Abstract
After the winemaking process, the residues formed are called wine lees, which represent a mixture of autolyzed yeasts deposited at the bottom of wine-storage tanks. Approximately 2.96 million tons of yeast result from the vinification of 49.4 million tons of grapes. The increased costs of removing these by-products from the wine industry, which is no longer required in the production process, offer us the opportunity to capitalize on various bioactive compounds through the circular economy concept and circular process. Wine lees resulting from the large-scale production of wine represent a raw material for the valorization of phenolic compounds, proteins, and polysaccharides, as well as pigments or organic compounds. The substantial nutrient resources available from wine lees are described extensively in this manuscript and range from vitamins, amino acids, and fatty acids to food supplements, edible packaging, or food products such as bakery products. This review article explores the emerging horizons of winery waste utilization, unveiling the abundance of bioactive compounds and their manifold applications across the industrial realm.
1. Introduction
Wine production involves stages such as grape cultivation, harvesting, fermentation, and maturation, resulting in residues such as wastewater and organic solid waste, also known as wine pomace or grape pomace. These wastes, composed of stalks, skins, seeds, and yeast cells, are potential environmental pollutants and are often improperly discarded. According to Borges et al., 2021, waste generation accounts for 20–30% of total wine production [1]. To minimize waste generation and promote reuse, studies have focused on the potential applications of organic solid waste in the food industry, in the context of circular economy [1]. Wine lees (WLs) are estimated to account for 14% of total waste generated during winemaking operations, resulting in over 1.26 million tons produced worldwide [2].
Wine lees are residues accumulated in containers after fermentation, storage, treatment, filtering, or centrifuging, as defined by the European Union Council Regulation (EC) 1493/9911. They are a heterogeneous mass deposited after must fermentation and various technological processes in wine manufacturing [3,4]. Wine lees can also be obtained from fining, racking, and decanting. The distinctive smell and color of wine lees can influence the product’s organoleptic qualities, which is why it is important to reach the optimal percentage of addition [1]. The quality of wine lees can be influenced by the grape variety, the quality of the grapes [5], the fermentation temperature, the yeast strain, the shelf life or the type of yeast [6,7], but also by environmental conditions, the region of origin, and their agronomic characteristics [8]. Wine lees are composed of solid and liquid fractions [9]. The liquid one contains ethanol, lactic acid, and acetic acid. The solid fraction is composed of microorganisms (mainly yeasts), cellulosic and hemicellulosic materials, proteins, organic acids, phenolic compounds, and minerals [8,10].
Yeast-based products and their cell walls are recognized for their rich content of probiotics and prebiotics. Supplementation with yeast cells or products derived from the yeast cell wall in chickens improves production performance. This is evidenced by increased body weight, improved feed conversion ratio, egg weight, fertility, hatching, and carcass yield [11]. New restrictions on the use of antibiotics in the food industry make alternatives such as yeast a valuable source of prebiotics and probiotics that ensure the health of birds [12]. Recently, it has been shown that yeast cell walls have been able to decrease the Salmonella bacterial load in the cecum of infected birds (both contact and seeded birds) [13]. Of course, negative effects on animals have also been recorded when supplementing with yeasts, especially in dairy cattle [14]. Currently, the most common use of spent yeast is for feeding animals. This is due primarily to the regulations that no longer allow the use of meat and bone meal in the diet of animals, following the adoption of EU Regulation No. 999/2001, dated 22 May 2001 [15].
The aim of this review is to collect information from literature about wine lees characteristics and their application in food industry but also other ways to reuse it in the circular-economy context. The article reviews the most recent information about composition of winery by-products, highlighting their potential as suitable source of bioactive compounds. Furthermore, the techniques used to process and isolate these molecules, the health benefits, and their uses in various sectors are described in this paper. The emphasis is placed to the functional properties of extracts, as this is a valuable characteristic and presents uses in various industrial fields.
2. Wine Lees: Source and Physicochemical Composition
Sustainable handling of agricultural by-products presents both opportunities and challenges for the sector’s industries [16]. Wine lees were extracted from red or white grapes and are regarded as a rich source of bioactive substances like polyphenols and one of the primary by-products of winery wastes [2,17]. They possess a diverse composition and presently account for 6% of the byproducts produced per ton of wine grapes [18]. Recently, the emphasis has been on increasing the efficiency of bioactive compounds’ extraction, using environmentally friendly and economical solvents. Positive effects on the yield and extraction time were achieved for microwave and ultrasound extraction methods. An alternative for bioactive compounds extraction is enzyme-assisted extraction and high hydrostatic pressure. On the other hand, to concentrate bioactive compounds as a source of phytochemicals, membrane technology can be used [19].
Fermentation was carried out at 18 °C, and the lees were collected, washed, and centrifuged. The phenolic extract was obtained using ultrasound-assisted methods in 70% ethanol, then evaporated and freeze-dried [20]. The drying methods of wine lees influence their quality and chemical composition. The study undertaken by Poulain et al. examined the impact of various drying processes and operating conditions on the antioxidant properties of white wine lees. Four white wine lees samples were dried using Spray Drying, Freeze Drying, and Vacuum Oven. The resulting powders had varying physical characteristics, with larger particle sizes. After drying, the antioxidant capacities of all lees were maintained, with 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay values ranging from 9.5 to 10.8 mg Trolox equivalent (TE)/g d.m. and Ferric Reducing Antioxidant Power (FRAP) values ranging from 31.2 to 37.3 mg TE/g d.m. Even after 12 months of storage at room temperature, the antioxidant properties remained unchanged. The highest yield was achieved using freeze drying, with larger particles and without affecting antioxidant capacity, demonstrating potential applications for antioxidants in food, nutraceuticals, pharmaceuticals, and cosmetics. This suggests that wine lees serve as a potential natural source of antioxidants for various fields [21,22].
2.1. Wine Lees Characterization
Wine lees are defined according to the European Regulation EEC No. 337/979 as “the sediment formed at the base of the deposit or barrel containing wine after fermentation, during storage or after performing authorized treatments to the product, as well as residues obtained from the filtration or centrifugation of said product” [18]. One ton of grapes generates approximately 6% of wine lees, resulting annually in 2.96 million tons of yeast from the vinification of 49.4 million tons of grapes [18]. When alcoholic fermentation stops, a sludge-like material precipitates at the bottom of the wine tanks, consisting of living or dead yeast cells, yeast residues or other particles, which is called wine yeast [23,24]. Although it can be harmful to the environment if not managed properly, few studies have focused on yeast biomass [25,26]. Many studies have investigated the extraction of ethanol [27], tartaric acid [18], polyphenols [28,29,30,31], the production of culture media [32], as a source of beta-glucans [23] or mannoproteins [33], nutrient-rich fermentation supplements for the production of microbial oil [19] to support a sustainable outcome for wine lees [31]. Red wine yeast is found in larger quantities than white wine yeast and is known for its antioxidant and antimicrobial properties [34,35]. Rather than the total amount of phenolics, the phenolic content of wine lees has been associated with their antibacterial action [36]. Table 1 presents the composition of wine lees.

Table 1.
The composition of wine lees.
Wine lees classification [8,31,40]:
- first and second fermentation lees formed during alcoholic and malolactic fermentations;
- aged wine lees formed during aging in wooden barrels;
- heavy lees (100 μm–2 μm, settling within 24 h) and light lees (<100 μm, 1–24 μm, in suspension at least 24 h after agitation).
Several factors influence the characteristics of wine yeast, including environmental conditions, the region of origin, agronomic characteristics, grape variety, the winemaking process, and aging time [8]. Wine lees, a sludge material accumulated at the bottom of wine tanks after alcoholic fermentation, contain significant amounts of polysaccharides, proteins, lipids, and other organic molecules. They are a source of polyphenols with antioxidant activity, including anthocyanins and flavonoids [41]. The most abundant phenolic compounds were found in wine lees, with ellagic acid being the most abundant and quercetin being the most abundant flavonol. The most representative concentration of anthocyanins was found in malvidin and malvidin-3-acetyl glucoside. Polyphenols, synthesized by plants in response to stress conditions, counteract free radicals and have beneficial health effects against oxidative stress, inflammatory activity, and neurodegenerative, cardiac, and metabolic diseases. They also have a protective effect on sight, promote the growth of probiotics, and modulate the gut microbiota, which can have beneficial effects on human health [41,42]. Wine lees are increasingly being added to animal feed more frequently to increase appetite, as they are a rich source of B vitamins [43].
The total acidity value of wine lees is 121.25 ± 11.25 mg g−1 tartaric acid, with a pH of 3.32 ± 0.02 [34].
Potassium, phosphorus, magnesium, and calcium are essential minerals for human health, mediating cellular signaling and electrical conduction. They are crucial for the nervous system and muscles and for strong bone structure and dental health. The mineral content of wine lees showed high levels of these elements, while microelements like iron, zinc, copper, and boron were present at lower levels [34]. Gümüş et al. identified in their study a total acidity of 121.25 ± 11.25 mg/g tartaric acid and a pH of 3.32 ± 0.02 [34].
2.2. Bioactive Compounds
Red wine lees are a significant source of phenolic compounds [7], which contain flavonoids, a group with aromatic rings that have antioxidant properties, inhibiting free radicals in the human organism due to their oxidation-reduction capacity [31,44]. The phenolic content of dried WL is high, with gallic acid, caffeic acid, myricetin, quercetin, syringic acid, and p-coumaric acid being major phenolic compounds [34]. Extraction is a critical stage in the recovery of phenolic compounds; hence, the extraction parameters must be carefully regulated to produce extracts rich in bioactive compounds [8]. The extraction of bioactive compounds from wine yeast can be achieved using various methods to ensure optimal yields. However, most studies focus on alcoholic fermentation yeast, and only a small portion on malolactic fermentation. The increasing demand for more effective recovery methods led to the development of new unconventional treatments. By lowering extraction time, process temperature, and solvent consumption, these novel techniques can enhance extraction efficiency while consuming less energy [22]. In principle, binary mixtures (e.g., ethanol-water) are used, and the most used extraction method is ultrasound method extract [45]. Other extraction methods used are microwave, conventional, membranes, enzymatic, or supercritical. The potential of wine yeasts as a source of bioactive compounds is highlighted, with the need for further research exploring alternative extraction technologies and combining methods [29,46]. As an environmentally friendly substitute for traditional solvents, natural deep eutectic solvents (NADESs) were studied by Bosiljkov et al. [47]. Choline chloride: malic acid is the most promising of the NADES studied, and it has been shown to be successful in extracting anthocyanins from wine lees when compared to traditional solvents [47]. Reig-Valor et al. investigated the extraction of polyphenols from wine lees using membrane technology. Ultrafiltration (UF) and nanofiltration (NF) membranes are used in a sequential filtration process under various operating conditions. According to the statistics, UF had a rejection rate of 54% and NF had a 90% rate [30]. Water was used as the solvent in a green liquid extraction process to recover phenolic acids, flavonoids, and related chemicals from wine lees. After microfiltering the resultant extract solution to eliminate microparticles, it underwent ultrafiltration (UF) employing membranes with molecular weight cut-offs (MWCOs) of 30 kDa and 5 kDa [48]. Ye et al. investigated the oxidation of phenolic compounds using the flavonoid model (it provides a simpler matrix for understanding chemical changes during oxidation), and all wine lees samples showed bioactivities, the oxidation treatment not having a significant impact on antioxidant activity [49]. From the anthocyanins group, malvidin-3-O-glucoside and malvidin-3-(6″-p-coumarylglucoside) were detected in higher concentrations. The main hydroxycinnamic acids identified in lees are tartaric, ferulic, p-coumaric, and caffeic acids [31].
3. Wine Lees Application in Food
The by-products resulting from the wine industry are considered environmental pollutants due to their high chemical oxygen demand (COD) and the high biological oxygen demand (BOD). On the other hand, due to their high phenolic content, as well as the desire to reduce the amount of waste, these by-products are increasingly used in the food industry as nutrient supplements, food ingredients or edible packaging [50]. According to reports, the way this waste is now managed—whether disposed of in a landfill or spread as compost in vineyards—has a detrimental effect on soil [51]. Figure 1 presents some of the applications of wine lees.
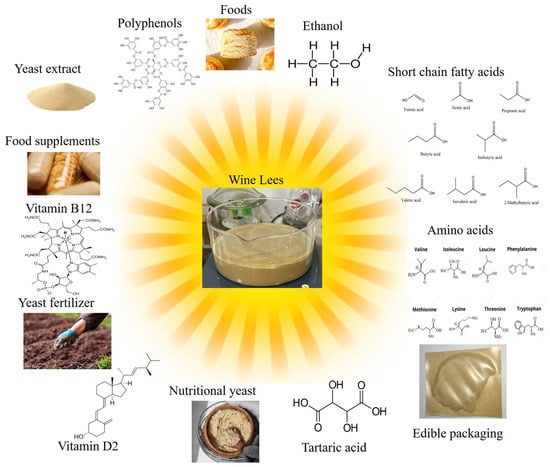
Figure 1.
Wine lees utilization in food and non-food application.
3.1. Muffins
Bianchi et al. [37] conducted a study using wine lees to replace oil (at 25%, 50%, 75%, and 100% of its weight) in muffin production. The technological, sensory, and nutritional characteristics were evaluated in muffins with the addition of wine lees in different proportions (25%, 50%, 75% and 100%), which were used as a fat substitute. Wine lees is a promising fat substitute due to its properties. The addition of wine lees influenced the increase in the apparent viscosity with the increase in the shear speed. The recommended amount of Amarone wine lees as a fat replacer in wheat-based muffins was found to be between 25% and 50% overall based on nutritional, technological, and sensory analyses. This resulted in a reduction in calories and an increase in dietary fiber content of the products without significantly compromising technological or sensorial aspects. The texture and sensory qualities of the muffin recipes with 75% and 100% WL, however, might be enhanced by adding more ingredients or using other mixing and whipping techniques [37]. Customer acceptability is key to the success of functional foods, particularly if they are made from by-products. In fact, the rejection of new applications has led to the necessity of evaluating consumers’ acceptance of novel technology and functional foods. Furthermore, the agri-food sector can only thrive if it effectively meets consumer demand [52].
3.2. Biscuits
To obtain the powder from wine lees, the by-product left over after the alcoholic fermentation was completed was used. The first-ranking lees were filtered and freeze-dried to a final moisture content of less than 3%. Three biscuit formulations were created to achieve nutritional claims of “source of fiber” and “high fiber” according to EC Regulation No. 1924/2006. The ingredients included wheat flour 00, wine lees, partially skimmed milk, olive oil, sugar, and ammonium bicarbonate. The process involved mixing, kneading, shaping, and cooking in a ventilated electric oven at 160 °C for 16 min [42]. The study found that the addition of wine lees to biscuits resulted in significantly higher moisture values than the control, possibly due to the presence of fiber. This increased water absorption capacity suggests a potential effect on the biscuits’ properties. The protein content also increased, reaching 9.57 g/100 g in the 20% substitution, similar to previous studies. The addition of wine lees led to an increase in dietary fiber, making the biscuits eligible to be labeled as “source of fiber” and “high fiber”. The replacement of wheat flour with wine lees significantly affected the total phenolic compound content and antioxidant activity of the biscuits, with the highest total phenolic compound content and antioxidant activity values observed in the 20% substitution formulation [42]. The study utilized wine lees to create fortified biscuits with polyphenols and dietary fiber. Wheat flour was replaced with either 10% or 20% wine lees, improving nutritional composition and increasing bioactive compounds. The fortified biscuits were found to be stable against oxidation, with reduced Enterobacteriaceae cell density and increased lactic acid bacteria (LAB) growth.
3.3. Cereal Bars
Borges et al. used natural and autolyzed biomass of wine yeast in their cereal bar formulations, and the results showed an increase in protein content at a concentration of 2.5% and 5%, with significant influence on organoleptic characteristics [1]. The increasing demand for functional ingredients makes cereal bars among the most suitable products for the addition of bioactive compounds. Thus, Amorim et al. formulated cereal bars with the addition of peptides and β-glucans from residual yeast [53]. The authors concluded that with an addition of 2% bioactive content, the 52 panelists evaluated the product favorably, expressing their intention to purchase. In addition to dates and dried skim milk, Khalil et al. (1984) successfully used yeast in the development of high-protein and high-energy bars to improve their nutritional quality [54].
3.4. Yogurt
Sharma and Aglawe found in their study that the addition of 16% fine wine lees to yogurt significantly improved its nutritional, functional, and rheological properties, with a 3 h absorbance duration being the most effective. Significant changes in pH, protein, and carbohydrate content were seen when processed fine wine lees were added to yogurt and the absorbance duration was measured. The pH, protein, and carbohydrate content increased with longer absorption times. The sample with optimal addition of wine lees was considered the one with a 20 g/kg addition, leading to highly favorable sensory characteristics [7].
3.5. Ice Cream
In a study conducted by Hwang et al., wine lees were assessed as a natural antioxidant that could enhance the rheological qualities of ice cream. Since the ice cream showed a decrease in pH, firmness, lightness, and the amount of freezable water, as well as an increase in viscosity and fat destabilization, the results of the analysis were compared to the control sample and revealed beneficial impacts. Additionally, the wine lees ice cream displayed adequate levels of phenolic and anthocyanin components [55]. Sharma et al. added different proportions of wine lees to ice cream, from 5 to 40 g per kg. Panelists observed that color, taste, texture, flavor, nutritional value, antioxidant activities, and rheological properties were improved. Given the phenolic content of wine lees, ice cream with the addition of wine lees may have nutraceutical value, with improved sensory properties and microbiological stability [6].
3.6. Food Aditives
The purpose of the study conducted by Prieto-Santiago et al. was to create functional juice in order to value peach fruit with wine lees. WLs were added to unpasteurized peach and grape juice at varying amounts and kept in a refrigerator. According to the study, WLs enhanced total soluble solids, polyphenol content, and antioxidant activity while also dramatically inhibiting Listeria monocytogenes. Total phenolic compound, pH, and titratable acidity did not change while being stored. A total of 57% of tasters gave the functional juice a positive rating, according to the study, which suggests that these agri-food items could be helpful in creating functional juices with longer shelf lives [56]. Wine lees have been tested as preservatives in meat products, replacing commonly used additives. Adding wine lees at 2.5 and 5% to deer burgers in a modified atmosphere at 4 °C countered lipid and protein oxidation due to increased phenolic content and antioxidant activity [57]. The study undertaken by Alarcón et al. analyzed deer burgers stored in modified-atmosphere packaging and evaluated them at different storage periods. The addition of lees reduced pH, color variations, antioxidant capacity, phenolic content, lipid and protein oxidation, and inhibited psychotrophic bacteria. The addition of wine lees increased the concentration of aldehydes, esters, acids, and other compounds, resulting in new odor and taste attributes, such as wine, bakery, and raisin notes, indicating an antioxidant and antimicrobial effect [39].
4. Applications of Wine Lees in Various Industries
4.1. Bioactive Peptides
Finding possible bioactive peptides from yeast extracts following in vitro digestion was the goal of the investigation conducted by Moreira et al. [25] A synthetic must was used to recover wine lees from Saccharomyces cerevisiae EC1118 and Starmerella bacillaris FRI751 as well as from sequential fermentation carried out with both strains. The hydrolyzed peptides were sequenced using Liquid Chromatography with tandem mass spectrometry (LC-MS/MS), and their possible bioactivity was deduced. With 275 bioactive fragments, the FRI751 extract had the strongest potential antihypertensive effect. All yeast extracts were found to have additional bioactivities, including immunomodulatory and antibacterial properties. Only the yeast extract from a single strain of S. bacillaris contained the putative anti-obesity bioactive peptide. This study creates new opportunities for winemaking by-products to be valued [25].
4.2. Short-Chain Fatty Acids
Short-chain fatty acids (SFCAs) are a group of fatty acids with at least six carbon atoms organized in a straight chain. Some advantageous properties include enhancing metabolic function, alleviating immunological dysfunction, and reducing the insulin resistance [58]. Five mutations were identified by Gajewski et al. [59] in baker’s yeast, which can lead to the production of fatty acids. Thus, by carrying out specific mutations at the level of fatty acid synthases (FAS), the production of SFCA increased by up to 464 mg/L. It is well known that yeasts undergo genetic drift during the fermentation process and to be able to adapt to environmental conditions and serial replications [60,61]. These types of resistant spent yeasts can represent an opportunity in achieving a minimally invasive protein engineering on ketoacyl synthase, acetyltransferase, and malonyl/palmitoyl transferase, or introducing other mutations in the production of fatty acids. Of course, SFCAs are also a performance indicator of fermentation; thus, higher concentrations of fatty acids are found in brewer’s yeast compared to live yeast (37.3 mmol/L compared to 35.1 mmol/L) [62].
4.3. Essential Amino Acids
It is remarkable that yeast is among the main alternatives in terms of the protein source, and studies like those by Ma et al. [63] provide concrete data for various applications in the food industry. Proteins are made up of 20 amino acids. Nine amino acids, such as histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine, are classified as essential amino acids because they cannot be synthesized by humans or other mammalian cells [64]. Proteins constitute between 29 and 65% of the dry biomass of yeast, and it is known to contain all the essential amino acids. Yeast autolysis leads to the hydrolysis of proteins into free amino acids and peptides that represent up to 40% of the total protein content [65]. The essential ones represent more than 40% of the total amino acids, having lysine as an abundant amino acid, which is poor in plant proteins [63]. Podpora et al. [66] carried out a comparative study between the essential amino acids from the yeast extract with those from whole egg, identifying close values, having a quantity of 125 mg/g of protein less in yeast (from 490 mg/g of protein to 365 mg/g). The following table compares the data provided by Podpora et al. regarding the content of essential amino acids in residual yeast with the daily requirement of amino acids according to the World Health Organization (WHO) (Table 2) [67]. Similar values (in mg/100 g of cell lysate) were also reported by Tao et al. [68] in a review regarding the future applications and perspectives of yeast extract.

Table 2.
Content of essential amino acids in residual yeast compared to WHO recommendations.
Taking into account the proteins profile in yeast between 29 and 65%, with a simple calculation, we can conclude that 58–130 g of yeast could provide the necessary 184 mg amino acids/kg/day for a healthy adult weighing 75 kg. Over time, the methods of extracting and valorizing yeast proteins have been improved and mainly use alkaline treatments [69], ionic liquids that can be easily removed by vacuum [70], by the use of a pulsed electric field combined with incubation at alkaline pH [71] or through high-pressure homogenization (100 MPa) and alkaline treatment, which becomes a milestone in the extraction yield with amazing values of 75 g protein/100 g powder containing 30 g essential amino acids [72].
4.4. Vitamin D2
Yeast has a high potential in terms of its use as a source of vitamin D2 [73]. Vitamin D is a fat-soluble vitamin with limited distribution in food products such as fish meat, mushrooms, or yeast [74,75]. This type of vitamin is especially found in two forms, as vitamin D3 (cholecalciferol), synthesized as a result of the action of UVB radiation on the skin (in fish meat or lanolin), or as vitamin D2 (ergocalciferol), obtained from plant sources (mushrooms, yeasts) [75]. Even if the absorption path is the same for the two forms (hepatic conversion and transition to the active form in the kidneys), the bioavailability of the vitamin D2 is poor due to the hydroxylation mechanism at carbon-25 [76]. This does not stop its wide use in higher quantities because it is a plant-based source and has gained popularity among people who follow certain diets, such as vegans, and need an intake of vitamin D. Large-scale production from yeast is not expensive; however, it requires, in the last stage of yeast cultivation, an exposure to ultraviolet radiation that allows for the conversion of ergosterol to ergocalciferol [73]. Irradiated yeast has been administered to animals daily since the 1900s to increase the potency of cow’s milk [77] or in comparative studies with D vitamins from cod liver to determine the growth rate of pigs [78]. Of course, studies on the use of vitamin D2 in the food industry have highlighted important data. Thus, Itkonen et al. concluded that the fortification of bakery products failed to increase the levels of 25(OH)D in human serum, and this was due to poor digestibility of the yeast matrix. The authors proposed the use of intact irradiated yeast or the cell walls without being embedded in various food matrices [79].
4.5. Vitamin B12 Extraction
Yeast is known to be rich in vitamins, especially vitamins from the B group [80]. Parlog et al. [81] managed to improve the extraction of five water-soluble vitamins from primary and secondary yeast (B1, B2, B3, B6, and folic acid), successfully separating them by HPLC-DAD within 25 min. Vitamin B12 (cyanocobalamin) is also a water-soluble vitamin with a complex chemical structure and a role in regulating the nervous system and participating in the synthesis and repair mechanism of deoxyribonucleic acid (DNA) [82]. The increased interest in yeast vitamin B12 extraction originates from the rising demand for plant-based diets (especially among vegetarians) as well as the limited and challenging methods used for its industrial extraction over the years from bacteria strains [83,84]. In an in-depth review of the potential applications of residual yeast, Marson et al. [85] noted the presence of cobalamin levels between 0.12 and 0.33 mg/100 g d.w. Taking into account that the daily requirement of vitamin B12 for a healthy adult is 1–4 μg [86], we can conclude that 1–3 g of dry yeast is sufficient to ensure the daily intake of cyanocobalamin. However, it must be taken into account that, when yeast is subjected to other types of extraction, such as that of polysaccharides, vitamins B6, B9, and B12 will be lost [68].
4.6. Nutritional Supplement
The aging wine lees process involves yeast cells from the bottom of barrels interacting with wine during fermentation [87]. This procedure causes yeasts to release polysaccharides, proteins, peptides, amino acids, and lipids, which improves the organoleptic qualities and quality of wine [33]. Aging wine lees show benefits due to yeast-cell-released mannoproteins, which promote tartaric and protein stabilization [31,88]. Onetto et al., 2024, suggest in their study that post-alcoholic-fermentation wine lees can be used as a nutritional supplement for grape must fermentation, improving fermentation timeframes and altering volatile compound production [89]. Kulhankova et al. found in their study that wine lees have a major impact of different doses of fermentation lees on the oxygen consumption and antioxidant activity of wine, which are crucial for the final product’s quality and consumer satisfaction. The study analyzed wines of the Grüner Veltliner variety with different wine lees proportions. Higher dosages of fermentation lees resulted in a greater rate of dissolved oxygen consumption; however, as early as the second day following bottling, a notable decrease was noted. Total polyphenol content and antioxidant activity also increased with the dose of yeast lees. The major negative parameter for the highest addition of lees was the concentration of volatile acids, which increased from 0.34 g L−1 to 0.45 g L−1. The addition of this substance significantly impacts the chemical composition and specific parameters of wine, specifically the quantity of reducing sugars, volatile acids, and glycerol [88,90]. White wines, sparkling wines, and occasionally red wines are aged using WLs. An enological process called “aging on lees” involves putting wines on their lees, which are leftover grapes and dead yeast cells. The wine’s flavor, structure, and color stability are all improved by aging on lees, raising the wine’s overall quality [35].
Maxe et al.’s study examines the molecular fingerprints and oxidative stability of Chardonnay Champagne base wines that have been stored on lees for a year in new oak barrels after two consecutive vintages. New oak barrels improve the wines’ oxidative stability, and the antioxidant metabolome of the wines—which is composed of nucleophilic and antiradical compounds—seems to be vintage- and barrel-aging dependent [91]. Yeast lees autolysis releases mannoproteins, which can interact with phenolic chemicals [33], improving the quality of the final product by reducing the astringency and bitterness, and led to a better structure and the color stability [8].
4.7. Food Packaging
Wine yeast extract has been used as a phenolic source to produce phenolic polymeric compounds with enhanced properties [57]. The incubation time affects the enzymatic oxidation process and the yield of polymers. Techniques such as HPLC (high-performance liquid chromatography), FTIR (Fourier transform infrared spectroscopy), MRI (magnetic resonance imaging), DSC (Differential Scanning Calorimeters), and TGA (Thermogravimetric Analysis) were used to evaluate the polymerization process [20]. The resulting polymers showed high antioxidant activity, the highest activity observed after one hour of enzymatic reaction [20]. Wine lees can improve the overall characteristics of the biopolymers while maintaining their bio-based origin [92]. Athanasiou et al. included wine lees in chitosan films demonstrating excellent antioxidant activity, and this approach can result in the creation of innovative food packaging films in the food sector [2].
4.8. Natural Colorant
The study carried out by Gümüş et al. aimed to evaluate the use of wine yeast as a natural colorant and flavoring agent in gelatin-based jellies. Wine yeast was analyzed for their content of anthocyanins, phenolic compounds, and minerals, with good results on superior color stability compared to the control sample, suggesting that wine lees can be used as a viable alternative flavoring agent in the production of jellies [32].
4.9. Other Application of Wine Lees
Wine lees can be used as an ingredient to develop a nanocosmetic formulation because it exhibits a higher moisturizing capability in colored hair, making it a promising raw material for the cosmetic hair industry [93]. The study conducted by Kaynarca, 2024 focuses on creating low-cost emulsions with high strength and adaptability to food applications using low gelation proportions [38]. The addition of WLs can create innovative products like 3D printers and limit lipid oxidation, expanding emulsion application areas [38].
Another utilization of wine lees is for animal nutrition due to their high content in polyunsaturated fatty acids [33]. The poultry industry is exploring alternatives to antibiotics to enhance production performance and reduce enteric diseases. Yeast and yeast fermentation-derived products have been used in feed for over a decade to improve livestock growth and efficiency. These bioactive molecules modulate the host’s immune response, reduce pathogen load, and alleviate the effects of enteric infections [12]. A biorefinery was developed to produce ethanol, calcium tartrate, yeast cells, and an extract high in antioxidants by using wine lees valorization [27,94,95].
5. Conclusions
Wine lees are an underappreciated by-product of the wine industry, and their reuse to obtain new products supports the bioeconomy, bringing benefits to both producers, consumers, and the environment. The evaluation and identification of the compounds present in wine yeast are important steps both to ensure a good integration into the finished products and to streamline some extraction methods for valuable compounds. The integral valorization of wine lees (both from the cell wall glucans and mannoproteins as well as from the intracellular proteins) has been demonstrated to be a highly appealing method for creating value-added products such as biscuits, yogurt, cereal bars, ice cream, food supplements, muffins, etc.
On the other hand, the large-scale use of raw residual yeast poses economic challenges due to the relatively rapid degradation rate; therefore, drying and processing immediately after disposal are key to increasing its shelf life. The only viable option would be to include wine lees after several stages of washing and conditioning or as an extract in liquid products, yet rigorous microbiological control is required.
Wine lees are still less studied than used brewer’s yeast, which is more widely spread worldwide and is found in larger quantities. More studies are needed to attest to the bioactive potential of the compounds in wine lees and also the behavior of this by-product in different food matrices.
Author Contributions
Conceptualization, A.C., A.D. and I.A.; methodology, A.C. and V.A.; formal analysis, L.C. and A.D.; investigation, A.C., A.D., L.C., V.A. and I.A.; resources, A.D. and L.C.; writing—original draft preparation, A.C. and I.A.; writing—review and editing, L.C. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Stefan cel Mare” University of Suceava and supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI, project number PN-IV-P8-8.3-ROMD-2023-0121, within PNCDI.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI, project number PN-IV-P8-8.3-ROMD-2023-0121, within PNCDI.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Borges, M.S.; Biz, A.P.; Bertolo, A.P.; Bagatini, L.; Rigo, E.; Cavalheiro, D. Enriched Cereal Bars with Wine Fermentation Biomass. J. Sci. Food Agric. 2021, 101, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, P.E.; Patila, M.; Fotiadou, R.; Chatzikonstantinou, A.V.; Stamatis, H. Valorization of Wine Lees: Assessment of Antioxidant, Antimicrobial and Enzyme Inhibitory Activity of Wine Lees Extract and Incorporation in Chitosan Films. Waste Biomass Valorization 2024, 15, 5657–5672. [Google Scholar] [CrossRef]
- Gómez, M.E.; Igartuburu, J.M.; Pando, E.; Rodríguez Luis, F.; Mourente, G. Lipid Composition of Lees from Sherry Wine. J. Agric. Food Chem. 2004, 52, 4791–4794. [Google Scholar] [CrossRef]
- Chioru, A.; Chirsanova, A.; Dabija, A.; Avrămia, I.; Boiştean, A.; Chetrariu, A. Extraction Methods and Characterization of β-Glucans from Yeast Lees of Wines Produced Using Different Technologies. Foods 2024, 13, 3982. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Wang, B.; Chen, J.; Wang, X. Parameter Calibration of Discrete Element Model of Wine Lees Particles. Appl. Sci. 2024, 14, 5281. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, R.; Azad, Z.R.A.A.; Adsule, P.G. Use of Fine Wine Lees for Value Addition in Ice Cream. J. Food Sci. Technol. 2015, 52, 592–596. [Google Scholar] [CrossRef]
- Sharma, A.K.; Aglawe, M.K. Addition of Processed Fine Wine Lees of Cabernet Sauvignon to Improve Nutraceutical Properties of Yoghurt. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 92, 141–147. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef]
- Cortés, A.; Moreira, M.T.; Feijoo, G. Integrated Evaluation of Wine Lees Valorization to Produce Value-Added Products. Waste Manag. 2019, 95, 70–77. [Google Scholar] [CrossRef]
- Balmaseda, A.; Miot-Sertier, C.; Lytra, G.; Poulain, B.; Reguant, C.; Lucas, P.; Nioi, C. Application of White Wine Lees for Promoting Lactic Acid Bacteria Growth and Malolactic Fermentation in Wine. Int. J. Food Microbiol. 2024, 413, 110583. [Google Scholar] [CrossRef]
- Rozmierska, J.; Stecka, K.M.; Kotyrba, D.; Piasecka-Jóźwiak, K. Preparation of Sedimented Wine Yeast Derived Products for Potential Application in Food and Feed Industry. Waste Biomass Valorization 2019, 10, 455–463. [Google Scholar] [CrossRef]
- Fathima, S.; Shanmugasundaram, R.; Sifri, M.; Selvaraj, R. Yeasts and Yeast-Based Products in Poultry Nutrition. J. Appl. Poult. Res. 2023, 32, 100345. [Google Scholar] [CrossRef]
- Kiros, T.G.; Gaydos, T.; Corley, J.; Raspoet, R.; Berghaus, R.; Hofacre, C. Effect of Saccharomyces Cerevisiae Yeast Products in Reducing Direct Colonization and Horizontal Transmission of Salmonella Heidelberg in Broilers. J. Appl. Poult. Res. 2019, 28, 23–30. [Google Scholar] [CrossRef]
- Schlabitz, C.; Neutzling Lehn, D.; Volken de Souza, C.F. A Review of Saccharomyces Cerevisiae and the Applications of Its Byproducts in Dairy Cattle Feed: Trends in the Use of Residual Brewer’s Yeast. J. Clean. Prod. 2022, 332, 130059. [Google Scholar] [CrossRef]
- Hlasny, J.; Hlásný, J. Série A/Series A-Review A Cause of Bovine Spongiform Encephalopathy (BSE) Related to the Feeding of Meat and Bone Meal (MBM) to British Cows Can Be Ruled out Based on Known Circumstances; Natioal Institutes of Health: Bethesda, MD, USA, 2024. [Google Scholar]
- García, Á.A.; Ruiz Palomar, C.; Hermosilla, D.; Gascó, A.; Muñoz, R.; de Godos, I. Improving the Anaerobic Digestion Process of Wine Lees by the Addition of Microparticles. Water 2024, 16, 101. [Google Scholar] [CrossRef]
- Chetrariu, A.; Avrămia, I.; Dabija, D.; Caisîn, L.; Malenchi, D.; Agapii, V.; Pavlicenco, N.; Oroian, M.-A.; Dabija, A. Wine Lees—Characteristics and Potential of Valorisation. Lucr. Științifice Ser. Agron. 2024, 67, 165–170. [Google Scholar]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Physicochemical and Nutritional Characterization of Winemaking Lees: A New Food Ingredient. Agronomy 2020, 10, 996. [Google Scholar] [CrossRef]
- Portilla Rivera, O.M.; Saavedra Leos, M.D.; Solis, V.E.; Domínguez, J.M. Recent Trends on the Valorization of Winemaking Industry Wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100415. [Google Scholar] [CrossRef]
- Athanasiou, P.E.; Gkountela, C.I.; Patila, M.; Fotiadou, R.; Chatzikonstantinou, A.V.; Vouyiouka, S.N.; Stamatis, H. Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging. Biomolecules 2024, 14, 323. [Google Scholar] [CrossRef]
- Poulain, B.; Hsein, H.; Tchoreloff, P.; Nioi, C. Effects of Different Drying Methods on the Antioxidant Properties of White Wine Lees. J. Bioprocess. Biotech. 2024, 14, 1–14. [Google Scholar]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef] [PubMed]
- De Iseppi, A.; Marangon, M.; Vincenzi, S.; Lomolino, G.; Curioni, A.; Divol, B. A Novel Approach for the Valorization of Wine Lees as a Source of Compounds Able to Modify Wine Properties. LWT 2021, 136, 110274. [Google Scholar] [CrossRef]
- Moreira, L.d.P.D.; Corich, V.; Jørgensen, E.G.; Devold, T.G.; Nadai, C.; Giacomini, A.; Porcellato, D. Potential Bioactive Peptides Obtained after in Vitro Gastrointestinal Digestion of Wine Lees from Sequential Fermentations. Food Res. Int. 2023, 176, 113833. [Google Scholar] [CrossRef]
- Felix, M.; Martínez, I.; Sayago, A.; Recamales, M.Á.F. Wine Lees: From Waste to O/W Emulsion Stabilizer. Innov. Food Sci. Emerg. Technol. 2021, 74, 102810. [Google Scholar] [CrossRef]
- Dimou, C.; Vlysidis, A.; Kopsahelis, N.; Papanikolaou, S.; Koutinas, A.A.; Kookos, I.K. Techno-Economic Evaluation of Wine Lees Refining for the Production of Value-Added Products. Biochem. Eng. J. 2016, 116, 157–165. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Palacios, V.; Barbero, G.F. Ultrasound-Assisted Extraction of Anthocyanins and Total Phenolic Compounds in Vitis vinifera L. “Tempranillo” Winemaking Lees. Vitis J. Grapevine Res. 2019, 58, 39–47. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Conidi, C.; Cassano, A. A Membrane-Assisted Green Strategy for Purifying Bioactive Compounds from Extracted White Wine Lees. Sep. Purif. Technol. 2024, 336, 126183. [Google Scholar] [CrossRef]
- Reig-Valor, M.J.; Rozas-Martínez, J.; López-Borrell, A.; Lora-García, J.; López-Pérez, M.F. Experimental Study of a Sequential Membrane Process of Ultrafiltration and Nanofiltration for Efficient Polyphenol Extraction from Wine Lees. Membranes 2024, 14, 82. [Google Scholar] [CrossRef]
- de Andrade Bulos, R.B.; da Gama Paz, F.; Machado, C.G.; Tavares, P.P.L.G.; de Souza, C.O.; Umsza-Guez, M.A. Scientific and Technological Research on the Use of Wine Lees. Food Prod. Process. Nutr. 2023, 5, 25. [Google Scholar] [CrossRef]
- De Iseppi, A.; Marangon, M.; Lomolino, G.; Crapisi, A.; Curioni, A. Red and White Wine Lees as a Novel Source of Emulsifiers and Foaming Agents. LWT 2021, 152, 112273. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of Lees in Wine Production: A Review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Gümüş, T.; Altan Kamer, D.D.; Kaynarca, G.B. Investigating the Potential of Wine Lees as a Natural Colorant and Functional Ingredient in Jelly Production. J. Sci. Food Agric. 2024, 104, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Winstel, D.; Marchal, A.; Nioi, C. Optimization of Extraction and Development of an LC-HRMS Method to Quantify Glutathione and Glutathione Disulfide in White Wine Lees and Yeast Derivatives. Food Chem. 2024, 439, 138121. [Google Scholar] [CrossRef]
- Ye, Z.; Qin, Y.; Harrison, R.; Hider, R.; Bekhit, A.E.D.A. Characterization of Bioactive Compounds in Lees from New Zealand Wines with Different Vinification Backgrounds. Antioxidants 2022, 11, 2335. [Google Scholar] [CrossRef]
- Bianchi, F.; Cervini, M.; Giuberti, G.; Simonato, B. The Potential of Wine Lees as a Fat Substitute for Muffin Formulations. Foods 2023, 12, 2584. [Google Scholar] [CrossRef]
- Kaynarca, G.B. Characterization and Molecular Docking of Sustainable Wine Lees and Gelatin-Based Emulsions: Innovative Fat Substitution. J. Sci. Food Agric. 2024, 104, 7429–7440. [Google Scholar] [CrossRef]
- Alarcón, M.; López-Viñas, M.; Pérez-Coello, M.S.; Díaz-Maroto, M.C.; Alañón, M.E.; Soriano, A. Effect of Wine Lees as Alternative Antioxidants on Physicochemical and Sensorial Composition of Deer Burgers Stored during Chilled Storage. Antioxidants 2020, 9, 687. [Google Scholar] [CrossRef]
- Delteil, D. Working with Lees: Key Elements to Wine Maturing in Grapegrower & Winemaker, 30th Technical Issue. Aust. N. Z. Grapegrow. Winemak. 2002, 30, 104–108. [Google Scholar]
- Siller-Sánchez, A.; Luna-Sánchez, K.A.; Bautista-Hernández, I.; Chávez-González, M.L. Use of Grape Pomace from the Wine Industry for the Extraction of Valuable Compounds with Potential Use in the Food Industry. Curr. Food Sci. Technol. Rep. 2024, 2, 7–16. [Google Scholar] [CrossRef]
- Caponio, G.R.; Miolla, R.; Vacca, M.; Difonzo, G.; De Angelis, M. Wine Lees as Functional Ingredient to Produce Biscuits Fortified with Polyphenols and Dietary Fibre. LWT 2024, 198, 115943. [Google Scholar] [CrossRef]
- Skračić, Ž.; Ljubenkov, I.; Mimica, N.; Generalić Mekinić, I. Valorizacija Nusproizvoda Proizvodnje Vina. Kem. Ind. 2023, 72, 247–255. [Google Scholar] [CrossRef]
- Jurcevic, I.L.; Dora, M.; Guberovic, I.; Petras, M.; Brncic, S.R.; Dikic, D. Polyphenols from Wine Lees as a Novel Functional Bioactive Compound in the Protection against Oxidative Stress and Hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar] [CrossRef]
- Chiselița, N.; Chiselița, O.; Efremova, N.; Beșliu, A. Valorization of the Red Wine Yeast Waste. Pol. J. Environ. Stud. 2024, 33, 1623–1630. [Google Scholar] [CrossRef]
- Melo, F.D.O.; Ferreira, V.C.; Barbero, G.F.; Correa, C.; Ferreira, D.S.E.; Umsza-Guez, M.A. Extraction of Bioactive Compounds from Wine Lees: A Systematic and Bibliometric Review. Molecules 2024, 29, 2060. [Google Scholar]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural Deep Eutectic Solvents and Ultrasound-Assisted Extraction: Green Approaches for Extraction of Wine Lees Anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Carretero, I.; Coves, J.R.; Pedrouso, A.; Castro-Barros, C.M.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M.; Sentellas, S. Recovery of Phenolic Compounds from Wine Lees Using Green Processing: Identifying Target Molecules and Assessing Membrane Ultrafiltration Performance. Sci. Total Environ. 2023, 857, 159623. [Google Scholar] [CrossRef]
- Ye, Z.; Shi, J.; Harrison, R.; Hider, R.; Bekhit, A.E.D.A. Studies on the Effect of Oxidation on Bioactivity of Phenolics and Wine Lees Extracts. Antioxidants 2023, 12, 931. [Google Scholar] [CrossRef]
- Zhijing, Y.; Shavandi, A.; Harrison, R.; Bekhit, A.E.D.A. Characterization of Phenolic Compounds in Wine Lees. Antioxidants 2018, 7, 48. [Google Scholar] [CrossRef]
- Moldes, A.B.; Vázquez, M.; Domínguez, J.M.; Díaz-Fierros, F.; Barral, M.T. Negative Effect of Discharging Vinification Lees on Soils. Bioresour. Technol. 2008, 99, 5991–5996. [Google Scholar] [CrossRef]
- Miolla, R.; Ottomano Palmisano, G.; Roma, R.; Caponio, F.; Difonzo, G.; De Boni, A. Functional Foods Acceptability: A Consumers’ Survey on Bread Enriched with Oenological By-Products. Foods 2023, 12, 2014. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.; Pereira, J.; Pinheiro, H.; Pacheco, M. Formulation and Consumer Acceptance of Cereal Bars with Functional Properties by the Incorporation of Peptides and β-Glucans from Spent Brewer’s Yeast; University Católica Portuguesa: Porto, Portugal, 2014. [Google Scholar]
- Khalil, J.K.; Sawaya, W.N.; Khatchadourian, H.A.; Safi, W.J. Fortification of Date Bars with Yeast Proteins and Dry Skim Milk. Can. Inst. Food Sci. Technol. J. 1984, 17, 131–136. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Shyu, Y.S.; Hsu, C.K. Grape Wine Lees Improves the Rheological and Adds Antioxidant Properties to Ice Cream. LWT 2009, 42, 312–318. [Google Scholar] [CrossRef]
- Prieto-Santiago, V.; Aguiló-Aguayo, I.; Bravo, F.I.; Mulero, M.; Abadias, M. Valorization of Peach Fruit and Wine Lees through the Production of a Functional Peach and Grape Juice. Foods 2024, 13, 1095. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef]
- Rekha, K.; Venkidasamy, B.; Samynathan, R.; Nagella, P.; Rebezov, M.; Khayrullin, M.; Ponomarev, E.; Bouyahya, A.; Sarkar, T.; Shariati, M.A. Short-Chain Fatty Acid: An Updated Review on Signaling, Metabolism, and Therapeutic Effects. Crit. Rev. Food Sci. Nutr. 2024, 64, 2461–2489. [Google Scholar]
- Gajewski, J.; Pavlovic, R.; Fischer, M.; Boles, E.; Grininger, M. Engineering Fungal de Novo Fatty Acid Synthesis for Short Chain Fatty Acid Production. Nat. Commun. 2017, 8, 14650. [Google Scholar] [CrossRef]
- Donnelly, D.; Blanchard, L.; Dabros, M.; O’Hara, S.; Brabazon, D.; Foley, G.; Freeland, B. Fed-Batch System for Propagation of Brewer’s Yeast. J. Am. Soc. Brew. Chem. 2022, 80, 190–200. [Google Scholar]
- Francois, J.M.; Formosa, C.; Schiavone, M.; Pillet, F.; Martin-Yken, H.; Dague, E. Use of Atomic Force Microscopy (AFM) to Explore Cell Wall Properties and Response to Stress in the Yeast Saccharomyces Cerevisiae. Curr. Genet. 2013, 59, 187–196. [Google Scholar] [CrossRef]
- Rakebrandt, M. Fermentation Performance of Brewers ‘yeast Compared to Live Yeast. Gas 2024, 4. [Google Scholar]
- Ma, C.; Xia, S.; Song, J.; Hou, Y.; Hao, T.; Shen, S.; Li, K.; Xue, C.; Jiang, X. Yeast Protein as a Novel Protein Source: Processing, Functional Properties, and Potential Applications in Foods. Innov. Food Sci. Emerg. Technol. 2024, 93, 103606. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sirisena, S.; Chan, S.; Roberts, N.; Dal Maso, S.; Gras, S.L.; Martin, G.J.O. Influence of Yeast Growth Conditions and Proteolytic Enzymes on the Amino Acid Profiles of Yeast Hydrolysates: Implications for Taste and Nutrition. Food Chem. 2024, 437, 137906. [Google Scholar] [CrossRef] [PubMed]
- Podpora, B.; Świderski, F.; Sadowska, A.; Rakowska, R.; Wasiak-Zys, G. Spent Brewer’s Yeast Extracts as a New Component of Functional Food. Czech J. Food Sci. 2016, 34, 554–563. [Google Scholar] [CrossRef]
- Who, J. Protein and Amino Acid Requirements in Human Nutrition. World Health Organ. Tech. Rep. Ser. 2007, 935, 1–265. [Google Scholar]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Kushnirov, V.V. Rapid and Reliable Protein Extraction from Yeast. Yeast 2000, 16, 857–860. [Google Scholar] [CrossRef]
- Ge, L.; Wang, X.-T.; Tan, S.N.; Tsai, H.H.; Yong, J.W.H.; Hua, L. A Novel Method of Protein Extraction from Yeast Using Ionic Liquid Solution. Talanta 2010, 81, 1861–1864. [Google Scholar] [CrossRef]
- Ganeva, V.; Angelova, B.; Galutzov, B.; Goltsev, V.; Zhiponova, M. Extraction of Proteins and Other Intracellular Bioactive Compounds from Baker’s Yeasts by Pulsed Electric Field Treatment. Front. Bioeng. Biotechnol. 2020, 8, 552335. [Google Scholar] [CrossRef]
- Lee, S.; Kim, E.; Jo, M.; Choi, Y.J. Characterization of Yeast Protein Isolates Extracted via High-pressure Homogenization and PH Shift: A Promising Protein Source Enriched with Essential Amino Acids and Branched-chain Amino Acids. J. Food Sci. 2024, 89, 900–912. [Google Scholar] [CrossRef]
- Kessi-Pérez, E.I.; González, A.; Palacios, J.L.; Martínez, C. Yeast as a Biological Platform for Vitamin D Production: A Promising Alternative to Help Reduce Vitamin D Deficiency in Humans. Yeast 2022, 39, 482–492. [Google Scholar] [CrossRef]
- Dereje, S.; Muradov, I.; Nazzal, S.; Nguyen, T. Cholecalciferol (D3) versus Ergocalciferol (D2) in Older Adults. Consult. Pharm. 2017, 32, 337–339. [Google Scholar] [PubMed]
- Benedik, E. Sources of Vitamin D for Humans. Int. J. Vitam. Nutr. Res. 2021, 92, 118–125. [Google Scholar] [PubMed]
- Avrămia, I.; Oroian, M.-A.; Oiţă, R.-C. A Review of Current Trends of Vitamin Identification and Quantification by Chromatography from Food Samples. J. Food Compos. Anal. 2024, 131, 106244. [Google Scholar] [CrossRef]
- Thomas, B.H.; MacLeod, F.L. Increasing the Vitamin D Potency of Cow’s Milk by the Daily Feeding of Irradiated Yeast or Irradiated Ergosterol. Science 1931, 73, 618–620. [Google Scholar]
- Bethke, R.M.; Burroughs, W.; Wilder, O.H.M.; Edgington, B.H.; Robison, W.L. The Comparative Efficacy of Vitamin D from Irradiated Yeast and Cod-Liver Oil for Growing Pigs, with Observations on Their Vitamin D Requirements; The Online Books Page: Philadelphia, PA, USA, 1946. [Google Scholar]
- Itkonen, S.T.; Pajula, E.T.; Dowling, K.G.; Hull, G.L.J.; Cashman, K.D.; Lamberg-Allardt, C.J.E. Poor Bioavailability of Vitamin D2 from Ultraviolet-Irradiated D2-Rich Yeast in Rats. Nutr. Res. 2018, 59, 36–43. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A. Nutritional Yeast Biomass: Characterization and Application. In Handbook of Food Bioengineering; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 9; pp. 237–270. ISBN 978-0-12-811440-7. [Google Scholar]
- Parlog, R.M.; Nicula, A.; Nicula, T.A.; Socaciu, C. The Optimization of Extraction and HPLC Analysis of Vitamins B from Yeast Products. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Agric. 2008, 65, 323–328. [Google Scholar]
- Zhou, Y.; He, A.; Xu, B. Natural Resources, Quantification, Microbial Bioconversion, and Bioactivities of Vitamin B12 for Vegetarian Diet. Food Chem. 2024, 463, 140849. [Google Scholar] [CrossRef]
- Lehner, S.; Boles, E. Development of Vitamin B12 Dependency in Saccharomyces Cerevisiae. FEMS Yeast Res. 2023, 23, foad020. [Google Scholar] [CrossRef]
- Niklewicz, A.; Smith, A.D.; Smith, A.; Holzer, A.; Klein, A.; McCaddon, A.; Molloy, A.M.; Wolffenbuttel, B.H.R.; Nexo, E.; McNulty, H. The Importance of Vitamin B12 for Individuals Choosing Plant-Based Diets. Eur. J. Nutr. 2023, 62, 1551–1559. [Google Scholar]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.P.; Hubinger, M.D. Spent Brewer’s Yeast as a Source of High Added Value Molecules: A Systematic Review on Its Characteristics, Processing and Potential Applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Halczuk, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T.; Karmańska, A.; Cieślak, M. Vitamin B12—Multifaceted In Vivo Functions and In Vitro Applications. Nutrients 2023, 15, 2734. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; García, J.F.; Sun, D.W. Advances in Wine Aging Technologies for Enhancing Wine Quality and Accelerating Wine Aging Process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Fia, G.; Zanoni, B.; Gori, C. A New Technique for Exploitation of Wine Lees. Agric. Agric. Sci. Procedia 2016, 8, 748–754. [Google Scholar] [CrossRef][Green Version]
- Onetto, C.; McCarthy, J.; Solomon, M.; Borneman, A.R.; Schmidt, S.A. Enhancing Fermentation Performance through the Reutilisation of Wine Yeast Lees. Oeno One 2024, 58, 7749. [Google Scholar] [CrossRef]
- Kulhankova, M.; Prusova, B.; Baron, M. Study of Oxygen in Wines with Different Proportions of Yeast Lees. Ital. J. Food Sci. 2024, 36, 44–52. [Google Scholar] [CrossRef]
- Maxe, C.; Romanet, R.; Parisot, M.; Gougeon, R.D.; Nikolantonaki, M. The Oxidative Stability of Champagne Base Wines Aged on Lees in Barrels: A 2-Year Study. Antioxidants 2024, 13, 364. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Effect of the Wine Lees Wastes as Cost-Advantage and Natural Fillers on the Thermal and Mechanical Properties of Poly(3-Hydroxybutyrate-Co-Hydroxyhexanoate) (PHBH) and Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate) (PHBV). J. Appl. Polym. Sci. 2020, 137, 48869. [Google Scholar] [CrossRef]
- De Souza, A.L.A.S.; Gomes, A.K.C.; Morgado, C.S.; Junior, E.R.; Simas, N.K.; Dos Santos, E.P.; Azevedo, A.D.; Gomes, A.C.C.; de Souza Bustamante Monteiro, M.S. Nanoemulsion with Wine Lees: A Green Approach. An. Acad. Bras. Cienc. 2024, 96, e20230373. [Google Scholar] [CrossRef]
- Ling-Niao, K.; Song-Tao, G.; Yang, Y.; Feng, F. Pyrolysis Mechanism and Pyrolysis Kinetics of Yellow Wine Lees. RSC Adv. 2024, 14, 16951–16959. [Google Scholar] [CrossRef]
- Zhang, N.; Hoadley, A.; Patel, J.; Lim, S.; Li, C. Sustainable Options for the Utilization of Solid Residues from Wine Production. Waste Manag. 2017, 60, 173–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).