The Effect of Edible Plant Oils on Increasing the Viability of Lacticaseibacillus rhamnosus GG During the Microencapsulation by Spray Drying Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals and Reagents
2.2. Bacterial Culture
2.3. Emulsions Characterization and Preparation
2.4. Spray Drying Process
2.5. Viability Rate of Lacticaseibacillus rhamnosus and Powder Storage
2.6. Differential Scanning Calorimetry (DSC)
2.7. Moisture Content
2.8. Water Activity
2.9. Particle Size of Powders
2.10. Powders Morphology
2.11. Color Measurement
2.12. Statistical Analysis
3. Results and Discussion
3.1. Viability Rate of Lacticaseibacillus rhamnosus After Spray Drying and During Powder Storage
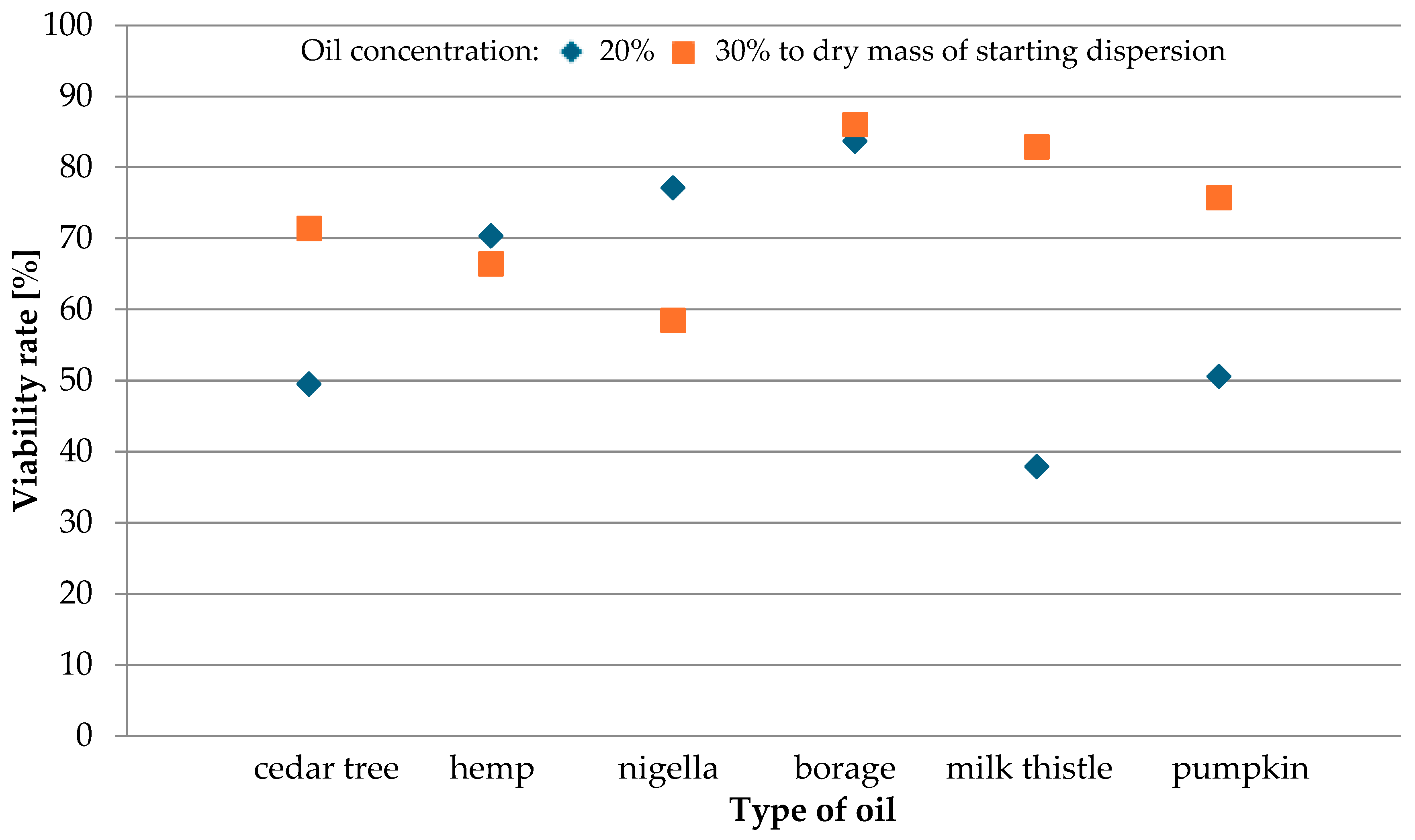
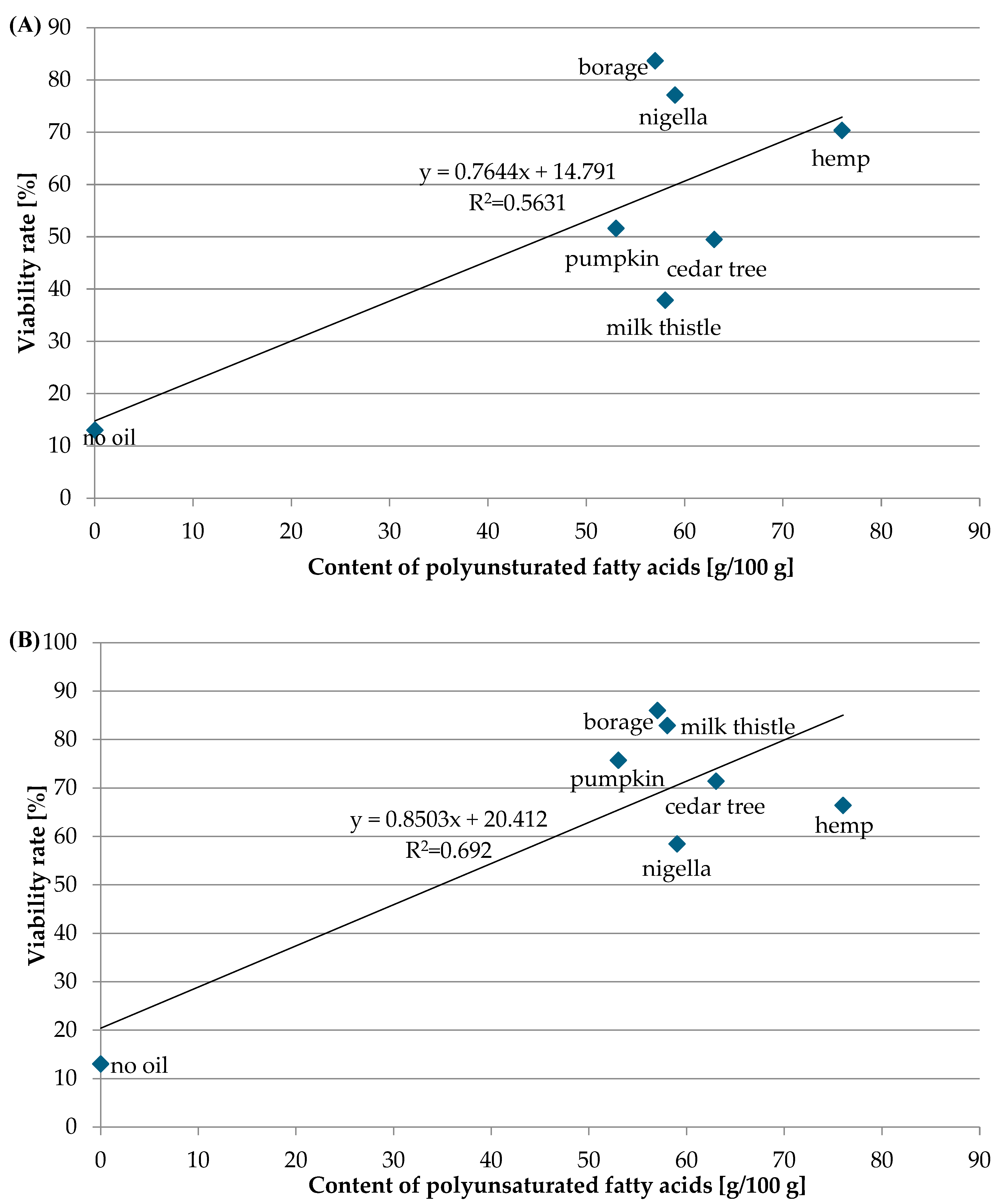
3.2. The Composition of Oil and Lacticaseibacillus rhamnosus Viability
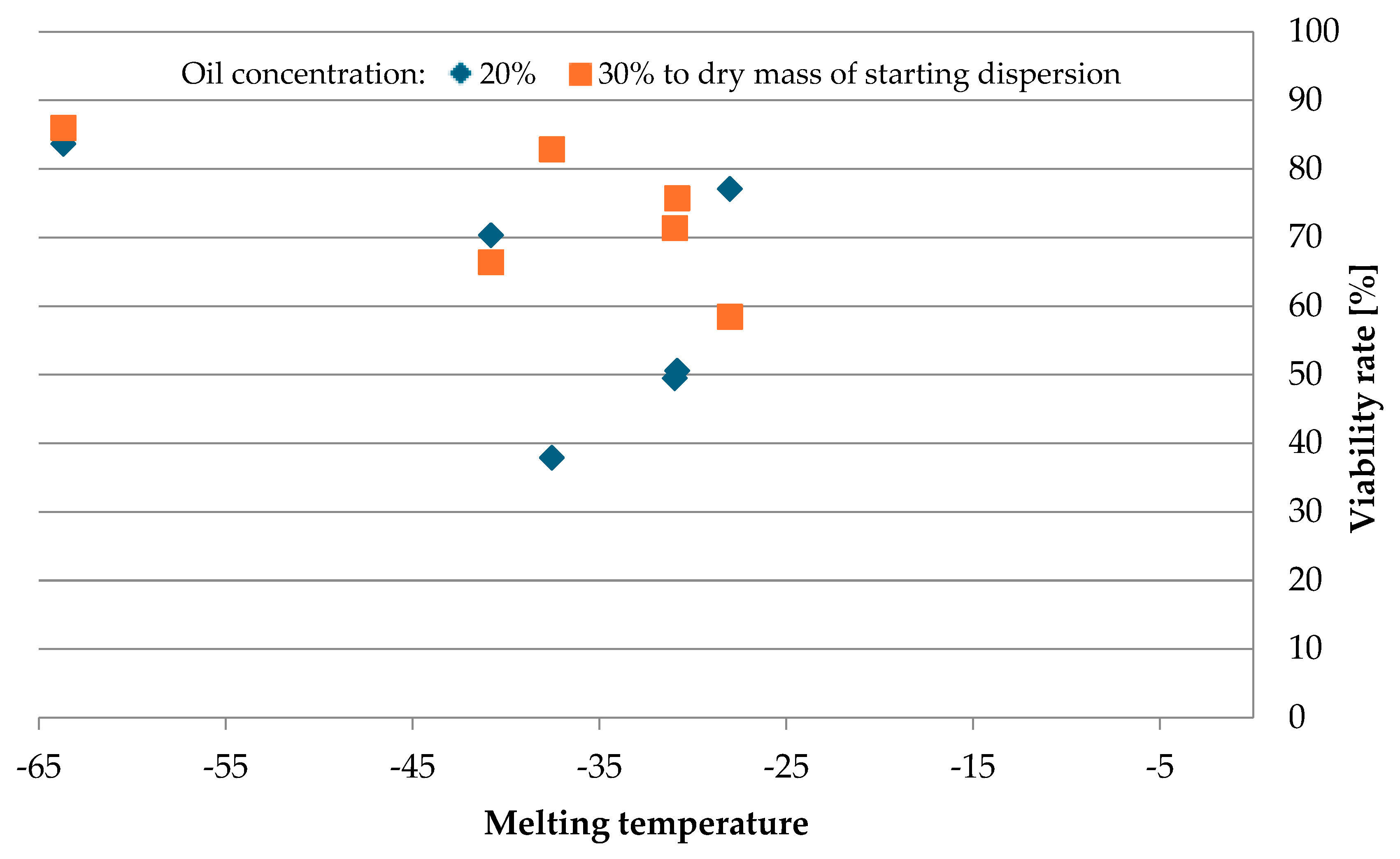
3.3. The Thermal Characterization of Oils Using Differential Scanning Calorimetry (DSC) and the Effect of the Melting Temperature of Oils on the Viability Rate of Bacteria Cells After Spray Drying
3.4. Moisture Content, Water Activity, and Particle Size D4,3 Parameter of Powders
3.5. Particle Size Distribution and Surface Morphology of Powders
3.6. Color Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. FAO/WHO Joint Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food (30 April 2002 and 1 May 2002); Scientific Research Publishing: London, ON, Canada, 2002; pp. 35–45. [Google Scholar]
- Champagne, C.P.; Gardner, N.J.; Roy, D. Challenges in the addition of probiotic cultures to foods. Crit. Rev. Food Sci. Nutr. 2005, 45, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Goh, Y.J.; Klaenhammer, T.R. Probiotics and Prebiotics. In Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington, DC, USA, 2019; pp. 831–854. [Google Scholar]
- Forrsten, S.D.; Sindelar, C.W.; Ouwehand, A.C. Probiotics from an industrial perspective. Anaerobe 2011, 17, 410–413. [Google Scholar] [CrossRef]
- Murua-Pagola, B.; Castro-Becerra, A.L.; Abadia-Garcia, L.; Castano-Tostado, E.; Amaya-Llano, S.L. Protective effect of a crosslinked starch by extrusion on the survival of Bifidobacterium breve ATCC 15700 in yogurt. J. Food Process. Preserv. 2021, 45, e15097. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Saeed, M.; Ahmed, A.; Ateeq, H.; Nadeem, M.T.; Tufail, T. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal conditions and in pasteurized grape juice. J. Food Process. Preserv. 2020, 44, 14346. [Google Scholar] [CrossRef]
- Thamacharoensuk, T.; Boonsom, T.; Tanasupawat, S.; Dumkliang, E. Optimization of microencapsulated Lactobacillus rhamnosus GG from whey protein and glutinous rice starch by spray drying. Key Eng. Mater. 2020, 859, 265–270. [Google Scholar] [CrossRef]
- Kailasapathy, K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT 2006, 39, 1221–1227. [Google Scholar] [CrossRef]
- Lai, K.; How, Y.; Pui, L. Microencapsulation of Lactobacillus rhamnosus GG with flaxseed mucilage using co-extrusion technique. J. Microencapsul. 2021, 38, 134–148. [Google Scholar] [CrossRef]
- Picot, A.; Lacroix, C. Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. Intern. Dairy J. 2004, 14, 505–515. [Google Scholar] [CrossRef]
- Lakkis, J.M. Encapsulation and Controlled Release Technologies in Food Systems; Blackwell Publication: Oxford, UK, 2007; pp. 1–254. [Google Scholar]
- Zuidam, N.J.; Shimoni, E. Overview of Microencapsulates for Use in Food Products or Processes and Methods to Make Them. In Encapsulation Technologies for Food Active Ingredients and Food Processing; Zuidam, N.J., Nedović, V.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–30. [Google Scholar]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef]
- Masters, K. Spray Drying Handbook, 5th ed.; Longman Scientific and Technical: Singapore, 1991; pp. 1–725. [Google Scholar]
- Masters, K. Spray Drying Handbook, 4th ed.; George Godwin Ltd.: London, UK, 1985; pp. 575–624. [Google Scholar]
- Hasheminya, S.M.; Dehghannya, J. Spray dryers: Applications, performance, essential parts and classifications. Int. J. Farming Allied Sci. 2013, 2, 756–759. [Google Scholar]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [PubMed]
- Ahmed, I.; Niazi, M.B.K.; Jahan, Z.; Naqvi, S.R. Effect of drying parameters on the physical, morphological and thermal properties of spray-dried inulin. J. Polym. Eng. 2018, 38, 775–783. [Google Scholar]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Filkova, I.; Huang, L.X.; Mujumdas, A.S. Industrial spray drying systems. In Handbook of Industrial Drying; Mujumdar, A.S., Ed.; CRC Press: New York, NY, USA, 2007; pp. 251–256. [Google Scholar]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavors and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar]
- Passos, M.L.; Birchal, V.S. Manipulating physical properties of powder. In Spray Drying Technology—Volume I; Woo, M.W., Mujumdar, A.S., Daud, W.R.W., Eds.; Springer: Cham, Switzerland, 2010; pp. 37–60. [Google Scholar]
- Vicente, J.; Pinto, J.; Menezes, J.; Gaspar, F. Fundamental analysis of particle formation in spray drying. Powder Technol. 2013, 247, 1–7. [Google Scholar] [CrossRef]
- Ananta, E.; Volkert, M.; Knorr, K. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int. Dairy J. 2005, 15, 399–409. [Google Scholar]
- Favaro-Trindade, C.S.; Heinemann, R.J.B.; Pedroso, D.L. Developments in probiotic encapsulation. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 1–8. [Google Scholar]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate–gum Arabic complex coacervates. J. Funct. Foods 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Mandal, S.; Hati, S.; Puniya, A.K.; Singh, R.; Singh, K. Development of synbiotic milk chocolate using encapsulated Lactobacillus casei NCDC 298. J. Food Process. Preserv. 2013, 37, 1031–1037. [Google Scholar] [CrossRef]
- Pech-Canul, A.d.l.C.; Ortega, D.; García-Triana, A.; González-Silva, N.; Solis-Oviedo, R.L. A brief review of edible coating materials for the microencapsulation of probiotics. Coatings 2020, 10, 197. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey proteins and its derivatives: Bioactivity, functionality, and current applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Vissoto, F.Z.; Montenegro, F.M.; Santos, J.M.; Oliveira, S.J.R. Avaliação da influência dos processos de lecitinação e de aglomeração nas propriedades físicas de achocolatado em pó. Ciênc. Technol. Aliment. 2006, 26, 666–671. [Google Scholar]
- Noreen, A.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar]
- Zhu, Y.; Wang, Z.; Bai, L.; Deng, J.; Zhou, Q. Biomaterial-based encapsulated probiotics for biomedical applications: Current status and future perspectives. Mater. Des. 2021, 210, 110018. [Google Scholar]
- Pavli, F.; Tassou, C.; Nychas, G.-J.E.; Chorianopoulos, N. Probiotic incorporation in edible films and coatings: Bioactive solution for functional foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef]
- Fedorowicz, A.; Bartkowiak, A. Wpływ wybranych substancji tłuszczowych, ich entalpii topnienia oraz kompozycji kwasów tłuszczowych na przeżywalność Lactobacillus rhamnosus GG podczas procesu suszenia rozpyłowego i przechowywania. Przemysł Spoz. 2023, 77, 32–39. [Google Scholar]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Othmana, N.Z.; Shadanb, N.H.; Ramlib, S.; Sarmidia, M.R. Study on the effects of Carbohydrate-Protein-Coconut Oil on the Viability of Lactobacillus bulgaricus during Spray Drying, Simulated Gastrointestinal Conditions and Unrefrigerated Storage by Simplex-Lattice Mixture Design. Chem. Eng. 2018, 63, 553–558. [Google Scholar]
- Liu, H.; Gong, J.; Chabot, D.; Miller, S.S.; Cui, S.W.; Ma, J.; Wang, Q. Protection of heat-sensitive probiotic bacteria during spray-drying by sodium caseinate stabilized fat particles. Food Hydrocoll. 2015, 51, 459–467. [Google Scholar] [CrossRef]
- Agudelo, J.; Cano, A.; González-Martínez, C.; Chiralt, A. Disaccharide incorporation to improve survival during storage of spray dried Lactiobacillus rhamnosus in whey protein-maltodextrin carriers. J. Funcitonal Foods 2017, 37, 416–423. [Google Scholar] [CrossRef]
- Rochat, T.; Miyoshi, A.; Gratadoux, J.J.; Duwat, P.; Sourice, S.; Azevedo, V.; Langella, P. High-level resistance to oxidative stress in Lactococcus lactis conferred by Bacillus subtilis catalase KatE. Microbiology 2005, 151, 3011–3018. [Google Scholar] [CrossRef]
- Velasco, L.; Ruiz-M’endez, M.V. 11—Sunflower oil minor constituents. In Sunflower (297-329); Martínez-Force, E., Dunford, N.T., Salas, J.J., Eds.; AOCS Press: Champaign, IL, USA, 2015. [Google Scholar] [CrossRef]
- Ngamekaue, N.; Dumrongchai, T.; Rodklongtan, A.; Chitprasert, P. Improving probiotic survival through encapsulation in coconut oil in whey protein isolate emulsions during spray drying and gastrointestinal digestion. LWT 2024, 198, 116061. [Google Scholar] [CrossRef]
- Fedorowicz, A.; Bartkowiak, A. The Influence of Different Butter Type, Their Fatty Acid Composition and Melting Enthalpy on the Viability Rate of Lacticaseibacillus rhamnosus GG Directly After the Spray-Drying Process and During Storage of Powders. Foods 2024, 13, 3803. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Che Man, Y.B. Differential scanning calorimetry analysis of edible oils: Comparison of thermal properties and chemical composition. J. Am. Oil Chem. Soc. 2000, 77, 143–155. [Google Scholar] [CrossRef]
- Yin, M.; Chen, M.; Yuan, Y.; Liu, F.; Zhong, F. Encapsulation of Lactobacillus rhamnosus GG in whey protein isolate-shortening oil and gum Arabic by complex coacervation: Enhanced the viability of probiotics during spray drying and storage. Food Hydrocoll. 2024, 146, 109252. [Google Scholar] [CrossRef]
- Liao, L.K.; Wei, X.Y.; Gong, X.; Li, J.H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus casei LK-1 by spray drying related to its stability and in vitro digestion. LWT Food Sci. Technol. 2017, 82, 82–89. [Google Scholar] [CrossRef]
- Viernstein, H.; Raffalt, J.; Polheim, D. Stabilisation of probiotic microorganisms: An overview of the techniques and some commercially available products. In Applications of Cell Immobilisation Biotechnology; Springer: Dordrecht, The Netherlands, 2005; pp. 439–453. [Google Scholar]
- Šipailienė, A.; Petraitytė, S. Encapsulation of probiotics: Proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Probiotics Antimicrob. Proteins 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Dinkçi, N.; Akdeniz, V.; Akalin, A.S. Survival of probiotics in functional foods during shelf life. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 201–233. [Google Scholar] [CrossRef]
- Barajas-Alvarez, P.; Gonzalez-Avila, M.; Espinosa-Andrews, H. Microencapsulation of Lactiobacillus rhamnosus HN001 by spray drying and its evaluation under gastrointestinal and storage conditions. LWT 2022, 153, 112485. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, J.; Torley, P.; Wang, D.; Niu, S. Measurement of particle diameter of Lactobacillus acidophilus microcapsule by spray drying and analysis on its microstructure. World J. Microbiol. Biotechnol. 2008, 24, 1349–1354. [Google Scholar] [CrossRef]
- Khem, S.; Small, D.M.; May, B.K. The behaviour of whey protein isolate in protecting Lactobacillus plantarum. Food Chem. 2016, 190, 717–723. [Google Scholar]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of acai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar]
- Kim, E.H.; Chen, X.D.; Pearce, D. Surface composition of industrial spray-dried milk powders. 2. Effects of spray drying conditions on the surface composition. J. Food Eng. 2009, 94, 169–181. [Google Scholar]
- Nunes, G.L.; de Araújo Etchepare, M.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; de Moraes Flores, É.M.; da Silva, C.D.B.; de Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Behboudi-Jobbehdar, S.; Soukoulis, C.; Yonekura, L.; Fisk, I. Optimization of Spray-Drying Process Conditions for the Production of Maximally Viable Microencapsulated L. acidophilus NCIMB 701748. Dry. Technol. 2013, 31, 1274–1283. [Google Scholar]
- Aniesrani Delfiya, D.S.; Thangavel, K.; Natarajan, N.; Kasthuri, R.; Kailappan, R. Microencapsulation of Turmeric Oleoresin by spray drying and in vitro release studies of microcapsules. J. Food Process Eng. 2015, 38, 37–48. [Google Scholar]
- Comunian, T.A.; Monterrey-Quintero, E.S.; Thomazini, M.; Balieiro, J.C.; Piccone, P.; Pittia, P.; Favaro-Trindade, C.S. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried chlorophyllide, a natural food colourant, using gum Arabic, maltodextrin and soy protein isolate-based carrier systems. Int. J. Food Sci. Technol. 2011, 46, 1259–1265. [Google Scholar]
- Bhagwat, A.; Bhushette, P.; Annapure, U.S. Spray drying studies of probiotic Enterococcus strains encapsulated with whey protein and maltodextrin. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 33. [Google Scholar] [CrossRef]
- De Castro-Cislaghi, F.P.; Silva, C.D.R.E.; Fritzen-Freire, C.B.; Lorenz, J.G.; Sant’Anna, E.S. Bifidobacterium Bb-12 microencapsulated by spray drying with whey: Survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. J. Food Eng. 2012, 113, 186–193. [Google Scholar]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar]
- Lindsey, D.T.; Wee, A.G. Perceptibility and acceptability of CIELAB color differences in computer-simulated teeth. J. Dent. 2007, 35, 593–599. [Google Scholar] [PubMed]
- Wojciech, M.; Maciej, T. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]

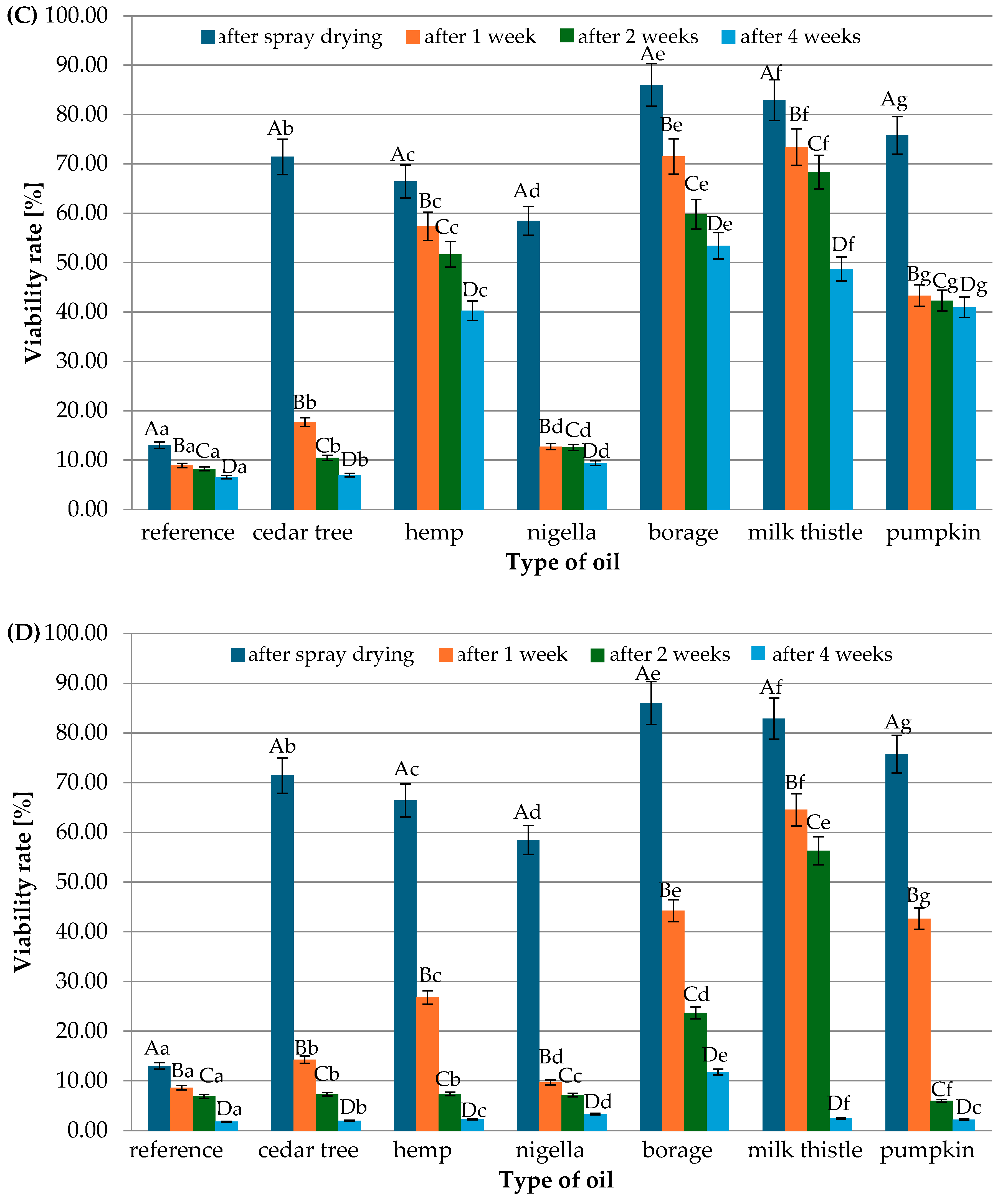
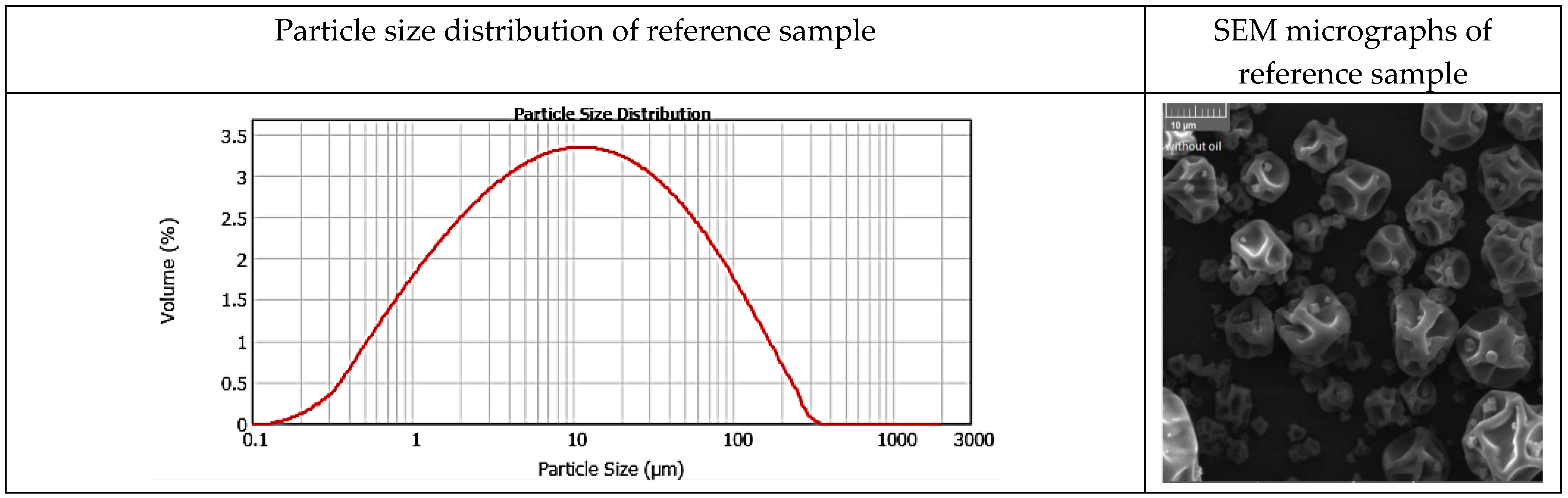
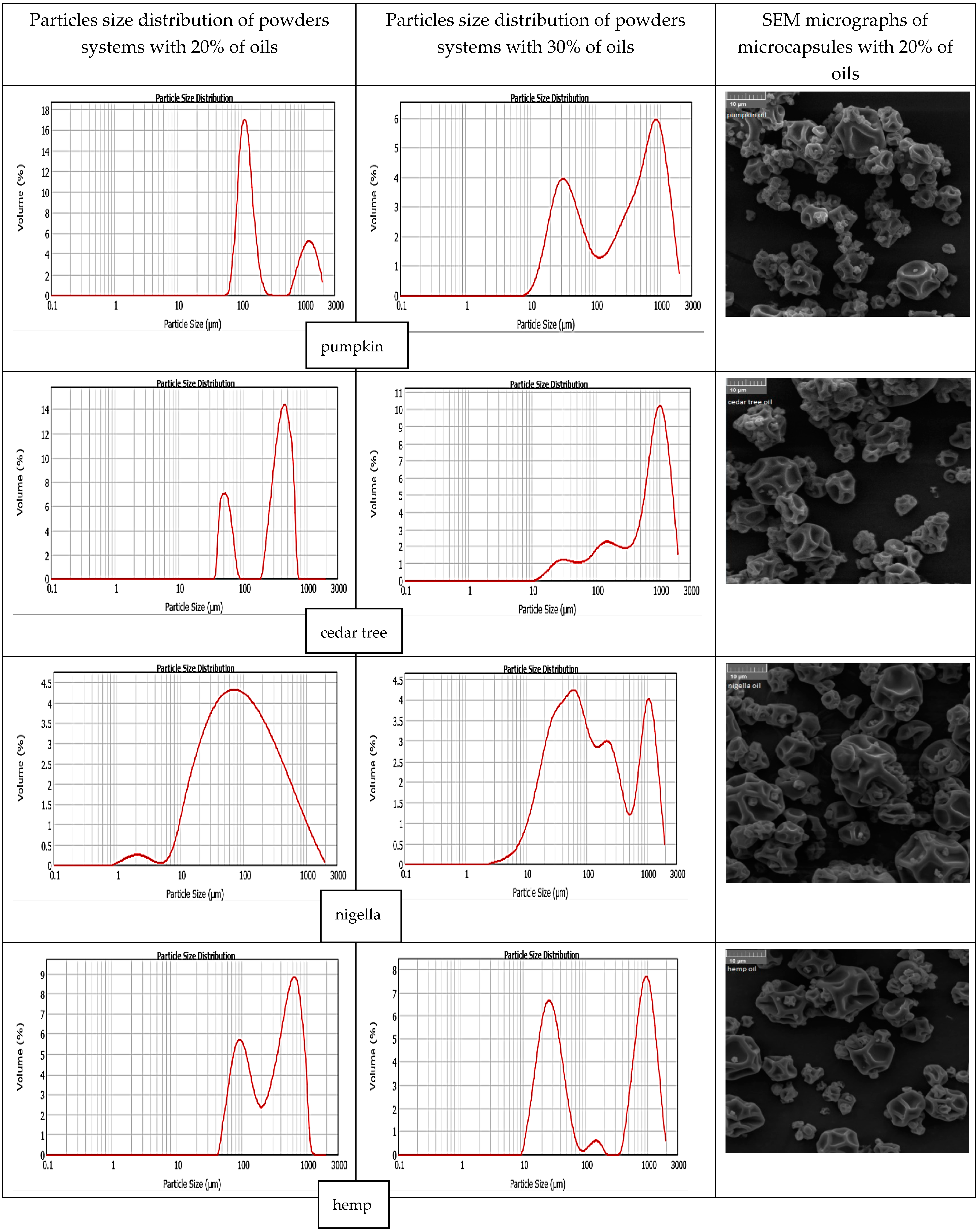
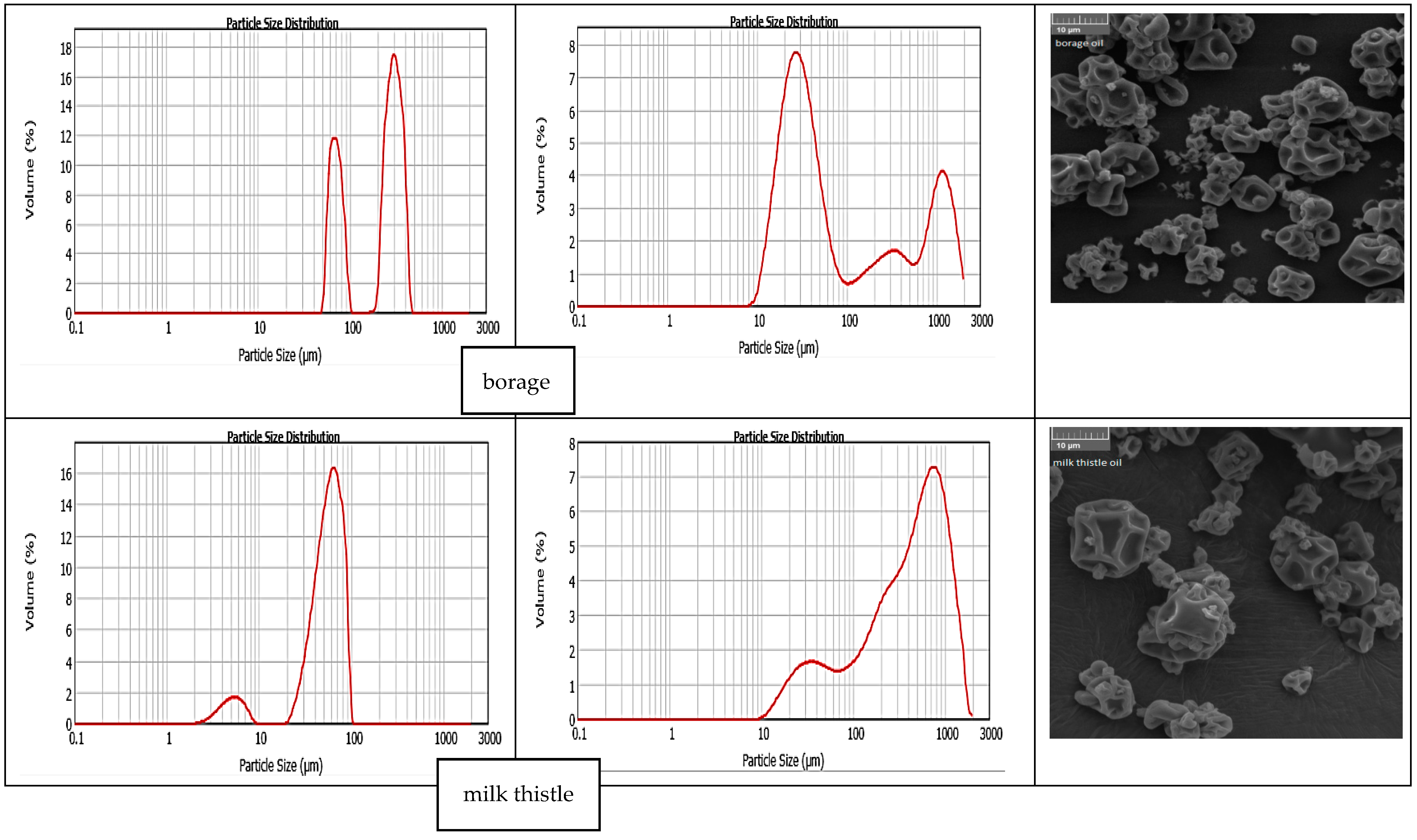
| Type of Oil | Saturated Fatty Acids | Monounsaturated Fatty Acids | Polyunsaturated Fatty Acids | Total Unsaturated Fatty Acids |
|---|---|---|---|---|
| Cedar tree | 7.0 | 25 | 63 | 88 |
| Hemp | 10 | 13 | 76 | 89 |
| Nigella | 16 | 24 | 59 | 83 |
| Borage | 16 | 27 | 57 | 84 |
| Milk thistle | 18 | 24 | 58 | 82 |
| Pumpkin | 19 | 28 | 53 | 81 |
| Type of Oil | Monounsaturated | Polyunsaturated | Vitamin E [mg] | Viability Rate of LGG [%] | |||
|---|---|---|---|---|---|---|---|
| Oleic Acid [g/100 g] | Linoleic Acid [g/100 g] | α-Linoleic Acid [g/100 g] | γ-Linolenic Acid [g/100 g] | 20% of Oil Content | 30% of Oil Content | ||
| Cedar tree | 25 | 43 | - | 20 | 70 | 49.42 | 71.43 |
| Hemp | - | 55 | 16 | - | 78 | 70.37 | 66.44 |
| Nigella | 24 | 56 | - | - | 78 | 77.13 | 58.49 |
| Borage | 18 | 37 | - | 20 | 78 | 83.69 | 86.03 |
| Milk thistle | 24 | 56 | - | - | 48 | 37.91 | 82.91 |
| Pumpkin | 26 | 52 | - | - | - | 51.64 | 75.77 |
| Type of Oil | Transition Temperature [°C] | |||
|---|---|---|---|---|
| Melting | Crystallization | |||
| 1 | 2 | 3 | 1 | |
| Cedar tree | −30.98 | −58.64 | ||
| Pumpkin | −30.85 | −14.45 | −7.64 | −65.60 |
| Borage | −63.70 | −27.32 | −9.61 | - |
| Nigella | −28.03 | −61.21 | ||
| Hemp | −40.81 | −29.91 | −63.17 | |
| Milk thistle | −37.55 | −24.71 | −3.89 | −67.10 |
| Type of Oil | Water Content | aw | D4,3 [µm] of Powders | |||
|---|---|---|---|---|---|---|
| 20% * | 30% * | 20% * | 30% * | 20% * | 30% * | |
| Reference sample | 4.04 ± 0.035 a | 0.120 ± 0.02 a | 29.4 ± 2.1 a | |||
| Pumpkin | 5.95 ± 0.017 b | 5.8 ± 0.026 b | 0.277 ± 0.003 b | 0.225 ± 0.002 b | 415.7 ± 2.8 b | 463.8 ± 0.8 b |
| Cedar tree | 4.7 ± 0.026 c | 5.9 ± 0.010 c | 0.181 ± 0.002 a | 0.224 ± 0.003 b | 433.2 ± 3.3 c | 724.5 ± 2.5 c |
| Nigella | 5.35 ± 0.010 d | 5.56 ± 0.017 d | 0.373 ± 0.002 c | 0.128 ± 0.001 c | 175.9 ± 2.6 d | 321.1 ± 1.9 d |
| Hemp | 4.82 ± 0.010 c | 2.72 ± 0.017 e | 0.151 ± 0.001 a | 0.118 ± 0.002 a | 396.2 ± 5.2 e | 498.8 ± 1.4 e |
| Borage | 5.9 ± 0.017 b | 5.41 ± 0.020 f | 0.335 ± 0.002 c | 0.258 ± 0.003 d | 222.8 ± 7.5 f | 321.5 ± 1.9 d |
| Milk thistle | 3.28 ± 0.010 e | 5.49 ± 0.026 g | 0.230 ± 0.01 d | 0.258 ± 0.001 d | 54.6 ± 2.7 g | 506.8 ± 2.0 f |
| Type of Oil | L* | a* | b* | ΔE | ||||
| 20% ** | 30% ** | 20% ** | 30% ** | 20% ** | 30% ** | 20% ** | 30% ** | |
| Reference sample | 89.64 ± 0.01 a | 0.26 ± 0.01 a | 13.70 ± 0.01 a | |||||
| Milk thistle | 88.35 b | 87.36 b | 0.85 b | 0.54 ± 0.01 b | 14.56 b | 13.70 ± 0.01 a | 1.20 | 1.49 |
| Pumpkin | 86.82 c | 87.26 c | 0.24 ± 0.01 a | 0.07 c | 12.08 c | 14.03 b | 2.07 | 1.55 |
| Cedar tree | 89.31 d | 86.62 d | 0.79 c | 0.63 d | 12.02 d | 12.92 c | 1.31 | 2.05 |
| Nigella | 87.08 e | 86.38 e | 0.50 ± 0.01 d | 0.57 e | 13.73 e | 13.20 ± 0.01 d | 1.66 | 2.14 |
| Hemp | 88.96 f | 82.63 f | 0.82 e | 0.13 ± 0.01 f | 13.02 f | 13.36 ± 0.01 e | 0.95 | 4.57 |
| Borage | 88.53 g | 75.61 g | 0.75 ± 0.01 f | 0.17 g | 12.46 g | 11.13 f | 1.24 | 9.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorowicz, A.; Bartkowiak, A. The Effect of Edible Plant Oils on Increasing the Viability of Lacticaseibacillus rhamnosus GG During the Microencapsulation by Spray Drying Process. Appl. Sci. 2025, 15, 3948. https://doi.org/10.3390/app15073948
Fedorowicz A, Bartkowiak A. The Effect of Edible Plant Oils on Increasing the Viability of Lacticaseibacillus rhamnosus GG During the Microencapsulation by Spray Drying Process. Applied Sciences. 2025; 15(7):3948. https://doi.org/10.3390/app15073948
Chicago/Turabian StyleFedorowicz, Alicja, and Artur Bartkowiak. 2025. "The Effect of Edible Plant Oils on Increasing the Viability of Lacticaseibacillus rhamnosus GG During the Microencapsulation by Spray Drying Process" Applied Sciences 15, no. 7: 3948. https://doi.org/10.3390/app15073948
APA StyleFedorowicz, A., & Bartkowiak, A. (2025). The Effect of Edible Plant Oils on Increasing the Viability of Lacticaseibacillus rhamnosus GG During the Microencapsulation by Spray Drying Process. Applied Sciences, 15(7), 3948. https://doi.org/10.3390/app15073948







