A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fermented Dairy, Dairy–Oat, and Oat Beverages
2.3. pH Measurement
2.4. Texture Analysis: Hardness and Adhesiveness
2.5. Syneresis Testing
2.6. Determination of Selected Carbohydrate Content
2.7. Enumeration of Starter Culture Populations
2.8. Statistical Analysis
3. Results
3.1. Fermentation Curve
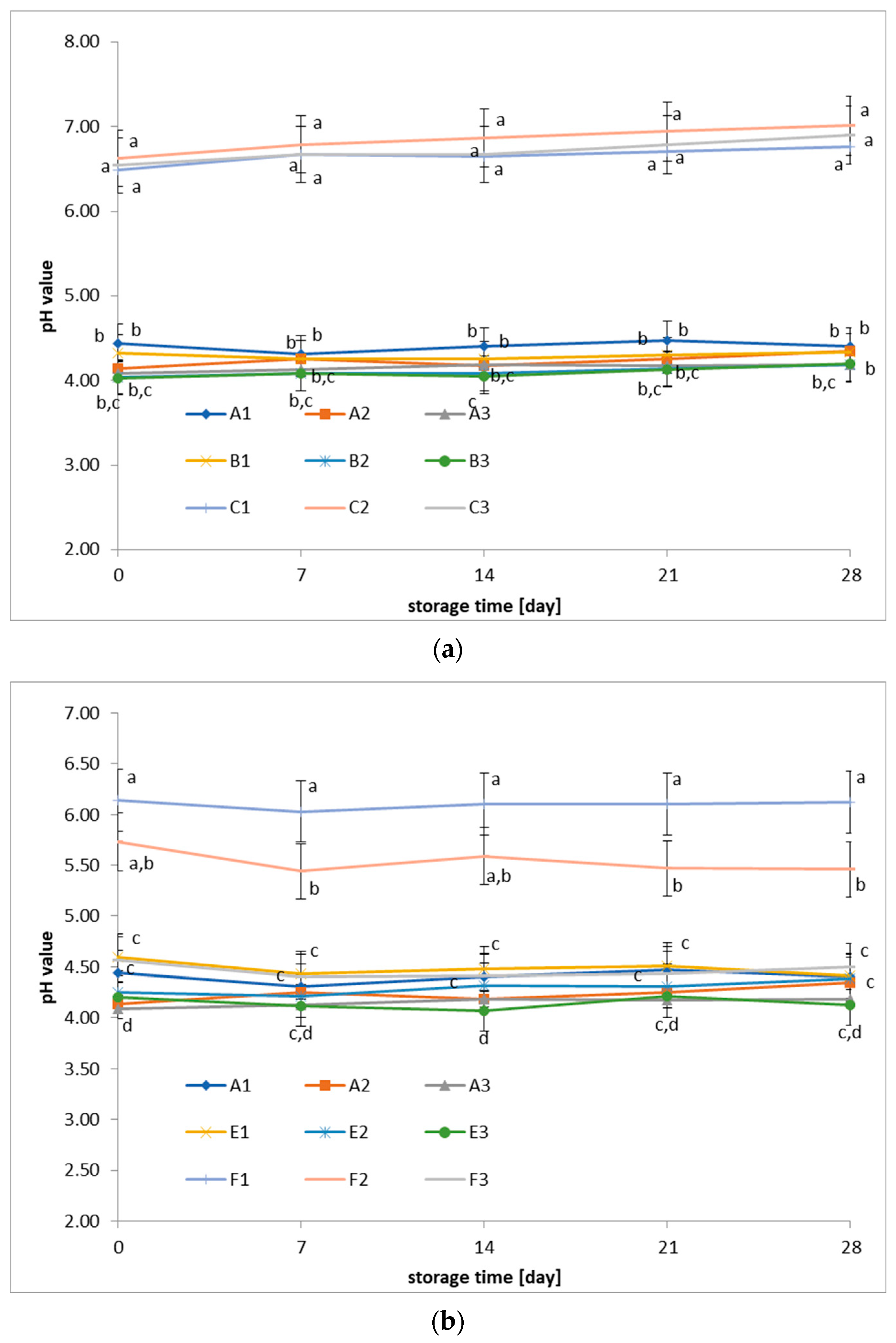
3.2. Changes in pH of Fermented Beverages During Cold Storage
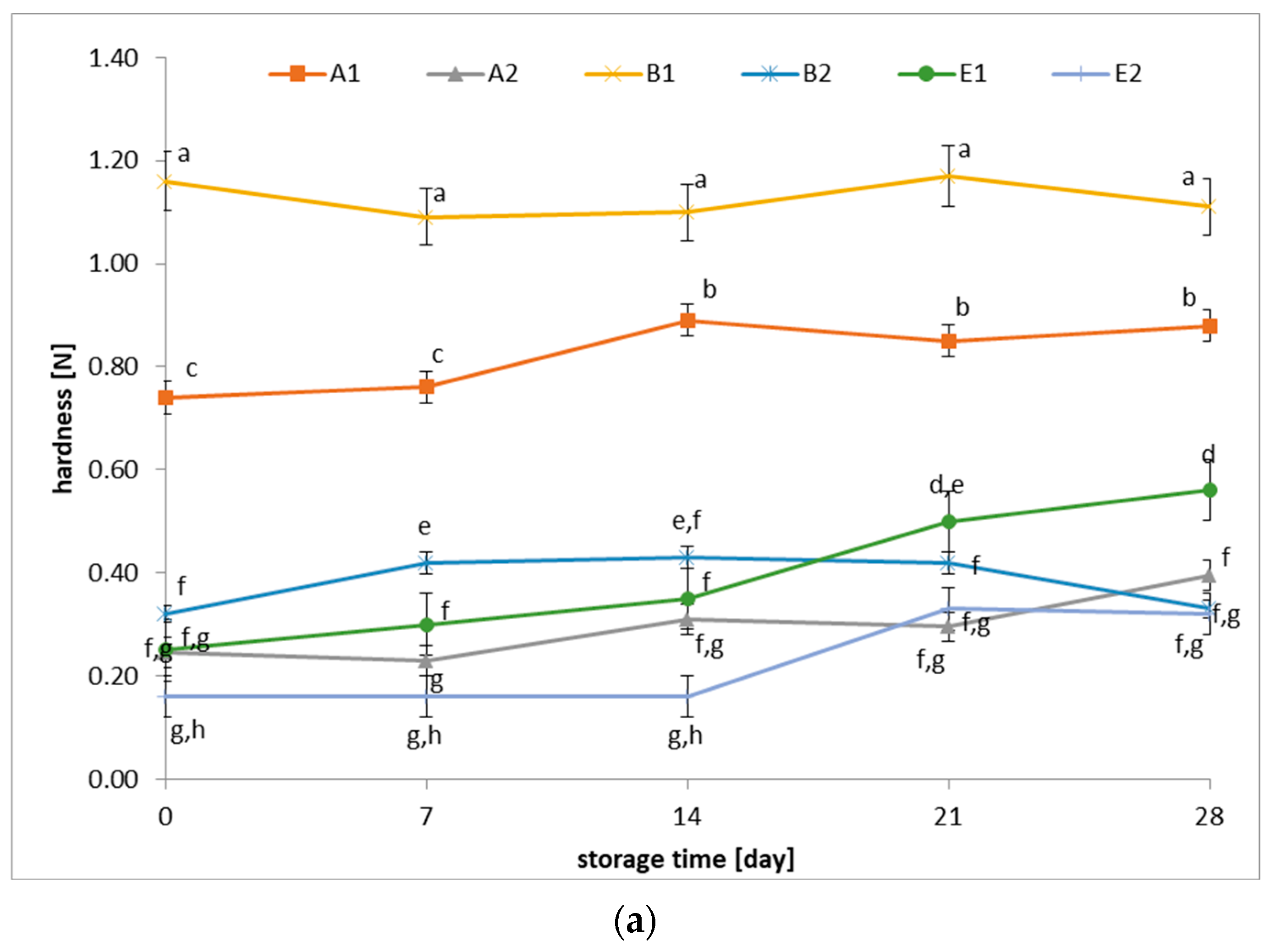
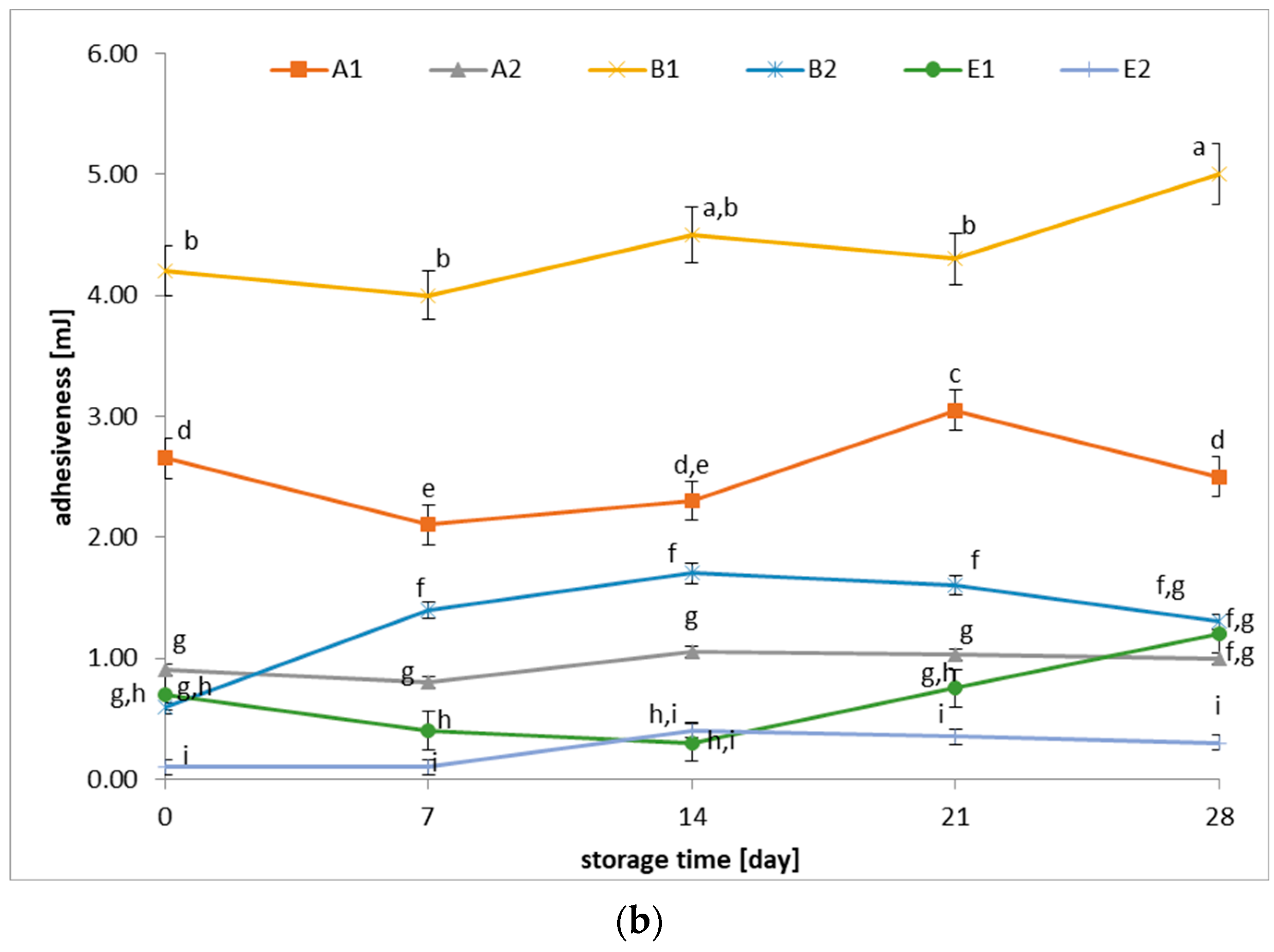
3.3. Changes in Hardness and Adhesiveness of Fermented Beverages
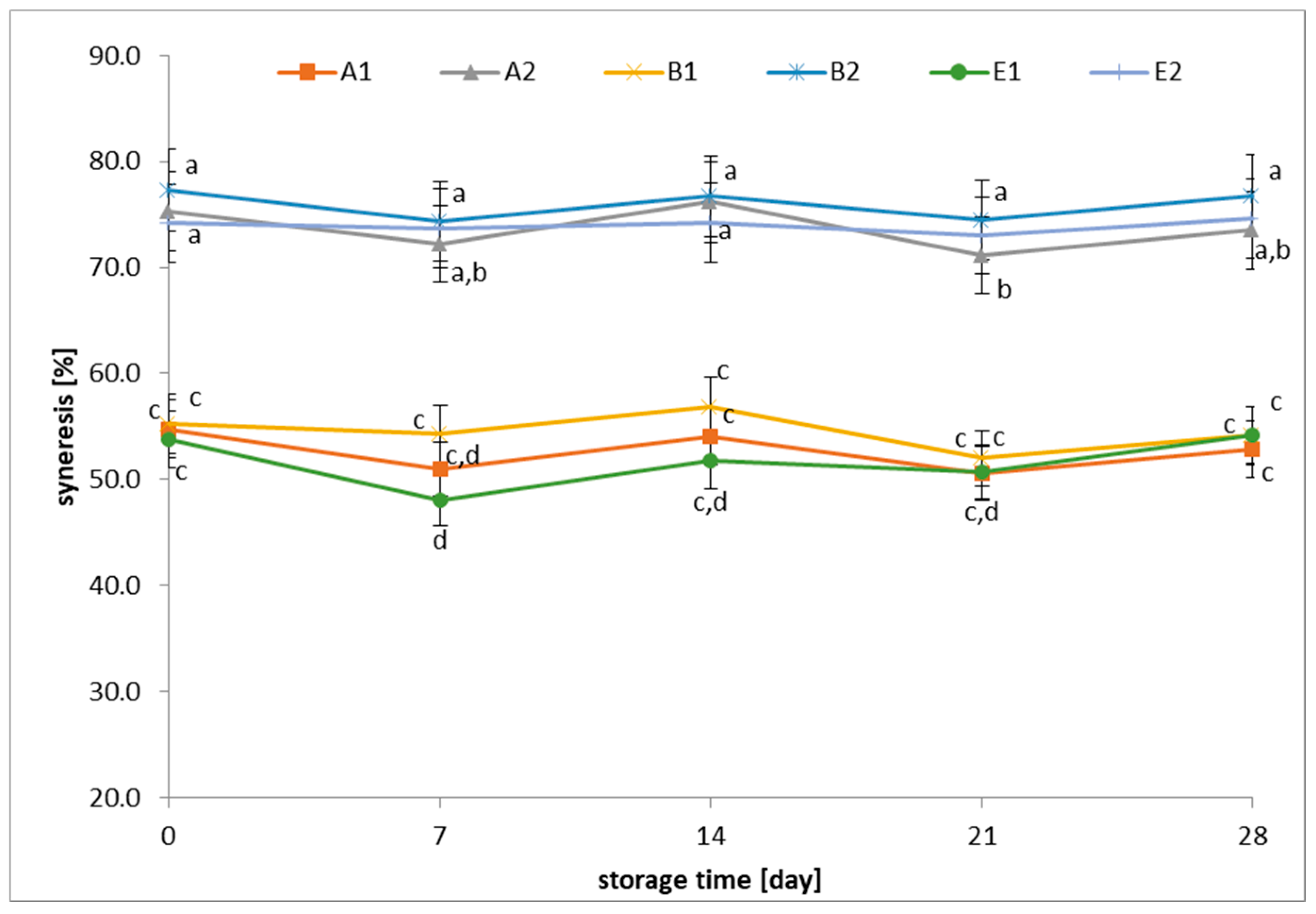
3.4. Changes in Syneresis of Fermented Beverages
3.5. Changes in the Content of Selected Carbohydrates in Fermented Beverages
3.6. Viability of Streptococcus thermophilus in Fermented Beverages
3.7. Viability of Lactobacillus delbrueckii subsp. bulgaricus in Fermented Beverages
3.8. Viability of L. plantarum 299v in Fermented Beverages
3.9. Viability of Lactobacillus acidophilus La-5 in Fermented Beverages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Clark, M. Global Diets Link Environmental Sustainability and Human Health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Karmaus, A.L.; Jones, W. Future Foods Symposium on Alternative Proteins: Workshop Proceedings. Trends Food Sci. Technol. 2021, 107, 124–129. [Google Scholar] [CrossRef]
- Damayanti, A.; Bahlawan, Z.A.S.; Fibriana, F.; Kayati, F.N. Review: Production Mechanism of Yogurt with Additional Plant-Based High Protein for Vegetarian Consumers. E3S Web Conf. 2024, 576, 04010. [Google Scholar] [CrossRef]
- D’Andrea, A.E.; Kinchla, A.J.; Nolden, A.A. A Comparison of the Nutritional Profile and Nutrient Density of Commercially Available Plant-Based and Dairy Yogurts in the United States. Front. Nutr. 2023, 10, 1195045. [Google Scholar] [CrossRef]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; Cabrera-Hidalgo, A.d.J.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toalá, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Erem, E.; Kilic-Akyilmaz, M. The Role of Fermentation with Lactic Acid Bacteria in Quality and Health Effects of Plant-Based Dairy Analogues. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13402. [Google Scholar] [CrossRef]
- Hu, Y.; Badar, I.H.; Zhang, L.; Yang, L.; Xu, B. Odor and Taste Characteristics, Transduction Mechanism, and Perceptual Interaction in Fermented Foods: A Review. Crit. Rev. Food Sci. Nutr. 2024, 1–19. [Google Scholar] [CrossRef]
- Saleem, G.N.; Gu, R.; Qu, H.; Bahar Khaskheli, G.; Rashid Rajput, I.; Qasim, M.; Chen, X. Therapeutic Potential of Popular Fermented Dairy Products and Its Benefits on Human Health. Front. Nutr. 2024, 11, 1328620. [Google Scholar] [CrossRef]
- Raungrusmee, S.; Kumar, S.R.; Anal, A.K. Probiotic Cereal-Based Food and Beverages, Their Production and Health Benefits. In Probiotics, Prebiotics and Synbiotics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 186–212. ISBN 978-1-119-70216-0. [Google Scholar]
- Angelov, A.; Yaneva-Marinova, T.; Gotcheva, V. Oats as a Matrix of Choice for Developing Fermented Functional Beverages. J. Food Sci. Technol. 2018, 55, 2351–2360. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, A.; Tomer, V.; Rasane, P.; Gupta, K. Development of Nutricereals and Milk-Based Beverage: Process Optimization and Validation of Improved Nutritional Properties. J. Food Process Eng. 2020, 43, e13025. [Google Scholar] [CrossRef]
- Popova, N.V.; Kalinina, I.V.; Vasiliev, A.K.; Kameneva, K.S. Evaluation of effectiveness and optimisation of the process of fermentation of oat drink with lactic acid microorganisms. Agrar. Sci. 2024, 2, 127–132. [Google Scholar] [CrossRef]
- Yu, Q.; Xia, X.; Qian, J.; Xu, Q.; Yu, H.; Guo, Y.; Xie, Y.; Yao, W.; Qian, H.; Cheng, Y. A Study on Physicochemical Properties and Flavor Profile of Oat Beverage Fermented by Lactococcus lactis. SSRN 2023. [Google Scholar] [CrossRef]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V®): Three Decades of Research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef]
- Lactobacillus Acidophilus, LA-5®—A Well-Documented Probiotic Strain. The Probiotics Institute by Chr. Hansen A/S. Available online: https://global.theprobioticsinstitute.com/en/chr-hansen-probiotics/la-5 (accessed on 15 February 2025).
- Süle, J.; Kõrösi, T.; Hucker, A.; Varga, L. Evaluation of Culture Media for Selective Enumeration of Bifidobacteria and Lactic Acid Bacteria. Braz. J. Microbiol. 2014, 45, 1023–1030. [Google Scholar] [CrossRef]
- Fang, X.; Ye, H.; Chen, M.; Chen, X.; Chen, M.; Chen, J.; Kong, W.; Wang, Q.; Zhang, Z. Effects of Co-Fermentation of Lactobacillus Plantarum and Inulin on Beany Flavor and Physicochemical Quality of Soy Yogurt in a Multiple Lactic Acid Bacteria Fermentation System. Cereal Chem. 2024, 101, 248–262. [Google Scholar] [CrossRef]
- Küçükgöz, K.; Franczak, A.; Borysewicz, W.; Kamińska, K.; Salman, M.; Mosiej, W.; Kruk, M.; Kołożyn-Krajewska, D.; Trząskowska, M. Impact of Lactic Acid Fermentation on the Organic Acids and Sugars of Developed Oat and Buckwheat Beverages. Fermentation 2024, 10, 373. [Google Scholar] [CrossRef]
- Šertović, E.; Sarić, Z.; Božanić, R.; Barać, M.; Barukčić, I.; Kostić, A. Fermentation of Cow’s Milk and Soy Milk Mixture with L. Acidophilus Probiotic Bacteria with Yoghurt Culture. In Proceedings of the 30th Scientific-Experts Conference of Agriculture and Food Industry, Sarajevo, Bosnia and Herzegovina, 26–27 September 2019; Brka, M., Omanović-Mikličanin, E., Karić, L., Falan, V., Toroman, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 251–259. [Google Scholar]
- Raj, S.K.; Ligimol, J.; Beena, A.K.; Rejeesh, R.; Aysha, C.H.; Divya, M.P. Assessment of pH Variations During Extended Period of Fermentation of Cow Milk with Different Lactic Acid Bacterial Cultures. In Prospective Research and Technological Advancements in Food and Health Sciences; Skyfox Publishing Group: Thanjavur, Indian, 2023. [Google Scholar]
- Khrundin, D.V.; Nikitina, E.V. Chemical, Textural and Antioxidant Properties of Oat-Fermented Beverages with Different Starter Lactic Acid Bacteria and Pectin. BioTech 2024, 13, 38. [Google Scholar] [CrossRef]
- Masiá, C.; Fernández-Varela, R.; Jensen, P.E.; Rahimi Yazdi, S. The Impact of Different Bacterial Blends on Texture and Flavour Development in Plant-Based Cheese. Future Foods 2023, 8, 100250. [Google Scholar] [CrossRef]
- Komalasari, H.; Karni, I.; Heldiyanti, R.; Arianto, A.R.; Rahayu, E.S. Effect of Inoculation Time for Indigenous Probiotic Bacteria Lactobacillus Plantarum DAD-13 on the Physiochemical and Organoleptic Properties of Yoghurt Drink: Pengaruh Waktu Inokulasi Bakteri Probiotik Indigenous Lactobacillus Plantarum DAD-13 Terhadap Sifat Fisikokimia Dan Organoleptik Yoghurt Drink. JITIPARI J. Ilm. Teknol. Dan Ind. Pangan UNISRI 2024, 9, 67–78. [Google Scholar] [CrossRef]
- Domínguez-Murillo, A.C.; Urías-Silvas, J.E. Fermented Coconut Jelly as a Probiotic Vehicle, Physicochemical and Microbiology Characterisation during an in Vitro Digestion. Int. J. Food Sci. Technol. 2023, 58, 45–52. [Google Scholar] [CrossRef]
- Gustaw, W.; Kozioł, J.; Radzki, W.; Skrzypczak, K.; Michalak-Majewska, M.; Sołowiej, B.; Sławińska, A.; Jabłońska-Ryś, E. The Effect of Addition of Selected Milk Protein Preparations on the Growth of Lactobacillus Acidophilus and Physicochemical Properties of Fermented Milk. Acta Sci. Pol. Technol. Aliment. 2016, 15, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Cao, Z.; Zhang, J.; Mu, G.; Jiang, S. Characteristics of the Mixed Yogurt Fermented from Cow–Soy Milk in the Presence of Transglutaminase. Foods 2024, 13, 2120. [Google Scholar] [CrossRef] [PubMed]
- Sim, E.A.; Kim, S.-Y.; Kim, S.; Mun, E.-G. Probiotic Potential and Enhanced Adhesion of Fermented Foods-Isolated Lactic Acid Bacteria to Intestinal Epithelial Caco-2 and HT-29 Cells. Microorganisms 2025, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Horáčková, Š.; Vrchotová, B.; Koval, D.; Omarova, A.; Sluková, M.; Štětina, J. Use of Lactiplantibacillus Plantarum for Dairy and Non-Dairy Fermented Products. Czech J. Food Sci. 2022, 40, 392–399. [Google Scholar] [CrossRef]
- El Bouchikhi, S.; Pagès, P.; El Alaoui, Y.; Ibrahimi, A.; Bensouda, Y. Syneresis Investigations of Lacto-Fermented Sodium Caseinate in a Mixed Model System. BMC Biotechnol. 2019, 19, 57. [Google Scholar] [CrossRef]
- Gedik, O.; Karahan, A.G. Physicochemical Properties and Survival Assessment of Potential Probiotics in a Novel Dairy Drink during Storage. Food Sci. Nutr. 2023, 11, 7803–7815. [Google Scholar] [CrossRef]
- Martins, A.R.; Monteiro, R.L.; Burkert, J.F.d.M.; Burkert, C.A.V. Simultaneous Enzymatic Hydrolysis and Lactic Fermentation to Obtain a Yogurt with Low Lactose Content. Ciênc. Agrotecnol. 2012, 36, 551–559. [Google Scholar] [CrossRef]
- Tadesse, B.T.; Zhao, G.; Kempen, P.; Solem, C. Consolidated Bioprocessing in a Dairy Setting─Concurrent Yoghurt Fermentation and Lactose Hydrolysis without Using Lactase Enzymes. J. Agric. Food Chem. 2022, 70, 11623–11630. [Google Scholar] [CrossRef]
- Schwab, C.; Gänzle, M. Lactic Acid Bacteria Fermentation of Human Milk Oligosaccharide Components, Human Milk Oligosaccharides and Galactooligosaccharides. FEMS Microbiol. Lett. 2011, 315, 141–148. [Google Scholar] [CrossRef]
- Gunkova, P.I.; Buchilina, A.S.; Maksimiuk, N.N.; Bazarnova, Y.G.; Girel, K.S. Carbohydrate Fermentation Test of Lactic Acid Starter Cultures. IOP Conf. Ser. Earth Environ. Sci. 2021, 852, 012035. [Google Scholar] [CrossRef]
- Andreevskaya, M.; Johansson, P.; Jääskeläinen, E.; Rämö, T.; Ritari, J.; Paulin, L.; Björkroth, J.; Auvinen, P. Lactobacillus Oligofermentans Glucose, Ribose and Xylose Transcriptomes Show Higher Similarity between Glucose and Xylose Catabolism-Induced Responses in the Early Exponential Growth Phase. BMC Genom. 2016, 17, 539. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Larsen, F.H.; Jensen, H.M.; Vogensen, F.K.; Engelsen, S.B. Real-Time Metabolomic Analysis of Lactic Acid Bacteria as Monitored by in Vitro NMR and Chemometrics. Metabolomics 2016, 12, 77. [Google Scholar] [CrossRef]
- Hettinga, K. Study Used Wrong Assumption about Galactose Content of Fermented Dairy Products. BMJ 2014, 349, g7000. [Google Scholar] [CrossRef]
- Wu, Q.; Cheung, C.K.W.; Shah, N.P. Towards Galactose Accumulation in Dairy Foods Fermented by Conventional Starter Cultures: Challenges and Strategies. Trends Food Sci. Technol. 2015, 41, 24–36. [Google Scholar] [CrossRef]
- Zha, M.; Li, K.; Zhang, W.; Sun, Z.; Kwok, L.-Y.; Menghe, B.; Chen, Y. Untargeted Mass Spectrometry-Based Metabolomics Approach Unveils Molecular Changes in Milk Fermented by Lactobacillus Plantarum P9. LWT 2021, 140, 110759. [Google Scholar] [CrossRef]
- Sumarna. Changes of Raffinose and Stachyose in Soy Milk Fermentation by Lactic Acid Bacteria from Local Fermented Foods of Indonesian. Malays. J. Microbiol. 2008, 4, 26–34. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Wang, T.; Wang, R.; Luo, X. Effects of Five Different Lactic Acid Bacteria on Bioactive Components and Volatile Compounds of Oat. Foods 2022, 11, 3230. [Google Scholar] [CrossRef]
- Klajn, V.M.; Ames, C.W.; da Cunha, K.F.; Lorini, A.; Hackbart, H.C.d.S.; Filho, P.J.S.; Cruxen, C.E.d.S.; Fiorentini, Â.M. Probiotic Fermented Oat Dairy Beverage: Viability of Lactobacillus Casei, Fatty Acid Profile, Phenolic Compound Content and Acceptability. J. Food Sci. Technol. 2021, 58, 3444–3452. [Google Scholar] [CrossRef]
- Chen, L.; Wu, D.; Schlundt, J.; Conway, P.L. Development of a Dairy-Free Fermented Oat-Based Beverage With Enhanced Probiotic and Bioactive Properties. Front. Microbiol. 2020, 11, 609734. [Google Scholar] [CrossRef]
- Zacarchenco, P.B.; Massaguer-Roig, S. Avaliação sensorial, microbiológica e de pós-acidificação durante a vida-de-prateleira de leites fermentados contendo Streptococcus thermophilus, Bifidobacterium longum e Lactobacillus acidophilus. Food Sci. Technol. 2004, 24, 674–679. [Google Scholar] [CrossRef]
- Gueimonde, M.; Reyes-Gavilán, C.G. Reduction of Incubation Time in Carbonated Streptococcus Thermophilus/Lactobacillus Acidophilus Fermented Milks as Affected by the Growth and Acidification Capacity of the Starter Strains. Milchwiss.-Milk Sci. Int. 2004, 59, 280–283. [Google Scholar]
- Huang, Y.-Y.; Lu, Y.-H.; Liu, X.-T.; Wu, W.-T.; Li, W.-Q.; Lai, S.-Q.; Aadil, R.M.; Rajoka, M.S.R.; Wang, L.-H.; Zeng, X.-A. Metabolic Properties, Functional Characteristics, and Practical Application of Streptococcus thermophilus. Food Rev. Int. 2024, 40, 792–813. [Google Scholar] [CrossRef]
- Souza, C.H.B.; Saad, S.M.I. Viability of Lactobacillus Acidophilus La-5 Added Solely or in Co-Culture with a Yoghurt Starter Culture and Implications on Physico-Chemical and Related Properties of Minas Fresh Cheese during Storage. LWT Food Sci. Technol. 2009, 42, 633–640. [Google Scholar] [CrossRef]
- Sarvari, F.; Mortazavian, A.M.; Fazei, M.R. Biochemical Characteristics and Viability of Probiotic and Yogurt Bacteria in Yogurt during the Fermentation and Refrigerated Storage. Appl. Food Biotechnol. 2014, 1, 55–61. [Google Scholar] [CrossRef]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the Mechanisms of Positive Microbial Interactions That Benefit Lactic Acid Bacteria Co-Cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Bedani, R.; Perego, P.; Converti, A.; Saad, S.M.I. L. Acidophilus La-5, Fructo-Oligosaccharides and Inulin May Improve Sensory Acceptance and Texture Profile of a Synbiotic Diet Mousse. LWT 2019, 105, 329–335. [Google Scholar] [CrossRef]
- Gullo, M.; Zotta, T. Chapter 18—Probiotics in Dairy Products: Microencapsulation and Delivery. In Advances in Dairy Microbial Products; Singh, J., Vyas, A., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 271–285. ISBN 978-0-323-85793-2. [Google Scholar]
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascón, A.; Dueñas, M.T.; López, P.; Spano, G. Lactobacillus Plantarum Strains for Multifunctional Oat-Based Foods. LWT Food Sci. Technol. 2016, 68, 288–294. [Google Scholar] [CrossRef]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of Substrate Composition and Inoculum on the Fermentation Kinetics and Flavour Compound Profiles of Potentially Non-Dairy Probiotic Formulations. LWT Food Sci. Technol. 2014, 55, 240–247. [Google Scholar] [CrossRef]

| Sample | Cow’s Milk | Dairy-Oat Beverage | Oat Beverage |
|---|---|---|---|
| A: Inoculated with the yogurt culture | A1 | A2 | A3 |
| B: Inoculated with the yogurt culture and L. plantarum 299v | B1 | B2 | B3 |
| C: Inoculated with the L. plantarum 299v culture | C1 | C2 | C3 |
| E: Inoculated with the yogurt culture and L. acidophilus La-5 | E1 | E2 | E3 |
| F: Inoculated with the acidophilus La-5 culture | F1 | F2 | F3 |
| Storage Time [Day] | 0 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|
| A1 | |||||
| glucose | 0.02 a ± 0.00 | 0.03 a ± 0.00 | 0.02 a ± 0.01 | 0.02 a ± 0.01 | 0.02 a ± 0.01 |
| galactose | 0.80 a ± 0.04 | 0.85 a ± 0.05 | 0.82 a ± 0.05 | 0.67 b ± 0.03 | 0.59 b ± 0.04 |
| lactose * | 2.52 a ± 0.13 | 2.46 a,b ± 0.15 | 2.44 a,b ± 0.14 | 2.35 b ± 0.12 | 1.89 c ± 0.19 |
| B1 | |||||
| glucose | 0.02 a ± 0.00 | 0.02 a ± 0.01 | 0.02 a ± 0.01 | 0.02 a ± 0.00 | 0.02 a ± 0.00 |
| galactose | 0.65 a ± 0.03 | 0.61 a ± 0.04 | 0.61 a ± 0.05 | 0.58 a ± 0.03 | 0.61 a ± 0.03 |
| lactose | 2.40 a ± 0.12 | 2.33 b ± 0.14 | 2.02 c ± 0.15 | 2.01 c ± 0.10 | 1.66 d ± 0.08 |
| C1 | |||||
| glucose | 0.02 a ± 0.01 | 0.02 a ± 0.01 | 0.02 a ± 0.00 | 0.02 a ± 0.01 | 0.02 a ± 0.00 |
| galactose | 0.60 a ± 0.04 | 0.52 a ± 0.05 | 0.44 b ± 0.02 | 0.40 b ± 0.03 | 0.38 b ± 0.02 |
| lactose | 2.45 a ± 0.12 | 2.37 a ± 0.13 | 2.21 b ± 0.11 | 2.00 c ± 0.11 | 1.93 c ± 0.10 |
| D1 | |||||
| glucose | 0.02 a ± 0.01 | 0.02 a ± 0.00 | 0.02 a ± 0.01 | 0.02 a ± 0.00 | 0.02 a ± 0.01 |
| galactose | 0.64 a ± 0.05 | 0.74 a ± 0.04 | 0.72 a ± 0.05 | 0.70 a ± 0.04 | 0.63 a ± 0.06 |
| lactose | 2.57 a ± 0.14 | 2.41 a ± 0.12 | 1.80 c ± 0.10 | 1.70 c ± 0.09 | 1.46 d ± 0.07 |
| E1 | |||||
| glucose | 0.02 a ± 0.01 | 0.02 a ± 0.01 | 0.00 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.01 |
| galactose | 0.92 a ± 0.05 | 0.92 a ± 0.06 | 0.93 a ± 0.05 | 0.88 a ± 0.04 | 0.89 a ± 0.05 |
| lactose | 2.55 a ± 0.13 | 2.40 a ± 0.15 | 2.22 a ± 0.11 | 2.11 a ± 0.11 | 1.67 b ± 0.09 |
| F1 | |||||
| glucose | 0.01 a ± 0.01 | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.01 |
| galactose | 0.83 a ± 0.04 | 0.90 a ± 0.05 | 0.80 a ± 0.06 | 0.76 a ± 0.04 | 0.78 a ± 0.06 |
| lactose | 2.52 a ± 0.13 | 2.56 a ± 0.17 | 2.47 a ± 0.16 | 2.33 a ± 0.07 | 1.43 b ± 0.13 |
| Storage Time [Day] | 0 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|
| A2 | |||||
| fructose | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.00 |
| glucose | 0.04 a ± 0.02 | 0.04 a ± 0.01 | 0.04 a ± 0.00 | 0.04 a ± 0.00 | 0.04 a ± 0.01 |
| galactose | 0.47 a ± 0.03 | 0.50 a ± 0.02 | 0.48 a ± 0.03 | 0.40 a,b ± 0.05 | 0.35 b ± 0.03 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.36 a ± 0.02 | 0.31 a,b ± 0.01 | 0.27 b ± 0.01 | 0.26 b ± 0.03 | 0.26 b ± 0.02 |
| lactose | 1.22 a ± 0.06 | 1.17 a ± 0.06 | 0.94 a ± 0.05 | 1.24 a ± 0.06 | 1.18 a ± 0.06 |
| raffinose | 0.49 a ± 0.00 | 0.49 a ± 0.01 | 0.44 a ± 0.01 | 0.42 a ± 0.01 | 0.40 a ± 0.01 |
| stachyose | 0.03 a ± 0.00 | 0.03 a ± 0.00 | 0.04 a ± 0.00 | 0.03 a ± 0.00 | 0.03 a ± 0.00 |
| B2 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.00 |
| glucose | 0.04 a ± 0.00 | 0.04 a ± 0.02 | 0.04 a ± 0.00 | 0.04 a ± 0.01 | 0.03 a ± 0.01 |
| galactose | 0.38 a ± 0.02 | 0.36 a,b ± 0.02 | 0.36 a,b ± 0.02 | 0.34 b ± 0.02 | 0.36 a,b ± 03 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.31 a ± 0.03 | 0.31 a ± 0.02 | 0.30 a,b ± 0.02 | 0.28 b ± 0.01 | 0.28 b ± 0.02 |
| lactose | 0.00 a ± 0.01 | 0.73 a ± 0.04 | 0.92 a ± 0.05 | 0.62 a ± 0.03 | 0.63 a ± 0.04 |
| raffinose | 0.32 a ± 0.02 | 0.35 a ± 0.02 | 0.34 a ± 0.02 | 0.34 a ± 0.03 | 0.31 a ± 0.02 |
| stachyose | 0.03 a ± 0.01 | 0.03 a ± 0.01 | 0.03 a ± 0.01 | 0.02 b ± 0.01 | 0.02 b ± 0.00 |
| C2 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 |
| glucose | 0.04 a ± 0.01 | 0.04 a ± 0.02 | 0.04 a ± 0.00 | 0.04 a ± 0.02 | 0.04 a ± 0.00 |
| galactose | 0.35 a ± 0.03 | 0.31 a ± 0.03 | 0.26 a,b ± 0.01 | 0.23 a,b ± 0.03 | 0.22 b ± 0.01 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.35 a ± 0.02 | 0.34 a ± 0.03 | 0.33 a,b ± 0.03 | 0.32 a,b ± 0.02 | 0.28 b ± 0.02 |
| lactose | 2.54 a ± 0.13 | 1.93 a ± 0.11 | 2.23 a ± 0.12 | 1.56 a ± 0.09 | 1.73 a ± 0.10 |
| raffinose | 0.35 a ± 0.02 | 0.34 a ± 0.03 | 0.34 a ± 0.03 | 0.33 a ± 0.02 | 0.34 a ± 0.05 |
| stachyose | 0.04 a ± 0.01 | 0.05 a ± 0.01 | 0.05 a ± 0.00 | 0.05 a ± 0.01 | 0.04 a ± 0.01 |
| D2 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.01 |
| glucose | 0.05 a ± 0.01 | 0.06 a ± 0.01 | 0.05 a ± 0.02 | 0.04 a ± 0.00 | 0.04 a ± 0.01 |
| galactose | 0.38 a ± 0.03 | 0.43 b ± 0.03 | 0.43 b ± 0.02 | 0.41 a,b ± 0.02 | 0.37 a ± 0.03 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.28 a ± 0.02 | 0.29 a ± 0.02 | 0.27 a,b ± 0.02 | 0.25 a,b ± 0.02 | 0.22 b ± 0.02 |
| lactose | 1.20 a ± 0.07 | 1.65 a ± 0.08 | 1.74 a ± 0.10 | 1.30 a ± 0.08 | 0.90 a ± 0.06 |
| raffinose | 0.31 a ± 0.02 | 0.32 a ± 0.03 | 0.33 a ± 0.03 | 0.33 a ± 0.03 | 0.32 a ± 0.04 |
| stachyose | 0.03 a ± 0.01 | 0.03 a ± 0.01 | 0.03 a ± 0.01 | 0.03 a ± 0.01 | 0.03 a ± 0.00 |
| E2 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.01 |
| glucose | 0.05 a ± 0.01 | 0.04 a ± 0.01 | 0.05 a ± 0.01 | 0.04 a ± 0.01 | 0.05 a ± 0.02 |
| galactose | 0.54 a ± 0.04 | 0.54 a ± 0.04 | 0.55 a ± 0.05 | 0.52 a ± 0.00 | 0.52 a ± 0.01 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.22 a,b ± 0.02 | 0.25 a ± 0.02 | 0.23 a,b ± 0.02 | 0.22 a,b ± 0.01 | 0.20 b ± 0.03 |
| lactose | 1.04 a ± 0.06 | 0.84 a ± 0.05 | 0.87 a ± 0.05 | 1.40 a ± 0.08 | 0.89 a ± 0.02 |
| raffinose | 0.28 a ± 0.01 | 0.28 a ± 0.02 | 0.26 a ± 0.03 | 0.28 a ± 0.03 | 0.26 a ± 0.01 |
| stachyose | 0.03 a ± 0.01 | 0.04 a ± 0.01 | 0.04 a ± 0.00 | 0.03 a ± 0.01 | 0.03 a ± 0.00 |
| F2 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.00 |
| glucose | 0.04 a ± 0.01 | 0.05 a ± 0.02 | 0.05 a ± 0.01 | 0.04 a ± 0.01 | 0.04 a ± 0.01 |
| galactose | 0.49 a ± 0.04 | 0.53 a ± 0.05 | 0.47 a,b ± 0.05 | 0.45 b ± 0.05 | 0.46 a,b ± 0.03 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.28 a ± 0.02 | 0.28 a ± 0.03 | 0.26 a,b ± 0.01 | 0.26 a,b ± 0.02 | 0.23 b ± 0.02 |
| lactose | 1.83 a ± 0.10 | 0.56 a ± 0.04 | 1.37 a ± 0.08 | 0.81 a ± 0.06 | 1.06 a ± 0.06 |
| raffinose | 0.45 a ± 0.02 | 0.43 a ± 0.02 | 0.41 a ± 0.02 | 0.44 a ± 0.02 | 0.46 a ± 0.02 |
| stachyose | 0.02 a ± 0.00 | 0.02 a ± 0.00 | 0.02 a ± 0.01 | 0.02 a ± 0.00 | 0.03 a ± 0.01 |
| Storage Time [Day] | 0 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|
| A3 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 00 | 0.01 a ± 0.00 |
| glucose | 0.02 a ± 0.00 | 0.03 a ± 0.00 | 0.02 a ± 0.00 | 0.02 a ± 0.01 | 0.02 a ± 0.01 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.54 a ± 0.04 | 0.47 a ± 0.02 | 0.41 b ± 0.03 | 0.39 b ± 0.03 | 0.35 c ± 0.03 |
| raffinose | 0.81 a ± 0.03 | 0.81 a ± 0.06 | 0.58 a ± 0.05 | 0.55 a ± 0.04 | 0.60 a ± 0.05 |
| stachyose | 0.06 a,b ± 0.01 | 0.06 a,b ± 0.01 | 0.07 a ± 0.01 | 0.05 c ± 0.01 | 0.06 a,b ± 0.01 |
| B3 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.02 a ± 0.01 |
| glucose | 0.02 a ± 0.01 | 0.03 a ± 0.00 | 0.02 a ± 0.01 | 0.02 a ± 0.02 | 0.02 a ± 0.01 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.46 a ± 0.02 | 0.46 a ± 0.03 | 0.46 a ± 0.03 | 0.40 b ± 0.03 | 0.39 b ± 0.03 |
| raffinose | 0.48 a ± 0.02 | 0.52 a ± 0.04 | 0.52 a ± 0.03 | 0.51 a ± 0.05 | 0.47 a ± 0.03 |
| stachyose | 0.06 a ± 0.01 | 0.06 a ± 0.00 | 0.06 a ± 0.01 | 0.03 b ± 0.01 | 0.04 b ± 0.00 |
| C3 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 |
| glucose | 0.03 a ± 0.00 | 0.04 a ± 0.01 | 0.04 a ± 0.01 | 0.03 a ± 0.01 | 0.02 b ± 0.01 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.55 a ± 0.04 | 0.51 a ± 0.04 | 0.50 a ± 0.05 | 0.45 b ± 0.05 | 0.35 c ± 0.06 |
| raffinose | 0.52 a ± 0.04 | 0.51 a ± 0.04 | 0.51 a ± 0.04 | 0.50 a ± 0.03 | 0.51 a ± 0.02 |
| stachyose | 0.08 a,b ± 0.01 | 0.09 a,b ± 0.01 | 0.10 a ± 0.01 | 0.09 a,b ± 0.01 | 0.07 b ± 0.02 |
| D3 | |||||
| fructose | 0.02 a ± 0.00 | 0.01 a ± 0.01 | 0.02 a ± 0.01 | 0.02 a ± 0.01 | 0.01 a ± 0.00 |
| glucose | 0.03 a ± 0.01 | 0.04 a ± 0.01 | 0.05 b ± 0.02 | 0.04 a ± 0.01 | 0.04 a ± 0.01 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.51 a ± 0.04 | 0.43 a ± 0.03 | 0.41 b ± 0.03 | 0.38 b ± 0.05 | 0.27 c ± 0.03 |
| raffinose | 0.47 a ± 0.04 | 0.48 a ± 0.04 | 0.50 a ± 0.03 | 0.50 a ± 0.04 | 0.48 a ± 0.02 |
| stachyose | 0.06 a ± 0.01 | 0.06 a ± 0.02 | 0.06 a ± 0.01 | 0.06 a ± 0.02 | 0.07 b ± 0.01 |
| E3 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 |
| glucose | 0.05 a ± 0.02 | 0.05 a ± 0.02 | 0.05 a ± 0.01 | 0.04 b ± 0.01 | 0.04 b ± 0.01 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.33 a ± 0.02 | 0.31 a,b ± 0.04 | 0.28 b ± 0.01 | 0.27 a,b ± 0.01 | 0.25 b ± 0.02 |
| raffinose | 0.34 a ± 0.03 | 0.35 a ± 0.03 | 0.32 a ± 0.02 | 0.34 a ± 0.03 | 0.31 a ± 0.03 |
| stachyose | 0.07 a,b ± 0.01 | 0.07 a,b ± 0.01 | 0.08 a ± 0.01 | 0.06 b ± 0.00 | 0.06 b ± 0.01 |
| F3 | |||||
| fructose | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.02 a ± 0.01 | 0.01 a ± 0.01 |
| glucose | 0.03 a ± 0.00 | 0.04 a ± 0.01 | 0.04 a ± 0.01 | 0.03 a ± 0.01 | 0.03 a ± 0.01 |
| maltose | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.00 a ± 0.00 |
| sucrose | 0.48 a ± 0.02 | 0.48 a ± 0.02 | 0.42 a,b ± 0.03 | 0.42 a,b ± 0.04 | 0.38 b ± 0.03 |
| raffinose | 0.68 a ± 0.03 | 0.40 a ± 0.02 | 0.23 a ± 0.04 | 0.43 a ± 0.04 | 0.30 a ± 0.04 |
| stachyose | 0.05 a ± 0.02 | 0.03 b ± 0.01 | 0.05 a ± 0.01 | 0.04 a,b ± 0.01 | 0.05 a ± 0.01 |
| Storage Time [Day] | 0 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|
| Sample | L. delbrueckii subsp. bulgaricus | ||||
| A1 | 7.7 a ± 0.1 | 8.1 a ± 0.1 | 7.5 a ± 0.2 | 7.4 a ± 0.2 | 7.4 a ± 0.2 |
| A2 | 8.0 a ± 0.2 | 7.6 a,b ± 0.2 | 7.2 b,c ± 0.2 | 6.9 c ± 0.1 | 6.9 c ± 0.1 |
| A3 | 7.7 a ± 0.2 | 7.2 a ± 0.2 | 7.2 a ± 0.1 | 7.2 a ± 0.1 | 7.0 a ± 0.2 |
| B1 | 7.0 a ± 0.1 | 7.1 a ± 0.1 | 7.5 a ± 0.1 | 7.0 a ± 0.2 | 6.8 a ± 0.1 |
| B2 | 7.4 a ± 0.1 | 7.7 a ± 0.1 | 7.0 a ± 0.1 | 7.2 a ± 0.1 | 7.3 a ± 0.2 |
| B3 | 7.9 a ± 0.2 | 7.6 a ± 0.2 | 7.4 a ± 0.2 | 7.6 a ± 0.1 | 7.3 a ± 0.2 |
| E1 | 8.9 a ± 0.2 | 8.1 a ± 0.1 | 8.0 a,b ± 0.2 | 8.4 a ± 0.2 | 7.6 b ± 0.1 |
| E2 | 8.6 a ± 0.1 | 7.9 b ± 0.2 | 8.0 a,b ± 0.1 | 8.5 a ± 0.2 | 8.0 a,b ± 0.2 |
| E3 | 7.9 a ± 0.2 | 8.8 a ± 0.1 | 8.3 a ± 0.1 | 8.1 a ± 0.2 | 8.0 a ± 0.2 |
| S. thermophilus | |||||
| A1 | 7.9 a ± 0.1 | 7.9 a ± 0.1 | 8.1 a ± 0.2 | 7.9 a ± 0.2 | 7.6 a ± 0.2 |
| A2 | 8.0 a ± 0.2 | 7.9 a ± 0.1 | 8.4 a ± 0.2 | 7.7 a ± 0.1 | 7.6 a ± 0.1 |
| A3 | 8.9 a ± 0.2 | 9.1 a ± 0.2 | 8.9 a ± 0.2 | 8.7 a ± 0.2 | 8.2 a ± 0.2 |
| B1 | 8.9 a ± 0.2 | 8.8 a ± 0.2 | 7.9 a,b ± 0.2 | 7.6 b ± 0.2 | 7.3 b ± 0.1 |
| B2 | 8.9 a ± 0.2 | 8.8 a ± 0.2 | 8.9 a ± 0.2 | 8.6 a ± 0.2 | 8.5 a ± 0.2 |
| B3 | 8.0 a ± 0.2 | 8.2 a ± 0.1 | 7.8 a ± 0.1 | 7.8 a ± 0.2 | 7.8 a ± 0.2 |
| E1 | 7.8 a ± 0.2 | 7.6 a ± 0.1 | 7.6 a ± 0.2 | 7.1 a,b ± 0.1 | 6.9 b ± 0.2 |
| E2 | 7.9 a ± 0.2 | 7.7 a ± 0.2 | 7.2 a ± 0.1 | 6.9 a ± 0.1 | 7.5 a ± 0.1 |
| E3 | 8.1 a ± 0.2 | 7.7 a ± 0.2 | 7.4 a,b ± 0.1 | 6.9 b ± 0.1 | 7.0 a,b ± 0.2 |
| L. plantarum 299v | |||||
| B1 | 6.7 a ± 0.2 | 6.3 a ± 0.1 | 5.9 a,b ± 0.2 | 5.5 b ± 0.2 | 6.2 a ± 0.1 |
| B2 | 6.2 a ± 0.1 | 6.3 a ± 0.1 | 5.5 a ± 0.2 | 5.6 a ± 0.2 | 5.8 a ± 0.2 |
| B3 | 6.7 a ± 0.1 | 6.7 a ± 0.2 | 5.5 b ± 0.2 | 5.2 b ± 0.1 | 5.6 a,b ± 0.1 |
| C1 | 6.2 a ± 0.1 | 6.9 a ± 0.2 | 5.8 a,b ± 0.2 | 5.7 b ± 0.1 | 5.7 b ± 0.2 |
| C2 | 6.7 a ± 0.2 | 6.0 a,b ± 0.1 | 5.8 b ± 0.2 | 5.7 b ± 0.1 | 5.0 c ± 0.1 |
| C3 | 6.7 a ± 0.2 | 6.3 a ± 0.2 | 6.0 a ± 0.1 | 5.7 b ± 0.2 | 5.9 a,b ± 0.2 |
| L. acidophilus La-5 | |||||
| E1 | 7.8 a ± 0.2 | 6.9 b ± 0.2 | 7.0 a ± 0.2 | 6.9 b ± 0.1 | 6.0 b ± 0.1 |
| E2 | 8.7 a ± 0.2 | 7.8 a,b ± 0.2 | 7.9 a ± 0.2 | 7.4 b ± 0.2 | 6.9 b ± 0.1 |
| E3 | 8.1 a ± 0.1 | 8.5 a ± 0.2 | 8.0 a,b ± 0.2 | 7.5 b ± 0.2 | 6.7 c ± 0.2 |
| F1 | 8.8 a ± 0.1 | 8.7 a ± 0.1 | 8.8 a ± 0.1 | 8.2 a,b ± 0.2 | 7.4 b ± 0.2 |
| F2 | 8.4 a ± 0.2 | 7.9 a ± 0.2 | 8.1 a ± 0.2 | 8.1 a ± 0.1 | 7.8 a ± 0.1 |
| F3 | 8.4 a ± 0.2 | 8.6 a ± 0.1 | 8.3 a,b ± 0.2 | 8.2 a,b ± 0.2 | 7.4 b ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziarno, M.; Zaręba, D.; Kowalska, E.; Florowski, T. A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages. Appl. Sci. 2025, 15, 3714. https://doi.org/10.3390/app15073714
Ziarno M, Zaręba D, Kowalska E, Florowski T. A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages. Applied Sciences. 2025; 15(7):3714. https://doi.org/10.3390/app15073714
Chicago/Turabian StyleZiarno, Małgorzata, Dorota Zaręba, Ewa Kowalska, and Tomasz Florowski. 2025. "A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages" Applied Sciences 15, no. 7: 3714. https://doi.org/10.3390/app15073714
APA StyleZiarno, M., Zaręba, D., Kowalska, E., & Florowski, T. (2025). A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages. Applied Sciences, 15(7), 3714. https://doi.org/10.3390/app15073714








