Illegal Nitrite Treatment of Red Tuna and Prolonged Storage: What About Other Food Safety Risks?
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment of Red Tuna with Nitrite Solutions
2.2. Chemicals and Analytical Methods
2.2.1. Histamine Analysis: HPLC Reagents, Equipment and Procedure
2.2.2. TVBN Determination
2.2.3. Nitrite/Nitrate, Sulfites, Biogenic Amines, and Ascorbic Acid Determinations
2.3. Microbiological Determinations
Enumeration of Selected Pathogens Added to Tuna
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Scientific Committee. Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA J. 2015, 13, 3982. [Google Scholar] [CrossRef]
- FAO Fisheries Report. FAO Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption Rome, 25–29 January 2010; FAO: Rome, Italy, 2011; ISBN 978-92-5-106999-8. [Google Scholar]
- Domingo, J.L.; Bocio, A.; Falcó, G.; Llobet, J.M. Benefits and risks of fish consumption Part I. A quantitative analysis of the intake of omega-3 fatty acids and chemical contaminants. Toxicology 2007, 230, 219–226. [Google Scholar] [CrossRef]
- Mercogliano, R.; Santonicola, S. Scombroid fish poisoning: Factor influencing the production of histamine in tuna supply chain. A review. LWT—Food Sci. Technol. 2019, 114, 108374. [Google Scholar] [CrossRef]
- Sáez-Hernández, R.; Antela, K.U.; Mauri-Aucejo, A.R.; Morales-Rubio, A.; Cervera, M.L. Smartphone-based colorimetric study of adulterated tuna samples. Food Chem. 2022, 389, 133063. [Google Scholar] [CrossRef]
- Niederer, M.; Lang, S.; Roux, B.; Stebler, T.; Hohl, C. Identification of nitrite treated tuna fish meat via the determination of nitrous oxide by head space-gas chromatography/mass spectrometry. F1000Research 2019, 8, 711. [Google Scholar] [CrossRef]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Volume L 354, pp. 16–33. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R1333 (accessed on 1 August 2024).
- Al-Bulushi, I.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic Amines in Fish: Roles in Intoxication, Spoilage, and Nitrosamine Formation—A Review. Crit. Rev. Food Sci. 2009, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.E.J. Naturally occurring nitrate and nitrite in foods in relation to infant methaemoglobinaemia. Food Cosmet. Toxicol. 1971, 9, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sihto, H.M.; Budi Susilo, Y.; Tasara, T.; Radström, P.; Stephan, R.; Schelin, J.; Johler, S. Effect of Sodium Nitrite and Regulatory Mutations Δagr, ΔsarA, and ΔsigB on the MRNA and Protein Levels of Staphylococcal Enterotoxin D. Food Control 2016, 65, 37–45. [Google Scholar] [CrossRef]
- Akyüz, M.; Ata, Ş. Determination of Low Level Nitrite and Nitrate in Biological, Food and Enviromental Samples by Gas Chromatography-Mass Spectrometry and Liquid Chromatography with Fluorescence Detection. Talanta 2009, 79, 900–904. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Arnau, J.; Carballo, J.; Aguirre, J.S.; Gratacós-Cubarsí, M.; Fernández, M. Effect of Nitrate and Nitrite on Listeria and Selected Spoilage Bacteria Inoculated in Dry-Cured Ham. Food Res. Int. 2017, 101, 82–87. [Google Scholar] [CrossRef]
- Bernardo, P.; Patarata, L.; Lorenzo, J.M.; Fraqueza, M.J. Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products. Foods 2021, 10, 3019. [Google Scholar] [CrossRef] [PubMed]
- Reinik, M.; Tamme, T.; Roasto, M.; Juhkam, K.; Jurtšenko, S.; Tenno, T.; Kiis, A. Nitrites, nitrates and N-nitrosoamines in Estoniancured meat products: Intake by Estonian children and adolescents. Food Addit. Contam. 2005, 22, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Guembe-García, M.; González-Ceballos, L.; Arnaiz, A.; Fernández-Muiño, M.A.; Sancho, M.T.; Osés, S.M.; Ibeas, S.; Rovira, J.; Melero, B.; Represa, C.; et al. Easy Nitrite Analysis of Processed Meat with Colorimetric Polymer Sensors and a Smartphone App. Appl. Mater. Interfaces 2022, 14, 37051–37058. [Google Scholar] [CrossRef]
- World Health Organization. Nitrate and Nitrite in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003; Volume 22, p. 21. [Google Scholar]
- EFSA. Assessment of the incidents of histamine intoxication in some EU countries. EFSA Support. Publ. 2017, 14, 1301E. [Google Scholar] [CrossRef]
- Reese, I.; Ballmer-Weber, B.; Beyer, K.; Fuchs, T.; Kleine-Tebbe, J.; Klimek, L.; Lepp, U.; Niggemann, B.; Saloga, J.; Schäfer, C.; et al. German guideline for the management of adverse reactions to ingested histamine. Allergo J. Int. 2017, 26, 72–79. [Google Scholar] [CrossRef]
- Lo Magro, S.; Summa, S.; Iammarino, M.; D’Antini, P.; Marchesani, G.; Chiaravalle, A.E.; Muscarella, M. A 5-years (2015–2019) control activity of an EU laboratory: Contamination of histamine in fish products and expo-sure assessment. Appl. Sci. 2020, 10, 8693. [Google Scholar] [CrossRef]
- Muscarella, M.; Lo Magro, S.; Campaniello, M.; Armentano, A.; Stacchini, P. Survey of histamine levels in 325 fresh fish and fish products collected in Puglia (Italy) by ELISA and HPLC with fluorimetric detection. Food Control 2013, 31, 211–217. [Google Scholar] [CrossRef]
- ISO 19343:2017; Microbiology of the Food Chain—Detection and Quantification of Histamine in Fish and Fishery Products—HPLC Method. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/64657.html (accessed on 2 August 2024).
- European Commission. 2019 Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 Laying Down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and Amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls; European Commission: Brussels, Belgium, 2019.
- Iammarino, M.; Di Taranto, A.; Cristino, M. Endogenous levels of nitrites and nitrates in wide consumption foodstuffs: Results of five years of official controls and monitoring. Food Chem. 2013, 140, 763–771. [Google Scholar] [CrossRef]
- Iammarino, M.; Di Taranto, A.; Ientile, A.R. Monitoring of sulphites levels in shrimps samples collected in Puglia (Italy) by ion-exchange chromatography with conductivity detection. Food Addit. Contam. Part. B 2013, 7, 84–89. [Google Scholar] [CrossRef]
- UNI/TS 11868:2022; Determinazione di Anidride Solforosa e suoi sali nei Prodotti Carnei ed Ittici—Metodo in Cromatografia Liquida a Scambio Ionico con Rivelazione Elettrochimica Conduttimetrica. Ente Italiano di Normazione (UNI): Milan, Italy, 2022.
- Palermo, C.; Muscarella, M.; Nardiello, D.; Iammarino, M.; Centonze, D. A multiresidual method based on ion-exchange chromatography with conductivity detection for the determination of biogenic amines in food and beverages. Anal. Bioanal. Chem. 2013, 405, 1015–1023. [Google Scholar] [CrossRef]
- Iammarino, M.; Di Taranto, A. Monitoring on the presence of ascorbic acid in not prepacked fresh meat preparations by a validated HPLC method. J. Food Res. 2012, 1, 22–31. [Google Scholar] [CrossRef]
- ISO 4833-1:2013/Amd 1:2022; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 16649-2:2010; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of β-Glucuronidase-Positive Escherichia coli. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 6888-2:2021/Amd 1:2023; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and other species)—Part 2: Method using Rabbit Plasma Fibrinogen Agar Medium. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 21872-1:2017/Amd 1:2023; Microbiology of the Food Chain—Horizontal Method for the Determination of Vibrio spp.—Part 1: Detection of Potentially Enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus—Amendment 1: Inclusion of Performance Testing of Culture Media and Reagents. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6579-1:2017/Amd 1:2020; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.—Amendment 1: Broader Range of Incubation Temperatures, Amendment to the Status of Annex D, and Correction of the Composition of MSRV and SC. International Organization for Standardization: Geneva, Switzerland, 2017.
- Liu, C.; Mou, J.; Su, Y.C. Behavior of Salmonella and Listeria monocytogenes in Raw Yellowfin Tuna during Cold Storage. Foods 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC). No. 1441/2007 of 5 December 2007 Amending Regulation (EC) No. 2073/2005 on Microbiological Criteria for Foodstuffs; Commission Regulation: Bruxelles, Belgium, 2007; Volume L322, pp. 12–29. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:322:0012:0029:EN:PDF (accessed on 2 August 2024).


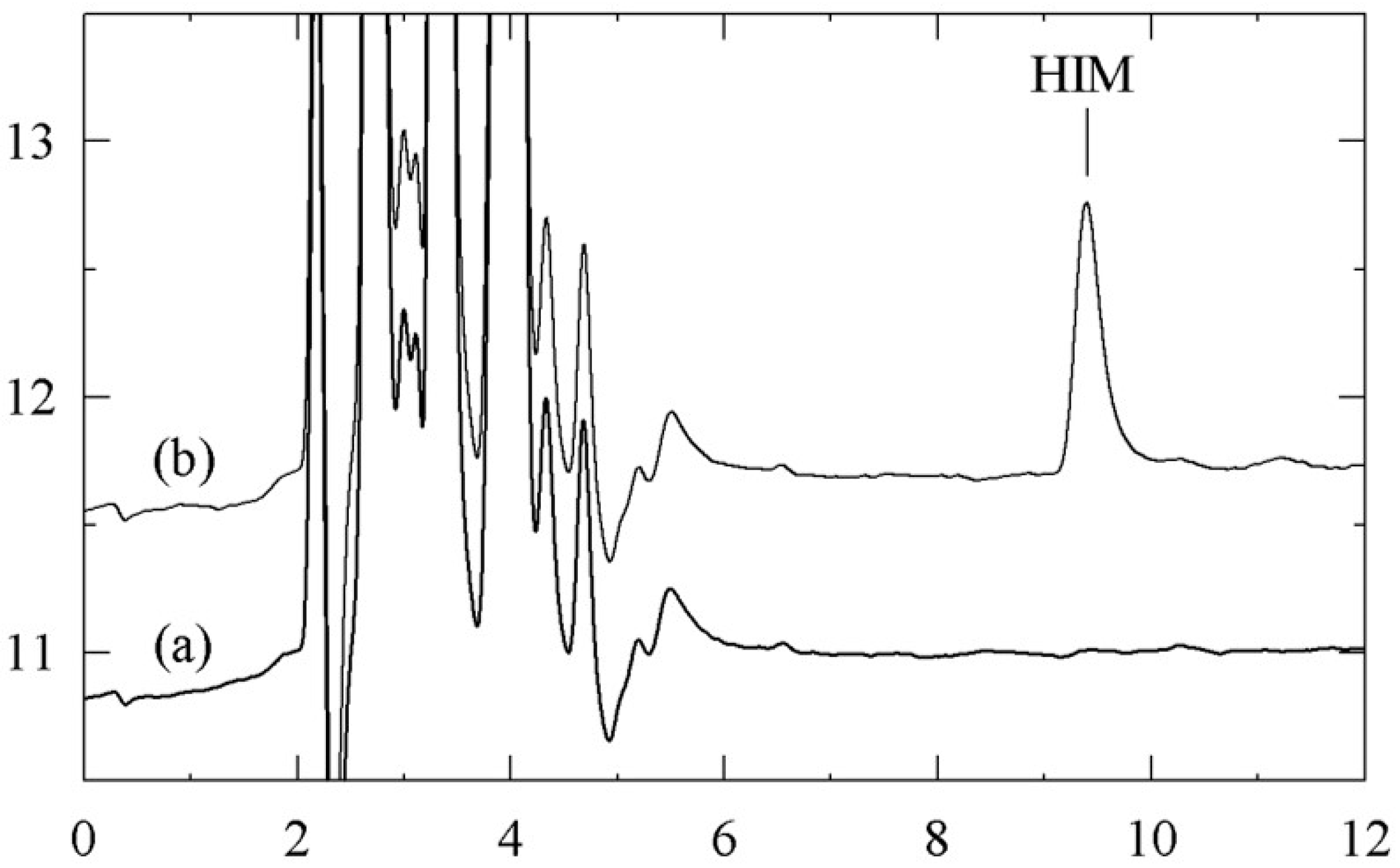
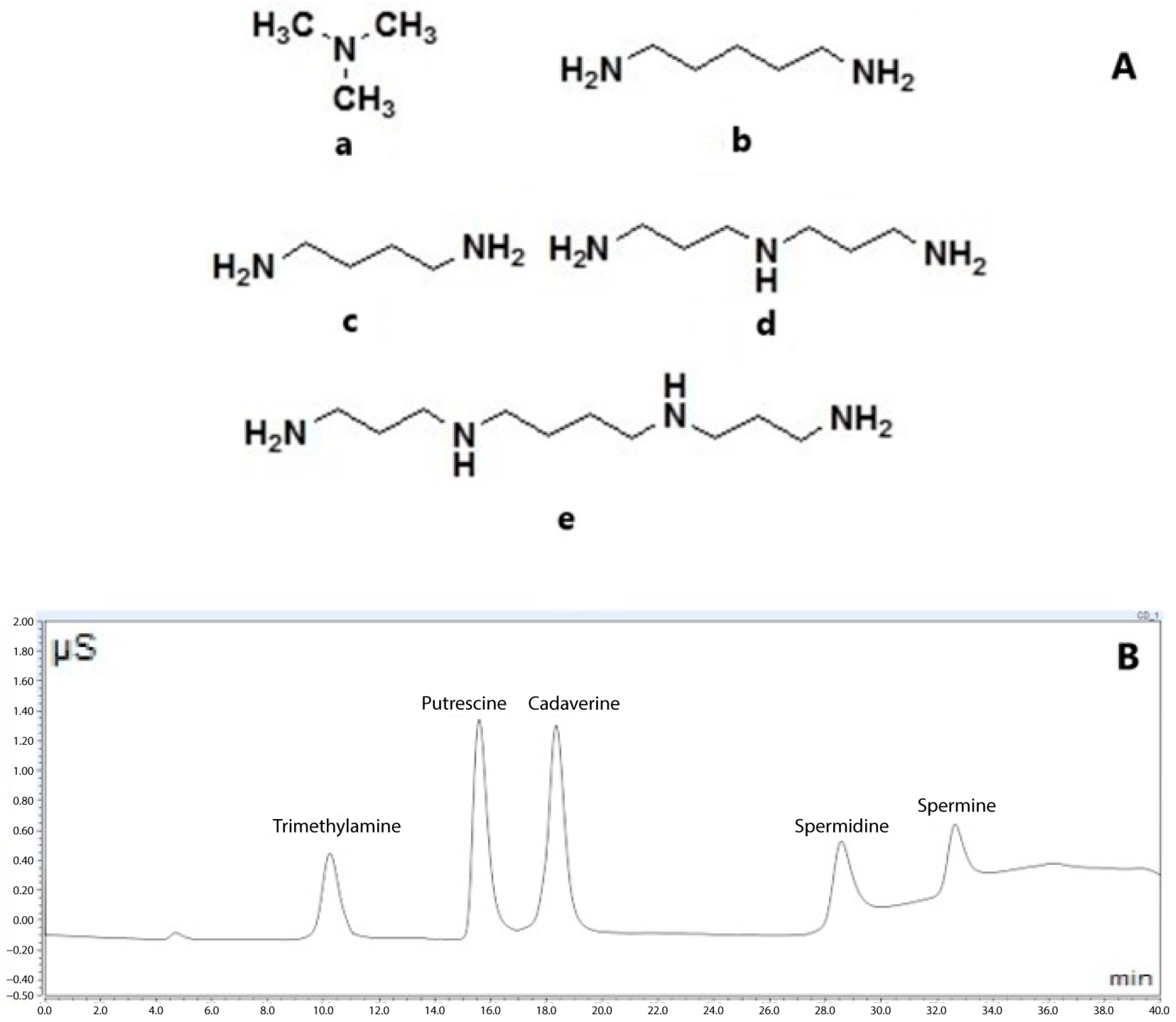
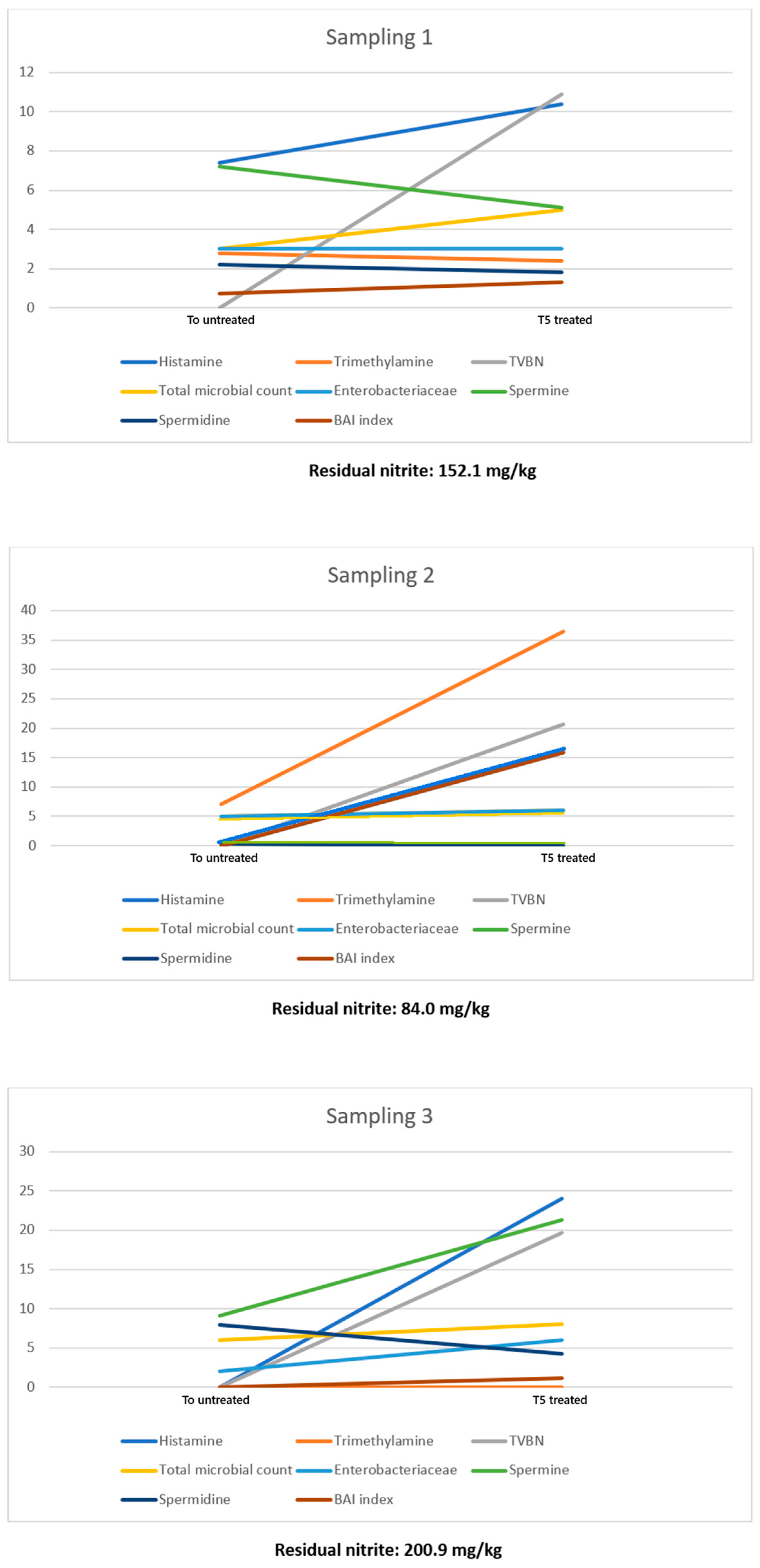
| Sampling 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Residual Nitrites = 152.1 mg/kg; Ascorbic Acid = 290.8 mg/kg; Sulfites = Absent | ||||||||||
| HIM (mg/kg) | Nitrites (mg/kg) | Nitrates (mg/kg) | Trimethylamine (mg/kg) | Cadaverine (mg/kg) | Putrescine (mg/kg) | Spermine (mg/kg) | Spermidine (mg/kg) | BAI Index | TVBN (mg/100 g) | |
| t0 untreated | 7.4 | 0 | 0 | 2.8 | 0 | 0 | 7.2 | 2.2 | 0.71 | 0 |
| t0 treated | 6.9 | 180.0 | 15.0 | 3.6 | 0 | 0 | 4.7 | 1.2 | 1.00 | 0 |
| t5gg untreated | 24.1 | 0 | 1.2 | 24.1 | 0 | 0 | 3.8 | 1.3 | 3.95 | 11.9 |
| t5gg treated | 10.4 | 152.1 | 76.0 | 2.4 | 0 | 0 | 5.1 | 1.8 | 1.32 | 10.9 |
| Sampling 2 | ||||||||||
| Residual Nitrites = 84.0 mg/kg. Ascorbic Acid = 200.3 mg/kg; Sulfites = Absent | ||||||||||
| HIM (mg/kg) | Nitrites (mg/kg) | Nitrates (mg/kg) | Trimethylamine (mg/kg) | Cadaverine (mg/kg) | Putrescine (mg/kg) | Spermine (mg/kg) | Spermidine (mg/kg) | BAI Index | TVBN (mg/100 g) | |
| t0 untreated | 0 | 5.6 | 0 | 7.1 | 0 | 0 | 0.3 | 0.4 | 0 | 0 |
| t0 treated | 0 | 74.2 | 5.7 | 2.1 | 0 | 0 | 5.6 | 0.6 | 0 | 0 |
| t5gg untreated | 29.6 | 0 | 0 | 12.4 | 0 | 0 | 3.1 | 0 | 7.22 | 24.0 |
| t5gg treated | 15.9 | 84.0 | 10.9 | 36.5 | 0 | 0 | 0 | 0 | 15.9 | 20.7 |
| Sampling 3 | ||||||||||
| Residual Nitrites = 200.9 mg/kg; Ascorbic Acid = 131.6 mg/kg; Sulfites = Absent | ||||||||||
| HIM (mg/kg) | Nitrites (mg/kg) | Nitrates (mg/kg) | Trimethylamine (mg/kg) | Cadaverine (mg/kg) | Putrescine (mg/kg) | Spermine (mg/kg) | Spermidine (mg/kg) | BAI Index | TVBN (mg/100 g) | |
| t0 untreated | 0 | 0 | 0 | 0 | 0 | 0 | 9.1 | 7.9 | 0 | 0 |
| t0 treated | 0 | 212.0 | 0 | 0 | 0 | 0 | 10.5 | 1.1 | 0 | 0 |
| t5gg untreated | 34.3 | 0 | 0 | 0 | 41.5 | 0 | 18.8 | 34.4 | 1.40 | 26.0 |
| t5gg treated | 24.0 | 200.9 | 37.0 | 0 | 0 | 5.4 | 21.3 | 4.2 | 1.11 | 19.7 |
| Sampling 1 | ||||||
|---|---|---|---|---|---|---|
| Total Microbial Count | Enterobacteriaceae | Staphylococci | Escherichia Coli | Salmonella | Vibrio | |
| t0 untreated | >103 CFU/g | >103 CFU/g | <10 CFU/g | absent | absent | absent |
| t0 treated | >103 CFU/g | >102 CFU/g | <10 CFU/g | absent | absent | absent |
| t5gg untreated | >105 CFU/g | >104 CFU/g | <10 CFU/g | absent | absent | absent |
| t5gg treated | >105 CFU/g | >103 CFU/g | <10 CFU/g | absent | absent | absent |
| Sampling 2 | ||||||
| Total Microbial Count | Enterobacteriaceae | Staphylococci | Escherichia Coli | Salmonella | Vibrio | |
| t0 untreated | >105 CFU/g | >105 CFU/g | <10 CFU/g | absent | absent | absent |
| t0 treated | >105 CFU/g | >105 CFU/g | <10 CFU/g | absent | absent | absent |
| t5gg untreated | >106 CFU/g | >106 CFU/g | <10 CFU/g | absent | absent | absent |
| t5gg treated | >106 CFU/g | >106 CFU/g | <10 CFU/g | absent | absent | absent |
| Sampling 3 | ||||||
| Total Microbial Count | Enterobacteriaceae | Staphylococci | Escherichia Coli | Salmonella | Vibrio | |
| t0 untreated | 7.0 × 106 CFU/g | 1.5 × 102 CFU/g | <10 CFU/g | absent | absent | absent |
| t0 treated | 6.2 × 106 CFU/g | 2.6 × 102 CFU/g | <10 CFU/g | absent | absent | absent |
| t5gg untreated | 1.0 × 108 CFU/g | 4.2 × 106 CFU/g | <10 CFU/g | absent | absent | absent |
| t5gg treated | 1.0 × 108 CFU/g | 4.0 × 106 CFU/g | <10 CFU/g | absent | absent | absent |
| Parameter | T0 (Control) Mean Value * | Nitrite-Treated Sample t5gg Mean Value * |
|---|---|---|
| Histamine (mg/kg) | 2.8 a | 16.8 b |
| TVBN (mg/100 g) | 0.0 a | 17.1 b |
| BAIindex | 0.0 a | 6.1 a |
| Total microbial count (CFU/g) | 2.4 × 106 a | 3.5 × 107 a |
| Enterobacteriaceae (CFU/g) | 1.0 × 105 a | 1.7 × 106 a |
| Sample | Salmonella t0 | Salmonella t5 | Sample | Morganella morganii t0 | Morganella morganii t5 |
|---|---|---|---|---|---|
| Untreated sample (contam. 102 CFU/g) | 3 × 10 CFU/g | 3 × 10 CFU/g | Untreated sample not contaminated | 3.0 × 104 CFU/g | 2.8 × 107 CFU/g |
| NO2− treated sample (contam. 102 CFU/g) | 2 × 10 CFU/g | 0 | Untreated sample (contam. 106 CFU/g) | 4.5 × 106 CFU/g | 2.7 × 107 CFU/g |
| Untreated sample (contam. 104 CFU/g) | 4 × 10 CFU/g | 1.5 × 102 CFU/g | NO2− treated sample (contam. 106 CFU/g) | 1.7 × 106 CFU/g | 9.0 × 105 CFU/g |
| NO2− treated sample (contam. 104 CFU/g) | 4 × 10 CFU/g | 2 × 10 CFU/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summa, S.; Iammarino, M.; Lo Magro, S.; D'Antini, P.; La Salandra, G.; Basanisi, M.G.; Nobili, G.; Berardi, G.; Langianese, M.E.; La Bella, G.; et al. Illegal Nitrite Treatment of Red Tuna and Prolonged Storage: What About Other Food Safety Risks? Appl. Sci. 2025, 15, 3975. https://doi.org/10.3390/app15073975
Summa S, Iammarino M, Lo Magro S, D'Antini P, La Salandra G, Basanisi MG, Nobili G, Berardi G, Langianese ME, La Bella G, et al. Illegal Nitrite Treatment of Red Tuna and Prolonged Storage: What About Other Food Safety Risks? Applied Sciences. 2025; 15(7):3975. https://doi.org/10.3390/app15073975
Chicago/Turabian StyleSumma, Simona, Marco Iammarino, Sonia Lo Magro, Pasqualino D'Antini, Giovanna La Salandra, Maria Grazia Basanisi, Gaia Nobili, Giovanna Berardi, Marco Emanuele Langianese, Gianfranco La Bella, and et al. 2025. "Illegal Nitrite Treatment of Red Tuna and Prolonged Storage: What About Other Food Safety Risks?" Applied Sciences 15, no. 7: 3975. https://doi.org/10.3390/app15073975
APA StyleSumma, S., Iammarino, M., Lo Magro, S., D'Antini, P., La Salandra, G., Basanisi, M. G., Nobili, G., Berardi, G., Langianese, M. E., La Bella, G., & Muscarella, M. (2025). Illegal Nitrite Treatment of Red Tuna and Prolonged Storage: What About Other Food Safety Risks? Applied Sciences, 15(7), 3975. https://doi.org/10.3390/app15073975







