A Review of Transcranial Electrical and Magnetic Stimulation Usefulness in Major Depression Disorder—Lessons from Animal Models and Patient Studies
Abstract
1. Introduction
1.1. Cognitive Decline in Depression
1.2. Structural Brain Abnormalities in Major Depressive Disorder
1.3. EEG/qEEG Recordings in Depression
2. Methods
- To investigate the neurobiological mechanisms through which tDCS and tMS modulate brain activity in the treatment of MDD.
- To explore findings from animal models and their translational relevance to clinical applications for MDD treatment.
- To provide recommendations for integrating tDCS and tMS into personalized treatment approaches for patients with treatment-resistant depression.
3. Brain Stimulation Techniques
3.1. The Effect of tDCS and tMS on Neuroplasticity
3.2. tDCS in Depression
3.3. Exploring tMS as a Treatment Option for Depression
3.4. tDCS and tMS in Animal Models for Depression
3.5. Combining tDCS and tMS with Other Therapeutic Approaches in Depression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monroe, S.M.; Harkness, K.L. Major depression and its recurrences: Life course matters. Annu. Rev. Clin. Psychol. 2022, 18, 329–357. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text revision; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; WHO/MSD/MER/2017.2; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef]
- Wang, J.; Luo, H.; Schülke, R.; Geng, X.; Sahakian, B.J.; Wang, S. Is transcranial direct current stimulation, alone or in combination with antidepressant medications or psychotherapies, effective in treating major depressive disorder? A systematic review and meta-analysis. BMC Med. 2021, 19, 319. [Google Scholar] [CrossRef] [PubMed]
- Borrione, L.; Bellini, H.; Razza, L.B.; Avila, A.G.; Baeken, C.; Brem, A.K.; Busatto, G.; Carvalho, A.F.; Chekroud, A.; Daskalakis, Z.J.; et al. Precision non-implantable neuromodulation therapies: A perspective for the depressed brain. Braz. J. Psychiatr. 2020, 42, 403–419. [Google Scholar] [CrossRef]
- Lam, R.W.; Kennedy, S.H.; Mclntyre, R.S.; Khullar, A. Cognitive dysfunction in major depressive disorder: Effects on psychosocial functioning and implications for treatment. Can. J. Psychiatry 2014, 59, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Cotta Ramusino, M.; Sinforiani, E.; Bernini, S.; Petrachi, R.; Costa, A. Cognitive impairment in depression: Recent advances and novel treatments. Neuropsychiatr. Dis. Treat 2019, 15, 1249–1258. [Google Scholar] [CrossRef]
- Zuckerman, H.; Pan, Z.; Park, C.; Brietzke, E.; Musial, N.; Shariq, A.S.; Iacobucci, M.; Yim, S.J.; Lui, L.; Rong, C.; et al. Recognition and Treatment of Cognitive Dysfunction in Major Depressive Disorder. Front. Psychiatry 2018, 9, 655. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Boggio, P.S.; De Raedt, R.; Benseñor, I.M.; Lotufo, P.A.; Namur, V.; Valiengo, L.C.; Vanderhasselt, M.A. Cognitive control therapy and transcranial direct current stimulation for depression: A randomized, double-blinded, controlled trial. J. Affect. Disord. 2014, 162, 43–49. [Google Scholar] [CrossRef]
- Grimm, S.; Beck, J.; Schuepbach, D.; Hell, D.; Boesiger, P.; Bermpohl, F.; Niehaus, L.; Boeker, H.; Northoff, G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biolog. Psychiatry 2008, 63, 369–376. [Google Scholar] [CrossRef]
- Qiu, L.; Lui, S.; Kuang, W.; Huang, X.; Li, J.; Zhang, J.; Chen, H.; Sweeney, J.A.; Gong, Q. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl. Psychiatry. 2014, 4, e378. [Google Scholar] [CrossRef]
- Wise, T.; Radua, J.; Via, E.; Cardoner, N.; Abe, O.; Adams, T.M.; Amico, F.; Cheng, Y.; Cole, J.H.; de Azevedo Marques Périco, C.; et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: Evidence from voxel-based meta-analysis. Mol. Psychiatry 2017, 22, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Auer, D.P.; Pütz, B.; Kraft, E.; Lipinski, B.; Schill, J.; Holsboer, F. Reduced glutamate in the anterior cingulate cortex in depression: An in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry 2000, 47, 305–313. [Google Scholar] [CrossRef]
- Bench, C.J.; Frith, C.D.; Grasby, P.M.; Friston, K.J.; Paulesu, E.; Frackowiak, R.S.; Dolan, R.J. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 1993, 31, 907–922. [Google Scholar] [CrossRef]
- George, M.S.; Wassermann, E.M.; Kimbrell, T.A.; Little, J.T.; Williams, W.E.; Danielson, A.L.; Greenberg, B.D.; Hallett, M.; Post, R.M. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: A placebo-controlled crossover trial. Am. J. Psychiatry 1997, 154, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Pfleiderer, B.; Michael, N.; Erfurth, A.; Ohrmann, P.; Homann, U.; Wolgast, M.; Fiebich, M.; Arolt, V.; Heindel, W. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatr. Res. Neuroimaging 2003, 122, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Strafella, A.P.; Paus, T.; Barrett, J.; Dagher, A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 2001, 21, 157. [Google Scholar] [CrossRef]
- Dubin, M. Imaging TMS: Antidepressant mechanisms and treatment optimization. Int. Rev. Psychiatry 2017, 29, 89–97. [Google Scholar] [CrossRef]
- Li, Q.; Fu, Y.; Liu, C.; Meng, Z. Transcranial direct current stimulation of the dorsolateral prefrontal cortex for treatment of neuropsychiatric disorders. Front. Behav. Neurosci. 2022, 16, 893955. [Google Scholar] [CrossRef]
- Gupta, A.; Burgess, R.; Drozd, M.; Gierula, J.; Witte, K.K.; Straw, S. The Surprise Question and clinician-predicted prognosis: Systematic review and meta-analysis. BMJ Support. Palliat. Care. 2024, 15, 12–35. [Google Scholar] [CrossRef]
- Iosifescu, D.V. Electroencephalography-derived biomarkers of antidepressant response. Harv. Rev. Psychiatry 2011, 19, 144–154. [Google Scholar] [CrossRef]
- Widge, A.S.; Bilge, M.T.; Montana, R.; Chang, W.; Rodriguez, C.I.; Deckersbach, T.; Carpenter, L.L.; Kalin, N.H.; Nemeroff, C.B. Electroencephalographic Biomarkers for Treatment Response Prediction in Major Depressive Illness: A Meta-Analysis. Am. J. Psychiatry 2019, 176, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Breda, V.; Freire, R. Repetitive Transcranial Magnetic Stimulation (rTMS) in Major Depression. Adv. Exp. Med. Biol. 2024, 1456, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Valiengo, L.; Baccaro, A.; Zanão, T.A.; de Oliveira, J.F.; Goulart, A.; Boggio, P.S.; Lotufo, P.A.; Benseñor, I.M.; Fregni, F. The sertraline vs. electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013, 70, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Dokos, S.; Ho, K.A.; Loo, C. A computational modeling study of transcranial direct current stimulation montages used in depression. Neuroimage 2014, 87, 332–344. [Google Scholar] [CrossRef]
- Bennabi, D.; Haffen, E. Transcranial Direct Current Stimulation (tDCS): A Promising Treatment for Major Depressive Disorder? Brain Sci. 2018, 8, 81. [Google Scholar] [CrossRef]
- Herrera-Melendez, A.L.; Bajbouj, M.; Aust, S. Application of Transcranial Direct Current Stimulation in Psychiatry. Neuropsychobiology 2020, 79, 372–383. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Priori, A. Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. 2003, 114, 589–595. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 15, 527. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2016, 2, 19–25. [Google Scholar] [CrossRef]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci 2008, 28, 14147–14155. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Transcranial magnetic stimulation and the human brain. Nature 2000, 406, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Siebner, H.R.; Funke, K.; Aberra, A.S.; Antal, A.; Bestmann, S.; Chen, R.; Classen, J.; Davare, M.; Di Lazzaro, V.; Fox, P.T.; et al. Transcranial magnetic stimulation of the brain: What is stimulated?—A consensus and critical position paper. Clin. Neurophysiol. 2022, 140, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Mutz, J.; Vipulananthan, V.; Carter, B.; Hurlemann, R.; Fu, C.H.Y.; Young, A.H. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: Systematic review and network meta-analysis. BMJ 2019, 364, l1079. [Google Scholar] [CrossRef]
- Goldberg, J.F. Electroconvulsive therapy: Still the gold standard for highly treatment-resistant mood disorders. CNS Spectrums. 2022, 27, 525–526. [Google Scholar] [CrossRef]
- Qi, S.; Cao, L.; Wang, Q.; Sheng, Y.; Yu, J.; Liang, Z. The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review. Biology 2024, 13, 790. [Google Scholar] [CrossRef]
- Ornello, R.; Caponnetto, V.; Ratti, S.; D’Aurizio, G.; Rosignoli, C.; Pistoia, F.; Ferrara, M.; Sacco, S.; D’Atri, A. Which is the best transcranial direct current stimulation protocol for migraine prevention? A systematic review and critical appraisal of randomized controlled trials. J. Headache Pain. 2021, 22, 144. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Fields, R.D.; Johansen-Berg, H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012, 15, 528–536. [Google Scholar] [CrossRef]
- Luque-Casado, A.; Perakakis, P.; Hillman, C.H.; Kao, S.C.; Llorens, F.; Guerra, P.; Sanabria, D. Differences in Sustained Attention Capacity as a Function of Aerobic Fitness. Med. Sci. Sports Exerc. 2016, 48, 887–895. [Google Scholar] [CrossRef]
- Ponsoni, A.; Damiani Branco, L.; Cotrena, C.; Milman Shansis, F.; Fonseca, R.P. The effects of cognitive reserve and depressive symptoms on cognitive performance in major depression and bipolar disorder. J. Affect. Disord. 2020, 274, 813–818. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Moffa, A.H.; Sampaio-Junior, B.; Borrione, L.; Moreno, M.L.; Fernandes, R.A.; Veronezi, B.P.; Nogueira, B.S.; Aparicio, L.V.M.; Razza, L.B.; et al. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression. N. Engl. J. Med. 2017, 376, 2523–2533. [Google Scholar] [CrossRef]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Froc, D.J.; Chapman, C.A.; Trepel, C.; Racine, R.J. Long-term depression and depotentiation in the sensorimotor cortex of the freely moving rat. J. Neurosci. 2000, 20, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; McEwen, B.S. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nat. Neurosci. 2012, 15, 689–695. [Google Scholar] [CrossRef]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.J.; Reiser, M.; Padberg, F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- McDonnell, M.D.; Abbott, D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput. Biol. 2009, 5, e1000348. [Google Scholar] [CrossRef]
- Kronberg, G.; Bridi, M.; Abel, T.; Bikson, M.; Parra, L.C. Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul. 2017, 10, 51–58. [Google Scholar] [CrossRef]
- Boggio, P.S.; Rigonatti, S.P.; Ribeiro, R.B.; Myczkowski, M.L.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int. J. Neuropsychopharmacol. 2008, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Nitsche, M.A.; Marcolin, M.A.; Rigonatti, S.P.; Pascual-Leone, A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006, 8, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Du, X.D.; Chu, H.C.; Liao, Y.Y.; Pan, W.; Li, Z.; Hung, G.C. Delayed effect of bifrontal transcranial direct current stimulation in patients with treatment-resistant depression: A pilot study. BMC Psychiatry 2019, 19, 180. [Google Scholar] [CrossRef]
- Dondé, C.; Amad, A.; Nieto, I.; Brunoni, A.R.; Neufeld, N.H.; Bellivier, F.; Poulet, E.; Geoffroy, P.A. Transcranial direct-current stimulation (tDCS) for bipolar depression: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 78, 123–131. [Google Scholar] [CrossRef]
- Loo, C.K.; Sachdev, P.; Martin, D.; Pigot, M.; Alonzo, A.; Malhi, G.S.; Lagopoulos, J.; Mitchell, P. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int. J. Neuropsychopharmacol. 2010, 13, 61–69. [Google Scholar] [CrossRef]
- Rigonatti, S.P.; Boggio, P.S.; Myczkowski, M.L.; Otta, E.; Fiquer, J.T.; Ribeiro, R.B.; Fregni, F. Transcranial direct stimulation and fluoxetine for the treatment of depression. Eur. Psychiatry 2008, 23, 74–76. [Google Scholar] [CrossRef]
- Woodham, R.D.; Selvaraj, S.; Lajmi, N.; Hobday, H.; Sheehan, G.; Ghazi-Noori, A.R.; Lagerberg, P.J.; Rizvi, M.; Kwon, S.S.; Orhii, P.; et al. Home-based transcranial direct current stimulation treatment for major depressive disorder: A fully remote phase 2 randomized sham-controlled trial. Nat. Med. 2024, 31, 87–95. [Google Scholar] [CrossRef]
- Woodham, R.; Rimmer, R.M.; Mutz, J.; Fu, C.H.Y. Is tDCS a potential first-line treatment for major depression? Int. Rev. Psychiatry 2021, 33, 250–265. [Google Scholar] [CrossRef]
- Rotheneichner, P.; Lange, S.; O’Sullivan, A.; Marschallinger, J.; Zaunmair, P.; Geretsegger, C.; Aigner, L.; Couillard-Despres, S. Hippocampal neurogenesis and antidepressive therapy: Shocking relations. Neural Plast. 2014, 2014, 723915. [Google Scholar] [CrossRef]
- Habert, J.; Katzman, M.A.; Oluboka, O.J.; McIntyre, R.S.; McIntosh, D.; MacQueen, G.M.; Khullar, A.; Milev, R.V.; Kjernisted, K.D.; Chokka, P.R.; et al. Functional Recovery in Major Depressive Disorder: Focus on Early Optimized Treatment. Prim. Care Companion CNS Disord. 2016, 18, 24746. [Google Scholar] [CrossRef]
- Morriss, R.; Briley, P.M.; Webster, L.; Abdelghani, M.; Barber, S.; Bates, P.; Brookes, C.; Hall, B.; Ingram, L.; Kurkar, M.; et al. Connectivity-guided intermittent theta burst versus repetitive transcranial magnetic stimulation for treatment-resistant depression: A randomized controlled trial. Nat. Med. 2024, 30, 403–413. [Google Scholar] [CrossRef]
- Kinjo, M.; Wada, M.; Nakajima, S.; Tsugawa, S.; Nakahara, T.; Blumberger, D.M.; Mimura, M.; Noda, Y. Transcranial magnetic stimulation neurophysiology of patients with major depressive disorder: A systematic review and meta-analysis. Psychol. Med. 2021, 51, 1–10. [Google Scholar] [CrossRef]
- Mishra, B.R.; Sarkar, S.; Praharaj, S.K.; Mehta, V.S.; Diwedi, S.; Nizamie, S.H. Repetitive transcranial magnetic stimulation in psychiatry. Ann. Indian Acad. Neurol. 2021, 14, 245–251. [Google Scholar] [CrossRef]
- Anderson, I.M.; Delvai, N.A.; Ashim, B.; Lewin, C.; Singh, V.; Sturman, D.; Strickland, P.L. Adjunctive fast repetitive transcranial magnetic stimulation in depression. Br. J. Psychiatry 2007, 190, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Thickbroom, G.; Rodger, J. Repetitive Transcranial Magnetic Stimulation of the Brain. Neuroscientist 2016, 23, 82–94. [Google Scholar] [CrossRef]
- Gouveia, A.; de Oliveira Beleza, R.; Steculorum, S.M. AgRP neuronal activity across feeding-related behaviours. Eur. J. Neurosci. 2021, 54, 7458–7475. [Google Scholar] [CrossRef] [PubMed]
- Peanlikhit, T.; Van Waes, V.; Pedron, S.; Risold, P.Y.; Haffen, E.; Etiévant, A.; Monnin, J. The antidepressant-like effect of tDCS in mice: A behavioral and neurobiological characterization. Brain Stimul. 2017, 10, 748–756. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Asgharian, A.F.; Vaghef, L. The effectiveness of high-frequency left DLPFC-rTMS on depression, response inhibition, and cognitive flexibility in female subjects with major depressive disorder. J. Psychiatr. Res. 2022, 149, 287–292. [Google Scholar] [CrossRef]
- Speer, A.M.; Wassermann, E.M.; Benson, B.E.; Herscovitch, P.; Post, R.M. Antidepressant efficacy of high and low frequency rTMS at 110% of motor threshold versus sham stimulation over left prefrontal cortex. Brain Stimul. 2014, 7, 36–41. [Google Scholar] [CrossRef]
- Jackson, M.P.; Bikson, M.; Liebetanz, D.; Nitsche, M. How to consider animal data in tDCS safety standards. Brain Stimul. 2017, 10, 1141–1142. [Google Scholar] [CrossRef]
- Chhatbar, P.Y.; Chen, R.; Deardorff, R.; Dellenbach, B.; Kautz, S.A.; George, M.S.; Feng, W. Safety and tolerability of transcranial direct current stimulation to stroke patients—A phase I current escalation study. Brain Stimul. 2017, 10, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Saidi, M.; Firoozabadi, S.M. Glial cells have more important role in tDCS-induced brain activities rather than neurons. Med. Hypotheses 2021, 153, 110615. [Google Scholar] [CrossRef]
- Monai, H.; Hirase, H. Astrocytes as a target of transcranial direct current stimulation (tDCS) to treat depression. Neurosci. Res. 2018, 126, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Milighetti, S.; Sterzi, S.; Fregni, F.; Hanlon, A.C.; Hayley, P.; Murphy, M.D.; Bundy, D.T.; Nudo, R.J.; Guggenmos, D.J. Effects of tDCS on spontaneous spike activity in a healthy ambulatory rat model. Brain Stimul. 2020, 13, 1566–1576. [Google Scholar] [CrossRef]
- Feng, S.F.; Shi, T.Y.; Fan-Yang; Wang, W.N.; Chen, Y.C.; Tan, Q.R. Long-lasting effects of chronic rTMS to treat chronic rodent model of depression. Behav. Brain Res. 2012, 232, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Waye, S.C.; Dinesh, O.C.; Hasan, S.N.; Conway, J.D.; Raymond, R.; Nobrega, J.N.; Blundell, J.; Bambico, F.R. Antidepressant action of transcranial direct current stimulation in olfactory bulbectomised adolescent rats. J. Psychopharmacol. 2021, 35, 1003–1016. [Google Scholar] [CrossRef]
- Fang, G.; Wang, Y. Transcranial direct current stimulation (tDCS) produce anti-anxiety response in acute stress exposure rats via activation of amygdala CB1R. Behav. Brain Res. 2021, 400, 113050. [Google Scholar] [CrossRef]
- Peng, D.; Shi, F.; Li, G.; Fralick, D.; Shen, T.; Qiu, M.; Liu, J.; Jiang, K.; Shen, D.; Fang, Y. Surface vulnerability of cerebral cortex to major depressive disorder. PLoS ONE 2015, 10, e0120704. [Google Scholar] [CrossRef]
- Sachdev, P.S.; McBride, R.; Loo, C.; Mitchell, P.M.; Malhi, G.S.; Croker, V. Effects of different frequencies of transcranial magnetic stimulation (TMS) on the forced swim test model of depression in rats. Biol. Psychiatry 2022, 51, 474–479. [Google Scholar] [CrossRef]
- Mardani, P.; Zolghadriha, A.; Dadashi, M.; Javdani, H.; Mousavi, S.E. Effect of medication therapy combined with transcranial direct current stimulation on depression and response inhibition of patients with bipolar disorder type I: A clinical trial. BMC Psychiatry 2021, 21, 579. [Google Scholar] [CrossRef]
- Guleken, Z.; Eskikurt, G.; Karamürsel, S. Investigation of the effects of transcranial direct current stimulation and neurofeedback by the continuous performance test. Neurosci. Lett. 2020, 716, 134648. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, G.; Cordero, P.; Khanal, R.; Himelhoch, S.S.; Rush, C.R. Optimally combining transcranial magnetic stimulation with antidepressants in major depressive disorder: A systematic review and Meta-analysis. J. Affect. Disord. 2024, 358, 432–439. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Leuchter, A.F. The effect of psychotropic drugs on cortical excitability and plasticity measured with transcranial magnetic stimulation: Implications for psychiatric treatment. J. Affect. Disord. 2019, 253, 126–140. [Google Scholar] [CrossRef]
- Bretlau, L.G.; Lunde, M.; Lindberg, L.; Undén, M.; Dissing, S.; Bech, P. Repetitive transcranial magnetic stimulation (rTMS) in combination with escitalopram in patients with treatment-resistant major depression: A double-blind, randomised, sham-controlled trial. Pharmacopsychiatry 2008, 41, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rossini, D.; Serretti, A.; Franchini, L.; Mandelli, L.; Smeraldi, E.; De Ronchi, D.; Zanardi, R. Sertraline versus fluvoxamine in the treatment of elderly patients with major depression: A double-blind, randomized trial. J. Clin. Psychopharmacol. 2005, 25, 471–475. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Benitez, J.; de Castella, A.; Daskalakis, Z.J.; Brown, T.L.; Kulkarni, J. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am. J. Psychiatry 2006, 163, 88–94. [Google Scholar] [CrossRef]
- Zhou, D.; Li, X.; Wei, S.; Yu, C.; Wang, D.; Li, Y.; Li, J.; Liu, J.; Li, S.; Zhuang, W.; et al. Transcranial Direct Current Stimulation Combined With Repetitive Transcranial Magnetic Stimulation for Depression: A Randomized Clinical Trial. JAMA Netw. Open. 2024, 7, e2444306. [Google Scholar] [CrossRef]
- Garcia-Toro, M.; Salva, J.; Daumal, J.; Andres, J.; Romera, M.; Lafau, O.; Echevarría, M.; Mestre, M.; Bosch, C.; Collado, C.; et al. High (20-Hz) and low (1-Hz) frequency transcranial magnetic stimulation as adjuvant treatment in medication-resistant depression. Psychiatry Res. 2006, 146, 53–57. [Google Scholar] [CrossRef]
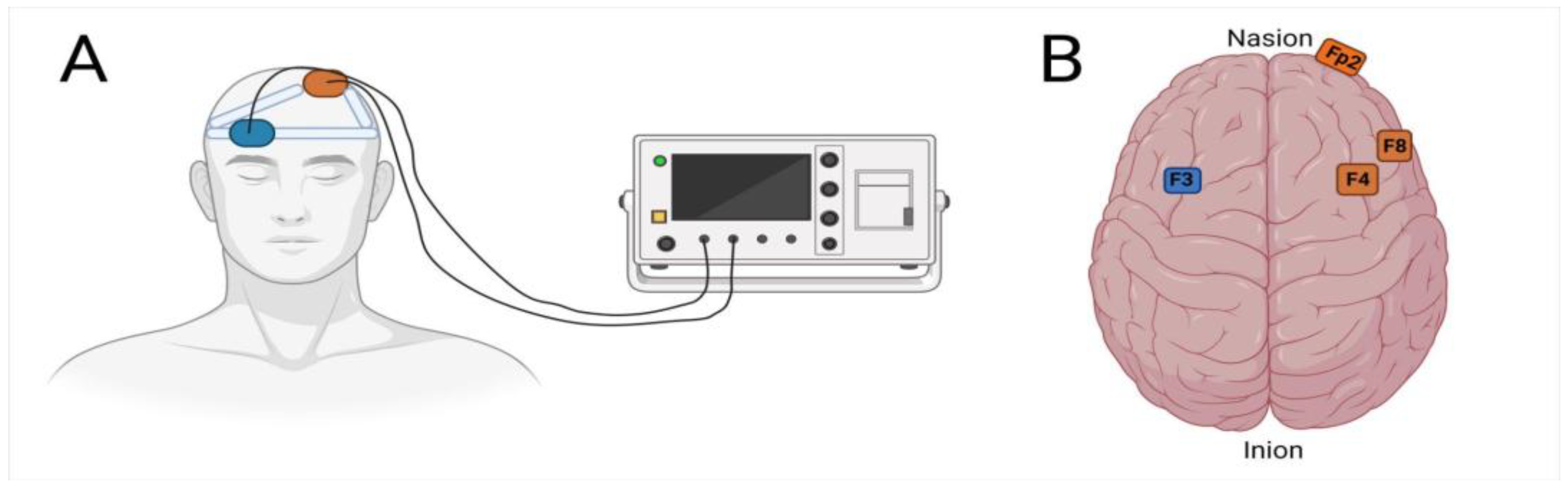
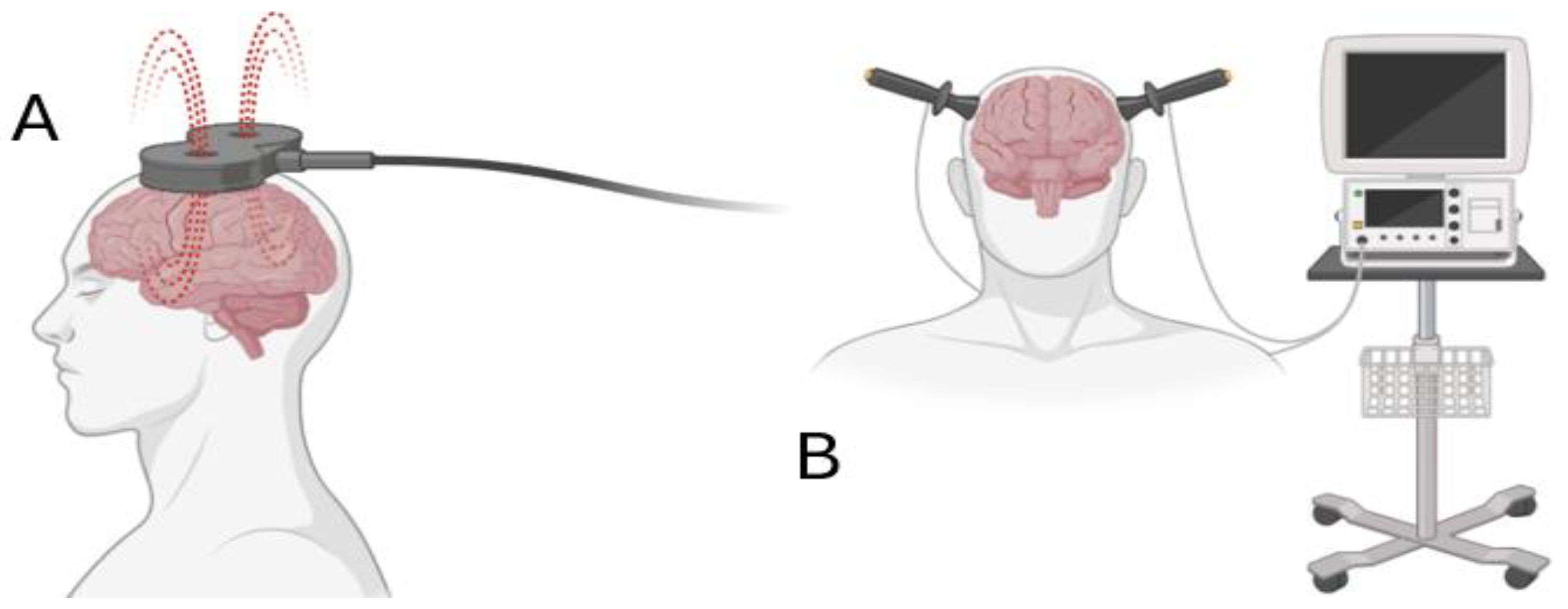
| Therapeutic Approach | Type of Depression | Depression Rating Scales | Outcome | References |
|---|---|---|---|---|
| 12 sessions; 2 mA for 30 min (anode F3; cathode F4) | Unipolar depression Bipolar depression | MADRS | Depression scores ↘ (6th and 8th week) Cognitive performance (e.g., paired association and social cognition) ↗ | [55] |
| 10 sessions; 2 mA, 20 min (anode F3, cathode Fp2) | Unipolar major depression H | HDRS, BDI | Depression scores ↘ (t1, immediately after treatment; t2, 15 d after the end of treatment; t3, 30 d after the end of treatment) | [53] |
| 15 sessions; 2 mA for 20 min (anode F3; cathode F4) | Unipolar depression Bipolar depression | HDRS, MADRS, BDI | Depression scores ↘ (10 sessions and 1 month) | [57] |
| 10 sessions; 2 mA for 20 min (anode F3; cathode F4) | Unipolar major Depression | BDI | Depression scores ↘ (2nd, 4th and 8th week) | [58] |
| 36 sessions (5 × 3 + 3 × 7) 2 mA for 30 min (anode F3; cathode F4) | Major depressive Disorder | HDRS, MADRS | Depression scores ↘ (10-week mean −4.01) | [59] |
| Therapeutic Approach | Type of Depression | Depression Rating Scales | Outcome | References |
|---|---|---|---|---|
| 13 sessions; 10 Hz for 4–6 weeks (left frontal rTMS) | Major depressive episode (on antidepressants) | MADRS HAD | MADRS effect size 0.86, ↘ HAD depression effect-size 0.92)↘ (4th and 6th week) at 12th week, a significant effect remained | [66] |
| 20 sessions 20 Hz for 2 weeks (DLPFC rTMS) | Major depressive disorder | BDI, Go/NoGo, WCST | Depression scores BDI↘ Enhanced accuracy and decrease reaction time at Go/NoGo task ↗ Errors and failures WCST↘ | [71] |
| 20 sessions 20 Hz for 2 weeks (left DLPFC rTMS) | Major Depression unipolar | HRSD-17 | Depression scores ↘ | [16] |
| 20 sessions 20 Hz or 5 Hz for 2 weeks (left DLPFC rTMS) | Medication resistant major depressive disorder or bipolar disorder | HRSD-17 | Depression scores ↘ Response rate for active rTMS was 60% ↗ | [72] |
| Study Details | Protocol | Test | Outcome | References |
|---|---|---|---|---|
| Adolescent male Sprague–Dawley rats | tDCS mPFC (AP: +2.2 mm to +4.7 mm) | Sucrose Preference Test (SPT), Open Field Test (OFT) | Additive tDCS—paroxetine therapeutic action | [79] |
| Adult male GEAS-W and Wistar | tDCS, Neocortex (AP: −1.5 mm, ML: ±3.0 mm) | The Open Field Test (OFT) | Active-tDCS shows anxiolytic and antidepressant effects in epileptic rats. | [68] |
| Adult female Swiss mice and adult male C57Bl/6 mice | tDCS, Frontal cortex | Forced Swim Test (FST) | tDCS induced long-lasting antidepressant-like effect | [69] |
| Male Sprague Dawley rats | tDCS, Frontal cortex | Open Field Test (OFT), Elevated Plus Maze Test (EPMT) | Anxiety reduction | [80] |
| Rat models of depression and cortex-derived astrocytes from newborn rats | tMS 1, 5, and 10 Hz | Open Field Test (OFT), Forced Swim Test (FST) Sucrose Preference Test (SPT) | Ameliorates depressive-like behavior and treats depression | [81] |
| Rat models of depression | rTMS 1, 5, 15, 25, 100 Hz, 1000 stimuli each | Forced Swim Test (FST) | Decreased immobility time in FST antidepressant effects | [82] |
| CUMS rat model of depression Male Sprague–Dawley rats | tMS 15 Hz for 3 wk, also venlafaxine | Sucrose Preference Test (SPT) Open Field Test (OFT), Forced Swim Test (FST) Novelty-suppressed feeding test | Long-lasting effects and induce neuroplasticity | [78] |
| Therapeutic Approach | Type of Depression 1 | Outcome | References |
|---|---|---|---|
| tDCS + Sertraline | MDD | Depressive symptoms ↘ | [25] |
| tDCS + Mood stabilizers; Lithium; Sodium valproate; Carbamazepine | BD | Depressive symptoms ↘ Response inhibition ability ↗ | [83] |
| tDCS + sertraline, hydrochloride, and escitalopram | MDD | Depressive symptoms ↘ response rate↗ | [4] |
| tDCS + Cognitive control therapy | MDD | Depressive symptoms ↘ Cognitive tasks ↗ | [10] |
| Therapeutic Approach | Type of Depression 1 | Outcome | References |
|---|---|---|---|
| tMS + Escitalopram 15 Hz: 15 sessions—20 mg | MRMD | Depressive symptoms ↘ HAM-D above 0.70 | [87] |
| rTMS + venlafaxine/sertraline/escitalopram 15 Hz 2 weeks | MDD | Depressive symptoms ↘ no difference between drugs | [88] |
| rTMS + SSRI/SNRI/Tricyclics Right 1 HZ + Left 10 Hz 10 sessions + further sessions up to 6 weeks if 10% reduction in MADRS weekly | MRMD | Depressive symptoms ↘ MADRS ↗ 7.7 | [89] |
| rTMS + clomipramine/sertraline/venlafaxine/reboxetine 1 Hz/2 Hz 10 sessions/2 weeks LF-TMS and HF-rTMS | MRMD | Depressive symptoms ↘ HamD no difference between drugs | [88] |
| tMS 15 Hz for 3 wk, also venlafaxine | MDD | Depressive symptoms ↘ no difference between drugs | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamfirache, F.; Dumitru, C.; Trandafir, D.-M.; Bratu, A.; Radu, B.M. A Review of Transcranial Electrical and Magnetic Stimulation Usefulness in Major Depression Disorder—Lessons from Animal Models and Patient Studies. Appl. Sci. 2025, 15, 4020. https://doi.org/10.3390/app15074020
Zamfirache F, Dumitru C, Trandafir D-M, Bratu A, Radu BM. A Review of Transcranial Electrical and Magnetic Stimulation Usefulness in Major Depression Disorder—Lessons from Animal Models and Patient Studies. Applied Sciences. 2025; 15(7):4020. https://doi.org/10.3390/app15074020
Chicago/Turabian StyleZamfirache, Florin, Cristina Dumitru, Deborah-Maria Trandafir, Andrei Bratu, and Beatrice Mihaela Radu. 2025. "A Review of Transcranial Electrical and Magnetic Stimulation Usefulness in Major Depression Disorder—Lessons from Animal Models and Patient Studies" Applied Sciences 15, no. 7: 4020. https://doi.org/10.3390/app15074020
APA StyleZamfirache, F., Dumitru, C., Trandafir, D.-M., Bratu, A., & Radu, B. M. (2025). A Review of Transcranial Electrical and Magnetic Stimulation Usefulness in Major Depression Disorder—Lessons from Animal Models and Patient Studies. Applied Sciences, 15(7), 4020. https://doi.org/10.3390/app15074020








