Elemental and Nutritional Characterisation with Vibrational Spectroscopy Analysis of Ulva sp., Gracilaria multipartita, and Sargassum muticum
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Layout
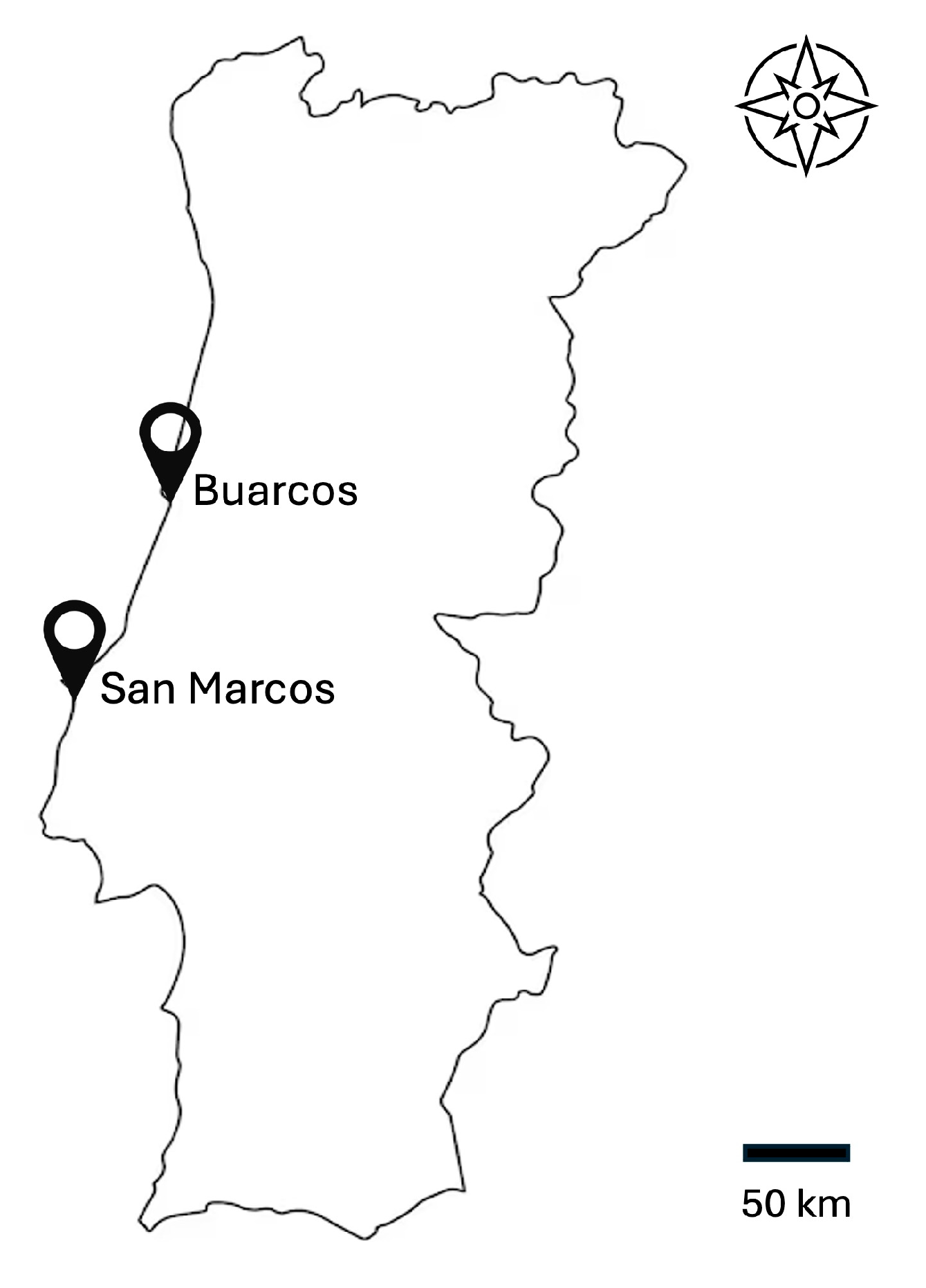
2.1.1. Sampling Collection
2.1.2. Sample Preparation
2.2. Chemical Analysis
2.2.1. Determination of Moisture and Dry Matter Content
2.2.2. Determination of Ash and Organic Matter Content
2.2.3. Determination of Crude Fat Content
2.2.4. Determination of Crude Fibre Content
2.2.5. Determination of the Total Nitrogen and Crude Protein Content
2.2.6. Determination of Carbohydrates
2.2.7. Determination of Energy
2.3. Mineral Analysis
2.3.1. Samples’ Digestion
2.3.2. Determination of Mineral Content Through Flame Atomic Absorption Spectrometry
2.4. Vibrational Spectroscopic Techniques
2.4.1. FT-NIR Spectroscopy
2.4.2. FTIR-ATR Spectroscopy
2.4.3. FT-Raman Spectroscopy
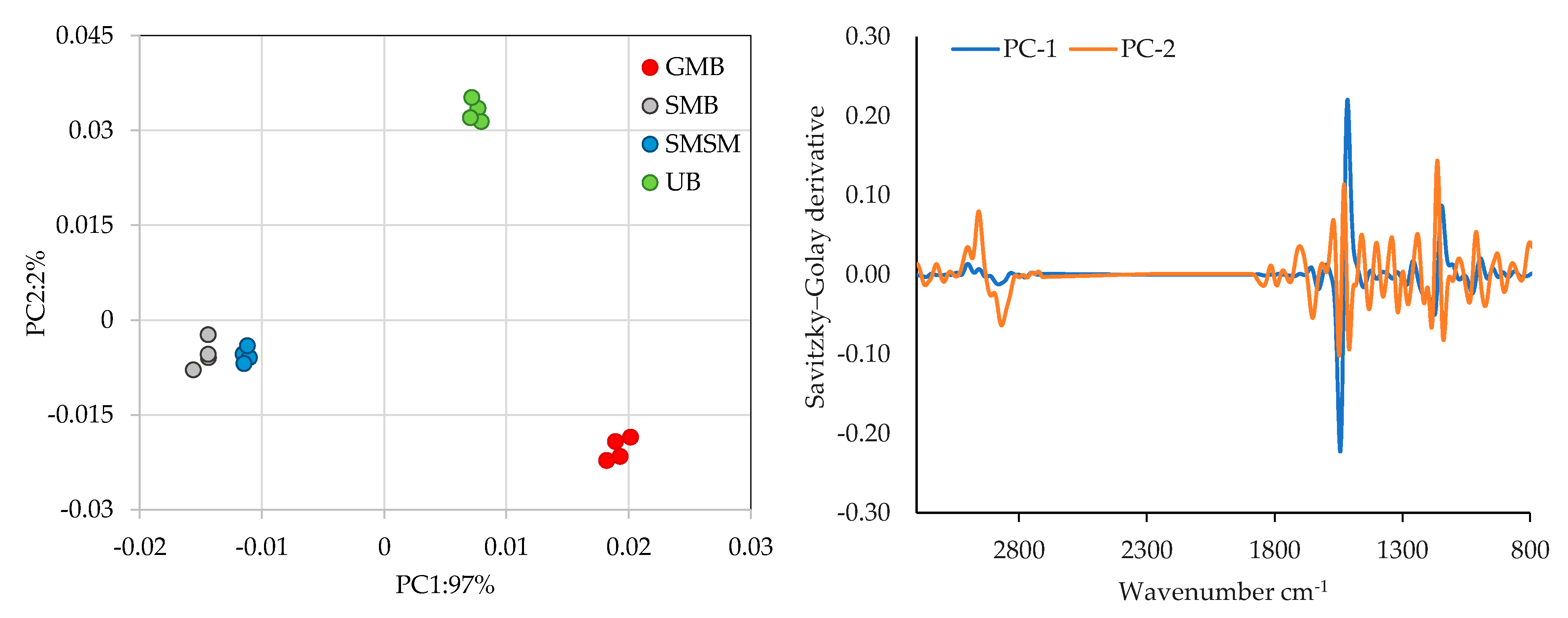
2.5. Multivariate Analysis
3. Results and Discussion
3.1. Nutritional Analysis
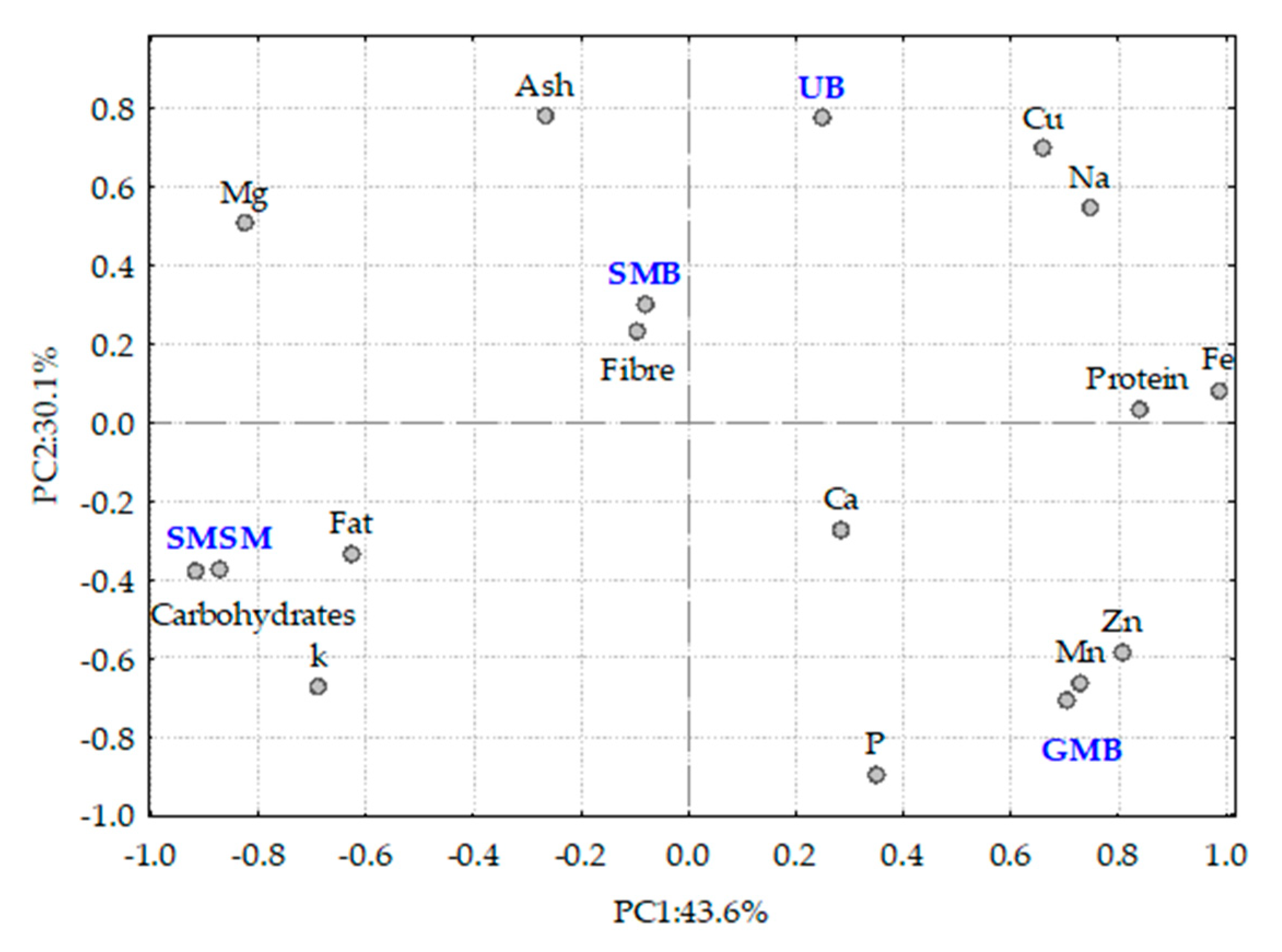
3.2. Data Analysis
3.3. Label Analysis
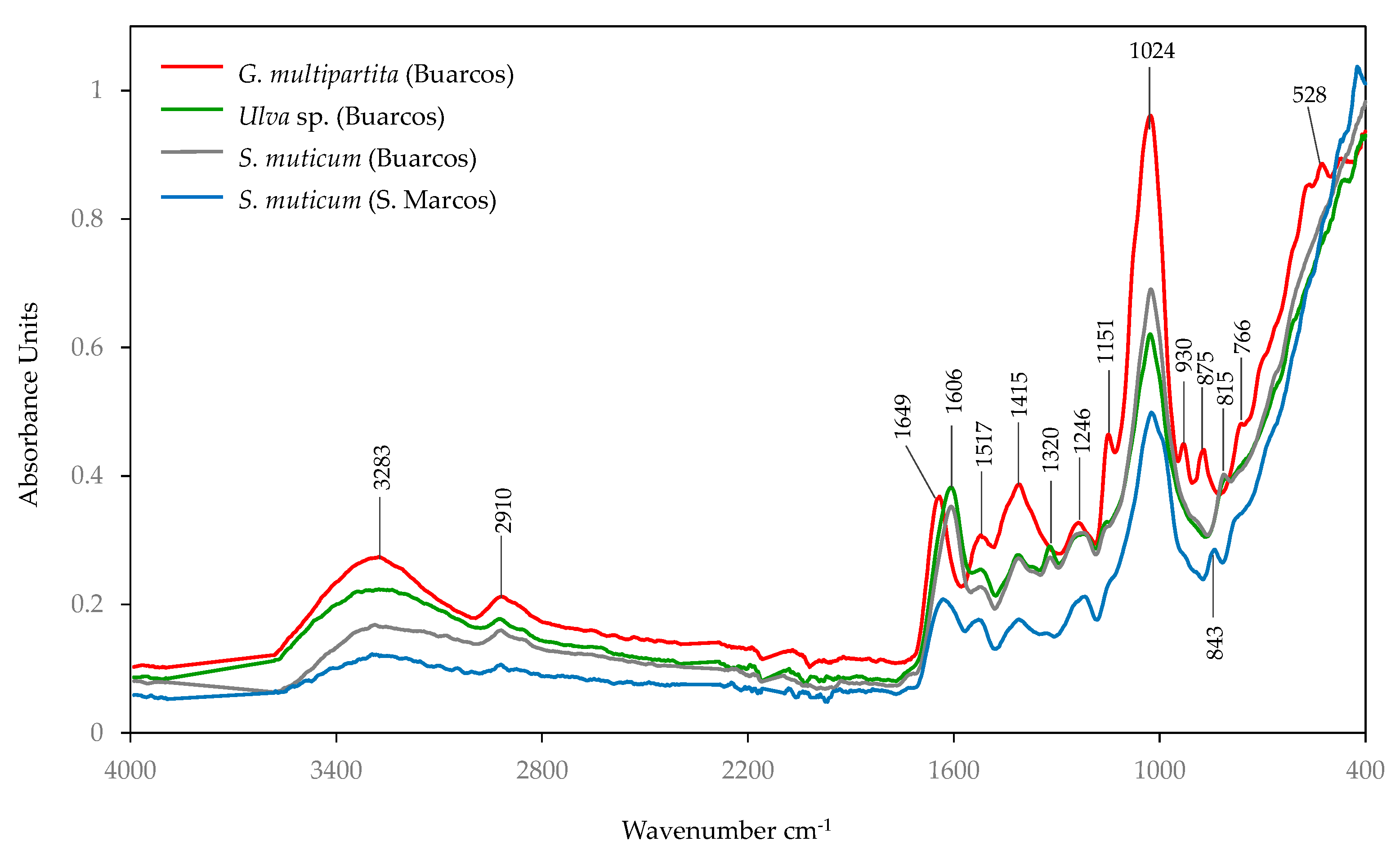
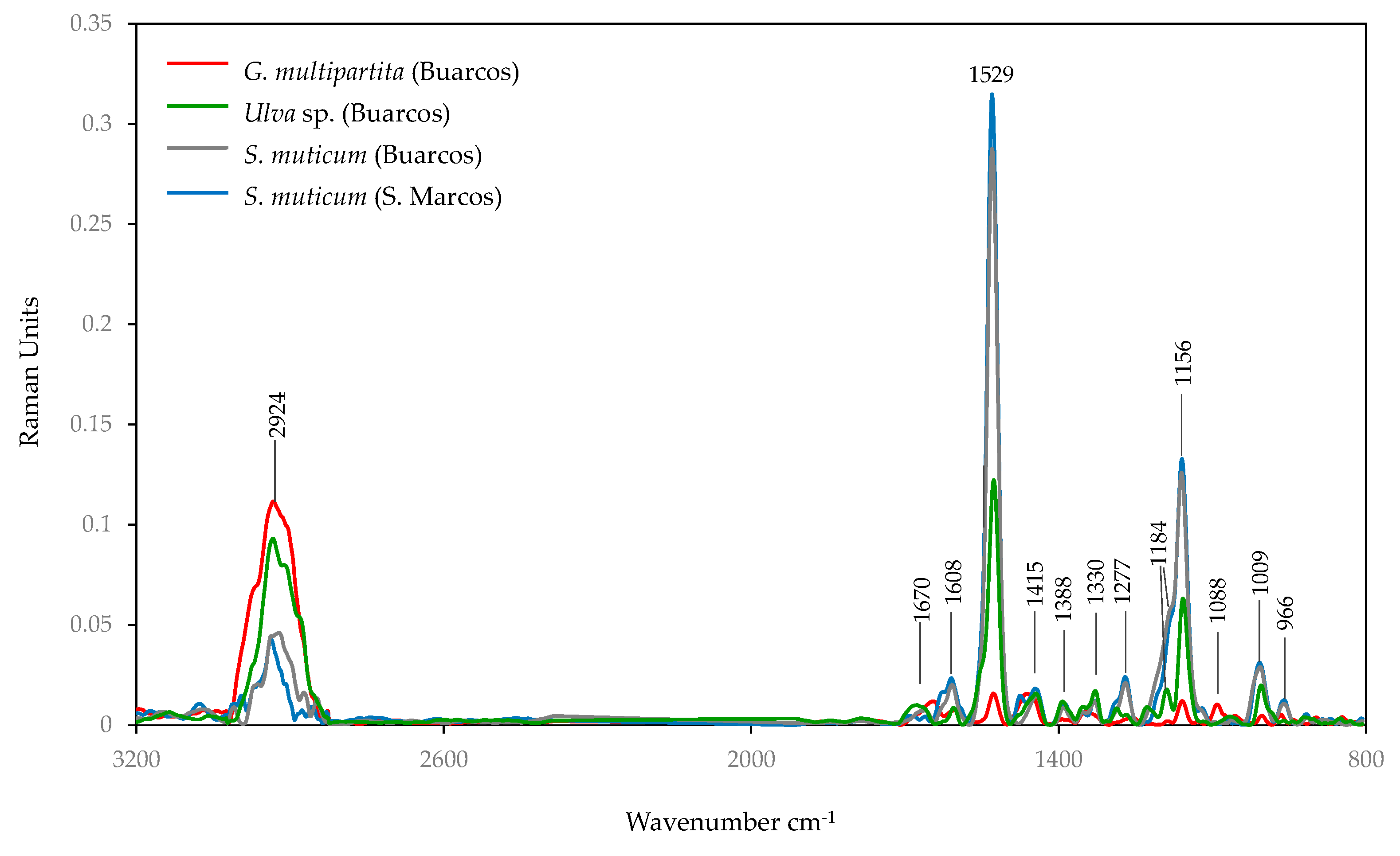
3.4. Spectroscopy Analysis
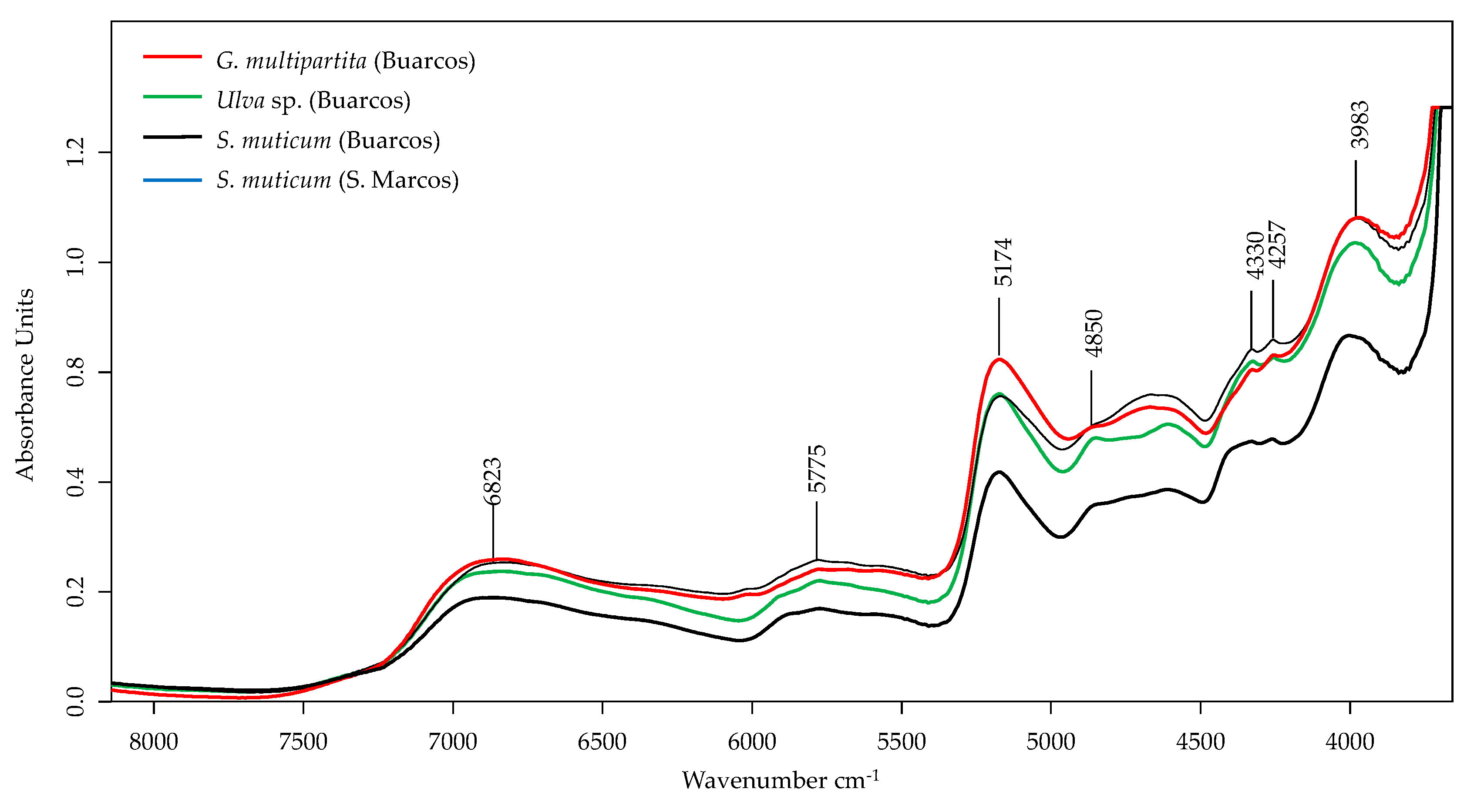
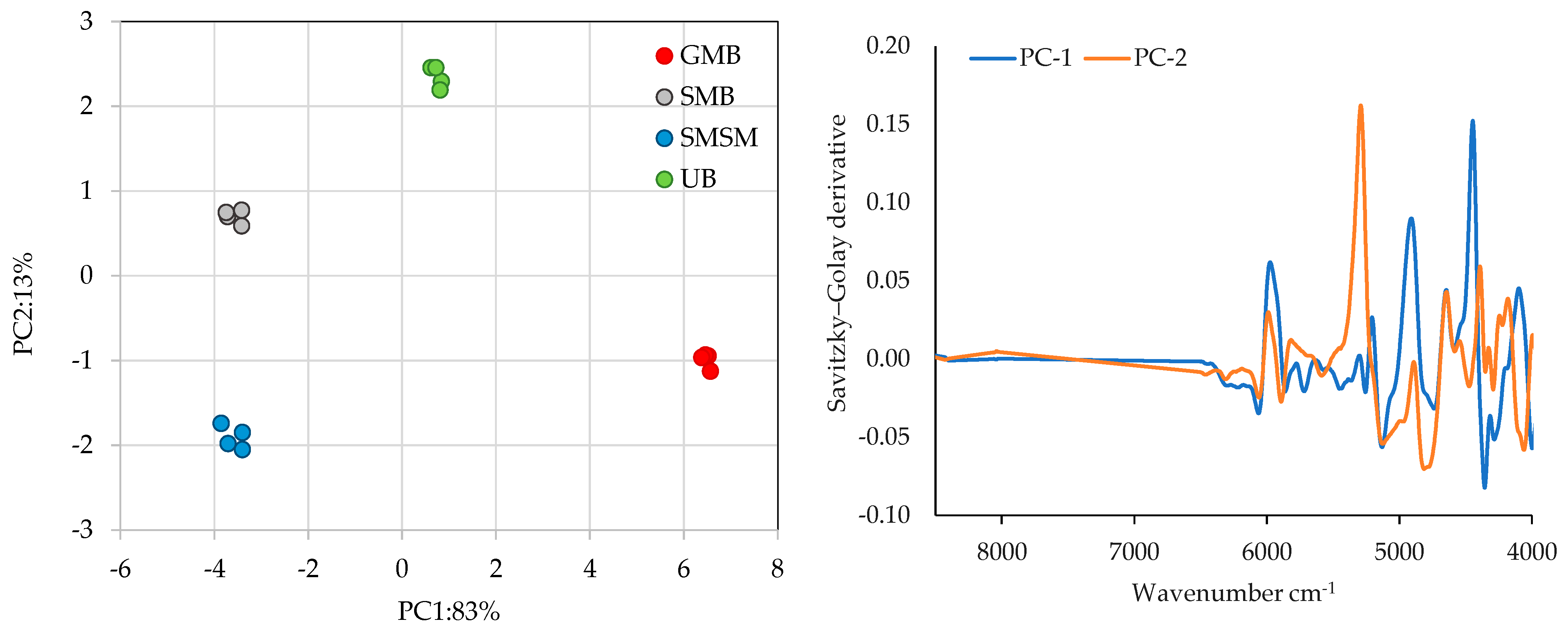
3.4.1. FT-NIR
3.4.2. FTIR-ATR
3.4.3. FT-RAMAN
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akila, N.; Jeyadoss, T. The Potential of Seaweed Liquid Fertilizer on the Growth and Antioxidant Enhancement of Helianthus annuus L. Orient. J. Chem. 2010, 26, 1353. [Google Scholar]
- Notowidjojo, L. Seaweed as Novel Food for Prevention and Therapy for Life Style Related Disease. World Nutr. J. 2021, 5, 1–5. [Google Scholar] [CrossRef]
- Pereira, L. Guia Ilustrado Das Macroalgas: Conhecer e Reconhecer Algumas Espécies Da Flora Portuguesa; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2009. [Google Scholar]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The New Tree of Eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Baptista, T.; Neves, M.; Mouga, T. Seasonal Changes in the Nutritional Composition of Agarophyton vermiculophyllum (Rhodophyta, Gracilariales) from the Center of Portugal. Foods 2021, 10, 1145. [Google Scholar] [CrossRef]
- Queirós, A.S.; Circuncisão, A.R.; Pereira, E.; Válega, M.; Abreu, M.H.; Silva, A.M.S.; Cardoso, S.M. Valuable Nutrients from Ulva rigida: Modulation by Seasonal and Cultivation Factors. Appl. Sci. 2021, 11, 6137. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper 441; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Dixit, D.C.; Reddy, C.R.K.; Balar, N.; Suthar, P.; Gajaria, T.; Gadhavi, D.K. Assessment of the Nutritive, Biochemical, Antioxidant and Antibacterial Potential of Eight Tropical Macro Algae Along Kachchh Coast, India as Human Food Supplements. J. Aquat. Food Prod. Technol. 2018, 27, 61–79. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Farag, M.A.; Abib, B.; Qin, Z.; Ze, X.; Ali, S.E. Dietary Macrominerals: Updated Review of Their Role and Orchestration in Human Nutrition throughout the Life Cycle with Sex Differences. Curr. Res. Food Sci. 2023, 6, 100450. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication. 2024. Available online: https://www.algaebase.org/ (accessed on 22 January 2025).
- Paopun, Y.; Thanomchat, P.; Roopkham, C.; Umroong, P.; Pan-Utai, W.; Satmalee, P.; Kosawatpat, P.; Thongdang, B.; Tamtin, M. Structural Development of Marine Green Alga (Ulva rigida C. Agardh, 1823) during Cultivation. Trends Sci. 2023, 20, 6747. [Google Scholar] [CrossRef]
- Womersley, H.B.S. The Marine Benthic Flora of Southern Australia Part I. In Flora of Australia Supplementary Series; State Herbarium South Australia: Adelaide, Australia, 1984; p. 533. ISBN 064256826X. [Google Scholar]
- Freitas, M.V.; Inácio, L.G.; Martins, M.; Afonso, C.; Pereira, L.; Mouga, T. Primary Composition and Pigments of 11 Red Seaweed Species from the Center of Portugal. J. Mar. Sci. Eng. 2022, 10, 1168. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Samaraweera, A.M.; Vidanarachchi, J.K.; Kurukulasuriya, M.S. Industrial Applications of Macroalgae. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- World Bank Group. Seaweed Aquaculture for Food Security, Income Generation and Environmental Health in Tropical Developing Countries; World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Rodríguez-Prieto, C.; Freshwater, D.W.; Hommersand, M.H. Gracilaria corallicola and G. multipartita (Gracilariales, Rhodophyta), Two Related Flattened European Species. Eur. J. Phycol. 2016, 51, 444–460. [Google Scholar] [CrossRef]
- Mouradi, A.; Chikhaoui, M.; Fekhaou, M.; Akallal, R.; Guessous, A.; Givernaud, T. Variabilité Interspécifique de Trois Algues Rouges: Hypnea Musciformis, Gracilaria multipartita et Gelidium sesquipedale (Rhodophycées) de La Côte Atlantique Marocaine. Afr. Sci. Rev. Int. Des. Sci. Technol. 2010, 2, 365–389. [Google Scholar] [CrossRef]
- Givernaud, T.; El Gourji, A.; Mouradi-Givernaud, A.; Lemoine, Y.; Chiadmi, N.; Givernaud, T.; El Gourji, A.; Mouradi-Givernaud, A.; Lemoine, Y.; Chiadmi, N. Seasonal Variations of Growth and Agar Composition of Harvested along the Atlantic Coast of Morocco. HyBio 1999, 398–399, 167–172. [Google Scholar] [CrossRef]
- Yende, S.; Harle, U.; Chaugule, B. Therapeutic Potential and Health Benefits of Sargassum species. Pharmacogn. Rev. 2014, 8, 1. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global Seaweed Farming and Processing in the Past 20 Years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-Value Products from Macroalgae: The Potential Uses of the Invasive Brown Seaweed, Sargassum muticum. Rev. Environ. Sci. Biotechnol. 2016, 15, 67–88. [Google Scholar] [CrossRef]
- Englen, A.H.; Serebryakova, A.; Ang, P.; Simmons, K.B. Climate-Driven Trophic Cascades Affecting Seabirds around the British Isles. In Oceanography and Marine Biology; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Blanco, A.; Larrinaga, A.R.; Neto, J.M.; Troncoso, J.; Méndez, G.; Domínguez-Lapido, P.; Ovejero, A.; Pereira, L.; Mouga, T.M.; Gaspar, R.; et al. Spotting Intruders: Species Distribution Models for Managing Invasive Intertidal Macroalgae. J. Environ. Manag. 2021, 281, 111861. [Google Scholar] [CrossRef]
- Shefer, S.; Israel, A.; Golberg, A.; Chudnovsky, A. Carbohydrate-Based Phenotyping of the Green Macroalga Ulva fasciata Using near-Infrared Spectrometry: Potential Implications for Marine Biorefinery. Bot. Mar. 2017, 60, 219–228. [Google Scholar] [CrossRef]
- Power, A.; Chapman, J.; Hoffman, L.; Cozzolino, D. Shining Light on Seaweed-the Utilization of Vibrational Spectroscopy and Machine Learning in the Seaweed Industry. Int. J. Food Sci. Technol. 2025, 60, vvaf012. [Google Scholar] [CrossRef]
- Ptak, S.H.; Sanchez, L.; Fretté, X.; Kurouski, D. Complementarity of Raman and Infrared Spectroscopy for Rapid Characterization of Fucoidan Extracts. Plant Methods 2021, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid Determination of Bulk Microalgal Biochemical Composition by Fourier-Transform Infrared Spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef]
- Cebi, N.; Bekiroglu, H.; Erarslan, A. Nondestructive Metabolomic Fingerprinting: FTIR, NIR and Raman Spectroscopy in Food Screening. Molecules 2023, 28, 7933. [Google Scholar] [CrossRef]
- Vandanjon, L.; Burlot, A.-S.; Zamanileha, E.F.; Douzenel, P.; Ravelonandro, P.H.; Bourgougnon, N.; Bedoux, G. The Use of FTIR Spectroscopy as a Tool for the Seasonal Variation Analysis and for the Quality Control of Polysaccharides from Seaweeds. Mar. Drugs 2023, 21, 482. [Google Scholar] [CrossRef]
- Domenighini, A.; Giordano, M. Fourier Transform Infrared Spectroscopy of Microalgae as a Novel Tool for Biodiversity Studies, Species Identification, and the Assessment of Water Quality. J. Phycol. 2009, 45, 522–531. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Wolfrum, E.J. Feasibility of Spectroscopic Characterization of Algal Lipids: Chemometric Correlation of NIR and FTIR Spectra with Exogenous Lipids in Algal Biomass. BioEnergy Res. 2010, 4, 22–35. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 20th ed.; Association Official Analytical: Gaithersburg, MA, USA, 2016. [Google Scholar]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical Composition of Red, Brown and Green Macroalgae from Buarcos Bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The Protein Content of Seaweeds: A Universal Nitrogen-to-Protein Conversion Factor of Five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- McLoughlin, C.; McKie, V.A.; McCleary, B. V Validation of the Test Method—Determination of Available Carbohydrates in Cereal and Cereal Products, Dairy Products, Vegetables, Fruit, and Related Food Products and Animal Feeds: Collaborative Study, Final Action 2020.07. J. AOAC Int. 2023, 106, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Velayudhan, D.E.; Kim, I.H.; Nyachoti, C.M. Characterization of Dietary Energy in Swine Feed and Feed Ingredients: A Review of Recent Research Results. Asian-Australas. J. Anim. Sci. 2015, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shurson, G.C.; Hung, Y.T.; Jang, J.C.; Urriola, P.E. Measures Matter—Determining the True Nutri-Physiological Value of Feed Ingredients for Swine. Animals 2021, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council on the provision of food information to consumers, of 25 October 2011. Of. J. Eur. Union 2011, 304, 16–83.
- Pedro, S.I.; Fernandes, T.A.; Luís, Â.; Antunes, A.M.M.; Gonçalves, J.C.; Gominho, J.; Gallardo, E.; Anjos, O. First Chemical Profile Analysis of Acacia Pods. Plants 2023, 12, 3486. [Google Scholar] [CrossRef]
- Taboada, C.; Millán, R.; Míguez, I. Composition, Nutritional Aspects and Effect on Serum Parameters of Marine Algae Ulva rigida. J. Sci. Food Agric. 2010, 90, 445–449. [Google Scholar] [CrossRef]
- Trigui, M.; Gasmi, L.; Zouari, I.; Tounsi, S. Seasonal Variation in Phenolic Composition, Antibacterial and Antioxidant Activities of Ulva rigida (Chlorophyta) and Assessment of Antiacetylcholinesterase Potential. J. Appl. Phycol. 2013, 25, 319–328. [Google Scholar] [CrossRef]
- Freitas, M.V.; Mouga, T.; Correia, A.P.; Afonso, C.; Baptista, T. New Insights on the Sporulation, Germination, and Nutritional Profile of Gracilaria gracilis (Rhodophyta) Grown under Controlled Conditions. J. Mar. Sci. Eng. 2021, 9, 562. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Nutritional and Functional Bioactivity Value of Selected Azorean Macroalgae: Ulva Compressa, Ulva rigida, Gelidium microdon, and Pterocladiella capillacea. J. Food Sci. 2017, 82, 1757–1764. [Google Scholar] [CrossRef]
- Soares, C.; Sousa, S.; Machado, S.; Vieira, E.; Carvalho, A.P.; Ramalhosa, M.J.; Morais, S.; Correia, M.; Oliva-Teles, T.; Domingues, V.F.; et al. Bioactive Lipids of Seaweeds from the Portuguese North Coast: Health Benefits versus Potential Contamination. Foods 2021, 10, 1366. [Google Scholar] [CrossRef]
- Kumari, P.; Bijo, A.J.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Fatty Acid Profiling of Tropical Marine Macroalgae: An Analysis from Chemotaxonomic and Nutritional Perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Health-Promoting Ingredients from Four Selected Azorean Macroalgae. Food Res. Int. 2016, 89, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.S. Development of Extraction and Quantification Technologies for the Main Nutritional Components of Macroalgae from the Azores Coast with a View to Their Utilisation as Food Supplements; Universidade dos Açores: Açores, Portugal, 2014. [Google Scholar]
- Milinovic, J. Nutritional Benefits of Edible Macroalgae from the Central Portuguese Coast: Inclusion of Low-Calorie ‘Sea Vegetables’ in Human Diet. Int. J. Environ. Sci. Nat. Resour. 2021, 28, 556250. [Google Scholar] [CrossRef]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional Value of Selected Macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef]
- Salman, A.; Ferreira, A.; Soares, J.; Souza, J. Methodologies for Evaluating Feed for Domestic Ruminants, 1st ed.; Embrapa: Porto Velho, Brazil, 2010. [Google Scholar]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction and Characterization of Protein from Irish Brown Seaweed Ascophyllum nodosum. Food Res. Int. 2017, 99, 1021–1027. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese Coast as a Source of Proteinaceous Material: Total and Free Amino Acid Composition Profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Zhou, A.Y.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Changes in Total Nitrogen and Amino Acid Composition of New Zealand Undaria Pinnatifida with Growth, Location and Plant Parts. Food Chem. 2015, 186, 319–325. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability: Food and Non-Food Applications; Academic Press Inc.: Cambridge, MA, USA, 2015. [Google Scholar]
- Mišurcová, L.; Machů, L.; Orsavová, J. Seaweed Minerals as Nutraceuticals. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 64. [Google Scholar]
- World Health Organization. Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Circuncisão, A.R.S. Analysing the Nutritional Profile of Ulva rigida Cultivated under Different Conditions in an Integrated Multi-Trophic Aquaculture System; University of Aveiro: Aveiro, Portugal, 2017. [Google Scholar]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Lobeer, A.; Zhang, W.; Irfath, M.; Su, P.; Edirisinghe, N.; Amaratunga, G. Comparative Analysis of Sugar and Mineral Content of Sargassum Spp. Collected from Different Coasts of Sri Lanka. J. Appl. Phycol. 2019, 31, 2643–2651. [Google Scholar] [CrossRef]
- Gamero-Vega, G.; Palacios-Palacios, M.; Quitral, V. Nutritional Composition and Bioactive Compounds of Red Seaweed: A Mini-Review. J. Food Nutr. Res. 2020, 8, 431–440. [Google Scholar] [CrossRef]
- Cardoso, S.; Carvalho, L.; Silva, P.; Rodrigues, M.; Pereira, O.; Pereira, L. Bioproducts from Seaweeds: A Review with Special Focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Adluri, R.S.; Zhan, L.; Bagchi, M.; Maulik, N.; Maulik, G. Comparative Effects of a Novel Plant-Based Calcium Supplement with Two Common Calcium Salts on Proliferation and Mineralization in Human Osteoblast Cells. Mol. Cell Biochem. 2010, 340, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Xavier, J. Study of Mineral and Nutritional Composition of Some Seaweeds Found along the Coast of Gulf of Mannar, India. Plant Sci. Today 2020, 7, 631–637. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Meynard, A.; López-Cristoffanini, C.; Latorre, N.; Kumar, M. Marine Metal Pollution and Effects on Seaweed Species. In Systems Biology of Marine Ecosystems; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Yates, A.A.; Schlicker, S.A.; Suitor, C.W. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-07279-3. [Google Scholar]
- Baghel, R.S.; Choudhary, B.; Pandey, S.; Pathak, P.K.; Patel, M.K.; Mishra, A. Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals. Foods 2023, 12, 3642. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Hagen Rødde, R.S.; Vårum, K.M.; Larsen, B.A.; Myklestad, S.M. Seasonal and Geographical Variation in the Chemical Composition of the Red Alga Palmaria palmata (L.) Kuntze. Bot. Mar. 2004, 47, 125–133. [Google Scholar] [CrossRef]
- Trigueros, E.; Amaro, F.; de Pinho, P.G.; Oliveira, A.P. Comprehensive Analysis of Dehydrated Edible Macroalgae: Volatile Compounds, Chemical Profiles, Biological Activities, and Cytotoxicity. J. Appl. Phycol. 2025, 37, 597–615. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Alalawy, A.I.; Almutairi, F.M.; Al-Duais, M.A.; Wang, J.; Salama, E.S. Identification and Characterization of Marine Seaweeds for Biocompounds Production. Environ. Technol. Innov. 2021, 24, 101848. [Google Scholar] [CrossRef]
- Campbell, M.; Ortuño, J.; Koidis, A.; Theodoridou, K. The Use of Near-Infrared and Mid-Infrared Spectroscopy to Rapidly Measure the Nutrient Composition and the in Vitro Rumen Dry Matter Digestibility of Brown Seaweeds. Anim. Feed. Sci. Technol. 2022, 285, 115239. [Google Scholar] [CrossRef]
- Manley, M. Near-Infrared Spectroscopy and Hyperspectral Imaging: Non-Destructive Analysis of Biological Materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [PubMed]
- Agelet, L.E.; Hurburgh, C.R. A Tutorial on near Infrared Spectroscopy and Its Calibration. Crit. Rev. Anal. Chem. 2010, 40, 246–260. [Google Scholar] [CrossRef]
- Simpson, M.B. Near-Infrared Spectroscopy for Process Analytical Chemistry: Theory, Technology and Implementation. In Process Analytical Technology; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar]
- Nunes, N.; Rosa, G.P.; Ferraz, S.; Barreto, M.C.; de Carvalho, M.A.A.P. Fatty Acid Composition, TLC Screening, ATR-FTIR Analysis, Anti-Cholinesterase Activity, and in Vitro Cytotoxicity to A549 Tumor Cell Line of Extracts of 3 Macroalgae Collected in Madeira. J. Appl. Phycol. 2020, 32, 759–771. [Google Scholar] [CrossRef]
- Xia, L.; Li, H.; Song, S. Cell Surface Characterization of Some Oleaginous Green Algae. J. Appl. Phycol. 2016, 28, 2323–2332. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR Spectroscopy for Rapid Determination of Lipid Accumulation in Response to Nitrogen Limitation in Freshwater Microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef]
- Cesário, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M.D. Marine Algal Carbohydrates as Carbon Sources for the Production of Biochemicals and Biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of Selected Seaweed Polysaccharides (Phycocolloids) by Vibrational Spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Niemi, C.; Mortensen, A.M.; Rautenberger, R.; Matsson, S.; Gorzsás, A.; Gentili, F.G. Rapid and Accurate Determination of Protein Content in North Atlantic Seaweed by NIR and FTIR Spectroscopies. Food Chem. 2023, 404, 134700. [Google Scholar] [CrossRef]
- Querido, W.; Kandel, S.; Pleshko, N. Applications of Vibrational Spectroscopy for Analysis of Connective Tissues. Molecules 2021, 26, 922. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Mironiuk, M.; Marycz, K. A Comprehensive Analysis of Biosorption of Metal Ions by Macroalgae Using ICP-OES, SEM-EDX and FTIR Techniques. PLoS ONE 2018, 13, e0205590. [Google Scholar] [CrossRef]
- Al-Shikaili, T.Y.; Thomasson, J.A.; Ge, Y.; Brown, L.; Brown, J. FTIR Transmission Spectroscopy for Measurement of Algae Neutral Lipids. Agric. Eng. Int. CIGR J. 2022, 24, 111–118. [Google Scholar]
- Yalçın, S.; Sezer, S.; Apak, R. Characterization and Lead(II), Cadmium(II), Nickel(II) Biosorption of Dried Marine Brown Macro Algae Cystoseira barbata. Environ. Sci. Pollut. Res. 2012, 19, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Adar, F. Carotenoids-Their Resonance Raman Spectra and How They Can Be Helpful in Characterizing a Number of Biological Systems. Spectroscopy 2017, 32, 12–20. [Google Scholar]
- Rodrigues, D.; Costa-Pinto, A.R.; Sousa, S.; Vasconcelos, M.W.; Pintado, M.M.; Pereira, L.; Rocha-Santos, T.A.P.; Da Costa, J.P.; Silva, A.M.S.; Duarte, A.C.; et al. Sargassum Muticum and Osmundea Pinnatifida Enzymatic Extracts: Chemical, Structural, and Cytotoxic Characterization. Mar. Drugs 2019, 17, 209. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of Seaweed Extracts from Laminaria and Ascophyllum nodosum spp. As Biostimulants in Zea mays L. Using a Combination of Chemical, Biochemical and Morphological Approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]


| Nutritional Composition | Taxon | |||
|---|---|---|---|---|
| Ulva sp.
(Buarcos) | G. multipartita (Buarcos) | S. muticum (Buarcos) | S. muticum (San Marcos) | |
| Moisture (g/100 g dw) | 7.58 ± 0.03 a | 6.07 ± 0.30 b | 7.77 ± 0.10 a | 5.56 ± 0.10 c |
| Dry Matter (g/100 g) | 92.42 ± 0.03 c | 93.93 ± 0.30 b | 92.23 ± 0.10 c | 94.44 ± 0.10 a |
| Ash (g/100 g dw) | 33.53 ± 0.09 a | 26.56 ±0.02 d | 28.42 ± 0.06 c | 30.17 ± 0.30 b |
| Organic Matter (g/100 g dw) | 66.47 ± 0.09 c | 73.44 ± 0.02 b | 71.58 ± 0.06 d | 69.83 ± 0.30 a |
| Crude Protein (g/100 g dw) | 18.05 ± 0.10 a | 18.43 ± 0.07 a | 10.25 ± 0.35 b | 10.13 ± 0.07 c |
| Crude Fat (g/100 g dw) | 0.11 ± 0.00 b | 0.14 ± 0.00 ab | 0.19 ± 0.01 a | 0.19 ± 0.05 ab |
| Crude Fibre (g/100 g dw) | 5.83 ± 0.02 b | 6.17 ± 0.35 b | 10.88 ± 0.20 a | 6.24 ± 1.34 b |
| Carbohydrates (g/100 g dw) | 42.48 ± 0.15 c | 48.70 ± 0.41 b | 50.33 ± 0.74 b | 53.28 ± 1.11 a |
| Energy (kcal/100 g dw) | 254.77 ± 0.09 c | 282.15 ± 1.37 a | 265.68 ± 0.07 b | 267.79 ± 2.44 b |
| Energy (kJ/100 g dw) | 1079.72 ± 0.39 c | 1195.89 ± 5.94 a | 1123.51 ± 0.36 c | 1134.81 ± 10.84 b |
| Mineral Composition | Taxon | |||
|---|---|---|---|---|
| Ulva sp. (Buarcos) | G. multipartita (Buarcos) | S. muticum (Buarcos) | S. muticum (San Marcos) | |
| Macronutrients (mg/g dw) | ||||
| Potassium (K) | 7.05 ± 0.53 c | 36.94 ± 1.02 b | 15.04 ± 0.42 c | 86.19 ± 9.06 a |
| Sodium (Na) | 73.80 ± 0.45 a | 64.07 ± 5.96 a | 73.52 ± 6.93 a | 29.36 ± 3.82 b |
| Calcium (Ca) | 8.99 ± 0.56 b | 17.55 ± 0.95 a | 21.22 ± 1.48 a | 11.05 ± 1.33 b |
| Magnesium (Mg) | 15.47 ± 0.96 ab | 7.89 ± 0.21 c | 13.6 ± 0.41 b | 17.21 ± 0.86 a |
| Phosphorus (P) | 1.13 ± 0.00 c | 2.17 ± 0.07 a | 1.07 ± 0.02 c | 1.57 ± 0.09 b |
| Trace elements (μg/g dw) | ||||
| Iron (Fe) | 221.21 ± 6.86 b | 238.61 ± 9.00 b | 279.52 ± 5.65 a | 116.30 ± 6.68 c |
| Zinc (Zn) | 8.37 ± 0.81 bc | 9.63 ± 0.43 b | 28.90 ± 1.10 a | 5.60 ± 0.34 c |
| Manganese (Mn) | 1.04 ± 0.16 c | 8.68 ± 0.96 b | 71.06 ± 1.08 a | 3.69 ± 0.14 c |
| Copper (Cu) | 20.76 ± 1.39 ab | 22.28 ± 0.78 a | 20.20 ± 0.17 ab | 17.86 ± 0.19 b |
| Na+/K+ ratio | 10.50 | 1.74 | 4.90 | 0.34 |
| Nutritional Composition | Adult Daily Reference Value | taxa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ulva sp. (Buarcos) | G. multipartita (Buarcos) | S. muticum (Buarcos) | S. muticum (San Marcos) | ||||||
| Nutrient/8 g | %RDI | Nutrient/8 g | %RDI | Nutrient/8 g | %RDI | Nutrient/8 g | %RDI | ||
| Energy (kcal) | 2000 | 20.38 | 1.02 | 21.24 | 1.13 | 21.24 | 1.06 | 21.42 | 1.07 |
| Energy (kJ) | 8400 | 86.37 | 1.02 | 89.81 | 1.13 | 89.81 | 1.06 | 90.78 | 1.07 |
| Total Lipids (g) | 70 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| Carbohydrates (g) | 260 | 3.40 | 1.31 | 3.89 | 1.50 | 4.02 | 1.55 | 3.74 | 1.44 |
| Sugars (g) | 90 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Proteins (g) | 50 | 1.44 | 2.88 | 1.47 | 2.94 | 0.82 | 1.64 | 0.81 | 1.62 |
| Fibre (g) > | 25 | 0.47 | 1.88 | 0.49 | 1.96 | 0.87 | 3.48 | 0.50 | 2.00 |
| Salt (g) < | * 6 | 1.48 | 0.25 | 1.28 | 0.20 | 1.47 | 0.25 | 0.59 | 0.10 |
| Minerals | Adult daily Reference Value | mg/8 g | %RDI | mg/8 g | %RDI | mg/8 g | %RDI | mg/8 g | %RDI |
| Ca (mg) | 800 | 71.90 | 9.00 | 140.40 | 17.50 | 169.70 | 21.20 | 88.40 | 11.10 |
| P (mg) | 700 | 9.02 | 1.30 | 17.40 | 2.50 | 8.50 | 1.20 | 12.56 | 1.80 |
| K (mg) | 3500 | 56.40 | 1.60 | 295.50 | 8.40 | 120.30 | 3.40 | 689.48 | 19.70 |
| Mg (mg) | 375 | 123.70 | 33.00 | 62.82 | 16.80 | 109.00 | 29.10 | 137.70 | 36.70 |
| Fe (mg) | 14 | 1.90 | 13.60 | 2.24 | 16.00 | 1.80 | 12.60 | 0.93 | 6.60 |
| Na (mg) | 2000 | 590.40 | 29.50 | 512.50 | 25.60 | 588.20 | 29.40 | 234.90 | 11.70 |
| Zn (mg) | 10 | 0.08 | 0.80 | 0.23 | 2.30 | 0.07 | 0.70 | 0.04 | 0.45 |
| Cu (mg) | 1 | 0.18 | 17.80 | 0.16 | 16.20 | 0.20 | 16.60 | 0.14 | 14.30 |
| Mn (mg) | 2 | 0.07 | 3.50 | 0.57 | 28.40 | 0.01 | 0.40 | 0.03 | 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouga, T.; Almeida, M.M.; Pitacas, F.I.; Rodrigues, A.M.; Vitória, C.; Anjos, O. Elemental and Nutritional Characterisation with Vibrational Spectroscopy Analysis of Ulva sp., Gracilaria multipartita, and Sargassum muticum. Appl. Sci. 2025, 15, 4212. https://doi.org/10.3390/app15084212
Mouga T, Almeida MM, Pitacas FI, Rodrigues AM, Vitória C, Anjos O. Elemental and Nutritional Characterisation with Vibrational Spectroscopy Analysis of Ulva sp., Gracilaria multipartita, and Sargassum muticum. Applied Sciences. 2025; 15(8):4212. https://doi.org/10.3390/app15084212
Chicago/Turabian StyleMouga, Teresa, Mariana M. Almeida, Filipa Inês Pitacas, António Moitinho Rodrigues, Cláudia Vitória, and Ofélia Anjos. 2025. "Elemental and Nutritional Characterisation with Vibrational Spectroscopy Analysis of Ulva sp., Gracilaria multipartita, and Sargassum muticum" Applied Sciences 15, no. 8: 4212. https://doi.org/10.3390/app15084212
APA StyleMouga, T., Almeida, M. M., Pitacas, F. I., Rodrigues, A. M., Vitória, C., & Anjos, O. (2025). Elemental and Nutritional Characterisation with Vibrational Spectroscopy Analysis of Ulva sp., Gracilaria multipartita, and Sargassum muticum. Applied Sciences, 15(8), 4212. https://doi.org/10.3390/app15084212








