S: EO1: 68.89, EO2: 78.74, EO3: 76.9 N: EO1: 86.47, EO2: 91.5, EO3: 92.25 Monoterpene hydrocarbons J: EO1: 31.12, EO2: 45.12, EO3: 67.24 S: EO1: 28.98, EO2: 14.51, EO3: 14.98 N: EO1: 3.89, EO2: 3.84, EO3: 3.65 Sesquiterpene hydrocarbons J: EO1: 5.31, EO2: 5.04, EO3: 5.11 S: EO1: 1.62, EO2: 2.09, EO3: 4.09 N: EO3: 3.14, EO2: 1.19, EO3: 0.98 Oxygenated sesquiterpenes J: 0 S: EO1: 0.16, EO2: 0.44, EO3: 1.58 N: EO1: 3.05, EO2: 1.9, EO3: 1.29 Others J: 0 S: EO1: 0.08, EO2: 3.23, EO3: 0.32 N: EO1: 1.5, EO2: 0.63, EO3: 0.65 | β-Pinene: J: EO1: 13.62, EO2: 19.44, EO3: 45.43; S: EO1: 12.75, EO2: 6.1 EO3: 5.79, N: EO1: 2.72, EO2: 2.51, EO3: 2.22 Eucalyptol: J: EO1: 16.46, EO2: 33.35, EO3: 25.88 S: EO1: 40.34, EO2: 63.91, EO3: 47.99 N: EO1: 17.15, EO2: 53.42, EO3: 53.85 Z-β-Ocimene: J: EO1: 9.89, EO2: 14.32, EO3: 10.75 S: EO1: 11.3, EO2: 5.18, EO3: 6.59 N: EO1: 0.35, EO2: 0.12, EO3: 0.15 cis-Pinocamphone: J: EO1: 41.69, EO2: 13.19 S: EO1: 18.41, EO2: 9.64, EO3: 21.53 N: EO1: 52.25, EO2: 25.99, EO3: 26.84 Sabinene: J: EO1: 1.63, EO2: 2.35, EO3: 2.08 trans-Pinocarveol: N: EO1: 2.02, EO2: 1.6, EO3: 1.42 trans-Pinocamphone: S: EO1: 7.14 N: EO1: 5.92, EO2: 2.6, EO3: 2.68 Methyl eugenol: S: EO2: 3.04 E-Caryophyllene: J: EO1: 1.1, EO2: 1.04, EO3: 2.17 Germacrene D: J: EO1: 3.27, EO2: 2.33, EO3: 1.27 Caryophyllene oxide: S: EO3: 1.4 N: EO1: 2.68, EO2: 1.69, EO3: 1.15 α-Pinene: J: EO2: 1.96, EO3: 2.76 S: EO1: 1.48 | [21] |
| EO1: 2015: 57 2016: 65 EO2: 2015: 52 2016: 62 EO3: 2015: 57 2016: 60 | EO1: 2015: 99.80 2016: 99.43 EO2: 2015: 99.24 2016: 99.40 EO3: 2015: 99.76 2016: 98.81 | Oxygenated monoterpenes: EO1: 2015: 50.73, 2016: 38.48 EO2: 2015: 53.03, 2016: 45.29 EO3: 2015: 54.04, 2016: 48.11 Monoterpene hydrocarbons: EO1: 2015: 13.77, 2016: 10.15 EO2: 2015: 13.78, 2016: 12.68 EO2: 2015: 12.34, 2016: 10.46 Sesquiterpene hydrocarbons: EO1: 2015: 20.79, 2016: 28.31 EO2: 2015: 19.22, 2016: 24.45 EO3: 2015: 19.76, 2016: 23.12 Oxygenated sesquiterpenes: EO1: 2015: 14.24, 2016: 21.08 EO2: 2015: 12.90, 2016: 15.91 EO3: 2015: 13.48, 2016: 16.00 Other compounds: EO1: 2015: 0.27, 2016: 1.41 EO2: 2015: 0.31, 2016: 1.07 EO3: 2015: 0.14, 2016: 1.12 | Isopinocamphone: EO1: 2015: 28.02, 2016: 20.05 EO2: 2015: 43.02, 2016: 33.33 EO3: 2015: 31.85, 2016: 21.26 Pinocamphone: EO1: 2015: 15.83, 2016: 12.17 EO2: 2015: 1.68, 2016: 3.45 EO3: 2015: 15.25, 2016: 19.62 β-Pinene: EO1: 2015: 7.13, 2016: 4.53 EO2: 2015: 6.95, 2016: 5.83 EO3: 2015: 7.49, 2016: 5.82 β-Phellandrene: EO1: 2015: 3.47, 2016: 2.99 EO2: 2015: 4.23, 2016: 4.37 EO3: 2015: 1.66, 2016: 1.46 δ-Elemene: EO1: 2015: 2.38, 2016: 2.73 EO2: 2015: 1.80, 2016: 2.36 EO3: 2015: 2.15, 2016: 2.34 β-Caryophyllene: EO1: 2015: 2.73, 2016: 3.36 EO2: 2015: 2.81, 2016: 4.13 EO3: 2015: 3.23, 2016: 3.87 Alloaromadendrene: EO1: 2015: 2.12, 2016: 3.28 EO2: 2015: 2.93, 2016: 2.93 EO3: 2015: 2.38, 2016: 2.47 Germacrene D: EO3: 2015: 4.34, 2016: 5.76 EO3: 2015: 3.85, 2016: 5.32 EO4: 2015: 4.11, 2016: 5.20 Bicyclogermacrene: EO1: 2015: 3.01, 2016: 4.15 EO2: 2015: 2.40, 2016: 2.91 EO3: 2015: 2.30, 2016: 2.43 Elemol: EO1: 2015: 8.74, 2016: 11.37 EO2: 2015: 8.04, 2016: 11.02 EO3: 2015: 8.77, 2016: 9.56 Spathulenol: EO1: 2015: 1.78, 2016: 1.72 EO2: 2015: 2.00, 2016: 0.90 EO3: 2015: 1.68, 2016: 1.14 | [37] |
| 46 | NM | NM | β-Myrcene: 10.16; β-Phellandrene: 49.79; Linalool: 10.04; α-Elemol: 3.62; Germacrene D: 2.79; Linalyl acetate: 2.46; Bicyclogermacrene: 2.08; Alloaromadendrene: 2.07 | [39] |
| Total Number of Identified Compounds | Total Identified Compounds (%) | Main Classes of Compounds (%) | Main Compounds (%) | Reference |
|---|---|---|---|---|
| 7 | 99.99 | NM | Estragole: 94.89; Limonene: 2.91 | [16] |
| 12 | 99.26 | NM | Estragole: 94; 1,8-Cineole: 3.33 | [44] |
| 7 | 95.4 | NM | Estragole: 83.; Limonene: 3.4; Spathulenol: 3.1; Caryophyllene oxide: 3.1 | [40] |
| EO1: 31 EO2: 45 EO3: 44 | 100 | Monoterpene hydrocarbons: EO1: 2.4, EO2: 4.9, EO3: 2.9 Oxygenated monoterpenoids: EO1: 1.0, EO2: 0.1, EO3: 0.1 Sesquiterpene hydrocarbons: EO1: 1.8, EO2: 2.5, EO3: 2.8 Oxygenated sesquiterpenoids: EO1: 0.2, EO2: 0.2, EO3: 0.3 Benzenoid aromatics: EO1: 94.1, EO2: 91.3, EO3: 92.9 Others: EO1: 0.5, EO2: 1.0, EO3: 0.9 | Estragole: EO1: 93.2, EO2: 88.4, EO3: 91.5; Limonene: EO1: 1.5, EO2: 4.9, EO3: 2.9; β-Caryophyllene: EO1: 1.2, EO2: 1.6, EO3: 1.9 | [41] |
| 13 | 100 | Oxygenated aliphatics: 1.98 Monoterpene hydrocarbons: 10.23 Oxygenated monoterpenes: 0.08 Sesquiterpene hydrocarbons: 3.04 Phenylpropanoids: 84.67 | Methyl chavicol: 82.03 ± 0.80; Limonene: 9.90 ± 0.09; β-Caryophyllene: 2.35 ± 0.02; 1-Octen-3-ol, acetate: 1.84 ± 0.01 | [19] |
| 17 | NM | NM | Methyl chavicol: 3.2; Menthone: 34.3; Isomenthone: 14.4; Pulegone: 9.1; Methyl eugenol: 3.1 | [42] |
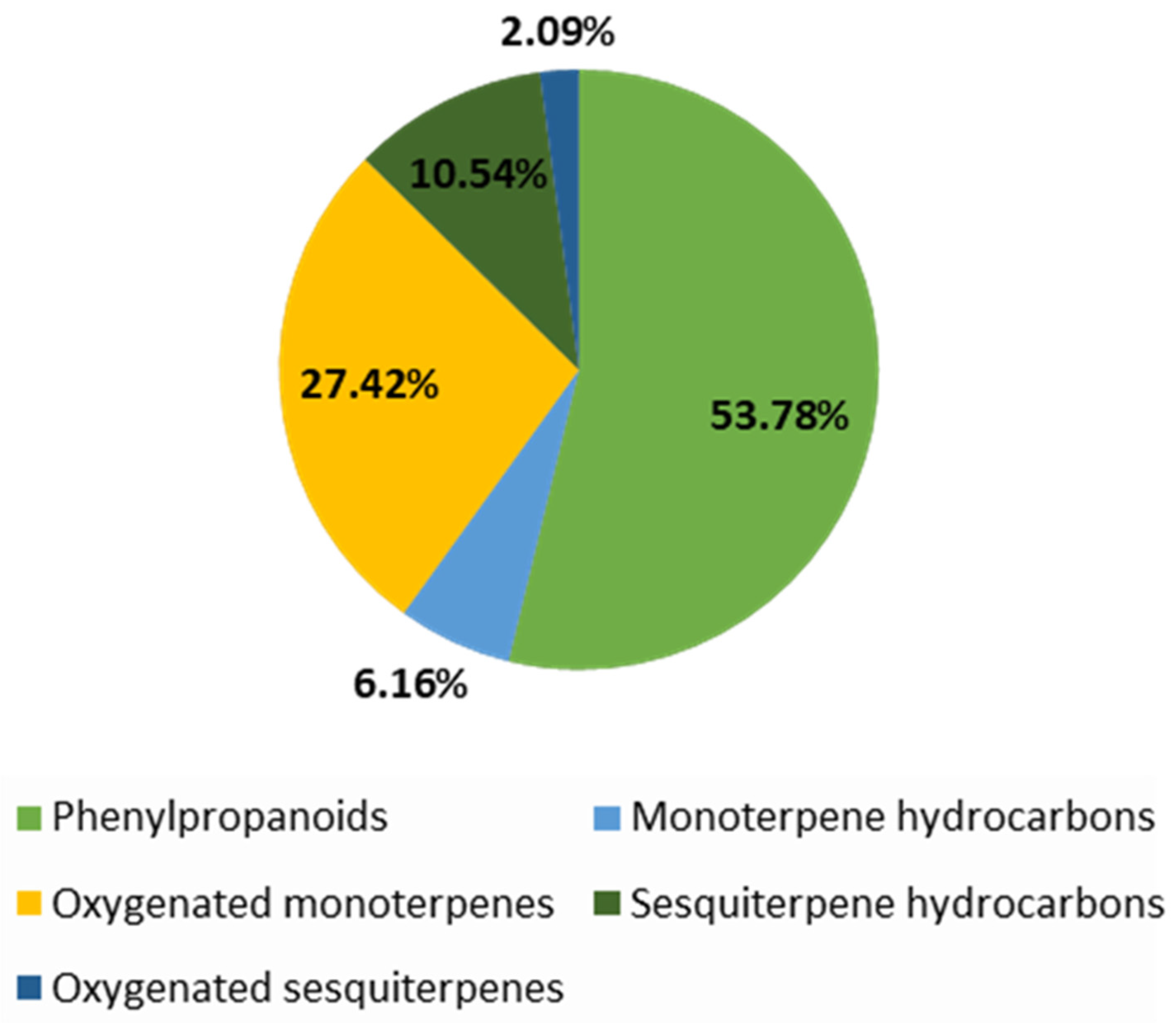
| 27 | 100 | Phenylpropanoids: 69.79 Sesquiterpene hydrocarbons: 19.19 Monoterpene hydrocarbons: 6.29 Oxygenated monoterpenes: 0.46 Oxygenated sesquiterpenes: 2.42 Other: 1.85 | Estragole: 63.27; Caryophyllene: 10.44; Limonene: 6.29; Methyl isoeugenol: 4.34; | [17] |
| 34 | 99.63 | Phenylpropanoids: 22.39 Sesquiterpene hydrocarbons: 9.23 Mnoterpene hydrocarbons: 8.69 Oxygenated monoterpenes: 54.51 Oxygenated sesquiterpenes: 3.96 Others: 0.85 | Estragole: 21.80; Menthone: 31.58; Pulegone: 21.44; Caryophyllene: 5.03 | |
| EO1: 10 EO2: 8 EO3: 8 | NM | NM | Estragole: EO1: 66.48, EO2: 30.16, EO3: 88.09; Limonene: EO1: 5.24, EO3: 8.01; Chavicol: EO1: 1.04, EO2: 14.22; Methyl eugenol: EO1: 9.77; Eugenol: EO2: 13.96; Benzaldehyde: EO1: 5.20, EO2: 2.44; Pentanol: EO1: 4.45, EO2: 3.13; Benzyl alcohol: EO2: 2.44; Phenyl ethyl alcohol: EO2: 20.19; Ethyl lactate: EO2: 4.53 | [43] |
| 43 | EO1: 96.32% EO2: 98.63% | - | Methyl chavicol: EO1: 78.75 ± 3.18, EO2: 81.02 ± 2.00; Limonene: EO1: 2.71 ± 0.12, EO2: 3.00 ± 0.12; 1,8-Cineole: EO1: 3.23 ± 0.03, EO2: 3.04 ± 0.07; Globulol: EO1: 1.43 ± 0.04, EO2: 1.67 ± 0.11 | [22] |
| Microorganism | MIC | MBC/MFC | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Bacterial concentration: 106 cfu/mL | [8] | |||||||
| Bacillus cereus ATCC 11778 | 14.20 μL/mL | 28.40 μL/mL | ||||||
| Escherichia coli ATCC 8739 | 227.25 μL/mL | 227.25 μL/mL | ||||||
| Salmonella Enteritidis ATCC 13076 | 227.25 μL/mL | 227.25 μL/mL | ||||||
| Staphylococcus aureus ATCC 25923 | 227.25 μL/mL | 227.25 μL/mL | ||||||
| Enterococcus faecalis ATCC 29212 | 454.50 μL/mL | 454.50 μL/mL | ||||||
| Pseudomonas aeruginosa ATCC 27853 | 454.50 μL/mL | 454.50 μL/mL | ||||||
| Staphylococcus epidermidis ATCC 12228 | 227.25 μL/mL | 227.25 μL/mL | ||||||
| Proteus hauseri ATCC 13315 | 454.50 μL/mL | 454.50 μL/mL | ||||||
| Bacterial concentration: 106 cfu/mL | [7] | |||||||
| Staphylococcus aureus ATCC 25923 | EO1: 10 mg/mL EO2: 5 mg/mL | EO1: 20 mg/mL EO2: 10 mg/mL | ||||||
| Bacillus subtilis ATCC 6633 | EO1:5 mg/mL EO2: 0.625 mg/mL | EO1: 5 mg/mL EO2: 2.5 mg/mL | ||||||
| Streptococcus pyogenes ATCC 19615 | EO1: 0.625 mg/mL EO2: 0.312 mg/mL | EO1:1.25 mg/mL EO2: 0.625 mg/mL | ||||||
| Escherichia coli ATCC 25922 | EO1: 5 mg/mL EO2: 5 mg/mL | EO1: 10 mg/mL EO2: 5 mg/mL | ||||||
| Proteus mirabilis ATCC 12453 | EO1: 5 mg/mL EO2: 5 mg/mL | EO1: 10 mg/mL EO2: 10 mg/mL | ||||||
| Klebsiella pneumoniae ATCC 13883 | EO1: 5 mg/mL EO2: 5 mg/mL | EO1: 10 mg/mL EO2: 10 mg/mL | ||||||
| Pseudomonas aeruginosa ATCC 9027 | EO1: 5 mg/mL EO2: 5 mg/mL | EO1: 10 mg/mL EO2: 10 mg/mL | ||||||
| Staphylococcus epidermidis ATCC 12228 | EO1: 5 mg/mL EO2: 2.5 mg/mL | EO1: 20 mg/mL EO2: 5 mg/mL | ||||||
| Micrococcus luteus ATCC 10240 | EO1: 2.5 mg/mL EO2: 2.5 mg/mL | EO1: 5 mg/mL EO2: 5 mg/mL | ||||||
| Streptococcus pneumoniae ATCC 49619 | EO1: 0.625 mg/mL EO2: 0.312 mg/mL | EO1: 1.25 mg/mL EO2: 0.625 mg/mL | ||||||
| Streptococcus mutans ATCC 25175 | EO1: 1.25 mg/mL EO2: 0.625 mg/mL | EO1: 1.25 mg/mL EO2: 1.25 mg/mL | ||||||
| Streptococcus pyogenes ATCC 19615 | EO1: 0.625 mg/mL EO2: 0.312 mg/mL | EO1: 1.25 mg/mL EO2: 0.625 mg/mL | ||||||
| Fungal concentration: 5 × 104 cfu/mL | ||||||||
| Candida albicans ATCC 102231 | EO1: 0.625 mg/mL EO2: 0.625 mg/mL | WF: 25 mg/mL PF: 2.5 mg/mL | ||||||
| Candida parapsilosis ATCC 102231 | EO1: 1.25 mg/mL EO2: 0.625 mg/mL | WF: 5 mg/mL PF: 1.25 mg/mL | ||||||
| Fungal concentration: 0.5 × 103–2.5 × 103 cfu/mL | [24] | |||||||
| 28 strains of Candida albicans: 1 × ATCC 10231 and 27 × clinical isolates | 128–256 μL/mL | 256–512 μL/mL | ||||||
| 8 strains of Candida glabrata: 1 × ATCC 90030 and 7 × clinical isolates | 512–1024 μL/mL | 1024–2048 μL/mL | ||||||
| 6 strains of Candida tropicalis: 1 × NBIMCC 23 and 5 × clinical isolates | 512–1024 μL/mL | 512–1024 μL/mL | ||||||
| 6 stains of Candida parapsilosis: 1 × ATCC 22019 and 5 × clinical isolates | 256–512 μL/mL | 512–1024 μL/mL | ||||||
| Candida krusei: 4 × clinical isolates | 128–256 μL/mL | 256–512 μL/mL | ||||||
| Fungal concentration: 1.0 × 106 spore/mL | [14] | |||||||
| Aspergillus niger ATCC 6275 | 52.20 mg/mL | 104.40 mg/mL | ||||||
| Aspergillus ochraceus ATCC 12066 | 26.10 mg/mL | 52.20 mg/mL | ||||||
| Aspergillus versicolor ATCC 11730 | 10.44 mg/mL | 26.10 mg/mL | ||||||
| Cladosporium cladosporioides ATCC 13276 | 10.44 mg/mL | 26.10 mg/mL | ||||||
| Cladosporium fulvum TK 5318 | 26.10 mg/mL | 26.10 mg/mL | ||||||
| Penicillium funiculosum ATCC 10509 | 52.20 mg/mL | 52.20 mg/mL | ||||||
| Penicillium ochrochloron ATCC 9112 | 26.10 mg/mL | 52.20 mg/mL | ||||||
| Trichoderma viride AM 5061 | 10.44 mg/mL | 26.10 mg/mL | ||||||
| Proteus vulgaris ATTC 13315 | 20 μg/mL | - | [28] | |||||
| Escherichia coli ATCC 35218 | 80 μg/mL | |||||||
| Staphylococcus aureus ATCC 25923 | 80 μg/mL | |||||||
| Staphylococcus aureus resistant | 30 μg/mL | |||||||
| Fungal concentration: 106 cfu/mL | [48] | |||||||
| Fusarium graminearum CCM F-683 | 0.4 mg/mL | - | ||||||
| Fusarium graminearum CCM 8244 | 0.4 mg/mL | - | ||||||
| Bacterial concentration: 1 × 107–1 × 108 cfu/mL | [31] | |||||||
| Staphylococcus aureus ATCC 25923 | 0.5 μL/mL | 0.5 μL/mL | ||||||
| Staphylococcus saprophyticus ATCC 13518 | 1 μL/mL | 1 μL/mL | ||||||
| Bacillus cereus ATCC 1247 | 1 μL/mL | 1 μL/mL | ||||||
| Escherichia coli ATCC 8739 | 4 μL/mL | 4 μL/mL | ||||||
| Pseudomonas aeruginosa ATCC 9027 | 4 μL/mL | 4 μL/mL | ||||||
| Fungal concentration: 1 ×107–1×108 cfu/mL | ||||||||
| Candida albicans ATCC 10231 | 1 μL/mL | 2 μL/mL | ||||||
| Aspergillus niger ATCC 16404 | 0.5 μL/mL | 8 μL/mL | ||||||
| Bacillus cereus ATCC 10876 | 4.1 mg/mL | - | [34] | |||||
| Bacillus subtilis ATCC 6633 | 4.1 mg/mL | |||||||
| Staphylococcus aureus ATCC 29213, ATCC 6538, ATCC 25923 | 4.1 mg/mL | |||||||
| Staphylococcus aureus ATCC 43300 | Resistant | |||||||
| Enterococcus faecalis ATCC 29212 | 4.1 mg/mL | |||||||
| Acinetobacter baumanni ATCC 19606 | 4.1 mg/mL | |||||||
| Pseudomonas aeruginosa ATCC 27853 | Resistant | |||||||
| Escherichia coli ATCC 25922 | 4.1 mg/mL | |||||||
| Klebsiella pneumoniae ATCC 13883 | 4.1 mg/mL | |||||||
| Staphylococcus epidermidis ATCC 12228 | 4.1 mg/mL | |||||||
| Clinical isolates: Staphylococcus aureus (IG22) | 4.1 mg/mL | |||||||
| Staphylococcus aureus (IG23) | 2.0 mg/mL | |||||||
| Staphylococcus aureus (IG24) | Resistant | |||||||
| Staphylococcus aureus (IG5) | Resistant | |||||||
| Staphylococcus epidermidis (IG1) | Resistant | |||||||
| Staphylococcus epidermidis (IG6) | 4.1 mg/mL | |||||||
| Serratia marcescens (IG) | 4.1 mg/mL | |||||||
| Acinetobacter baumannii (BS1) MDR | 4.1 mg/mL | |||||||
| Acinetobacter baumannii (BS2) MDR | 4.1 mg/mL | |||||||
| Acinetobacter baumannii (BS3) MDR | 4.1 mg/mL | |||||||
| Klebsiella pneumoniae (BS1) MDR | Resistant | |||||||
| Klebsiella pneumoniae (BS2) MDR | 4.1 mg/mL | |||||||
| Bacterial concentration: 106 cfu/mL | [36] | |||||||
| MDR clinical isolates: | ||||||||
| Wound swab isolates: | ||||||||
| Staphylococcus aureus | 22.15 mg/mL | 44.3 mg/mL | ||||||
| Streptococcus pyogenes | 88.60 mg/mL | 88.60 mg/mL | ||||||
| Enterococcus faecalis | 22.15 mg/mL | 22.15 mg/mL | ||||||
| Escherichia coli | 44.3 mg/mL | 44.3 mg/mL | ||||||
| Pseudomonas aeruginosa | 44.3 mg/mL | 88.60 mg/mL | ||||||
| Acinetobacter sp. | 22.15 mg/mL | 22.15 mg/mL | ||||||
| Proteus Mirabilis | 44.3 mg/mL | 88.6 mg/mL | ||||||
| Nasal swab isolates: | ||||||||
| Klebsiella sp. | 88.6 mg/mL | 88.6 mg/mL | ||||||
| Streptococcus pneumoniae | 22.15 mg/mL | 22.15 mg/mL | ||||||
| Staphylococcus aureus | 22.15 mg/mL | 22.15 mg/mL | ||||||
| Throat swab isolates: | ||||||||
| Streptococcus pyogenes | 22.15 mg/mL | 22.15 mg/mL | ||||||
| Escherichia coli | 44.3 mg/mL | 44.3 mg/mL | ||||||
| Sputum isolates: | ||||||||
| Pseudomonas aeruginosa 1 | 11.08 mg/mL | 11.08 mg/mL | ||||||
| Pseudomonas aeruginosa 2 | 44.30 mg/mL | 88.60 mg/mL | ||||||
| Klebsiella sp. | 11.08 mg/mL | 22.15 mg/mL | ||||||
| Aspirate isolates: | ||||||||
| Escherichia coli | 44.30 mg/mL | 44.3 mg/mL | ||||||
| Fungal concentration: 106 spores/mL | [39] | |||||||
| 6 fruits from which Cladosporium cladosporioides was isolated: | 7 days of incubation | 14 days of incubation | ||||||
| KMi-1034 | 500 µL/L | >500 µL/L | ||||||
| KMi-1035 | 500 µL/L | >500 µL/L | ||||||
| KMi-1036 | 500 µL/L | 500 µL/L | ||||||
| KMi-1037 | 250 µL/L | 500 µL/L | ||||||
| KMi-1038 | 500 µL/L | >500 µL/L | ||||||
| Bacterial concentration: 107–108 cfu/mL | [21] | |||||||
| J | S | N | J | S | N | |||
| Staphylococcus aureus ATCC 25923 | EO1 | 38.5 mg/mL | 64.0 mg/mL | 8.8 mg/mL | 154.0 mg/mL | 64.0 mg/mL | 70.5 mg/mL | |
| EO2 | 39.4 mg/mL | 65.5 mg/mL | 72.5 mg/mL | 157.5 mg/mL | 65.5 mg/mL | 72.5 mg/mL | ||
| EO3 | 160.0 mg/mL | 66.0 mg/mL | 37.8 mg/mL | 160.0 mg/mL | 66.0 mg/mL | 75.5 mg/mL | ||
| Bacillus cereus ATCC 11778 | EO1 | 9.6 mg/mL | 64.0 mg/mL | 70.5 mg/mL | 9.6 mg/mL | 64.0 mg/mL | 70.5 mg/mL | |
| EO2 | 39.4 mg/mL | 65.5 mg/mL | 72.5 mg/mL | 39.4 mg/mL | 65.5 mg/mL | 72.5 mg/mL | ||
| EO3 | 40.0 mg/mL | 66.0 mg/mL | 37.8 mg/mL | 40.0 mg/mL | 66.0 mg/mL | 75.5 mg/mL | ||
| Enterococcus faecalis ATCC 19433 | EO1 | 38.5 mg/mL | 8.0 mg/mL | 8.8 mg/mL | 77.0 mg/mL | 64.0 mg/mL | 70.5 mg/mL | |
| EO2 | 78.8 mg/mL | 8.2 mg/mL | 9.1 mg/mL | 78.8 mg/mL | 65.5 mg/mL | 72.5 mg/mL | ||
| EO3 | 160.0 mg/mL | 16.5 mg/mL | 9.4 mg/mL | 160.0 mg/mL | 66.0 mg/mL | 75.5 mg/mL | ||
| Salmonella enteritidis ATCC 13076 | EO1 | 9.6 mg/mL | 16.0 mg/mL | 8.8 mg/mL | 9.6 mg/mL | 64.0 mg/mL | 70.5 mg/mL | |
| EO2 | 19.7 mg/mL | 32.8 mg/mL | 9.1 mg/mL | 39.4 mg/mL | 65.5 mg/mL | 72.5 mg/mL | ||
| EO3 | 80.0 mg/mL | 66.0 mg/mL | 18.9 mg/mL | 80.0 mg/mL | 66.0 mg/mL | 75.5 mg/mL | ||
| Escherichia coli ATCC 25922 | EO1 | 77.0 mg/mL | 32.0 mg/mL | 35.3 mg/mL | 77.0 mg/mL | 64.0 mg/mL | 70.5 mg/mL | |
| EO2 | 78.8 mg/mL | 16.4 mg/mL | 18.1 mg/mL | 78.8 mg/mL | 65.5 mg/mL | 72.5 mg/mL | ||
| EO3 | 160.0 mg/mL | 16.5 mg/mL | 18.9 mg/mL | 160.0 mg/mL | 66.0 mg/mL | 75.5 mg/mL | ||
| Enterobacter aerogenes ATCC 13048 | EO1 | 38.5 mg/mL | 64.0 mg/mL | 70.5 mg/mL | 38.5 mg/mL | 64.0 mg/mL | 70.5 mg/mL | |
| EO2 | 39.4 mg/mL | 65.5 mg/mL | 72.5 mg/mL | 78.8 mg/mL | 65.5 mg/mL | 72.5 mg/mL | ||
| EO3 | 40.0 mg/mL | 66.0 mg/mL | 75.5 mg/mL | 80.0 mg/mL | 66.0 mg/mL | 75.5 mg/mL | ||
| Pseudomonas aeruginosa ATCC 9027 | EO1 | 38.5 mg/mL | 16.0 mg/mL | 8.8 mg/mL | 38.5 mg/mL | 16.0 mg/mL | 8.8 mg/mL | |
| EO2 | 157.5 mg/mL | 32.8 mg/mL | 9.1 mg/mL | 157.5 mg/mL | 32.8 mg/mL | 9.1 mg/mL | ||
| EO3 | 160.0 mg/mL | 16.5 mg/mL | 9.4 mg/mL | 160.0 mg/mL | 16.5 mg/mL | 18.9 mg/mL | ||
| Fungal concentration: 107–108 cfus/mL | ||||||||
| J | S | N | J | S | N | |||
| Candida albicans ATCC 24433 | EO1 | 2.4 mg/mL | 8.0 mg/mL | 4.4 mg/mL | 2.4 mg/mL | 16.0 mg/mL | 17.6 mg/mL | |
| EO2 | 19.7 mg/mL | 8.2 mg/mL | 4.5 mg/mL | 19.7 mg/mL | 32.8 mg/mL | 18.1 mg/mL | ||
| EO3 | 10.0 mg/mL | 8.3 mg/mL | 4.7 mg/mL | 160.0 mg/mL | 33.0 mg/mL | 9.4 mg/mL | ||
| Bacterial concentration: 2 × 108 ufc/mL | [47] 1 | |||||||
| EO1 | EO2 | EO3 | EO4 | EO5 | ||||
| Staphylococcus aureus ATCC 6538 | >500 µg/mL | 400 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | - | ||
| Bacillus subtilis ATCC 6633 | >500 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | |||
| Escherichia coli ATCC 8739 | >500 µg/mL | 400 µg/mL | >500 µg/mL | 400 µg/mL | 500 µg/mL | |||
| Klebsiella pneumoniae NCIMB 9111 | >500 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | |||
| Salmonella Typhimurium ATCC 14028 | >500 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | |||
| Pseudomonas aeruginosa ATCC 9027 | >500 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | >500 µg/mL | |||
| Fungal concentration: 2 × 106 ufc/mL | ||||||||
| EO1 | EO2 | EO3 | EO4 | EO5 | - | |||
| Candida albicans ATCC 10231 | 500 µg/mL | 500 µg/mL | 500 µg/mL | 500 µg/mL | 500 µg/mL | |||
| Microorganism | Inhibition Zone of Microorganism Growth | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Bacterial concentration: 1 × 108 ufc/mL | [27] | |||||||
| µg of EO/5 mm disc 1 | ||||||||
| 93 µg | 185 µg | 463 µg | ||||||
| Bacillus cereus DSM 4313 | Not active | Not active | 0.63(±0.06) cm | |||||
| Bacillus cereus DSM 4384 | Not active | Not active | 0.60(±0.05) cm | |||||
| Escherichia coli DSM 8579 | Not active | Not active | 0.77(±0.12) cm | |||||
| Enterococcus faecalis DSM 2352 | Not active | Not active | 0.60(±0.04) cm | |||||
| Staphylococcus aureus DSM 25923 | Not active | Not active | 0.60(±0.00) cm | |||||
| Fungal concentration: 1 × 106 ufc/mL | ||||||||
| µg of EO/5 mm | ||||||||
| 93 µg | 185 µg | 463 µg | ||||||
| Penicillium simplicissimum DSM 1097 | 0.60(±0.00) cm | 0.77(±0.6) cm | 0.27(±0.06) cm | |||||
| Aureobasidium pullulans DSM 62074 | 0.43(±0.06) cm | 0.53(±0.06) cm | 0.80(±0.00) cm | |||||
| Penicillium citrinum DSM 1997 | 0.50(±0.00) cm | 0.60(±0.00) cm | 0.73(±0.06) cm | |||||
| Penicillium aurantiogriseum DSM 2429 | 0.27(±0.06) cm | 0.30(±0.00) cm | 1.03(±0.06) cm | |||||
| Penicillium expansum DSM 1994 | Not active | Not active | Not active | |||||
| Debaryomyces hansenii DSM 70238 | Not active | Not active | Not active | |||||
| Proteus vulgaris ATTC 13315 | 5 ± 0.2 mm | [28] | ||||||
| Escherichia coli ATCC 35218 | 11 ± 0.3 mm | |||||||
| Staphylococcus aureus ATCC 25923 | 8 ± 0.24 mm | |||||||
| Staphylococcus aureus resistant | 4 ± 0.23 mm | |||||||
| 5 mm mycelial disc (10 days old) | [48] | |||||||
| Media with EOs (concentration) | ||||||||
| 100 μg/mL | 500 μg/mL | 1000 μg/mL | ||||||
| Fusarium graminearum CCM F-683 | Day 10 | 49.00 ± 2.00 mm | 42.00 ± 2.65 mm | 37.67 ± 1.15 mm | ||||
| Fusarium graminearum CCM 8244 | 50.00 ± 0.00 mm | 40.67 ± 1.15 mm | 39.33 ± 0.58 mm | |||||
| Bacterial concentration: 106 ufc/mL | [26] 2 | |||||||
| 6 mm discs containing | ||||||||
| 5 μL | 15 µL | 10 µL | 20 µL | |||||
| Staphylococcus aureus ATCC 25923 | 6.93 ± 0.26 mm | 12.62 ± 0.69 mm | 13.91 ± 0.28 mm | 16.16 ± 0.33 mm | ||||
| Salmonella Typhimurium ATCC 14028 | 6.69 ± 0.31 mm | 9.03 ± 0.22 mm | 14.66 ± 0.69 mm | 18.16 ± 0.29 mm | ||||
| Pseudomonas aeruginosa ATCC 25923 | 7.06 ± 0.17 mm | 8.32 ± 0.32 mm | 9.21 ± 0.34 mm | 10.81 ± 0.17 mm | ||||
| Escherichia coli ATCC 25922 | 6.76 ± 0.35 mm | 9.09 ± 0.3 mm | 14.84 ± 0.35 mm | 18.13 ± 0.29 mm | ||||
| Klebsiella pneumoniae ATCC 13882 | 16.58 ± 0.28 mm | 17.6 ± 0.46 mm | 18.59 ± 0.48 mm | 19.51 ± 0.45 mm | ||||
| Enterococcus faecalis ATCC 29212 | 10.4 ± 0.44 mm | 12.73 ± 0.3 mm | 13.64 ± 0.36 mm | 15.02 ± 0.37 mm | ||||
| Fungal concentration: 106 ufc/mL | ||||||||
| 6 mm discs containing the following EO volumes | ||||||||
| 5 μL | 15 µL | 10 µL | 20 µL | |||||
| Candida albicans ATCC 10231 | 15.56 ± 0.3 mm | 19.89 ± 0.4 mm | 25.82 ± 0.32 mm | 29.81 ± 0.33 mm | ||||
| 5 mm mycelial disc (7 days old) | [30] | |||||||
| Media with EOs (concentrations) | ||||||||
| 0.125% | 0.25% | 0.375% | 0.5% | |||||
| Aspergillus flavus isolated from rotted and injured fruits | Day 2 | 260.28 ± 0.5 mm2 | 173.52 ± 0.5 mm2 | 173.52 ± 0.5 mm2 | 129.53 ± 0.5 mm2 | |||
| Day 4 | 1711.06 ± 0.5 mm2 | 2418.65 ± 0.5 mm2 | 1976.76 ± 0.5 mm2 | 1474.75 ± 0.5 mm2 | ||||
| Day 6 | 5204.55 ± 0.5 mm2 | 3002.36 ± 0.5 mm2 | 2534.25 ± 0.5 mm2 | 1516.52 ± 0.5 mm2 | ||||
| Bacterial concentration: 1 × 107–1 × 108 cfu/mL | [31] | |||||||
| 6 mm disc containing the following EO volumes | ||||||||
| 1 µL | 2 µL | |||||||
| Staphylococcus aureus ATCC 25923 | 10 | 20 | ||||||
| Staphylococcus saprophyticus ATCC 13518 | 7 | 16 | ||||||
| Bacillus cereus ATCC 1247 | 9 | 19 | ||||||
| Escherichia coli ATCC 8739 | - | 8 | ||||||
| Pseudomonas aeruginosa ATCC 9027 | - | - | ||||||
| Fungal concentration: 1 × 107–1 × 108 cfu/mL | ||||||||
| 6 mm disc containing the following EO volumes | ||||||||
| 1 µL | 2 µL | |||||||
| Candida albicans ATCC 10231 | - | - | ||||||
| Aspergillus niger ATCC 16404 | - | 10 µL | ||||||
| Mycelial plugs, 8 mm diameter 5 mm discs containing 10 µL of EO | [35] | |||||||
| EO concentration | ||||||||
| 25% | 50% | 75% | 100% | |||||
| Fusarium oxysporum ZUM 2407 | Day 10 | insignificant compared with the control | 43.6% | 62.4% | 82.4% | |||
| Microorganism | MIC | MBC/MFC | Reference | ||
|---|---|---|---|---|---|
| Bacterial concentration: 1.5 × 106 cfu/mL | [40] | ||||
| Staphylococcus aureus ATCC 6538 | 125 µg/mL | 125 µg/mL | |||
| Escherichia coli ATCC 43894 | 500 µg/mL | 1000 µg/mL | |||
| Bacillus cereus BC 6830 | 125 µg/mL | 250 µg/mL | |||
| Bacillus subtilis ATCC 6633, | 62.5 µg/mL | 125 µg/mL | |||
| Salmonella Enteritidis ATCC 13076 | 250 µg/mL | 250 µg/mL | |||
| Salmonella Typhimurium ATCC 13311 | 250 µg/mL | 500 µg/mL | |||
| Listeria monocytogenes ATCC 19118 | 150 µg/mL | 250 µg/mL | |||
| Fungal concentration: 106 spore/mL | |||||
| Food-borne fungal strains: | 20 µL EO | 5 µL EO | |||
| Aspergillus flavus | 200 ppm | 400 ppm | |||
| Aspergillus niger | 400 ppm | 800 ppm | |||
| Bacterial concentration: 1.5 × 108 cfu/mL | [17] | ||||
| ABF | AF | ABF | AF | ||
| Escherichia coli ATCC 25922 | 18.72 ± 6.86 μL/mL | 18.72 ± 6.86 μL/mL | 22.68 ± 0.00 μL/mL | 18.72 ± 6.86 μL/mL | |
| Salmonella Enteritidis ATCC 13076 | 30.99 ±14.39 μL/mL | 22.68 ± 0.00 μL/mL | 47.62 ± 0.00 μL/mL | 22.68 ± 0.00 μL/mL | |
| Staphylococcus aureus ATCC 6538P | 22.68 ± 0.00 μL/mL | 10.80 ± 0.00 μL/mL | 47.62 ± 0.00 μL/mL | 18.72 ± 6.86 μL/mL | |
| Listeria monocytogenes ATCC 19114 | 22.68 ± 0.00 μL/mL | 22.68 ± 0.00 μL/mL | 22.68 ± 0.00 μL/mL | 22.68 ± 0.00 μL/mL | |
| Fungal concentration: 107 spores/mL | [49] 1 | ||||
| Aspergillus fumigates (PTCC 5009) | 1000 μL/mL | - | |||
| Aspergillus flavus (PTCC 5006) | 500 μL/mL | 2000 μL/mL | |||
| Fusarium solani (PTCC 5284) | 500 μL/mL | 2000 μL/mL | |||
| Microorganisms | Inhibition Zone of Microorganism Growth | Reference | |||
|---|---|---|---|---|---|
| 0.1 mL of the bacterial suspension/plate; concentration 1.5 × 106 cfu/mL 20 µL of EO/6 mm disc | [40] | ||||
| EO concentration 10–15 mg/mL | |||||
| Staphylococcus aureus ATCC 6538 | 16.33 ± 0.88 mm | ||||
| Escherichia coli ATCC 43894 | 9 ± 0.58 mm | ||||
| Bacillus cereus BC 6830, | 14.67 ± 0.33 mm | ||||
| Bacillus subtilis ATCC 6633, | 19.33 ± 1.85 mm | ||||
| Salmonella Enteritidis ATCC 13076 | 12.67 ± 1.2 mm | ||||
| Salmonella Typhimurium ATCC 13311 | 11 ± 1.15 mm | ||||
| Listeria monocytogenes ATCC 19118 | 15.67 ± 1.2 mm | ||||
| 100 µL of the fungal suspension/plate; concentration: 106 spores/mL 10 µL of EO dissolved in methanol | |||||
| EO concentration | |||||
| 1 mg/mL | 2.5 mg/mL | 5 mg/mL | 10 mg/mL | ||
| Foodborne fungal strains: Aspergillus flavus | 13.2 ± 1 mm | 18.67 ± 0.88 mm | 21.33 ± 0.67 mm | 25.33 ± 1.2 mm | |
| Aspergillus niger | 12 ± 0.78 mm | 15.3 ± 0.3 mm | 19.67 ± 1.2 mm | 22.67 ± 0.67 mm | |
| Fungal concentration: 108 spores/mL 10 µL of EO/6 mm disc | [49] | ||||
| EO concentration | |||||
| 250 μL/mL | 500 μL/mL | 1000 μL/mL | 2000 μL/mL | ||
| Aspergillus fumigates (PTCC 5009) | - | 7 mm | 16 mm | 21 mm | |
| Aspergillus flavus (PTCC 5006) | 7 mm | 12 mm | 18 mm | 24 mm | |
| Fusarium solani (PTCC 5284) | 7 mm | 11 mm | 19 mm | 28 mm | |
| 200 µL of the bacterial suspension/plate; concentration: 1.5 × 108 CFU/mL | [19] | ||||
| 50 µL of EO; concentration: 0.1 mL/mL | |||||
| Staphylococcus aureus ATCC 6538 | 25.7 ± 0.5 mm | ||||
| Bacillus cereus ATCC 10876 | 12.3 ± 0.2 mm | ||||
| Candida albicans ATCC 10231 | 16.5 ± 0.5 mm | ||||
| Saccharomyces cerevisiae ATCC 9763 | 16.3 ± 0.4 mm | ||||
| Bacterial concentration: 1.5 × 108 CFU/mL EO concentration: 10 µL on discs with a diameter of 6 mm 25 µL on discs with a diameter of 9 mm 100 µL in glass cylinders with a diameter of 6 mm | [43] | ||||
Staphylococcus aureus hospital flora | Flower EO: 18 mm Leaf EO: 9 mm Whole plant EO: 10.5 mm | ||||
| Name of the Compound | Foods in Which the Compounds Are Restricted | Maximum Level mg/kg | Reference |
|---|---|---|---|
| Estragole | Dairy products | 50 | [52] |
| Processed fruits and vegetables, nuts, and seeds | 50 | ||
| Fish products | 50 | ||
| Non-alcoholic beverages | 10 | ||
| Methyl eugenol | Dairy products | 25 | |
| Meat preparations and meat products | 15 | ||
| Fish preparations and fish products | 10 | ||
| Soups and sauces | 60 | ||
| Ready-to-eat savories | 20 | ||
| Non-alcoholic beverages | 1 | ||
| Pulegone | Mint/peppermint-containing confectionery, except micro breath-freshening confectionery | 250 | |
| Micro breath-freshening confectionery | 2000 | ||
| Chewing gum | 350 | ||
| Mint/peppermint-containing non-alcoholic beverages | 20 | ||
| Mint/peppermint-containing alcoholic beverages | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, A.L.; Pop, C.R.; Mitrea, L.; Cătunescu, G.M.; Vârban, R.; Lipșa, F.D.; Rusu, C.C.; Rotar, A.M. Antimicrobial Potential of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze Essential Oils for Food Applications: A Review of Their Chemical Compositions and Antimicrobial Efficacy. Appl. Sci. 2025, 15, 4772. https://doi.org/10.3390/app15094772
Nistor AL, Pop CR, Mitrea L, Cătunescu GM, Vârban R, Lipșa FD, Rusu CC, Rotar AM. Antimicrobial Potential of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze Essential Oils for Food Applications: A Review of Their Chemical Compositions and Antimicrobial Efficacy. Applied Sciences. 2025; 15(9):4772. https://doi.org/10.3390/app15094772
Chicago/Turabian StyleNistor, Alina L., Carmen R. Pop, Laura Mitrea, Giorgiana M. Cătunescu, Rodica Vârban, Florin D. Lipșa, Crina Claudia Rusu, and Ancuța M. Rotar. 2025. "Antimicrobial Potential of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze Essential Oils for Food Applications: A Review of Their Chemical Compositions and Antimicrobial Efficacy" Applied Sciences 15, no. 9: 4772. https://doi.org/10.3390/app15094772
APA StyleNistor, A. L., Pop, C. R., Mitrea, L., Cătunescu, G. M., Vârban, R., Lipșa, F. D., Rusu, C. C., & Rotar, A. M. (2025). Antimicrobial Potential of Hyssopus officinalis L. and Agastache foeniculum (Pursh) Kuntze Essential Oils for Food Applications: A Review of Their Chemical Compositions and Antimicrobial Efficacy. Applied Sciences, 15(9), 4772. https://doi.org/10.3390/app15094772










