Effects of Different Drying Methods on Structural Characterization, Rheological Properties, Antioxidant and Hypolipidemic Activities of Polysaccharides from Fig (Ficus carica L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Drying Experiments
2.3. Extraction Procedure of FPs
2.4. The Chemical Compositions of FPs
2.4.1. Sugar, Protein and Glucuronic Acid Analysis
2.4.2. Monosaccharide Analysis
2.4.3. Molecular Weight Analysis
2.5. Characterizations of FPs
2.5.1. Fourier-Transform Infrared (FT-IR) Spectrum Analysis
2.5.2. X-Ray Diffraction (XRD) Analysis
2.5.3. Scanning Electron Microscopy (SEM) Analysis
2.6. Rheological Characterization
2.7. Antioxidant Studies In Vitro
2.7.1. DPPH Radical Scavenging Activity
2.7.2. ABTS Radical Scavenging Activity
2.7.3. Ferric Reducing Antioxidant Power
2.8. Hypolipidemic Activity In Vitro
2.8.1. Cholesterol Binding Capacity Assay
2.8.2. Cholate Binding Capacity Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Yield, Neutral Sugar, Glucuronic Acid and Protein Content of FPs
3.2. Monosaccharide Composition of FPs
3.3. Molecular Weight of FPs
3.4. FT-IR Spectroscopy of FPs
3.5. XRD Examination of FPs
3.6. SEM Analysis of FPs
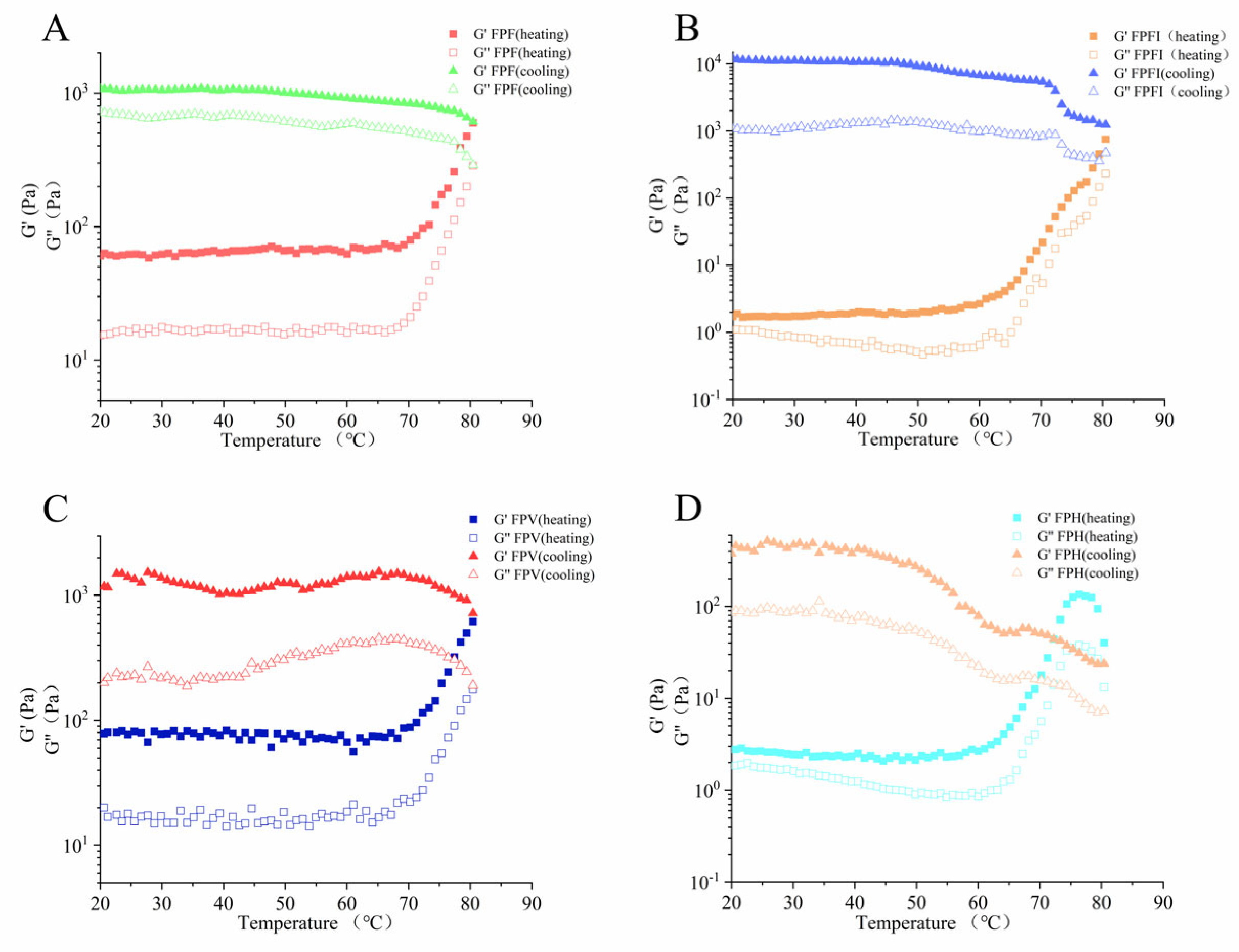
3.7. Rheological Characterization of FPs
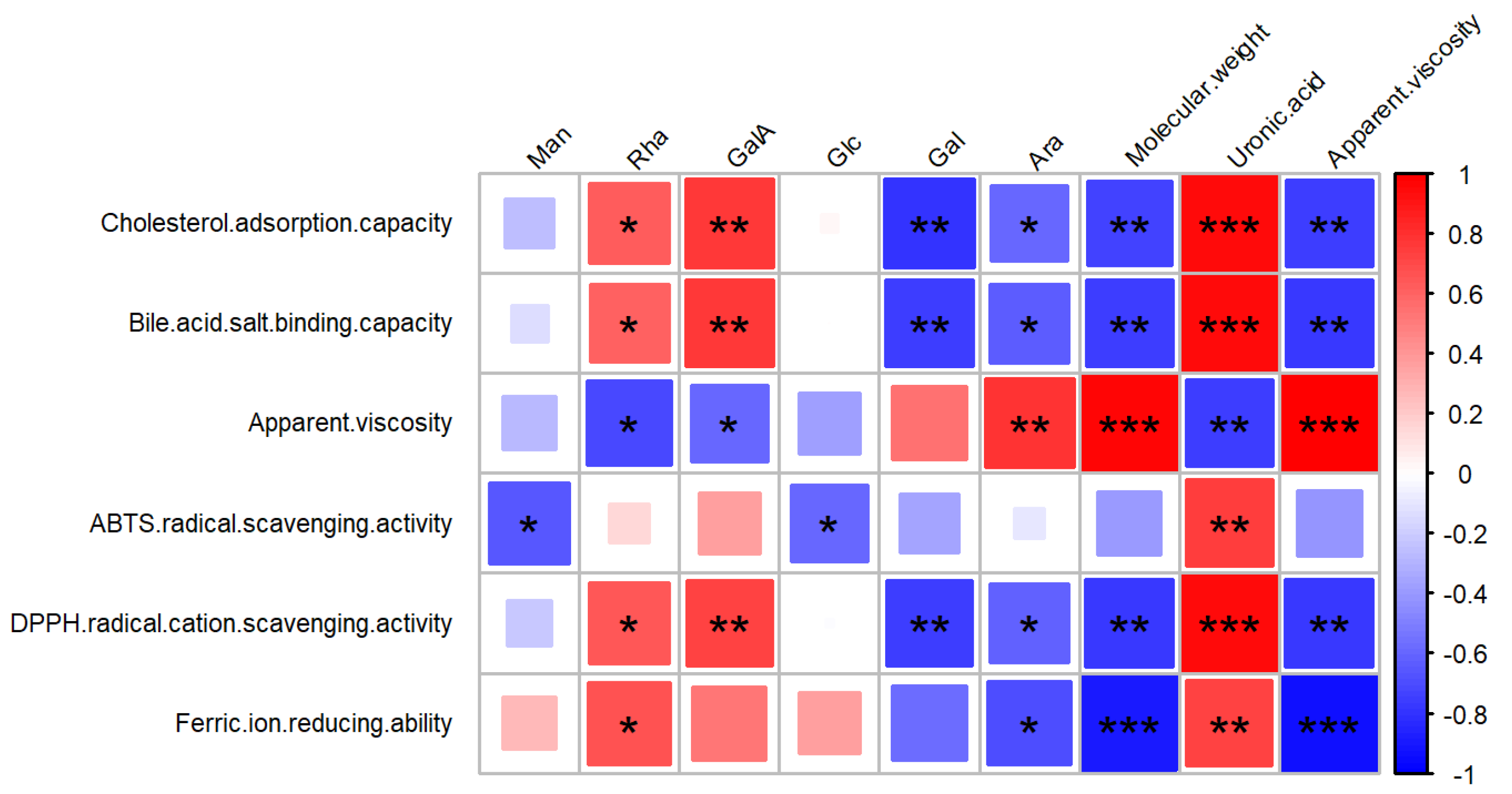
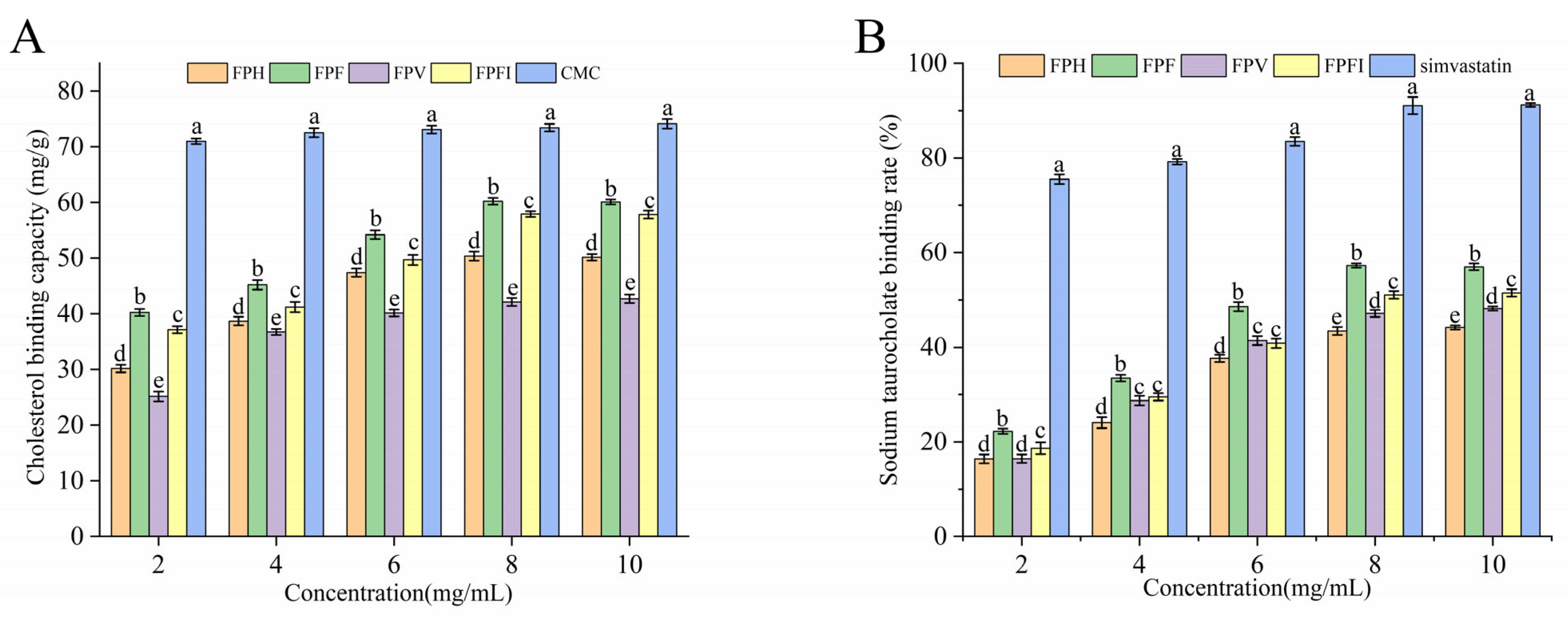
3.8. Antioxidant Activity of FPs
3.9. Sodium Taurocholate Binding Capacity In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teruel-Andreu, C.; Issa-Issa, H.; Noguera-Artiaga, L.; Sendra, E.; Hernández, F.; Cano-Lamadrid, M. Volatile profile of breba and fig fruits (peel and pulp) from different Ficus carica L. varieties. Sci. Hortic. 2024, 328, 112892. [Google Scholar] [CrossRef]
- Lansky, E.P.; Paavilainen, H.M.; Pawlus, A.D.; Newman, R.A. Ficus spp. (fig): Ethnobotany and potential as anticancer and anti-inflammatory agents. J. Ethnopharmacol. 2008, 119, 195–213. [Google Scholar] [CrossRef]
- Ayuso, M.; Carpena, M.; Taofiq, O.; Albuquerque, T.G.; Simal-Gandara, J.; Oliveira, M.B.P.P.; Prieto, M.A.; Ferreira, I.C.F.R.; Barros, L. Fig “Ficus carica L.” and its by-products: A decade evidence of their health-promoting benefits towards the development of novel food formulations. Trends Food Sci. Technol. 2022, 127, 1–13. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant Activities and Anthocyanin Content of Fresh Fruits of Common Fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Thangavel, P.; Saravanakumar, I.; Sundaram, M.K.; Rathinam, B.; Muthuvijayan, V. Preparation and characterization of a jelly fig (Ficus awkeotsang Makino) polysaccharide-based bioactive 3D scaffold for improved vascularization and skin tissue engineering applications. Int. J. Biol. Macromol. 2024, 259, 129199. [Google Scholar] [CrossRef]
- Chen, R.; Li, H.; Li, S.; Jin, C.; Lu, J. Extraction optimization, preliminary characterization and immunological activity of polysaccharides from figs. Int. J. Biol. Macromol. 2015, 72, 185–194. [Google Scholar] [CrossRef]
- Chen, H.-H.; Shyu, Y.-T.; Wu, S.-J. Physicochemical characteristics and retardation effects on in vitro starch digestibility of non-starch polysaccharides in jelly-fig (Ficus pumila L. var. awkeotsang). LWT 2023, 180, 114688. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Guo, Y.; Jiang, Y.; Wen, L.; Yang, B. New insights of fig (Ficus carica L.) as a potential function food. Trends Food Sci. Technol. 2023, 140, 104146. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Ma, X.; Li, X.; Guo, Z.; Liu, Y.; Zheng, S. Carboxylated nanodiamond-mediated NH2-PLGA nanoparticle-encapsulated fig polysaccharides for strongly enhanced immune responses in vitro and in vivo. Int. J. Biol. Macromol. 2020, 165, 1331–1345. [Google Scholar] [CrossRef]
- Wang, C.; Jing, S.; Hou, D.; Zhu, B.; Yang, Y.; Yu, J.; Liu, L.; Bai, J.; Xu, H.; Kou, L. X-rays irradiation maintains redox homeostasis and regulates energy metabolism of fresh figs (Ficus carica L. Siluhongyu). Food Chem. 2024, 438, 138067. [Google Scholar] [CrossRef]
- Çavdaroğlu, E.; Yemenicioğlu, A. Utilization of stalk waste separated during processing of sun-dried figs (Ficus carica) as a source of pectin: Extraction and determination of molecular and functional properties. LWT 2022, 154, 112624. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, L.; Song, G.; Jiang, W.; Mu, L.; Li, J. Ficus carica polysaccharide extraction via ultrasound-assisted technique: Structure characterization, antioxidant, hypoglycemic and immunomodulatory activities. Ultrason. Sonochem. 2023, 101, 106680. [Google Scholar] [CrossRef] [PubMed]
- Panza, O.; Conte, A.; Del Nobile, M.A. Recycling of fig peels to enhance the quality of handmade pasta. LWT 2022, 168, 113872. [Google Scholar] [CrossRef]
- Yuan, Q.; He, Y.; Xiang, P.-Y.; Huang, Y.-J.; Cao, Z.-W.; Shen, S.-W.; Zhao, L.; Zhang, Q.; Qin, W.; Wu, D.-T. Influences of different drying methods on the structural characteristics and multiple bioactivities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2020, 147, 1053–1063. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Zhang, Y.; Ai, Z.; Lei, D.; Pei, Y.; Liu, Y. Effect of different drying techniques on drying characteristics, physical quality, and active components of Citri reticulatae pericarpium, and the correlation between physiochemical quality. Ind. Crops Prod. 2023, 204, 117350. [Google Scholar] [CrossRef]
- An, K.; Wu, J.; Xiao, H.; Hu, T.; Yu, Y.; Yang, W.; Xiao, G.; Xu, Y. Effect of various drying methods on the physicochemical characterizations, antioxidant activities and hypoglycemic activities of lychee (Litchi chinensis Sonn.) pulp polysaccharides. Int. J. Biol. Macromol. 2022, 220, 510–519. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, P.; Qu, Z.; Bai, D.; Gao, X.; Zhao, C.; Chen, J.; Gao, W. Physicochemical characterizations of polysaccharides from Angelica Sinensis Radix under different drying methods for various applications. Int. J. Biol. Macromol. 2019, 121, 381–389. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, physicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, M.; Liu, F.; Feng, X.; Ibrahim, S.A.; Cheng, L.; Huang, W. Effects of freeze drying and hot-air drying on the physicochemical properties and bioactivities of polysaccharides from Lentinula edodes. Int. J. Biol. Macromol. 2020, 145, 476–483. [Google Scholar] [CrossRef]
- Hou, S.; Tan, M.; Chang, S.; Zhu, Y.; Rong, G.; Wei, G.; Zhang, J.; Zhao, B.; Zhao, Q.-S. Effects of different processing (Paozhi) on structural characterization and antioxidant activities of polysaccharides from Cistanche deserticola. Int. J. Biol. Macromol. 2023, 245, 125507. [Google Scholar] [CrossRef]
- Han, L.-C.; Jin, T.-X. Effects of combined vacuum and heat pump drying on drying characteristics and physicochemical properties of pineapple. LWT 2024, 192, 115727. [Google Scholar] [CrossRef]
- Chao, E.; Li, J.; Fan, L. Influence of combined freeze-drying and far-infrared drying technologies on physicochemical properties of seed-used pumpkin. Food Chem. 2023, 398, 133849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, L.; Qiao, Y.; Wang, C.; Shi, D.; An, K.; Hu, J. Effects of ultrahigh pressure and ultrasound pretreatments on properties of strawberry chips prepared by vacuum-freeze drying. Food Chem. 2020, 303, 125386. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, J.; Zhu, J.; Huang, C.; Bi, S.; Song, L.; Hu, X.; Yu, R. Structural characterization and immunomodulatory activity of a novel polysaccharide from Ficus carica. Food Funct. 2018, 9, 3930–3943. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Zhang, H.; Ma, X. Effects of ultrasonic-enzymatic-assisted ethanol precipitation method on the physicochemical characteristics, antioxidant and hypoglycemic activities of Tremella fuciformis polysaccharides. Ultrason. Sonochem. 2023, 101, 106682. [Google Scholar] [CrossRef]
- Tepsongkroh, B.; Thaihuttakij, C.; Supawong, S.; Jangchud, K. Impact of high pressure pre-treatment and hot water extraction on chemical properties of crude polysaccharide extract obtained from mushroom (Volvariella volvacea). Food Chem. X 2023, 19, 100864. [Google Scholar] [CrossRef]
- Zhang, Z.-f.; Lv, G.-y.; Song, T.-t.; Xu, Z.-w.; Wang, M.-y. Effects of different extraction methods on the structural and biological properties of Hericium coralloides polysaccharides. Food Chem. 2024, 445, 138752. [Google Scholar] [CrossRef]
- Qiao, Y.; Shen, Y.; Jiang, H.; Li, D.; Li, B. Structural characterization, antioxidant and antibacterial activity of three pectin polysaccharides from blueberry. Int. J. Biol. Macromol. 2024, 262, 129707. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Wang, C.-W.; Zhang, J.; Xu, P.-P.; Xue, Y.-T.; Wang, Q. Effects of different extraction methods on physicochemical characteristics and bioactivities of fig (Ficus carica L.) leaves polysaccharides. Arab. J. Chem. 2023, 16, 105319. [Google Scholar] [CrossRef]
- Deng, J.; Min, J.; Zhang, Y.; You, R.; Zhang, Z.; Hu, Y.; Chen, X.; Cheng, S.; Ma, X.; Zhang, S. Preparation, characterization and cytotoxicity assessment of a novel selenized polysaccharide from Morchella sextelata. Int. J. Biol. Macromol. 2024, 265, 131100. [Google Scholar] [CrossRef]
- Akbal, A.; Şahin, S.; Güroy, B. Optimization of ultrasonic-assisted extraction of polysaccharides from Ulva rigida and evaluation of their antioxidant activity. Algal Res. 2024, 77, 103356. [Google Scholar] [CrossRef]
- Ayimbila, F.; Siriwong, S.; Chaiyama, V.; Srihanant, N.; Keawsompong, S. Comparative study of bio-functional profile and bioactivities of polysaccharides from Ganoderma lucidum and Ganoderma neo-japonicum. Biocatal. Agric. Biotechnol. 2023, 53, 102875. [Google Scholar] [CrossRef]
- Li, X.-G.; Zhang, F.-Y.; Jiang, C.-X.; Jiang, J.; Hou, Y.-H.; Zhang, J.-W. Structural analysis, in vitro antioxidant and lipid-lowering activities of purified Tremella fuciformis polysaccharide fractions. Process Biochem. 2023, 133, 99–108. [Google Scholar] [CrossRef]
- Ma, Q.; Santhanam, R.K.; Xue, Z.; Guo, Q.; Gao, X.; Chen, H. Effect of different drying methods on the physicochemical properties and antioxidant activities of mulberry leaves polysaccharides. Int. J. Biol. Macromol. 2018, 119, 1137–1143. [Google Scholar] [CrossRef]
- Shang, H.; Cao, Z.; Zhang, H.; Guo, Y.; Zhao, J.; Wu, H. Physicochemical characterization and in vitro biological activities of polysaccharides from alfalfa (Medicago sativa L.) as affected by different drying methods. Process Biochem. 2021, 103, 39–49. [Google Scholar] [CrossRef]
- Xiang, G.; Sun, H.; Chen, Y.; Guo, H.; Liu, Y.; Li, Y.; Lu, C.; Wang, X. Antioxidant and hypoglycemic activity of tea polysaccharides with different degrees of fermentation. Int. J. Biol. Macromol. 2023, 228, 224–233. [Google Scholar] [CrossRef]
- Chen, G.-J.; Hong, Q.-Y.; Ji, N.; Wu, W.-N.; Ma, L.-Z. Influences of different drying methods on the structural characteristics and prebiotic activity of polysaccharides from bamboo shoot (Chimonobambusa quadrangularis) residues. Int. J. Biol. Macromol. 2020, 155, 674–684. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Zhu, W.; Wang, Z. Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Res. Int. 2013, 50, 633–640. [Google Scholar] [CrossRef]
- Fu, Y.; Feng, K.-L.; Wei, S.-Y.; Xiang, X.-R.; Ding, Y.; Li, H.-Y.; Zhao, L.; Qin, W.; Gan, R.-Y.; Wu, D.-T. Comparison of structural characteristics and bioactivities of polysaccharides from loquat leaves prepared by different drying techniques. Int. J. Biol. Macromol. 2020, 145, 611–619. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wu, L.-X.; Qiao, Z.-R.; Cai, W.-D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef]
- Wu, B.; Qiu, C.; Guo, Y.; Zhang, C.; Li, D.; Gao, K.; Ma, Y.; Ma, H. Comparative Evaluation of Physicochemical Properties, Microstructure, and Antioxidant Activity of Jujube Polysaccharides Subjected to Hot Air, Infrared, Radio Frequency, and Freeze Drying. Agriculture 2022, 12, 1606. [Google Scholar] [CrossRef]
- Jiang, F.; Sheng, Y.; Wang, F.; Pan, H.; Chen, W.; Kong, F. Characterization and biological activity of acidic sugarcane leaf polysaccharides by microwave-assisted hot alkali extraction. Food Biosci. 2023, 54, 102852. [Google Scholar] [CrossRef]
- Fimbres-Olivarria, D.; Carvajal-Millan, E.; Lopez-Elias, J.A.; Martinez-Robinson, K.G.; Miranda-Baeza, A.; Martinez-Cordova, L.R.; Enriquez-Ocaña, F.; Valdez-Holguin, J.E. Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula sp. Food Hydrocoll. 2018, 75, 229–236. [Google Scholar] [CrossRef]
- Ghosh, S.; Abdullah, M.F. Extraction of polysaccharide fraction from cadamba (Neolamarckia cadamba) fruits and evaluation of its in vitro and in vivo antioxidant activities. Int. J. Biol. Macromol. 2024, 279, 135564. [Google Scholar] [CrossRef]
- Lo, T.C.-T.; Chang, C.A.; Chiu, K.-H.; Tsay, P.-K.; Jen, J.-F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. A comparison study on polysaccharides extracted from Laminaria japonica using different methods: Structural characterization and bile acid-binding capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [CrossRef]
- Ma, Y.; Liao, Z.; Kang, X.; Li, C.; Hu, G. Physicochemical properties, structural characterization, and in vitro binding capacity of polysaccharide fractions from the fruiting bodies of Lentinula edodes. J. Food Meas. Charact. 2024, 18, 6858–6871. [Google Scholar] [CrossRef]






| Item | FPF | FPV | FPFI | FPH |
|---|---|---|---|---|
| Extraction yields (%) | 2.49 ± 0.16 a | 1.66 ± 0.13 c | 1.94 ± 0.12 b | 1.57 ± 0.21 c |
| Neutral sugar (%) | 60.04 ± 0.80 b | 62.84 ± 0.93 a | 58.09 ± 0.94 c | 63.29 ± 0.97 a |
| Uronic acid (%) | 25.56 ± 0.54 a | 14.34 ± 0.49 c | 19.84 ± 0.29 b | 13.51 ± 0.34 c |
| Protein (%) | 0.96 ± 0.18 b | 0.98 ± 0.10 b | 1.13 ± 0.07 b | 1.33 ± 0.04 a |
| Monosaccharides Composition (Molar Ratio, %) | FPF | FPV | FPFI | FPH |
|---|---|---|---|---|
| Mannose | 2.84 ± 0.17 c | 2.87 ± 0.21 c | 4.03 ± 0.12 a | 3.29 ± 0.18 b |
| Rhamnose | 8.35 ± 0.11 a | 6.86 ± 0.03 c | 8.38 ± 0.09 a | 7.64 ± 0.14 b |
| Glucuronic acid | nd | nd | nd | nd |
| Galacturonic acid | 24.35 ± 0.14 a | 11.32 ± 0.12 d | 18.69 ± 0.15 b | 16.26 ± 0.21 c |
| Glucose | 9.55 ± 0.13 c | 8.55 ± 0.15 d | 13.46 ± 0.18 a | 12.44 ± 0.09 b |
| Galactose | 17.64 ± 0.05 d | 21.30 ± 0.12 a | 18.37 ± 0.06 c | 20.47 ± 0.11 b |
| Xylose | nd | nd | nd | nd |
| Arabinose | 37.26 ± 0.15 c | 49.10 ± 0.07 a | 37.07 ± 0.18 c | 39.89 ± 0.17 b |
| Molecular Weight (Da) | FPF | FPV | FPFI | FPH |
|---|---|---|---|---|
| Number average molecular weight (Mn) | 8.572 × 104 (±0.959%) c | 1.088 × 105 (±1.982%) b | 5.047 × 104 (±1.145%) d | 1.124 × 105 (±1.245%) a |
| Peak molecular weight (Mp) | 4.240 × 104 (±1.036%) b | 6.484 × 104 (±0.805%) a | 4.711 × 104 (±1.621%) b | 6.792 × 104 (±1.005%) a |
| Weight average molecular weight (Mw) | 1.457 × 105 (±1.456%) c | 1.664 × 105 (±1.749%) b | 8.473 × 104 (±2.034%) d | 1.798 × 105 (±1.469%) a |
| Mw/Mn | 1.700 ± 0.01 a | 1.530 ± 0.02 b | 1.679 ± 0.02 a | 1.599 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Wu, J.; Yang, M.; Liang, J.; Sun, L.; Jia, M.; Sun, R. Effects of Different Drying Methods on Structural Characterization, Rheological Properties, Antioxidant and Hypolipidemic Activities of Polysaccharides from Fig (Ficus carica L.). Appl. Sci. 2025, 15, 4215. https://doi.org/10.3390/app15084215
Zhao G, Wu J, Yang M, Liang J, Sun L, Jia M, Sun R. Effects of Different Drying Methods on Structural Characterization, Rheological Properties, Antioxidant and Hypolipidemic Activities of Polysaccharides from Fig (Ficus carica L.). Applied Sciences. 2025; 15(8):4215. https://doi.org/10.3390/app15084215
Chicago/Turabian StyleZhao, Guojian, Jingya Wu, Mingguan Yang, Jing Liang, Lei Sun, Ming Jia, and Rui Sun. 2025. "Effects of Different Drying Methods on Structural Characterization, Rheological Properties, Antioxidant and Hypolipidemic Activities of Polysaccharides from Fig (Ficus carica L.)" Applied Sciences 15, no. 8: 4215. https://doi.org/10.3390/app15084215
APA StyleZhao, G., Wu, J., Yang, M., Liang, J., Sun, L., Jia, M., & Sun, R. (2025). Effects of Different Drying Methods on Structural Characterization, Rheological Properties, Antioxidant and Hypolipidemic Activities of Polysaccharides from Fig (Ficus carica L.). Applied Sciences, 15(8), 4215. https://doi.org/10.3390/app15084215





