Positron Emission Tomography in Cerebral Amyloid Angiopathy: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions
2.1.1. Cerebral Amyloid Angiopathy (CAA)
2.1.2. Positron Emission Tomography with Amyloid Tracers
2.2. Objectives
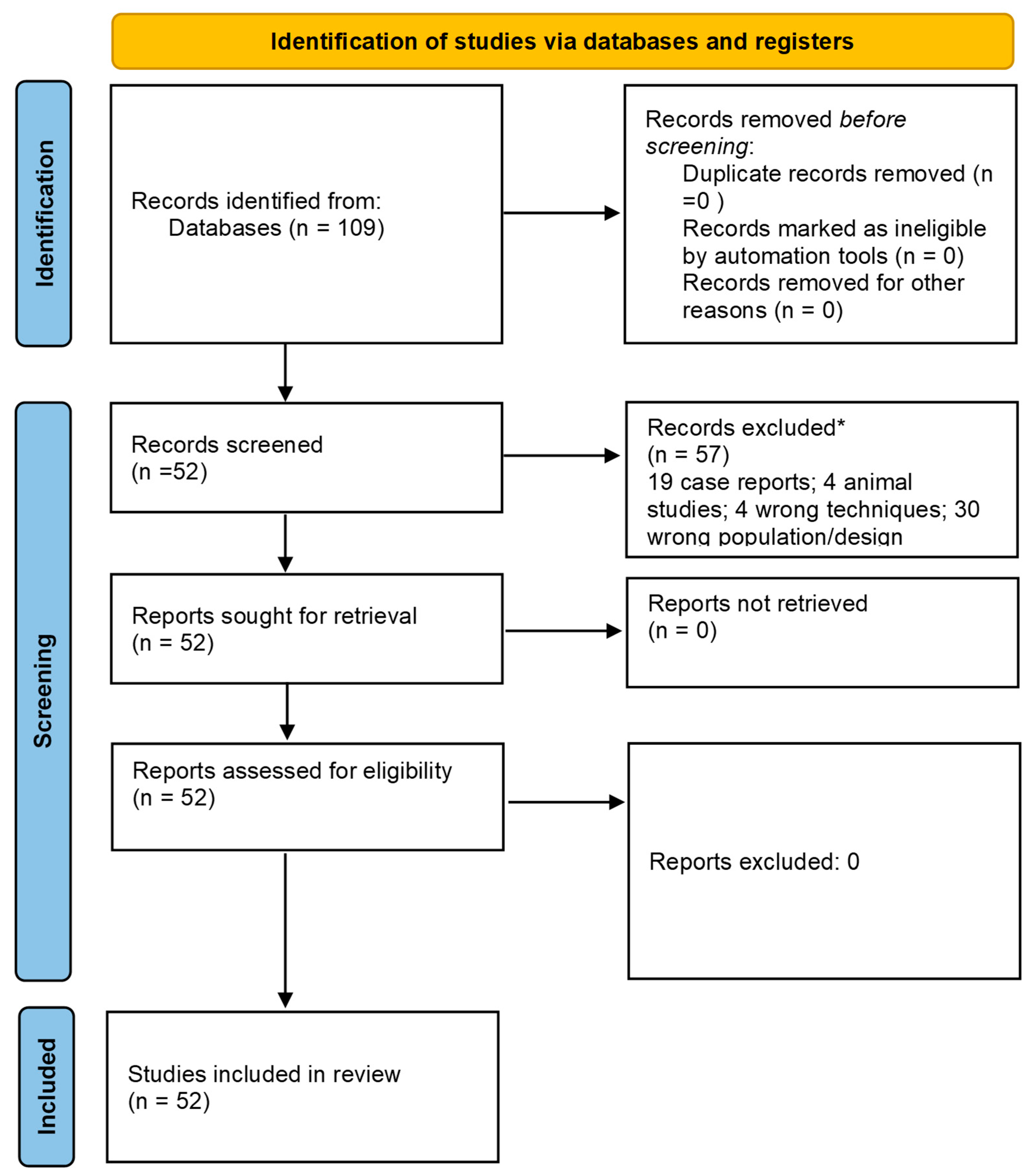
2.3. Literature Search
3. Results
3.1. Overview
3.2. CAA-Related ICH and SAH
3.3. Pathology-Proven CAA
3.4. Hereditary CAA
3.5. CAA-Related Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jokinen, H.; Koikkalainen, J.; Laakso, H.M.; Melkas, S.; Nieminen, T.; Brander, A.; Korvenoja, A.; Rueckert, D.; Barkhof, F.; Scheltens, P.; et al. Global Burden of Small Vessel Disease-Related Brain Changes on MRI Predicts Cognitive and Functional Decline. Stroke 2020, 51, 170–178. [Google Scholar] [CrossRef]
- Jansma, A.; de Bresser, J.; Schoones, J.W.; van Heemst, D.; Akintola, A.A. Sporadic cerebral small vessel disease and cognitive decline in healthy older adults: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2024, 44, 660–679. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef]
- Jäkel, L.; De Kort, A.M.; Klijn, C.J.M.; Schreuder, F.H.B.M.; Verbeek, M.M. Prevalence of cerebral amyloid angiopathy: A systematic review and meta-analysis. Alzheimers Dement. 2022, 18, 10–28. [Google Scholar] [CrossRef]
- Thal, D.R.; Attems, J.; Ewers, M. Spreading of amyloid, tau, and microvascular pathology in Alzheimer’s disease: Findings from neuropathological and neuroimaging studies. J. Alzheimers Dis. 2014, 42 (Suppl. S4), S421–S429. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Charidimou, A.; Farid, K.; Baron, J.C. Amyloid-PET in sporadic cerebral amyloid angiopathy: A diagnostic accuracy meta-analysis. Neurology 2017, 89, 1490–1498. [Google Scholar] [CrossRef]
- Piazza, F.; Winblad, B. Amyloid-Related Imaging Abnormalities (ARIA) in Immunotherapy Trials for Alzheimer’s Disease: Need for Prognostic Biomarkers? J. Alzheimers Dis. 2016, 52, 417–420. [Google Scholar] [CrossRef]
- Loeffler, D.A. Antibody-Mediated Clearance of Brain Amyloid-β: Mechanisms of Action, Effects of Natural and Monoclonal Anti-Aβ Antibodies, and Downstream Effects. J. Alzheimers Dis. Rep. 2023, 7, 873–899. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Jack, C.R., Jr.; Black, S.E.; Frosch, M.P.; Greenberg, S.M.; Hyman, B.T.; Scheltens, P.; Carrillo, M.C.; Thies, W.; Bednar, M.M.; et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011, 7, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Piazza, F.; Greenberg, S.M.; Savoiardo, M.; Gardinetti, M.; Chiapparini, L.; Raicher, I.; Nitrini, R.; Sakaguchi, H.; Brioschi, M.; Billo, G.; et al. Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: Implications for amyloid-modifying therapies. Ann. Neurol. 2013, 73, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Zedde, M.; Pascarella, R.; Piazza, F. CAA-ri and ARIA: Two Faces of the Same Coin? AJNR Am. J. Neuroradiol. 2023, 44, E13–E14. [Google Scholar] [CrossRef]
- Antolini, L.; Di Francesco, J.C.; Zedde, M.; Basso, G.; Arighi, A.; Shima, A.; Cagnin, A.; Caulo, M.; Carare, R.O.; Charidimou, A.; et al. Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study. Neurology 2021, 97, e1809–e1822. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Linn, J.; Halpin, A.; Demaerel, P.; Ruhland, J.; Giese, A.D.; Dichgans, M.; van Buchem, M.A.; Bruckmann, H.; Greenberg, S.M. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010, 74, 1346–1350. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Grabowski, T.; Gurol, M.E.; Skehan, M.E.; Nandigam, R.K.; Becker, J.A.; Garcia-Alloza, M.; Prada, C.; Frosch, M.P.; Rosand, J.; et al. Detection of isolated cerebrovascular beta-amyloid with Pittsburgh compound B. Ann. Neurol. 2008, 64, 587–591. [Google Scholar]
- Clark, C.M.; Schneider, J.A.; Bedell, B.J.; Beach, T.G.; Bilker, W.B.; Mintun, M.A.; Pontecorvo, M.J.; Hefti, F.; Carpenter, A.P.; Flitter, M.L.; et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 2011, 305, 275–283. [Google Scholar] [CrossRef]
- Papanastasiou, G.; Rodrigues, M.A.; Wang, C.; Heurling, K.; Lucatelli, C.; Al-Shahi Salman, R.; Wardlaw, J.M.; van Beek, E.J.R.; Thompson, G. Pharmacokinetic modelling for the simultaneous assessment of perfusion and 18F-flutemetamol uptake in cerebral amyloid angiopathy using a reduced PET-MR acquisition time: Proof of concept. Neuroimage 2021, 225, 117482. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Farid, K.; Charidimou, A.; Baron, J.C. Amyloid positron emission tomography in sporadic cerebral amyloid angiopathy: A systematic critical update. Neuroimage Clin. 2017, 15, 247–263. [Google Scholar] [CrossRef]
- Charidimou, A.; Farid, K.; Tsai, H.H.; Tsai, L.K.; Yen, R.F.; Baron, J.C. Amyloid-PET burden and regional distribution in cerebral amyloid angiopathy: A systematic review and meta-analysis of biomarker performance. J. Neurol. Neurosurg. Psychiatry 2018, 89, 410–417. [Google Scholar] [CrossRef]
- Romoli, M.; Marinoni, G.; Tagliabue, L.; Capozza, A.; Matteucci, F.; Mattone, V.; Longoni, M.; Cenni, P.; Ruggiero, M.; Rifino, N.; et al. 18FFlutemetamol-PET Aided Classification of Cerebral Amyloid Angiopathy: A Multicenter Study. Neurology 2024, 103, e209719. [Google Scholar] [CrossRef]
- Michiels, L.; Dobbels, L.; Demeestere, J.; Demaerel, P.; Van Laere, K.; Lemmens, R. Simplified Edinburgh and modified Boston criteria in relation to amyloid PET for lobar intracerebral hemorrhage. Neuroimage Clin. 2022, 35, 103107. [Google Scholar] [CrossRef]
- Tsai, H.H.; Pasi, M.; Tsai, L.K.; Huang, C.C.; Chen, Y.F.; Lee, B.C.; Yen, R.F.; Gurol, M.E.; Jeng, J.S. Centrum Semiovale Perivascular Space and Amyloid Deposition in Spontaneous Intracerebral Hemorrhage. Stroke 2021, 52, 2356–2362. [Google Scholar] [CrossRef]
- Tsai, H.H.; Pasi, M.; Tsai, L.K.; Chen, Y.F.; Lee, B.C.; Tang, S.C.; Fotiadis, P.; Huang, C.Y.; Yen, R.F.; Jeng, J.S.; et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: A PiB-PET study. Neurology 2019, 92, e774–e781. [Google Scholar] [CrossRef]
- Yost, M.; Fiebelkorn, C.A.; Rabinstein, A.A.; Klaas, J.; Aakre, J.A.; Brown, R.D., Jr.; Mielke, M.M.; Knopman, D.S.; Lowe, V.; Petersen, R.C.; et al. Incidence of Convexal Subarachnoid Hemorrhage in the Elderly: The Mayo Clinic Study of Aging. J. Stroke Cerebrovasc. Dis. 2019, 28, 104451. [Google Scholar] [CrossRef]
- Kim, J.; Na, H.K.; Shin, J.H.; Kim, H.J.; Seo, S.W.; Seong, J.K.; Na, D.L. Atrophy patterns in cerebral amyloid angiopathy with and without cortical superficial siderosis. Neurology 2018, 90, e1751–e1758. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.C.; Farid, K.; Dolan, E.; Turc, G.; Marrapu, S.T.; O’Brien, E.; Aigbirhio, F.I.; Fryer, T.D.; Menon, D.K.; Warburton, E.A.; et al. Diagnostic utility of amyloid PET in cerebral amyloid angiopathy-related symptomatic intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2014, 34, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Raposo, N.; Planton, M.; Payoux, P.; Péran, P.; Albucher, J.F.; Calviere, L.; Viguier, A.; Rousseau, V.; Hitzel, A.; Chollet, F.; et al. Enlarged perivascular spaces and florbetapir uptake in patients with intracerebral hemorrhage. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Planton, M.; Saint-Aubert, L.; Raposo, N.; Payoux, P.; Salabert, A.S.; Albucher, J.F.; Olivot, J.M.; Péran, P.; Pariente, J. Florbetapir Regional Distribution in Cerebral Amyloid Angiopathy and Alzheimer’s Disease: A PET Study. J. Alzheimers Dis. 2020, 73, 1607–1614. [Google Scholar] [CrossRef]
- Gokcal, E.; Horn, M.J.; Becker, J.A.; Das, A.S.; Schwab, K.; Biffi, A.; Rost, N.; Rosand, J.; Viswanathan, A.; Polimeni, J.R.; et al. Effect of vascular amyloid on white matter disease is mediated by vascular dysfunction in cerebral amyloid angiopathy. J. Cereb. Blood Flow Metab. 2022, 42, 1272–1281. [Google Scholar] [CrossRef]
- Raposo, N.; Planton, M.; Péran, P.; Payoux, P.; Bonneville, F.; Lyoubi, A.; Albucher, J.F.; Acket, B.; Salabert, A.S.; Olivot, J.M.; et al. Florbetapir imaging in cerebral amyloid angiopathy-related hemorrhages. Neurology 2017, 89, 697–704. [Google Scholar] [CrossRef]
- Ly, J.V.; Singhal, S.; Rowe, C.C.; Kempster, P.; Bower, S.; Phan, T.G. Convexity Subarachnoid Hemorrhage with PiB Positive Pet Scans: Clinical Features and Prognosis. J. Neuroimaging 2015, 25, 420–429. [Google Scholar] [CrossRef]
- Gurol, M.E.; Becker, J.A.; Fotiadis, P.; Riley, G.; Schwab, K.; Johnson, K.A.; Greenberg, S.M. Florbetapir-PET to diagnose cerebral amyloid angiopathy: A prospective study. Neurology 2016, 87, 2043–2049. [Google Scholar] [CrossRef]
- Jang, H.; Jang, Y.K.; Kim, H.J.; Werring, D.J.; Lee, J.S.; Choe, Y.S.; Park, S.; Lee, J.; Kim, K.W.; Kim, Y.; et al. Clinical significance of amyloid β positivity in patients with probable cerebral amyloid angiopathy markers. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1287–1298. [Google Scholar] [CrossRef]
- Pyun, J.M.; Kang, M.J.; Baek, S.J.; Lee, K.; Park, Y.H.; Kim, S.Y. Magnetic Resonance Imaging-Negative Cerebral Amyloid Angiopathy: Cerebrospinal Fluid Amyloid-β42 over Amyloid Positron Emission Tomography. J. Prev. Alzheimers Dis. 2024, 11, 1041–1046. [Google Scholar] [CrossRef]
- McCarter, S.J.; Lesnick, T.G.; Lowe, V.; Mielke, M.M.; Constantopoulos, E.; Rabinstein, A.A.; Przybelski, S.A.; Botha, H.; Jones, D.T.; Ramanan, V.K.; et al. Cerebral Amyloid Angiopathy Pathology and Its Association with Amyloid-β PET Signal. Neurology 2021, 97, e1799–e1808. [Google Scholar] [CrossRef] [PubMed]
- Buciuc, M.; Duffy, J.R.; Machulda, M.M.; Spychalla, A.J.; Gunter, J.L.; Jack, C.R., Jr.; Giannini, C.; Raghunathan, A.; Dickson, D.W.; Josephs, K.A.; et al. Association of amyloid angiopathy with microbleeds in logopenic progressive aphasia: An imaging-pathology study. Eur. J. Neurol. 2021, 28, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, Y.; Yazaki, M.; Oguchi, K.; Ezawa, N.; Yoshinaga, T.; Yamada, M.; Yahikozawa, H.; Watanabe, M.; Kametani, F.; Ikeda, S. Cerebral amyloid angiopathy in posttransplant patients with hereditary ATTR amyloidosis. Neurology 2016, 87, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Oguchi, K.; Mochizuki, Y.; Takasone, K.; Ezawa, N.; Matsushima, A.; Katoh, N.; Yazaki, M.; Sekijima, Y. Distribution and progression of cerebral amyloid angiopathy in early-onset V30M (p.V50M) hereditary ATTR amyloidosis. Amyloid 2023, 30, 109–118. [Google Scholar] [CrossRef]
- Chatterjee, P.; Fagan, A.M.; Xiong, C.; McKay, M.; Bhatnagar, A.; Wu, Y.; Singh, A.K.; Taddei, K.; Martins, I.; Gardener, S.L.; et al. Presymptomatic Dutch-Type Hereditary Cerebral Amyloid Angiopathy-Related Blood Metabolite Alterations. J. Alzheimers Dis. 2021, 79, 895–903. [Google Scholar] [CrossRef]
- Schultz, A.P.; Kloet, R.W.; Sohrabi, H.R.; van der Weerd, L.; van Rooden, S.; Wermer, M.J.H.; Moursel, L.G.; Yaqub, M.; van Berckel, B.N.M.; Chatterjee, P.; et al. Amyloid imaging of dutch-type hereditary cerebral amyloid angiopathy carriers. Ann. Neurol. 2019, 86, 616–625. [Google Scholar] [CrossRef]
- Huang, C.Y.; Hsiao, I.T.; Lin, K.J.; Huang, K.L.; Fung, H.C.; Liu, C.H.; Chang, T.Y.; Weng, Y.C.; Hsu, W.C.; Yen, T.C.; et al. Amyloid PET pattern with dementia and amyloid angiopathy in Taiwan familial AD with D678H APP mutation. J. Neurol. Sci. 2019, 398, 107–116. [Google Scholar] [CrossRef]
- Carmona-Iragui, M.; Fernández-Arcos, A.; Alcolea, D.; Piazza, F.; Morenas-Rodriguez, E.; Antón-Aguirre, S.; Sala, I.; Clarimon, J.; Dols-Icardo, O.; Camacho, V.; et al. Cerebrospinal Fluid Anti-Amyloid-β Autoantibodies and Amyloid PET in Cerebral Amyloid Angiopathy-Related Inflammation. J. Alzheimers Dis. 2016, 50, 1–7. [Google Scholar] [CrossRef]
- Renard, D.; Collombier, L.; Demattei, C.; Wacongne, A.; Charif, M.; Ayrignac, X.; Azakri, S.; Gaillard, N.; Boudousq, V.; Lehmann, S.; et al. Cerebrospinal Fluid, MRI, and Florbetaben-PET in Cerebral Amyloid Angiopathy-Related Inflammation. J. Alzheimers Dis. 2018, 61, 1107–1117. [Google Scholar] [CrossRef]
- Renard, D.; Tatu, L.; Collombier, L.; Wacongne, A.; Ayrignac, X.; Charif, M.; Boukriche, Y.; Chiper, L.; Fourcade, G.; Azakri, S.; et al. Cerebral Amyloid Angiopathy and Cerebral Amyloid Angiopathy-Related Inflammation: Comparison of Hemorrhagic and DWI MRI Features. J. Alzheimers Dis. 2018, 64, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Salman, R.A.; Biessels, G.J.; van Buchem, M.; Cordonnier, C.; Lee, J.M.; Montaner, J.; Schneider, J.A.; Smith, E.E.; Vernooij, M.; et al. Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol. 2014, 13, 419–428. [Google Scholar] [CrossRef] [PubMed]
- De Kort, A.M.; Verbeek, M.M.; Schreuder, F.H.B.M.; Klijn, C.J.M.; Jäkel, L. Prevalence of Cerebral Amyloid Angiopathy Pathology and Strictly Lobar Microbleeds in East-Asian Versus Western Populations: A Systematic Review and Meta-Analysis. J. Stroke 2024, 26, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Gamez, J.E.; Singh, U.; Sadowsky, C.H.; Villena, T.; Sabbagh, M.N.; Beach, T.G.; Duara, R.; Fleisher, A.S.; Frey, K.A.; et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 Imaging and neuritic plaque density. JAMA Neurol. 2015, 72, 287–294. [Google Scholar] [CrossRef]
- Re-Read Study to Compare the Brain Uptake of [18F] Flutemetamol with Brain Neuritic Plaque Density Determined Postmortem NCT02090855. Available online: https://clinicaltrials.gov/ct2/show/NCT02090855?term=NCT02090855&rank=1 (accessed on 20 July 2014).
- Mirra, S.S.; Heyman, A.; McKeel, D.; Sumi, S.M.; Crain, B.J.; Brownlee, L.M.; Vogel, F.S.; Hughes, J.P.; van Belle, G.; Berg, L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 41, 479–486. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Buckley, C.J.; Heurling, K.; Sherwin, P.; Jones, P.A.; Zanette, M.; Mathis, C.A.; Klunk, W.E.; Chakrabarty, A.; Ironside, J.; et al. Post-mortem histopathology underlying β-amyloid PET imaging following flutemetamol F 18 injection. Acta Neuropathol. Commun. 2016, 4, 130. [Google Scholar] [CrossRef]
| Diagnostic Category | Criteria |
|---|---|
| Definite CAA | Full post-mortem examination demonstrating the following:
|
| Probable CAA with supporting pathology | Clinical data and pathologic tissue (evacuated hematoma or cortical biopsy) demonstrating the following:
|
| Probable CAA | Clinical data and MRI or CT demonstrating the following:
|
| Possible CAA | Clinical data and MRI or CT demonstrating the following:
|
| Reference | Population | Details | Findings | Comments |
|---|---|---|---|---|
| Farid et al., 2017 [25] | Probable CAA: 129 Possible CAA: 65 Healthy controls (HCs): 30 | Probable CAA was diagnosed using Boston criteria [18] and amyloid PET imaging with [11C]PiB tracer. Possible CAA was diagnosed with suspected but unconfirmed CAA, due to lack of definitive imaging or pathology. HCs were matched by age and sex to CAA patients for comparison. | - Significantly higher global [11C]PiB PET uptake in CAA patients compared to controls and deep ICH patients. - Regional amyloid predominance in occipital lobes. | The focus was global and regional amyloid deposition in CAA. The findings support the diagnostic utility of amyloid PET in identifying CAA-specific amyloid patterns. Occipital predominance aids in differentiating CAA from other amyloid-related diseases, such as AD. |
| Charidimou et al., 2018 [26] | CAA (total): 155 AD: 320 HC: 80 | CAA includes both probable and possible CAA cases; amyloid PET imaging was conducted with different tracers. AD patients were included for comparison of amyloid patterns between CAA and AD populations. Controls were used to establish baseline amyloid uptake and regional distribution. | - Global amyloid burden higher in CAA vs. controls (effect size: 1.18, 95% CI: 1.08–1.28, p < 0.0001). - Occipital-to-global uptake ratio significantly higher in CAA vs. AD (mean ratio: 1.10, 95% CI: 1.03–1.19, p = 0.009). | A systematic review and meta-analysis of amyloid PET in CAA and AD. The findings confirm global and regional amyloid deposition patterns as key diagnostic markers. The findings highlight the occipital-to-global ratio as a differentiating feature between CAA and AD, improving diagnostic specificity. |
| Charidimou et al., 2017 [9] | Probable CAA: 52 AD: 75 HC: 20 | Probable CAA was defined by Boston criteria [18], and confirmed by imaging findings (including MRI and [11C]PiB PET). AD patients were included to investigate differences in amyloid regionality, particularly occipital involvement. HCs were used to analyze regional amyloid patterns compared to CAA patients. | - Occipital lobe amyloid deposition disproportionately higher compared to other cortical regions. - Regional amyloid patterns correlated with clinical manifestations (e.g., lobar hemorrhages, visual disturbances). | - The findings establish the occipital amyloid burden as a hallmark of CAA. - The findings suggest that regional amyloid patterns can predict clinical symptoms, and may guide targeted interventions or prognosis in patients with CAA. |
| Reference | Probable CAA/ICH | Possible CAA | Controls | Key Findings |
|---|---|---|---|---|
| Gokcal et al., 2022 [36] | 58 | 20 | 32 | Probable CAA patients showed a 1.5-fold increase in occipital amyloid PET SUVR compared to controls (p < 0.01). |
| Romoli et al., 2024 [27] | 42 | 15 | 30 | Probable CAA patients had a 40% higher occipital SUVR compared to Alzheimer’s patients (p < 0.001), which correlated with recurrent lobar ICH. |
| Kim et al., 2018 [32] | 64 | 25 | 35 | Out of all probable CAA cases, 73% showed occipital-predominant amyloid uptake, compared to 18% of Alzheimer’s cases (p < 0.001). |
| Baron et al., 2014 [33] | 40 | 18 | 28 | A strong correlation was found between occipital amyloid uptake and lobar microbleeds (r = 0.62, p < 0.001). |
| Tsai et al., 2021 [29] | 52 | 22 | 29 | An increased regional amyloid burden in probable CAA predicted ICH recurrence with 85% sensitivity and 82% specificity. |
| Raposo et al., 2017 [37] | 48 | 16 | 31 | Probable CAA patients showed a 2-fold higher occipital-to-global SUVR compared to possible CAA patients (p < 0.01). |
| Ly et al., 2015 [38] | 39 | 12 | 25 | Amyloid PET uptake was significantly higher in probable CAA with lobar microbleeds compared to hypertensive ICH (p < 0.001). |
| Yost et al., 2019 [31] | 46 | 15 | 24 | Amyloid PET differentiated probable CAA from hypertensive ICH with an AUC of 0.87 (95% CI: 0.81–0.92, p < 0.001). |
| Michiels et al., 2022 [28] | 57 | 23 | 30 | The occipital-to-global amyloid ratio was higher in probable CAA than Alzheimer’s disease (mean SUVR 1.8 ± 0.3 vs. 1.2 ± 0.4, p < 0.001). |
| Gurol et al., 2016 [39] | 44 | 18 | 26 | Probable CAA patients showed a significant amyloid burden (SUVR 1.6 ± 0.2) compared to controls (p < 0.001), which was correlated with ICH. |
| Tsai et al., 2019 [30] | 51 | 20 | 29 | A higher amyloid burden was found in recurrent ICH cases (SUVR 1.7 ± 0.3, p = 0.02); the occipital SUVR was elevated in probable CAA. |
| Planton et al., 2020 [35] | 48 | 14 | 28 | Amyloid PET had 87% sensitivity and 78% specificity for diagnosing probable CAA in ICH patients compared to controls. |
| Jang et al., 2019 [40] | 38 | 10 | 20 | The occipital SUVR was 50% higher in probable CAA patients compared to hypertensive ICH patients (p < 0.001). |
| Raposo et al., 2019 [34] | 47 | 18 | 27 | Regional amyloid deposition was found to be correlated with ICH burden and distribution (r = 0.58, p < 0.01). |
| Study | Probable CAA with ICH (N) | Possible CAA (N) | Controls (Healthy/AD) | Pathological Data | Key Findings |
|---|---|---|---|---|---|
| Pyun et al., 2024 [41] | 56 | 18 | 34 | Higher amyloid deposition in occipital regions; SUVR 1.65 ± 0.2 (p < 0.001). | PET imaging predicted ICH recurrence with 83% sensitivity. Occipital-to-global SUVR distinguished CAA from AD. |
| Buciuc et al., 2021 [43] | 62 | 20 | 30 | Regional amyloid correlated with cortical microbleeds (r = 0.58, p < 0.01). | Amyloid burden highest in occipital lobes in CAA patients, correlating with disease severity and microbleeds. |
| McCarter et al., 2021 [42] | 59 | 21 | 28 | Occipital-dominant SUVR in probable CAA (1.7 ± 0.3) vs. AD. Atrophy patterns also noted in posterior regions. | PET accurately differentiated probable CAA from AD with AUC of 0.89 (p < 0.001). |
| Kim et al., 2018 [32] | 48 | 16 | 30 | cSS associated with increased occipital amyloid burden. | Probable CAA with cSS showed higher occipital amyloid and greater atrophy in posterior cortices compared to non-cSS. |
| Reference | Population | Hereditary CAA | Imaging Findings | PET Findings |
|---|---|---|---|---|
| Sekijima et al., 2016 [44] | 20 patients with hereditary ATTR amyloidosis and 20 healthy controls | ATTR amyloidosis | Increased cortical cSS in patients (45% vs. 0% in controls); higher number of cerebral microbleeds in patients (median 18 vs. 0 in controls) | Higher Pittsburgh Compound B—[11C]Pib—retention in patients, indicating increased amyloid deposition |
| Chatterjee et al., 2021 [46] | 50 patients with hereditary Dutch-type CAA and matched controls | Dutch-type mutation | Extensive cortical microinfarcts (detected via advanced imaging) and higher white matter hyperintensities (WMHs); consistent presence of CMBs | Elevated amyloid load shown by [11C]PiB PET |
| Schultz et al., 2019 [47] | 12 patients with hereditary Dutch-type CAA and 12 controls | Dutch-type mutation | Increased prevalence of cSS (50% vs. 0%) and higher WMH volume | Increased PiB retention in patients, correlating with cSS and WMH volume |
| Huang et al., 2019 [48] | 15 patients with hereditary CAA due to APP duplication and matched controls | APP duplication | Severe WMHs, numerous cortical CMBs, and cSS | Increased PiB uptake in affected patients, with amyloid deposition extending beyond vessels |
| Takahashi et al., 2023 [45] | 15 patients with hereditary Dutch-type CAA and 15 controls | ATTR amyloidosis | Increased WMHs and presence of cSS in 40% of patients | Higher PiB retention in patients, consistent with amyloid pathology |
| Reference | Population | Imaging Findings | PET Findings |
|---|---|---|---|
| Carmona-Iragui et al., 2016 [49] | 12 patients with CAA-ri | MRI: leukoencephalopathy; multiple cortical and subcortical hyperintensities on FLAIR; microbleeds on GRE sequences | Not specified |
| Renard et al., 2018 [50] | 8 patients with CAA-ri | MRI: asymmetric white matter hyperintensities, cortical swelling, leptomeningeal enhancement; microbleeds on SWI | Amyloid PET: elevated uptake in cortical regions |
| Renard et al., 2018 [51] | 10 patients with CAA-ri | MRI: extensive white matter hyperintensities, cortical edema, leptomeningeal enhancement; microbleeds on GRE | Amyloid PET: increased cortical uptake; FDG-PET: hypometabolism in affected regions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedde, M.; Piazza, F.; Pascarella, R. Positron Emission Tomography in Cerebral Amyloid Angiopathy: A Scoping Review. Appl. Sci. 2025, 15, 3973. https://doi.org/10.3390/app15073973
Zedde M, Piazza F, Pascarella R. Positron Emission Tomography in Cerebral Amyloid Angiopathy: A Scoping Review. Applied Sciences. 2025; 15(7):3973. https://doi.org/10.3390/app15073973
Chicago/Turabian StyleZedde, Marialuisa, Fabrizio Piazza, and Rosario Pascarella. 2025. "Positron Emission Tomography in Cerebral Amyloid Angiopathy: A Scoping Review" Applied Sciences 15, no. 7: 3973. https://doi.org/10.3390/app15073973
APA StyleZedde, M., Piazza, F., & Pascarella, R. (2025). Positron Emission Tomography in Cerebral Amyloid Angiopathy: A Scoping Review. Applied Sciences, 15(7), 3973. https://doi.org/10.3390/app15073973






