Evaluating Changes in the VOC Profile of Different Types of Food Products After Electron Beam Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Samples and Sample Preparation for Analysis
2.2. Irradiation
2.3. Dosimetric Control
2.4. Equipment
2.5. GC-MS Analysis
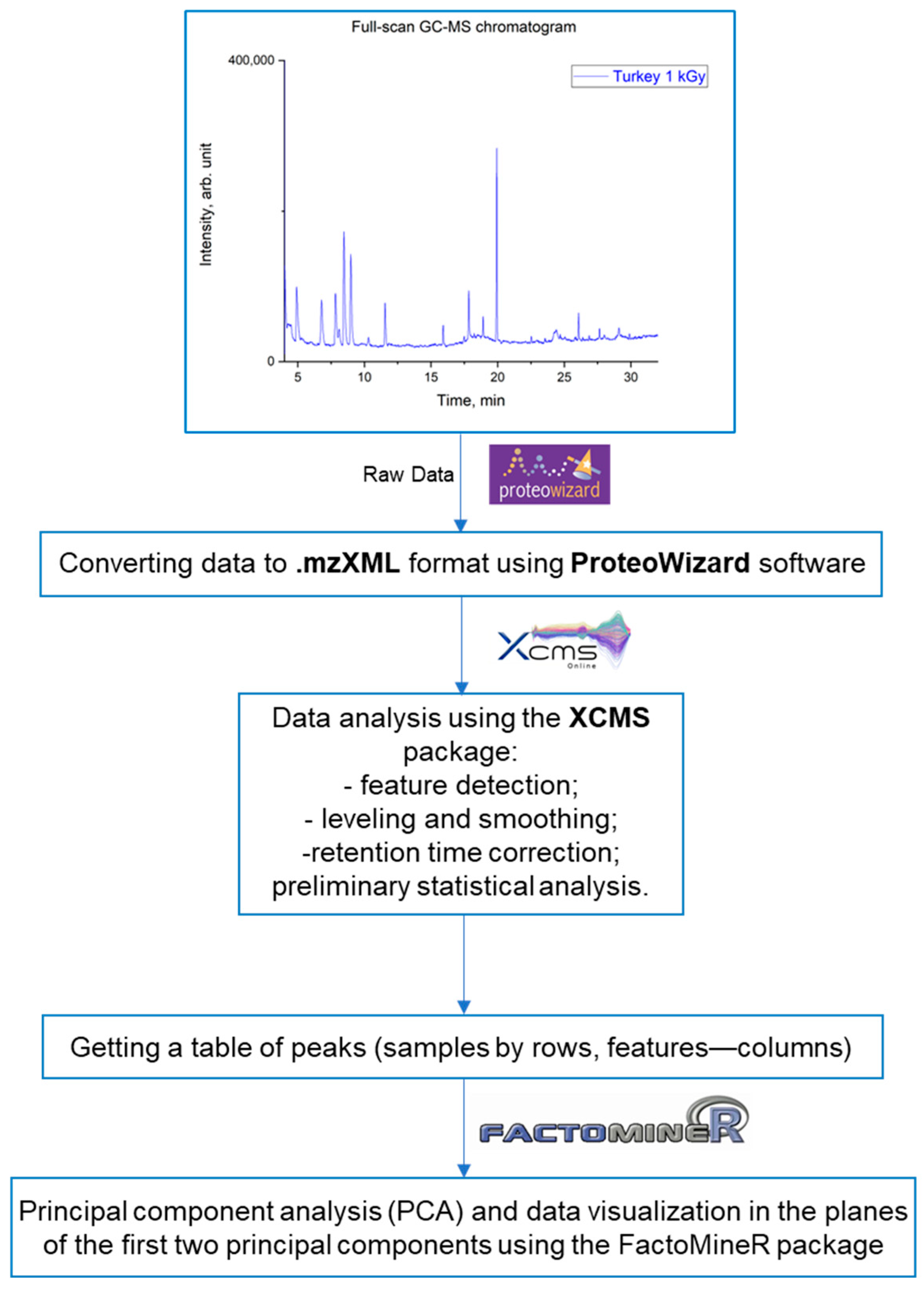
2.6. Chemometric Analysis
3. Results and Discussion
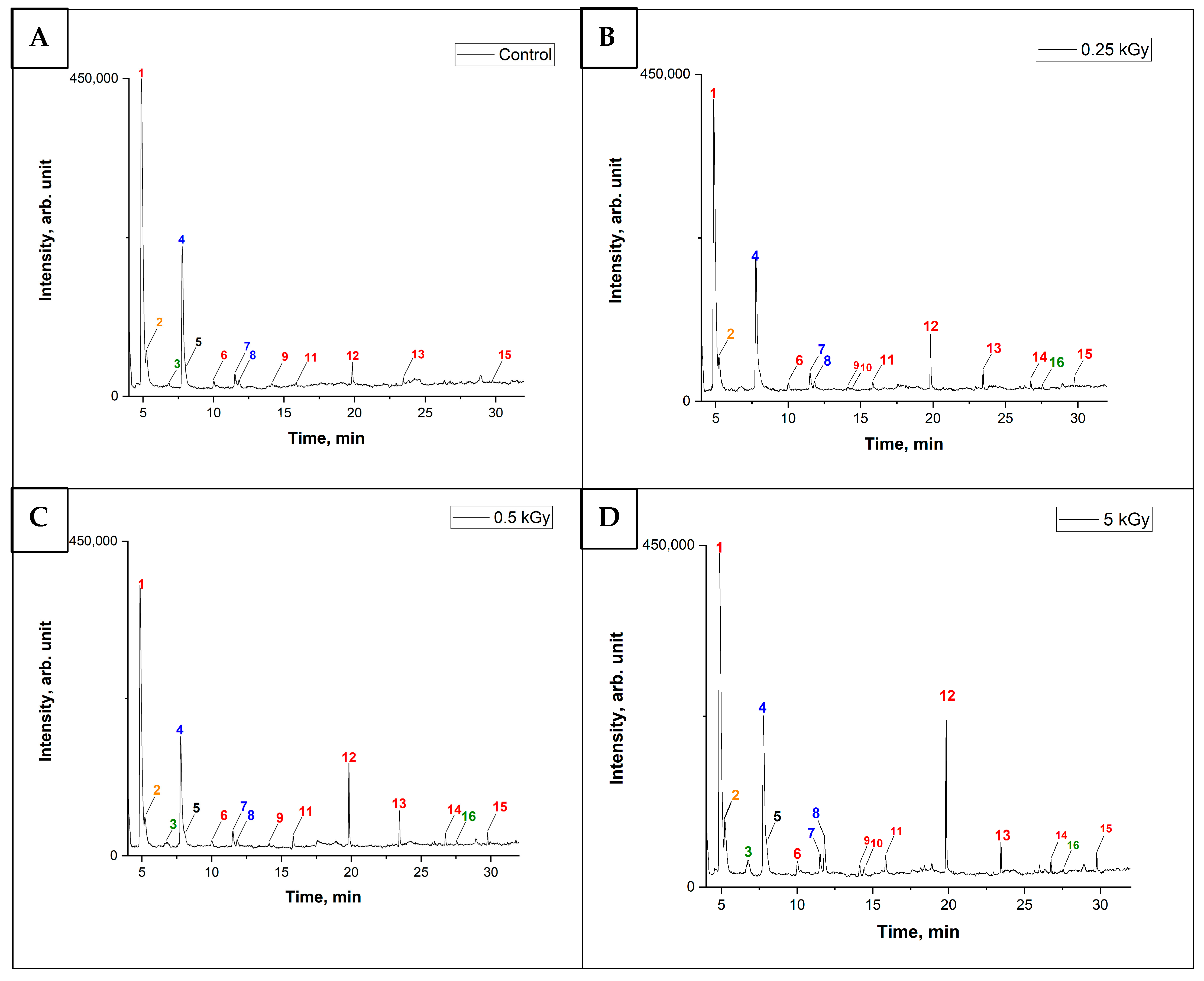
3.1. Optimization of GC-MS Analysis Conditions
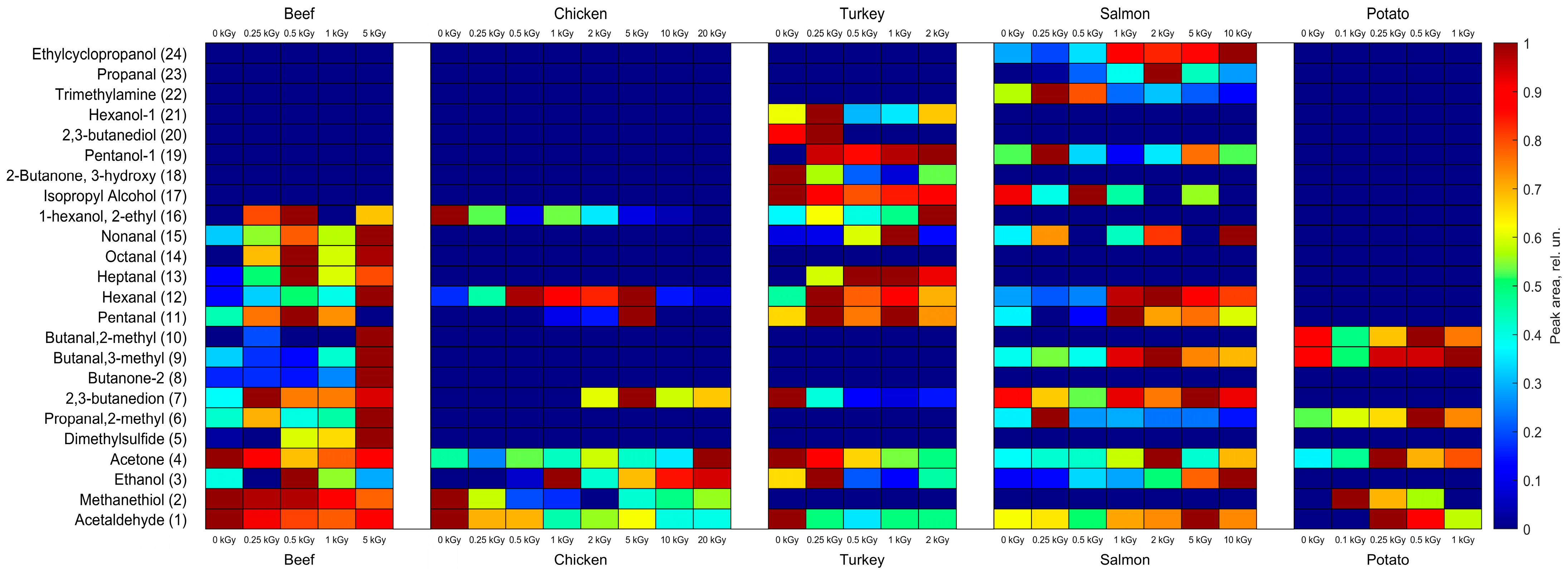
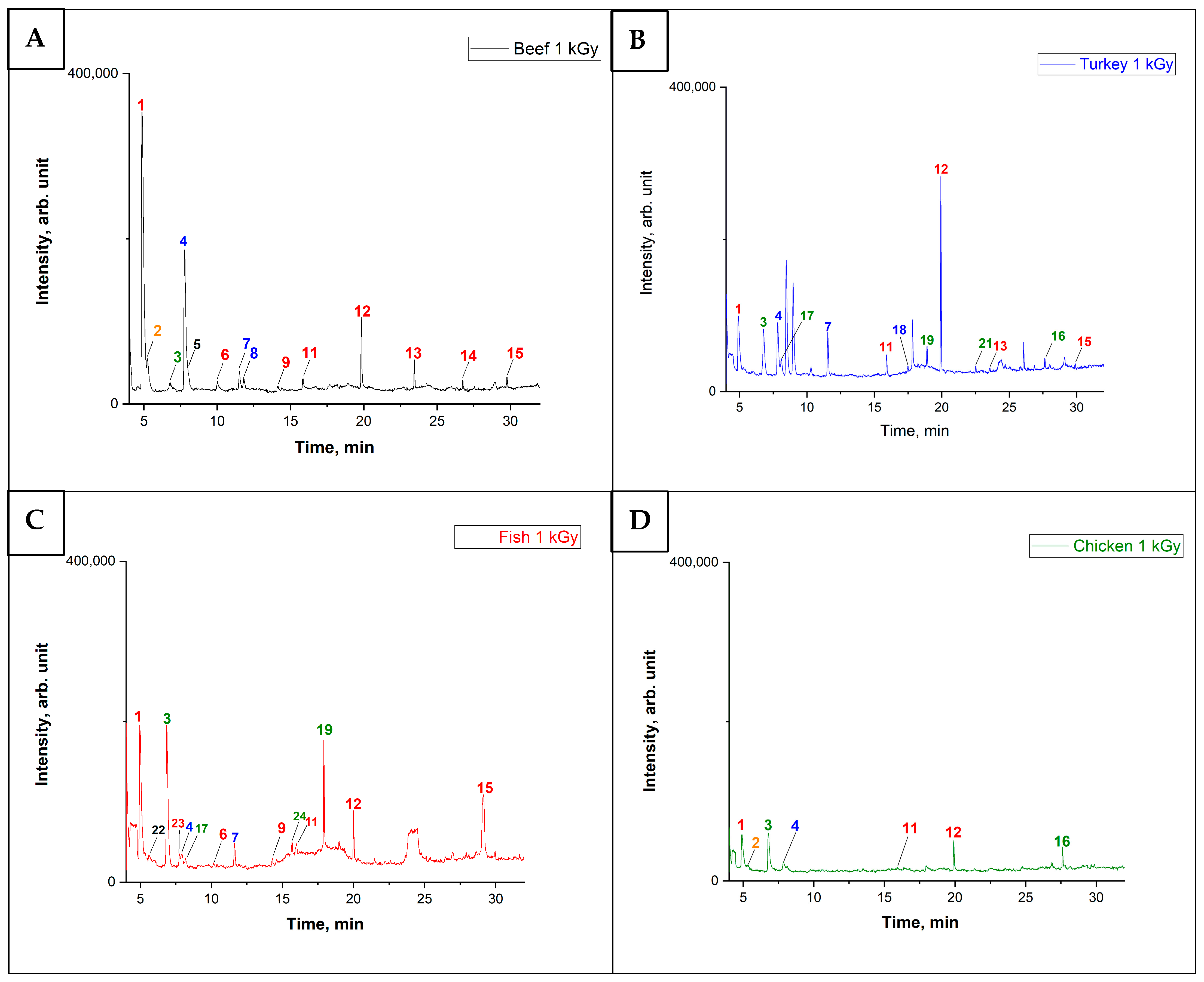
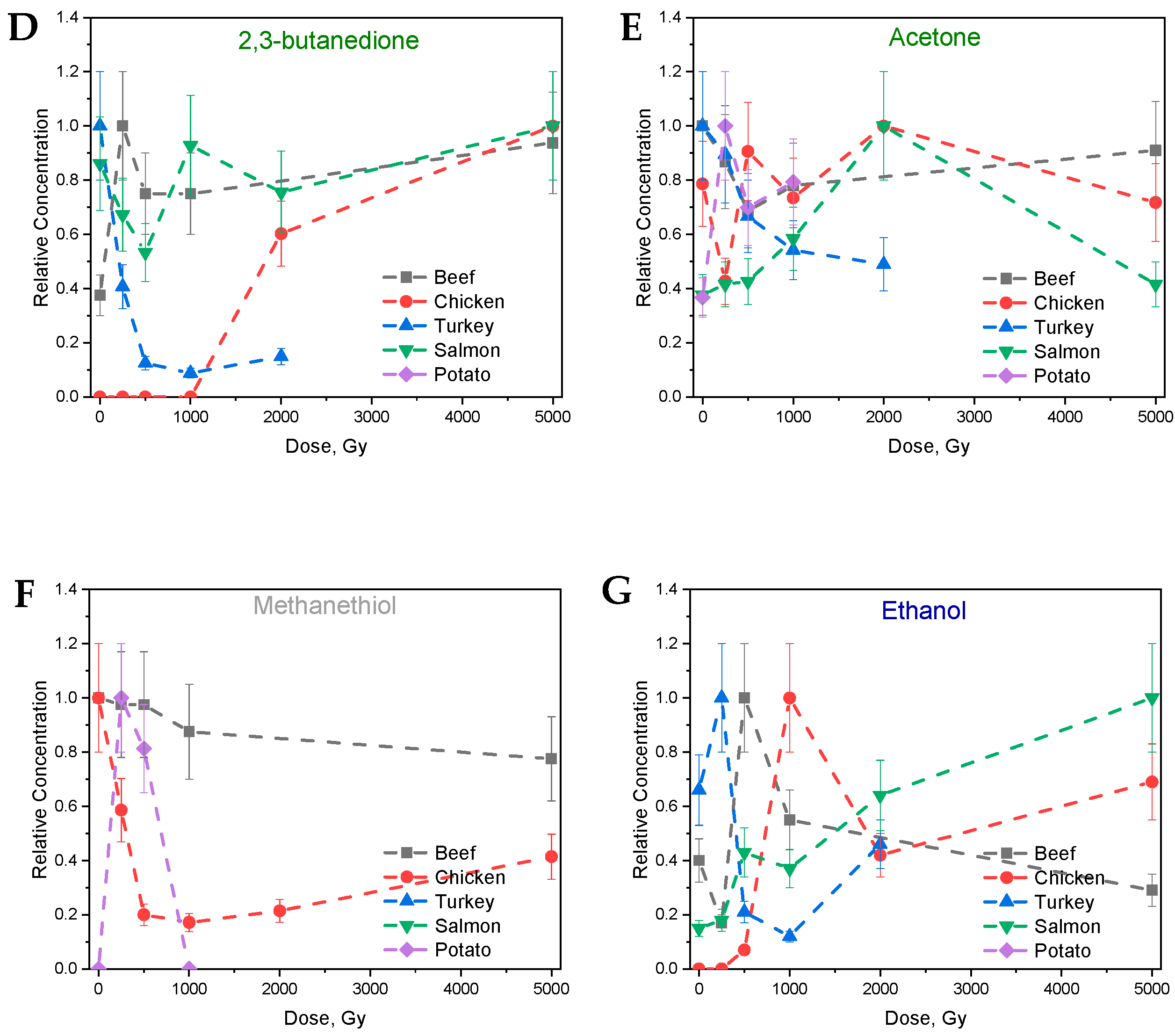
3.2. The Behavior of VOCs in the Food Samples After Irradiation
3.2.1. Aldehydes
3.2.2. Ketones
3.2.3. Alcohols
3.2.4. Other Compounds
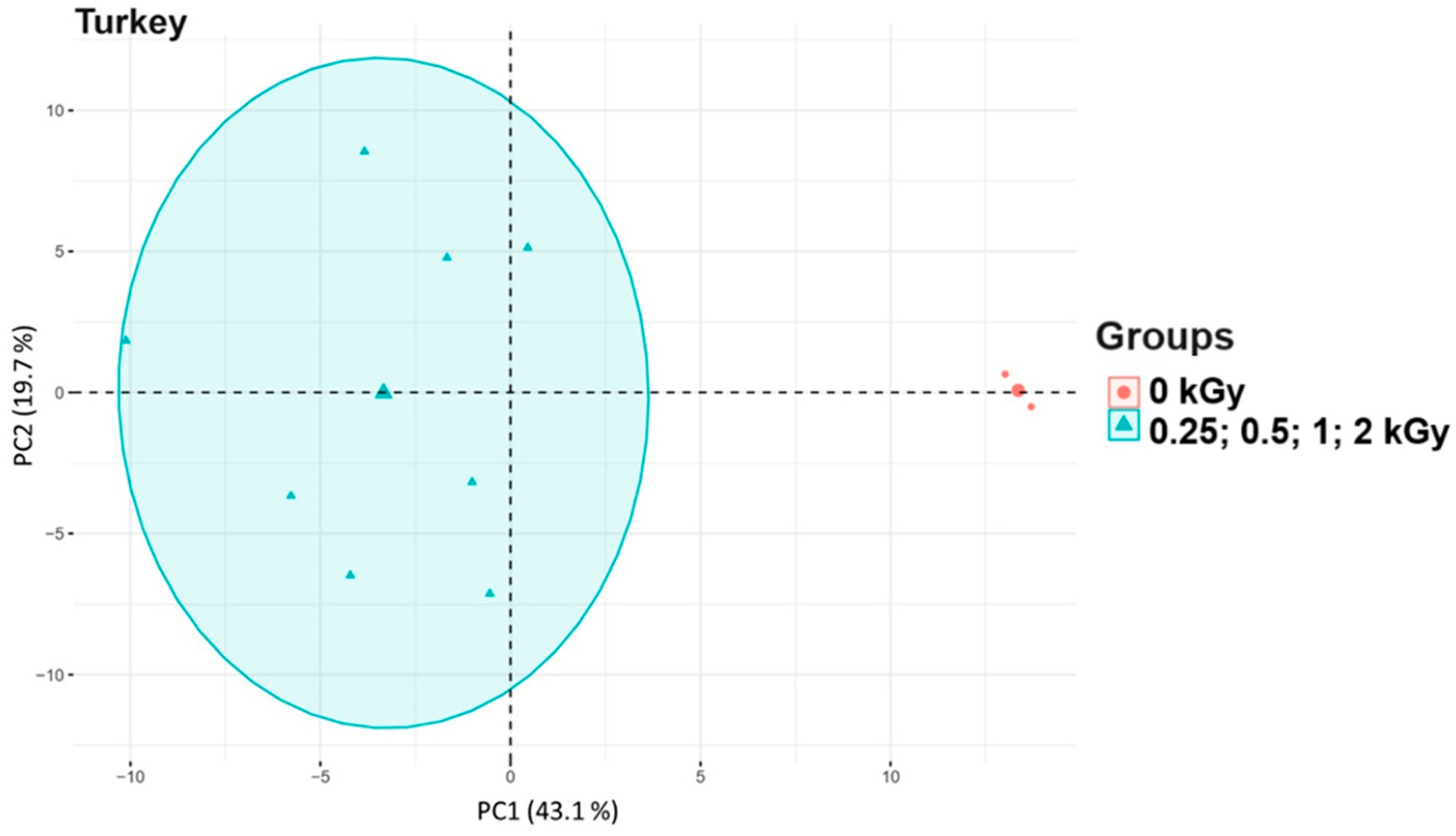
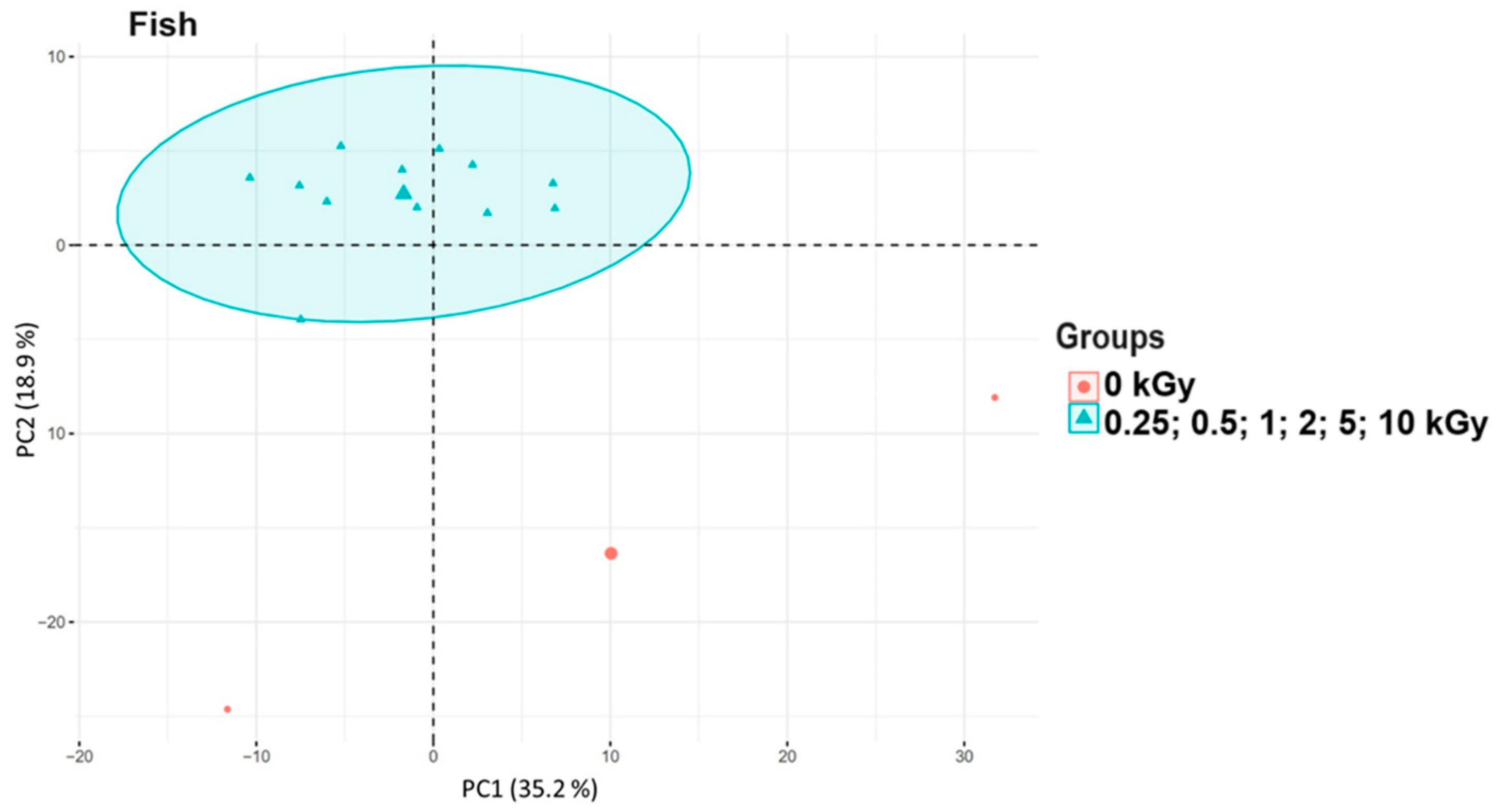
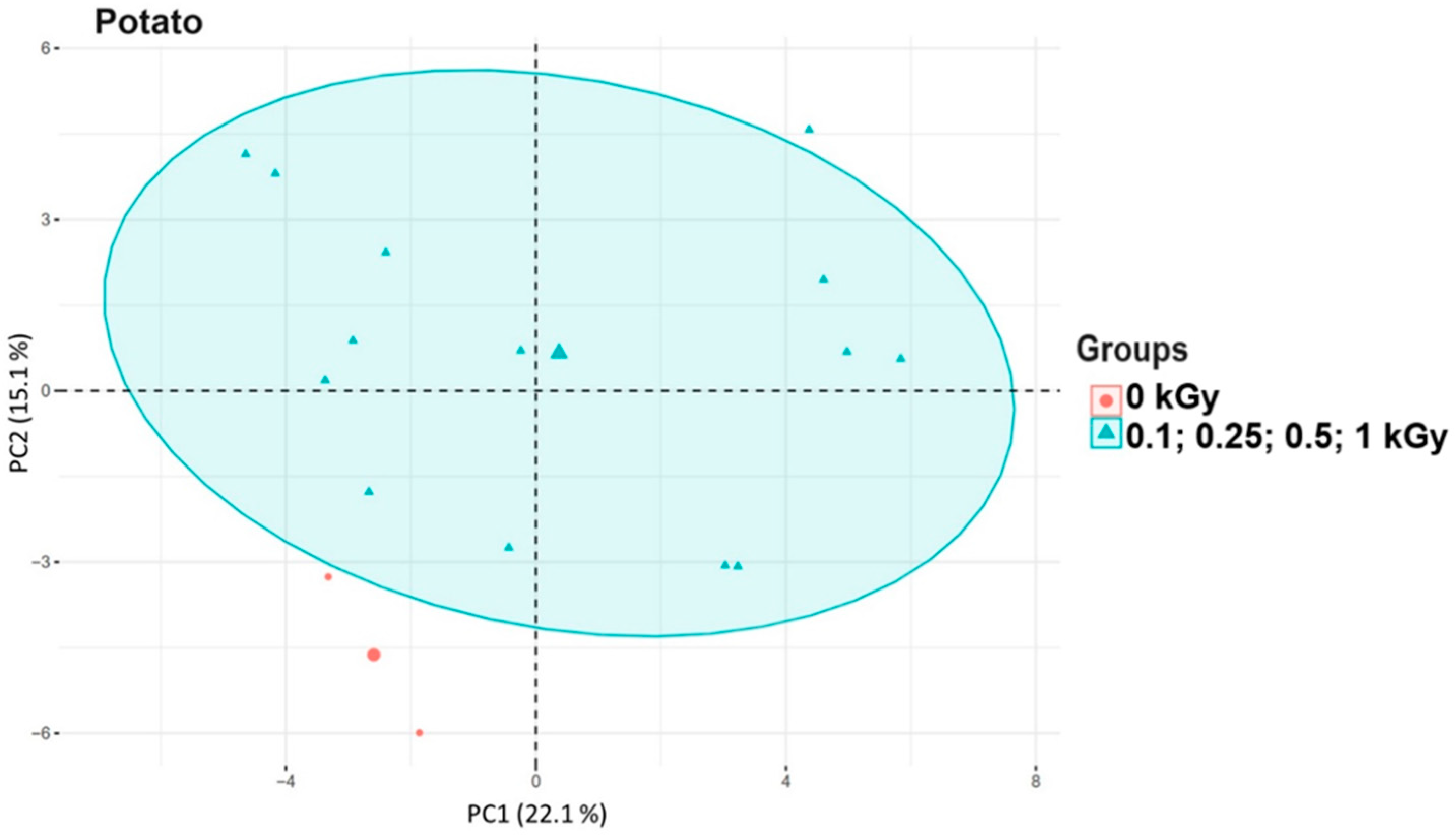
3.3. Principal Component Analysis of Food Samples After Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| VOCs | Retention Time, min | Similarity Search *, %/Peak Area, TIC × 105 | ||||
|---|---|---|---|---|---|---|
| Acetaldehyde (1) | Dose 0 kGy | Dose 0.25 kGy | Dose 0.5 kGy | Dose 1 kGy | Dose 5 kGy | |
| 4.88 | 98/44 ± 9 | 98/41 ± 8 | 98/36 ± 7 | 98/35 ± 7 | 98/39 ± 8 | |
| Methanethiol (2) | 5.23 | 95/4.0 ± 1.2 | 93/3.9 ± 1.2 | 94/3.9 ± 1.2 | 94/3.5 ± 1.1 | 96/3.1 ± 0.9 |
| Ethanol (3) | 6.85 | 76/0.26 ± 0.09 | ND 1 | 77/0.65 ± 0.23 | 76/0.36 ± 0.13 | 85/0.19 ± 0.07 |
| Acetone (4) | 7.77 | 96/18 ± 4 | 97/16 ± 3 | 97/13 ± 3 | 97/14 ± 3 | 97/17 ± 3 |
| Dimethylsulfide (5) | 7.93 | 94/0.083 ± 0.029 | ND | 89/2.1 ± 0.6 | 90/2.3 ± 0.7 | 94/3.5 ± 1.1 |
| Propanal,2-methyl (6) | 10.02 | 86/0.42 ± 0.15 | 93/0.70 ± 0.25 | 85/0.40 ± 0.14 | 87/0.46 ± 0.16 | 93/1.0 ± 0.4 |
| 2,3-butanedione (7) | 11.51 | 94/0.60 ± 0.21 | 94/1.6 ± 0.5 | 94/1.2 ± 0.4 | 93/1.2 ± 0.4 | 95/1.5 ± 0.5 |
| Butanone-2 (8) | 11.80 | 82/0.45 ± 0.16 | 88/0.48 ± 0.17 | 82/0.41 ± 0.14 | 88/0.72 ± 0.25 | 96/2.8 ± 0.8 |
| Butanal,3-methyl (9) | 14.12 | 80/0.24 ± 0.08 | 81/0.13 ± 0.05 | 84/0.10 ± 0.04 | 82/0.31 ± 0.11 | 86/0.74 ± 0.26 |
| Butanal,2-methyl (10) | 14.43 | ND | 77/0.098 ± 0.034 | ND | ND | 87/0.49 ± 0.15 |
| Pentanal (11) | 15.84 | 78/0.30 ± 0.11 | 87/0.51 ± 0.18 | 90/0.67 ± 0.23 | 85/0.49 ± 0.17 | 90/0.86 ± 0.30 |
| Hexanal (12) | 19.83 | 92/1.2 ± 0.4 | 94/2.9 ± 0.9 | 95/4.5 ± 1.4 | 95/3.5 ± 1.1 | 95/8.9 ± 2.7 |
| Heptanal (13) | 23.45 | 85/0.25 ± 0.09 | 96/1.0 ± 0.3 | 97/2.0 ± 0.6 | 94/1.2 ± 0.4 | 96/1.6 ± 0.5 |
| Octanal (14) | 26.75 | ND | 89/0.42 ± 0.15 | 93/0.61 ± 0.21 | 87/0.36 ± 0.13 | 91/0.60 ± 0.21 |
| Nonanal (15) | 29.77 | 89/0.25 ± 0.09 | 92/0.43 ± 0.15 | 92/0.61 ± 0.21 | 89/0.45 ± 0.16 | 91/0.78 ± 0.27 |
| 1-hexanol, 2-ethyl (16) | 27.54 | ND | 80/0.20 ± 0.07 | 86/0.25 ± 0.09 | ND | 89/0.17 ± 0.06 |
| VOCs | Retention Time, min | Similarity Search *, %/Peak Area, TIC × 105 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose 0 kGy | Dose 0.25 kGy | Dose 0.5 kGy | Dose 1 kGy | Dose 2 kGy | Dose 5 kGy | Dose 10 kGy | Dose 20 kGy | ||
| Acetaldehyde (1) | 4.88 | 97/71 ± 14 | 97/50 ± 10 | 97/50 ± 10 | 96/32 ± 6 | 97/40 ± 8 | 97/44 ± 9 | 95/28 ± 6 | 96/28 ± 6 |
| Methanethiol (2) | 5.23 | 88/7.0 ± 2.1 | 87/4.1 ± 1.2 | 84/1.4 ± 0.4 | 90/1.2 ± 0.4 | ND 1 | 80/2.9 ± 0.9 | 95/3.4 ± 1.1 | 84/3.9 ± 1.2 |
| Ethanol (3) | 6.78 | ND | ND | 87/2.5 ± 0.8 | 97/37 ± 7 | 97/15 ± 3 | 97/25 ± 5 | 98/31 ± 6 | 97/35 ± 7 |
| Acetone (4) | 7.77 | 93/9.2 ± 2.7 | 89/5.0 ± 1.5 | 91/11 ± 2 | 91/8.6 ± 2.3 | 85/12 ± 2 | 93/8.4 ± 2.5 | 90/7.0 ± 0.2.1 | 93/20 ± 4 |
| 2,3-Butanedione (7) | 11.56 | ND | ND | ND | ND | 87/4.7 ± 1.4 | 91/7.8 ± 2.3 | 92/4.6 ± 1.4 | 83/5.3 ± 1.6 |
| Pentanal (11) | 15.91 | ND | 87/2.8 ± 0.8 | 82/7.2 ± 2.2 | 81/0.65 ± 0.23 | 82/0.10 ± 0.04 | 86/0.66 ± 0.23 | ND | ND |
| Hexanal (12) | 19.92 | 90/12 ± 2 | 93/33 ± 7 | 93/72 ± 14 | 91/65 ± 13 | 92/61 ± 18 | 92/73 ± 22 | 91/11 ± 3 | 86/5.9 ± 1.8 |
| 1-hexanol, 2-ethyl (16) | 27.62 | 95/16 ± 3 | 95/8.6 ± 2.6 | 86/1.5 ± 0.5 | 92/8.7 ± 2.6 | 87/5.7 ± 1.7 | 87/1.5 ± 0.5 | 87/0.74 ± 0.26 | ND |
| VOCs | Retention Time, min | Similarity Search *, %/Peak Area, TIC × 105 | ||||
|---|---|---|---|---|---|---|
| Acetaldehyde (1) | Dose 0 kGy | Dose 0.25 kGy | Dose 0.5 kGy | Dose 1 kGy | Dose 2 kGy | |
| 4.90 | 98/12 ± 2 | 98/6.1 ± 1.8 | 97/4.3 ± 1.3 | 97/6.1 ± 1.8 | 98/6.0 ± 1.8 | |
| Ethanol (3) | 6.79 | 98/21 ± 4 | 98/32 ± 6 | 96/6.8 ± 2.0 | 94/3.8 ± 1.1 | 97/15 ± 3 |
| Acetone (4) | 7.83 | 96/9.6 ± 2.9 | 97/8.6 ± 2.6 | 97/6.4 ± 1.9 | 97/5.2 ± 1.6 | 96/4.7 ± 1.4 |
| Isopropyl Alcohol (17) | 8.09 | 86/1.9 ± 0.6 | 93/1.7 ± 0.5 | 92/1.5 ± 0.5 | 90/1.6 ± 0.5 | 89/1.7 ± 0.5 |
| 2,3-butanedione (7) | 11.55 | 98/33 ± 7 | 98/13 ± 3 | 98/4.1 ± 1.2 | 97/2.9 ± 0.9 | 98/4.9 ± 1.5 |
| Pentanal (11) | 15.18 | 88/0.73 ± 0.26 | 91/1.1 ± 0.3 | 90/0.83 ± 0.29 | 90/1.1 ± 0.3 | 89/0.80 ± 0.28 |
| 2-Butanone, 3-hydroxy (18) | 17.49 | 96/3.0 ± 0.9 | 93/1.7 ± 0.5 | 86/0.65 ± 0.23 | 83/0.24 ± 0.08 | 91/1.6 ± 0.5 |
| Pentanol-1 (19) | 18.91 | ND 1 | 86/0.94 ± 0.33 | 86/0.85 ± 0.3 | 90/0.96 ± 0.34 | 88/0.99 ± 0.35 |
| Hexanal (12) | 19.93 | 96/0.48 | 96/10.4 ± 3.1 | 95/8.1 ± 2.4 | 96/9.3 ± 2.8 | 95/7.3 ± 2.2 |
| 2,3-butanediol (20) | 20.77 | 82/2.2 ± 0.7 | 89/2.5 ± 0.8 | ND | ND | ND |
| Hexanol-1 (21) | 22.52 | 87/0.57 ± 0.20 | 91/0.93 ± 0.33 | 85/0.28 ± 0.10 | 83/0.33 ± 0.12 | 91/0.63 ± 0.22 |
| 1-hexanol, 2-ethyl (16) | 27.64 | 83/0.32 ± 0.11 | 90/0.54 ± 0.19 | 84/0.35 ± 0.12 | 88/0.42 ± 0.15 | 92/0.87 ± 0.3 |
| Heptanal (13) | 23.56 | ND | 81/0.077 ± 0.025 | 87/0.13 ± 0.05 | 90/0.13 ± 0.05 | 89/0.12 ± 0.04 |
| Nonanal (15) | 29.89 | 81/0.23 ± 0.08 | 85/0.25 ± 0.09 | 83/1.5 ± 0.5 | 84/2.5 ± 0.8 | 86/0.35 ± 0.11 |
| VOCs | Retention Time, min | Similarity Search *, %/Peak Area, TIC × 105 | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose 0 kGy | Dose 0.25 kGy | Dose 0.5 kGy | Dose 1 kGy | Dose 2 kGy | Dose 5 kGy | Dose 10 kGy | ||
| Acetaldehyde (1) | 4.98 | 98/140 ± 30 | 98/140 ± 30 | 98/110 ± 20 | 98/160 ± 30 | 98/160 ± 30 | 98/220 ± 40 | 98/160 ± 30 |
| Trimethylamine (22) | 5.55 | 94/11 ± 2 | 93/20 ± 4 | 87/16 ± 3 | 80/4.5 ± 1.4 | 85/6.2 ± 1.9 | 82/4.2 ± 1.3 | 88/2.4 ± 0.7 |
| Ethanol (3) | 6.88 | 98/63 ± 13 | 98/75 ± 15 | 98/180 ± 40 | 98/160 ± 30 | 98/270 ± 50 | 98/430 ± 90 | 98/550 ± 110 |
| Propanal (23) | 7.75 | ND 1 | 87/0.26 ± 0.09 | 91/2.5 ± 0.8 | 92/4.4 ± 1.3 | 95/11 ± 3 | 88/4.9 ± 1.5 | 85/3.1 ± 0.9 |
| Acetone (4) | 7.95 | 84/3.8 ± 1.1 | 86/4.2 ± 1.3 | 87/4.3 ± 1.3 | 92/5.9 ± 1.8 | 89/10.1 ± 3.0 | 80/4.2 ± 1.3 | 90/7.0 ± 2.1 |
| Isopropyl Alcohol (17) | 8.21 | 86/5.6 ± 1.7 | 88/2.4 ± 0.7 | 88/6.1 ± 1.8 | 85/2.8 ± 0.8 | ND | 87/3.4 ± 1.0 | ND |
| Propanal, 2-methyl (6) | 10.20 | 86/3.2 ± 1.0 | 87/8.9 ± 2.7 | 87/2.4 ± 0.7 | 91/2.6 ± 0.8 | 83/2.1 ± 0.6 | 85/2.1 ± 0.6 | 93/1.3 ± 0.4 |
| 2,3-butanedion (7) | 11.64 | 93/16 ± 3 | 95/12 ± 2 | 93/9.6 ± 2.9 | 93/17 ± 3 | 94/14 ± 3 | 95/18 ± 4 | 93/17 ± 3 |
| Butanal, 3-metlyl- (9) | 14.28 | 80/3.2 ± 1.0 | 82/3.2 ± 1.0 | 80/2.3 ± 0.7 | 82/5.5 ± 1.7 | 84/5.9 ± 1.8 | 78/4.4 ± 1.3 | 79/4.1 ± 1.2 |
| Ethylcyclopropanol (24) | 15.60 | 87/2.1 ± 0.6 | 86/1.4 ± 0.4 | 88/2.5 ± 0.8 | 87/6.6 ± 2.0 | 88/6.1 ± 1.8 | 87/6.3 ± 1.9 | 88/7.3 ± 2.2 |
| Pentanal (11) | 15.79 | 83/3.1 ± 0.9 | ND | 80/1.0 ± 0.4 | 83/8.5 ± 2.6 | 86/6.1 ± 1.8 | 81/6.5 ± 2.0 | 80/5.1 ± 1.5 |
| Pentanol-1 (19) | 17.87 | 84/1.1 ± 0.3 | 79/2.1 ± 0.6 | 86/0.70 ± 0.25 | 79/0.23 ± 0.08 | 85/0.74 ± 0.26 | 88/1.6 ± 0.5 | 91/1.1 ± 0.3 |
| Hexanal (12) | 19.80 | 88/6.6 ± 2.0 | 90/4.9 ± 1.5 | 89/5.9 ± 1.8 | 91/2.3 ± 0.7 | 93/24 ± 5 | 93/21 ± 4 | 92/19 ± 4 |
| Nonanal (15) | 29.80 | 81/1.6 ± 0.5 | 84/3.2 ± 1.0 | ND | 84/1.9 ± 0.6 | 87/3.6 ± 1.1 | ND | 90/4.4 ± 1.3 |
| VOCs | Retention Time, min | Similarity Search *, %/Peak Area, TIC × 105 | ||||
|---|---|---|---|---|---|---|
| Acetaldehyde (1) | Dose 0 kGy | Dose 0.1 kGy | Dose 0.25 kGy | Dose 0.5 kGy | Dose 1 kGy | |
| 4.80 | ND 1 | ND | 80/1.9 ± 0.6 | 79/1.7 ± 0.5 | 79/1.1 ± 0.3 | |
| Methanethiol (2) | 5.26 | ND | 80/4.6 ± 1.4 | 83/3.2 ± 1.0 | 87/2.6 ± 0.8 | ND |
| Acetone (4) | 7.80 | 88/9.4 ± 2.8 | 91/12 ± 2 | 95/26 ± 5 | 94/18 ± 4 | 93/20 ± 4 |
| Propanal,2-methyl (6) | 9.94 | 92/4.5 ± 1.4 | 86/5.1 ± 1.5 | 88/5.6 ± 1.7 | 89/8.5 ± 2.6 | 84/6.3 ± 1.9 |
| Butanal,3-methyl (9) | 14.1 | 81/3.3 ± 1.0 | 79/1.9 ± 0.6 | 80/3.6 ± 1.1 | 84/3.6 ± 1.1 | 76/3.8 ± 1.1 |
| Butanal,2-methyl (10) | 14.4 | 84/3.7 ± 1.1 | 80/2.0 ± 0.6 | 77/2.8 ± 0.8 | 87/4.1 ± 1.2 | 79/3.1 ± 0.9 |
References
- Hallman, G.J. Control of stored product pests by ionizing radiation. J. Stored Prod. Res. 2013, 52, 36–41. [Google Scholar] [CrossRef]
- Lizikova, M.S. The role of the IAEA in solving problems in the field of food and agriculture. Agrar. Land Law 2020, 7, 111–115. [Google Scholar]
- IAEA Report 2023. Available online: https://www.iaea.org/sites/default/files/gc/gc67-inf4_rus_1.pdf (accessed on 10 December 2024).
- Mshelia, R.D.; Dibal, N.I.; Chiroma, S.M. Food irradiation: An effective but under-utilized technique for food preservations. J. Food Sci. Technol. 2023, 60, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Mir, S.A.; Pala, S.A. Enhancing food safety and stability through irradiation: A review. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 371–378. [Google Scholar]
- Gautam, S.; Tripathi, J. Food Processing by Irradiation–An effective technology for food safety and security. Indian J. Exp. Biol. 2016, 54, 700–707. [Google Scholar]
- Joshua Ajibola, O. An overview of irradiation as a food preservation technique. Nov. Res. Microbiol. J. 2020, 4, 779–789. [Google Scholar]
- Indiarto, R.; Pratama, A.W.; Sari, T.I.; Theodora, H.C. Food irradiation technology: A review of the uses and their capabilities. Int. J. Eng. Trends Technol. 2020, 68, 91–98. [Google Scholar] [CrossRef]
- Yang, J.; Pan, M.; Han, R.; Yang, X.; Liu, X.; Yuan, S.; Wang, S. Food irradiation: An emerging processing technology to improve the quality and safety of foods. Food Rev. Int. 2023, 40, 2321–2343. [Google Scholar] [CrossRef]
- Chulikova, N.; Malyuga, A.; Borshchegovskaya, P.; Zubritskaya, Y.; Ipatova, V.; Chernyaev, A.; Yurov, D.; Zolotov, S.; Nikitchenko, A.; Bliznyuk, U.; et al. Electron Beam Irradiation to Control Rhizoctonia solani in Potato. Agriculture 2023, 13, 1221. [Google Scholar] [CrossRef]
- Bliznyuk, U.; Avdyukhina, V.; Borshchegovskaya, P.; Bolotnik, T.; Ipatova, V.; Nikitina, Z.; Nikitchenko, A.; Rodin, I.; Studenikin, F.; Chernyaev, A.; et al. Effect of electron and X-ray irradiation on microbiological and chemical parameters of chilled turkey. Sci. Rep. 2020, 12, 750. [Google Scholar] [CrossRef]
- Yurna, D.A.; Frolov, D.I. Technologies for safe conservation of products of animal origin. Innov. Technol. Technol. 2023, 10, 44–51. [Google Scholar]
- Chauhan, S.K.; Kumar, R.; Nadanasabapathy, S.; Bawa, A.S. Detection methods for irradiated foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 4–16. [Google Scholar] [CrossRef]
- Zeb, A.; Ullah, F. A Simple Spectrophotometric Method for the Determination of Thiobarbituric Acid Reactive Substances in Fried Fast Foods. J. Anal. Methods Chem. 2016, 2016, 9412767. [Google Scholar] [CrossRef] [PubMed]
- Rieger, P.H. Electron spin resonance: Analysis and interpretation. In Royal Society of Chemistry; RSC Publishing: London, UK, 2007. [Google Scholar]
- Ukai, M.; Kameya, H.; Nakamura, H.; Shimoyama, Y. An electron spin resonance study of dry vegetables before and after irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 1417–1422. [Google Scholar] [CrossRef]
- Barba, F.J.; Roohinejad, S.; Ishikawa, K.; Leong, S.Y.; Bekhit, A.E.D.A.; Saraiva, J.A.; Lebovka, N. Electron spin resonance as a tool to monitor the influence of novel processing technologies on food properties. Trends Food Sci. Technol. 2020, 100, 77–87. [Google Scholar] [CrossRef]
- Jung, Y.K.; Lee, J.Y.; Kang, T.S.; Jo, C.H.; Lee, J.H.; Choi, J.D.; Kwon, K.S. Detection of irradiated food using photostimulated luminescence and thermoluminescence. Korean J. Food Sci. Technol. 2016, 48, 399–404. [Google Scholar] [CrossRef]
- Murthy, K.V.R.; Rey, L.; Belon, P. Photoluminescence and thermally stimulated luminescence characteristics of rice flour. J. Lumin. 2007, 122, 279–283. [Google Scholar] [CrossRef]
- Wahyono, T.; Firmansyah, A.M.; Febrisiantosa, A.; Setiyawan, A.I.; Karimy, M.F.; Trinugraha, A.C.; Sholikin, M.M.; Benita, A.M.; Jayanegara, A. Thiobarbituric Acid Reactive Substances (TBARS) and quality of poultry meat as affected by electron beam irradiation: A meta-analysis study. In Developing Modern Livestock Production in Tropical Countries; CRC Press: Boca Raton, FL, USA, 2023; pp. 34–38. [Google Scholar]
- Khalid, W.; Arshad, M.S.; Yasin, M.; Imran, A.; Ahmad, M.H. Quality characteristics of gamma irradiation and kale leaf powder treated ostrich and chicken meat during storage. Int. J. Food Prop. 2021, 24, 1335–1348. [Google Scholar] [CrossRef]
- Feiner, G. Meat and Fat. In Salami; Academic Press: Cambridge, MA, USA, 2016; pp. 3–30. [Google Scholar]
- Parke, D.N.; Rogers, R.W.; Althen, T.G.; Martin, J.M. Meat Irradiation. Prof. Anim. Sci. 2005, 21, 75–80. [Google Scholar] [CrossRef]
- Nam, H.A.; Ramakrishnan, S.R.; Kwon, J.H. Effects of electron-beam irradiation on the quality characteristics of mandarin oranges (Citrus unshiu (Swingle) Marcov) during storage. Food Chem. 2019, 286, 338–345. [Google Scholar] [CrossRef]
- Feng, X.; Moon, S.; Lee, H.; Ahn, D.U. Effect of irradiation on the parameters that influence quality characteristics of uncured and cured cooked turkey meat products. Poult. Sci. 2016, 95, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Lee-Rangel, H.A.; Mendoza-Martinez, G.D.; Diaz de León-Martínez, L.; Relling, A.E.; Vazquez-Valladolid, A.; Palacios-Martínez, M.; Roque-Jiménez, J.A. Application of an electronic nose and HS-SPME/GC-MS to determine volatile organic compounds in fresh mexican cheese. Foods 2022, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Peng, J.; Wang, T.; Zhou, T.; Zhao, H.; Zhang, T.; Yang, Z. Overcoming the limits of cross-sensitivity: Pattern recognition methods for chemiresistive gas sensor array. Nano-Micro Lett. 2024, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart Gas Sensors: Recent Developments and Future Prospective. Nano-Micro Lett. 2025, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Park, J.H.; Kim, J.K.; Park, H.Y.; Yoon, Y.S.; Eun, J.B.; Song, B.S. Rapid Identification Method for Gamma-Irradiated Soybeans Using Gas Chromatography–Mass Spectrometry Coupled with a Headspace Solid-Phase Microextraction Technique. J. Agric. Food Chem. 2020, 68, 2803–2815. [Google Scholar] [CrossRef]
- Zianni, R.; Mentana, A.; Tomaiuolo, M.; Campaniello, M.; Iammarino, M.; Centonze, D.; Palermo, C. Volatolomic approach by HS-SPME/GC–MS and chemometric evaluations for the discrimination of X-ray irradiated mozzarella cheese. Food Chem. 2020, 423, 136239. [Google Scholar] [CrossRef]
- Rababah, T.; Hettiarachchy, N.S.; Horax, R.; Cho, M.J.; Davis, B.; Dickson, J. Thiobarbituric acid reactive substances and volatile compounds in chicken breast meat infused with plant extracts and subjected to electron beam irradiation. Poult. Sci. 2006, 85, 1107–1113. [Google Scholar] [CrossRef]
- Li, C.; He, L.; Jin, G.; Ma, S.; Wu, W.; Gai, L. Effect of different irradiation dose treatment on the lipid oxidation, instrumental color and volatiles of fresh pork and their changes during storage. Meat Sci. 2017, 128, 68–76. [Google Scholar] [CrossRef]
- Bliznyuk, U.; Borshchegovskaya, P.; Bolotnik, T.; Chernyaev, A.; Ipatova, V.; Nikitchenko, A.; Shinkarev, O.; Yurov, D.; Khmelevskiy, O.; Rodin, I. Research into Gas Chromatography–Mass Spectrometry (GC-MS) for Ensuring the Effect of 1 MeV-Accelerated Electrons on Volatile Organic Compounds in Turkey Meat. Separations 2022, 9, 227. [Google Scholar] [CrossRef]
- Taghvaei, M.; Sommers, C.; Ceric, O.; Hussain, F.; Yucel, U.; Smith, J.S. Solid-phase micro extraction of food irradiation marker 2-dodecylcyclobutanone (2-DCB) from chicken jerky treated with glycerol. J. Food Sci. 2020, 85, 2608–2614. [Google Scholar] [CrossRef]
- Rodionova, O.E.; Pomerantsev, A.L. Chemometrics: Achievements and prospects. Adv. Chem. 2006, 75, 302–321. [Google Scholar]
- Bi, K.; Zhang, D.; Qiu, T.; Huang, Y. GC-MS fingerprints profiling using machine learning models for food flavor prediction. Processes 2019, 8, 23. [Google Scholar] [CrossRef]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Almenara, X.; Siuzdak, G. Metabolomics data processing using XCMS. In Computational Methods and Data Analysis for Metabolomics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 11–24. [Google Scholar]
- Gromski, P.S.; Xu, Y.; Hollywood, K.A.; Turner, M.L.; Goodacre, R. The influence of scaling metabolomics data on model classification accuracy. Metabolomics 2015, 11, 684–695. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Z.; Tang, C.; Liao, W.; Yu, Y.; Li, G.; Yang, Y.; An, T. Malodorous gases production from food wastes decomposition by indigenous microorganisms. Sci. Total Environ. 2020, 717, 137175. [Google Scholar] [CrossRef]
- Dinh, T.; To, K.; Schilling, M. Fatty Acid Composition of Meat Animals as Flavor Precursors. Meat Muscle Biol. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Kosowska, M.; Majcher, M.A.; Fortuna, T.; Majcher, M.A. Volatile Compounds in Meat and Meat Products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Kim, Y.; Kemp, R.; Samuelsson, L. Effects of dry aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168–176. [Google Scholar] [CrossRef]
- Zareian, M.; Böhner, N.; Loos, H.M.; Silcock, P.; Bremer, P.; Beauchamp, J. Evaluation of Volatile Organic Compound Release in Modified Atmosphere-Packaged Minced Raw Pork in Relation to Shelf-Life. Food Packag. Shelf Life 2018, 18, 51–61. [Google Scholar] [CrossRef]
- Bhadury, D.; Nolvachai, Y.; Marriott, P.J.; Tanner, J.; Tuck, K.L. Detection of Volatiles from Raw Beef Meat from Different Packaging Systems Using Solid-Phase Microextraction GC–Accurate Mass Spectrometry. Foods 2021, 10, 2018. [Google Scholar] [CrossRef] [PubMed]
- Legako, J.; Cramer, T.; Yardley, K.; Murphy, T.; Gardner, T.; Chail, A.; Pitcher, L.; MacAdam, J. Retail stability of three beef muscles from grass-, legume-, and feedlot-finished cattle. J. Anim. Sci. 2018, 96, 2238–2248. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research Update on the Impact of Lactic Acid Bacteria on the Substance Metabolism, Flavor, and Quality Characteristics of Fermented Meat Products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Oliveira, I.; Silva, J.A.; Martins, C.; Ventanas, J.; García, C. Implementation of multivariate techniques for the selection of volatile compounds as indicators of sensory quality of raw beef. Food Sci. Technol. 2015, 52, 3887–3898. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Głąbska, D.; Brodowska, M.; Sun, D.V.; Wierzbicka, A. Volatilecompounds and fatty acids profile in Longissimus dorsi muscle frompigs fed with feed containing bioactive components. LWT Food Sci. Technol. 2016, 67, 112–117. [Google Scholar] [CrossRef]
- Servillo, L.; D’onofrio, N.; Giovane, A.; Casale, R.; Cautela, D.; Castaldo, D.; Iannaccone, F.; Neglia, G.; Campanile, G.; Balestrieri, M.L. Ruminant meat and milk contain delta-valerobetaine, another precursor of trimethylamine N-oxide (TMAO) like gamma-butyrobetaine. Food Chem. 2018, 260, 193–199. [Google Scholar] [CrossRef]



| GC-MS Parameters | Value |
|---|---|
| Column | VF-624 MS (60 m × 0.32 mm, film thickness 1.8 µm) |
| Split mode | 1:1 |
| Evaporation chamber temperature | 250 °C |
| Column temperature | 40 °C (5 min) → (6 °C/min) 220 °C (5 min) |
| Carrier gas | Helium |
| Carrier gas flow rate | 1.5 cm3/min |
| Interface temperature 220 °C | 220 °C |
| Ion source temperature | 230 °C |
| Ionization mode | Electron ionization |
| Electron energy | 70 eV |
| Measurement mode | Scanning |
| Scanning range | m/z 33–350 |
| Scanning speed | 3.3 scan/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprunenko, A.; Bolotnik, T.; Ikhalaynen, Y.; Ipatova, V.; Bliznyuk, U.; Borshchegovskaya, P.; Yurov, D.; Bolotnik, N.; Kozlova, E.; Chernyaev, A.; et al. Evaluating Changes in the VOC Profile of Different Types of Food Products After Electron Beam Irradiation. Appl. Sci. 2025, 15, 1333. https://doi.org/10.3390/app15031333
Oprunenko A, Bolotnik T, Ikhalaynen Y, Ipatova V, Bliznyuk U, Borshchegovskaya P, Yurov D, Bolotnik N, Kozlova E, Chernyaev A, et al. Evaluating Changes in the VOC Profile of Different Types of Food Products After Electron Beam Irradiation. Applied Sciences. 2025; 15(3):1333. https://doi.org/10.3390/app15031333
Chicago/Turabian StyleOprunenko, Anastasia, Timofey Bolotnik, Yuri Ikhalaynen, Victoria Ipatova, Ulyana Bliznyuk, Polina Borshchegovskaya, Dmitry Yurov, Nadezhda Bolotnik, Elena Kozlova, Alexander Chernyaev, and et al. 2025. "Evaluating Changes in the VOC Profile of Different Types of Food Products After Electron Beam Irradiation" Applied Sciences 15, no. 3: 1333. https://doi.org/10.3390/app15031333
APA StyleOprunenko, A., Bolotnik, T., Ikhalaynen, Y., Ipatova, V., Bliznyuk, U., Borshchegovskaya, P., Yurov, D., Bolotnik, N., Kozlova, E., Chernyaev, A., Ananieva, I., & Rodin, I. (2025). Evaluating Changes in the VOC Profile of Different Types of Food Products After Electron Beam Irradiation. Applied Sciences, 15(3), 1333. https://doi.org/10.3390/app15031333









