Abstract
Asthma is a respiratory condition affecting millions of individuals worldwide, often exacerbated by poor management and worsening weather conditions. As healthcare and weather data continue to expand, identifying the most appropriate and sustainable artificial intelligence (AI) models for asthma care has become a challenging task. Additionally, the integration of multi-modal data through advanced pre-processing and feature selection techniques has emerged as a critical innovation in developing more effective and robust models. This study examines the current state and potential of AI methods in respiratory care, utilizing available data sources to enhance outcomes. The novelty of this work highlights the progression from classical to advanced models, including machine learning, deep learning, and ChatGPT, applied to diverse data in asthma analysis, while outlining key challenges and discussing potential solutions and future directions. The aim of the study is to highlight how machine learning, deep learning, and hybrid model architectures contribute to effective asthma classification, while also demonstrating ChatGPT’s potential as a reliable support tool for physicians in asthma management and administration. It is projected that the review’s findings on key challenges and opportunities will provide insights and uncover potential research directions in asthma assessment through the application of AI models.
1. Introduction
A prevalent respiratory and chronic airway inflammation disease known as asthma affects 300 million individuals globally, which is expected to reach 400 million in 2025. The growing number of affected people raises global concern and hence imposes a burden on hospitals and communities. Poor asthma control results in excessive consumption of medical resources, including misuse of antibiotics [1,2,3]. Even with established guidelines for asthma management, the count of uncontrolled asthma cases continues to escalate [2]. The reason could lie in the inadequate adherence to the recognized protocols. The symptoms of the disease include persistent cough, chest discomfort, dyspnea, wheezing, and limited airflow, specifically in the early morning and at night. The severity of the symptoms is influenced by allergens, dust exposure, extreme weather conditions, and respiratory infections [4]. Asthma prevalence has shown continuously increasing trends worldwide, with an example demonstrated by Borna E. et al. [5] between 2008 and 2016 in the Swedish population. Several environmental factors have been proven to significantly affect asthma. These include particulate material (PM), carbon dioxide (CO2), nitrogen dioxide (NO2), temperature (temp), and humidity, in addition to the individual’s lifestyle. The symptoms vary in intensity and become severe due to inadequate management. Asthma patients commonly need to visit the hospital every 3–6 months so that the physician can adjust their treatment amid further disease control and efficient management [6]. The intensity of the disease may also be associated with health-related quality of life [4].
1.1. Impact of Artificial Intelligence on Healthcare
Artificial intelligence (AI) algorithms are expected to transform the future of healthcare, with applications in medical imaging analysis, drug discovery, remote patient care, rehabilitation, and diagnostics [7]. AI provides an extensive scale of optimized models for predicting diagnoses from medical images. For example, scientists at Google [8] exploited 128,175 retinal fundus images to diagnose diabetic retinopathy. Other examples include radiographs [9], magnetic resonance imaging scans [10,11], and ultrasound images [12] where deep learning (DL), a sub-group of machine learning (ML), was applied for reliable medical diagnostics. Likewise, drug discovery from the genomics data is becoming popular and revolutionizing pharmacology [13]. The intent is to identify the most appropriate medication for individuals, focusing on those that efficiently target specific proteins. Some examples include predicting the drug target interaction in [14] and androstane receptor using DeepSnap models [15]. Moreover, the correlation of stress with asthma has been explored in [16], demonstrating potential AI application in understanding asthma triggers. The recent AI technology, i.e., generative pre-trained transformer (GPT) models that are advanced versions of transformer and natural language processing (NLP) algorithms, has proven helpful for physicians in creating personalized rehabilitation plans for elderly patients [17]. However, it has some limitations, i.e., lacking information sources and bioethical considerations. Similarly, memory consumption, performance, and lack of trust in patients and professionals are common concerns.
1.2. Artificial Intelligence in Asthma Management
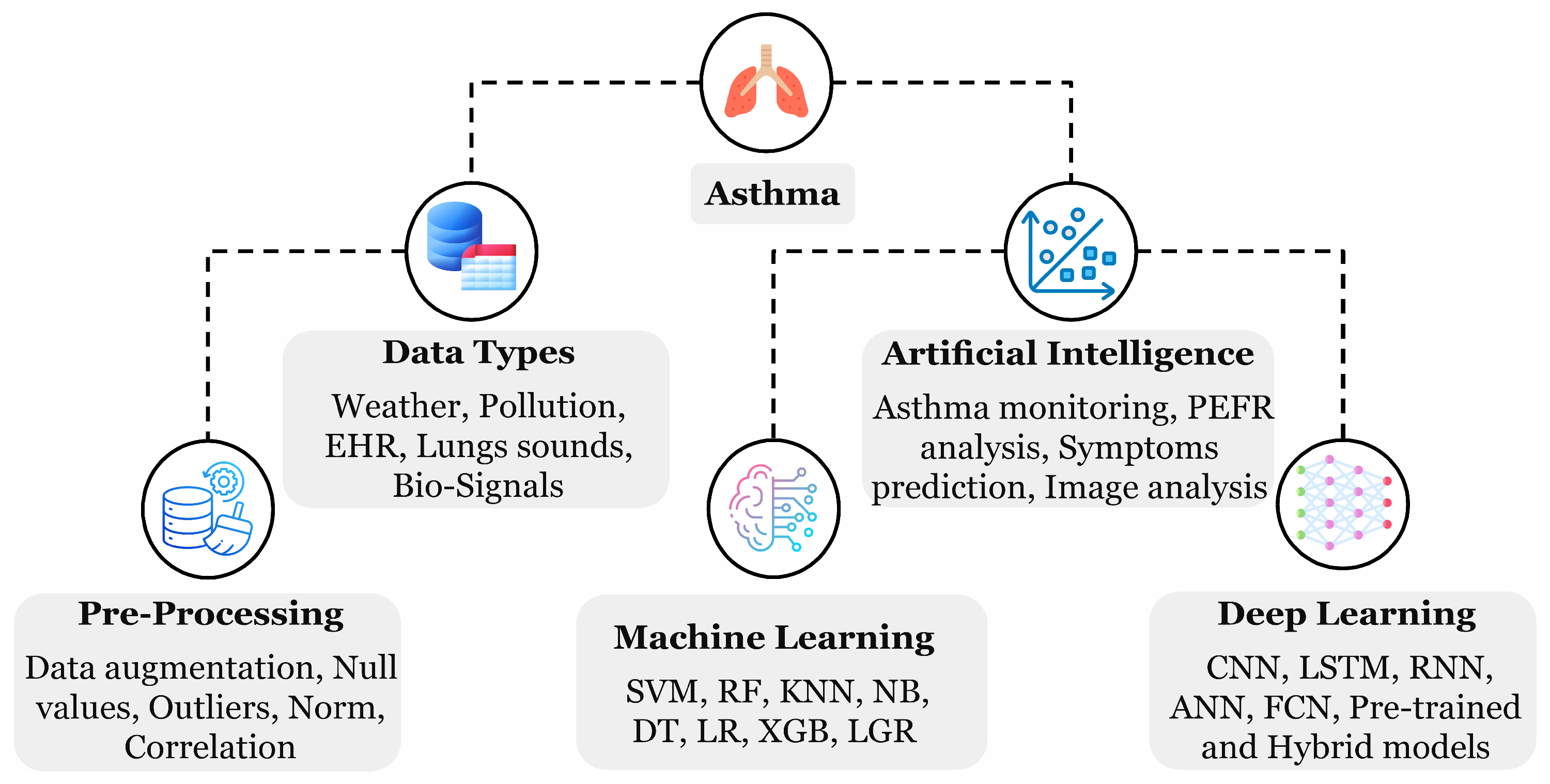
Diverse ML- and DL-oriented applications and methods have been introduced for asthma management as depicted in Figure 1. AI can assist in monitoring asthma through weather and environmental monitoring for safe route recommendations, predicting event triggers using peak expiratory flow rate analysis, predicting relevant symptoms, and analyzing chest imaging data, including X-rays and CT scans. The highlighted techniques extensively support real-time monitoring and tailored treatment strategies, precisely with the help of AI-emerged wearable devices [18]. Asthma is exceptionally rich in providing beneficial data with respect to algorithms training; such data could have various forms, including biomedical signals detected from the human skin surface, cough sound recordings through devices, electronic health records (EHRs) from the hospitals, and weather and environmental factors measured through the sensors. A patient can record their data by themselves multiple times daily to enrich the dataset using a mechanical peak expiratory flow device, wearable devices, and cough sound recordings. Enormous training data can produce a robust AI model for the diagnosis. A study by Gang Y. et al. [3] highlighted several ML models effective in asthma management using EHR data, including CatBoost, which handles categorical features, logistic regression (LR), which used probability prediction, a support vector machine (SVM) that locates the optimal hyperplane, and naive Bayes (NB) which assumes feature independence. A parallel trend for the growing adaptation of AI methods in finding asthma trigger events has been observed in the review work by Konstantinos P. et al. [1] spanning the last decade. Nevertheless, some limitations still exist, including biased algorithms, sharing of larger datasets, patients’ privacy, and ethical considerations that need to be addressed.
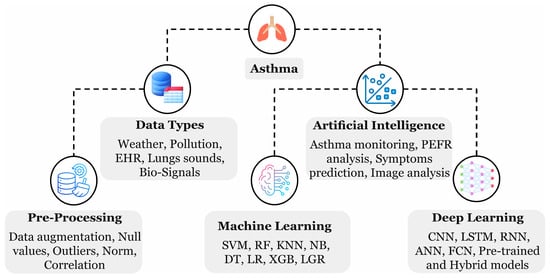
Figure 1.
A hierarchical diagram illustrating the key components of asthma analysis, including data and AI methods, as highlighted during the review process.
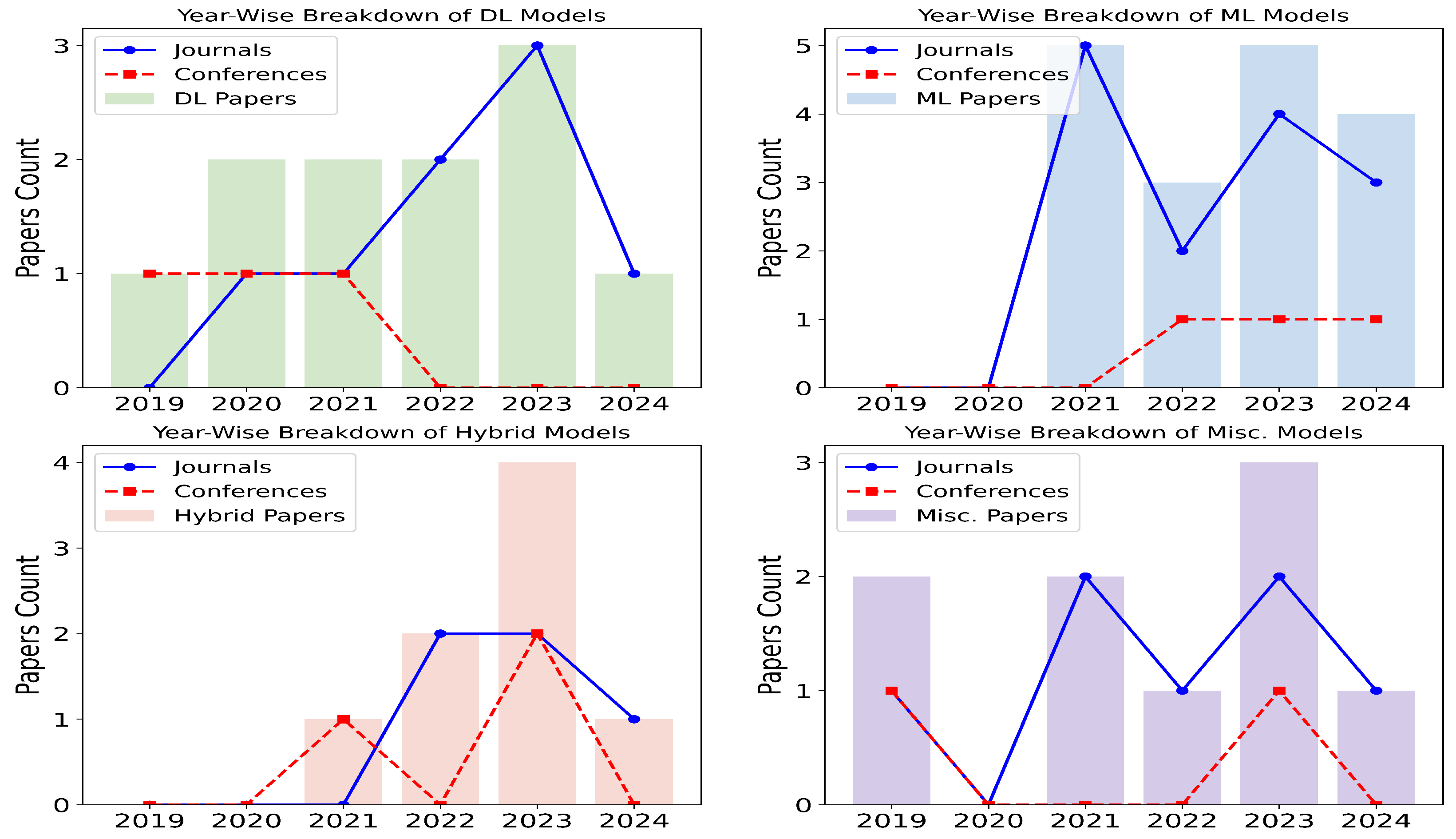
Figure 2 illustrates that most of the articles that qualified for this review were published in journals that focus on in-depth research and analytics. This review, conducted between 2019 and 2024, includes qualified articles that meet the criteria outlined in Table 1 and Figure 3. DL models have remained the most prominent over the years, consistently being favored for their performance. ML models, however, have gained increasing popularity over time, likely due to their computational efficiency, as they do not require powerful graphical processing units. Hybrid models have also seen a rise in usage, as they combine the strengths of both DL and ML. For example, DL models are often used to extract features, while ML models are employed for classification tasks. The miscellaneous category includes other statistical methods, such as knowledge graphs, linear discriminant analysis, and Gaussian mixture models, as well as review-based works.
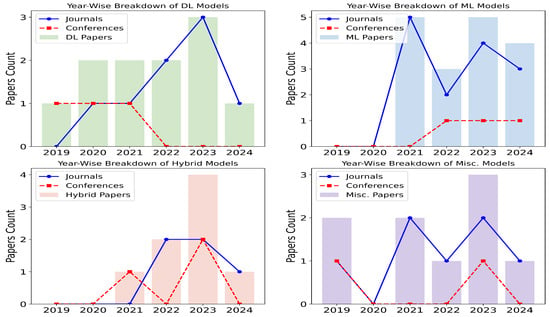
Figure 2.
Proportion of research articles published in journals and conferences represented in line alongside the breakdown of ML, DL, hybrid, and Misc. (statistical) models depicted in bars with years on x-axis and count on y-axis.

Table 1.
Scoring table depicting the scale and description assigned to each article.
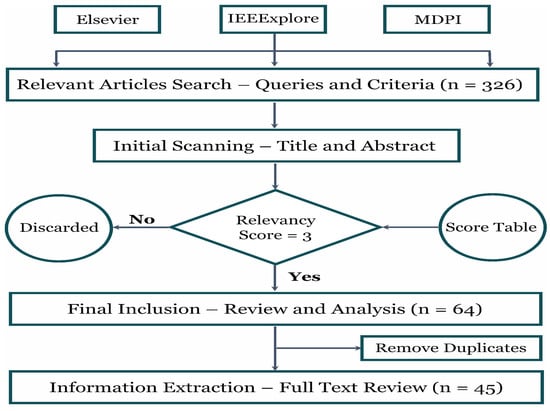
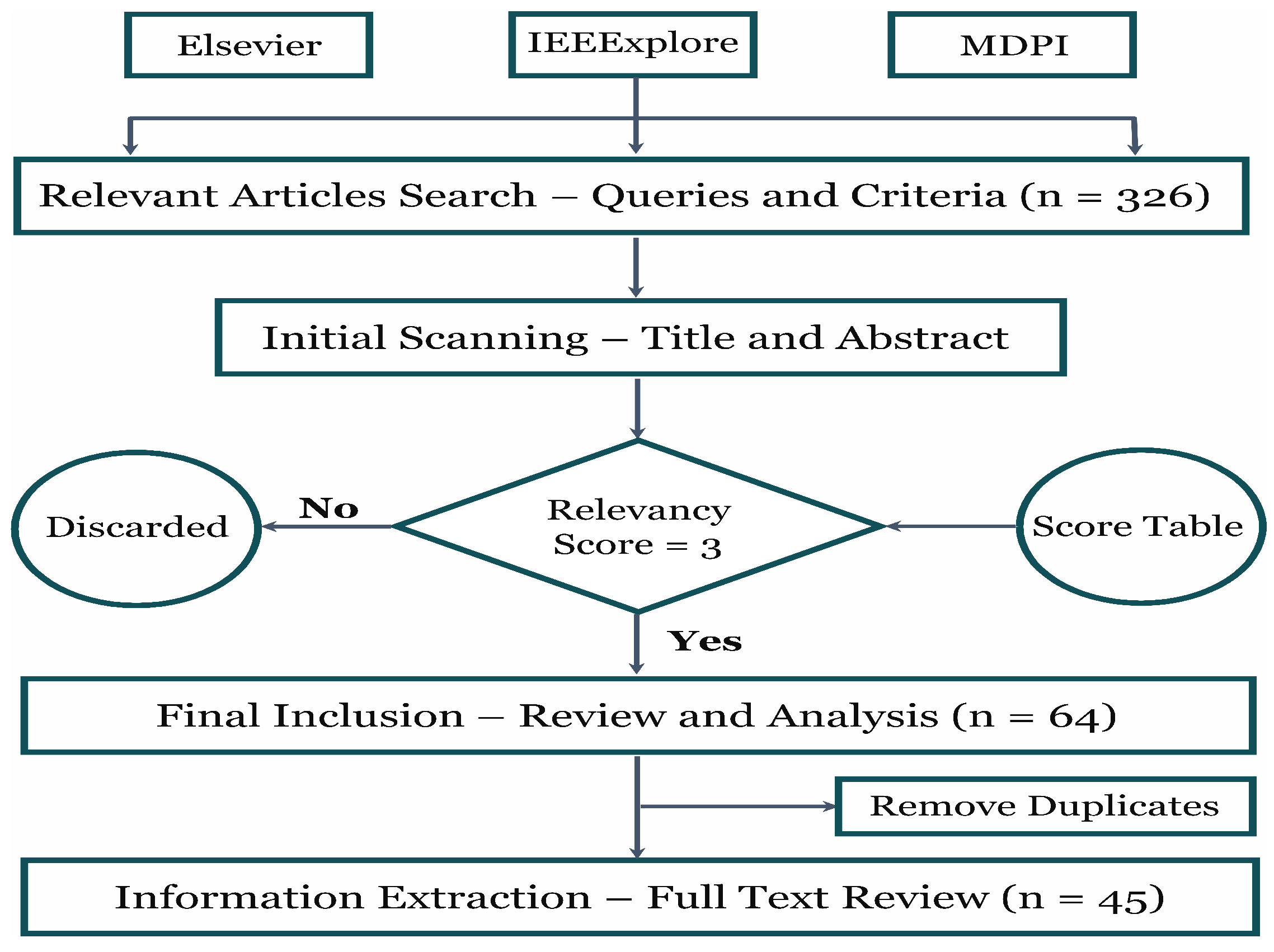
Figure 3.
The methodology adopted in this review involved analyzing three research databases, beginning with preliminary screening and concluding with specific criteria for final selection.
1.3. Review Assessment
This review highlights the role of AI models in asthma prediction and their overall impact. The key strengths and limitations of the included literature are as follows:
1.3.1. Strengths
- The included literature identifies recent trends in AI algorithms for asthma diagnosis using diverse data types, such as signals, cough sounds, weather, environment, and EHRs.
- Presents the performance achieved so far in asthma diagnosis with multi-modal data and essential pre-processing techniques.
- Explores latest technology, i.e., ChatGPT’s role in asthma management and administration, focusing on its effectiveness as a support tool for physicians.
- Highlights the challenges and opportunities of implementing AI models in this context and suggests possible future directions.
- Proposes a framework for asthma management based on the existing literature techniques.
1.3.2. Limitations
- This review considered articles from selected databases, including Elsevier, IEEE, and MDPI, which are mostly indexed in Scopus. However, Scopus itself was not directly searched, which may have limited access to additional journals, such as those from Springer.
- The model comparison primarily focuses on accuracy, as not all of the included literature reported the same evaluation metrics, making direct comparisons difficult.
- The included literature reveals a limitation in the detailed analysis of deep learning model architectures for asthma classification using imaging data, since the primary focus was on structured medical data.
- The review does not extensively cover synthetic data generation or augmentation techniques, such as the Synthetic Minority Oversampling Technique, as these methods require a separate in-depth discussion.
1.4. Review Eligibility Criteria
This section outlines the criteria used to include and exclude existing studies for this review, ensuring relevance and consistency in the analysis.
Figure 3 shows that the number of articles within the ‘last five years’ under the ‘computer science’ subject was collected from three well-known databases, including Elsevier, IEEExplore, and MDPI. A relevance table scoring ‘1 to 5’ was established to smooth the inclusion and exclusion criteria. Articles with scores were selected for the final review. Likewise, duplicate articles were removed during the final stage. The Excel sheet with a range of columns, i.e., data type, database, disease, pre-processing techniques, AI models, performance, the purpose of the study, remarks, article type, and year of publication, was maintained for each database in which the extracted information was stored during the full-text review of every article. Finally, the acquired details were utilized to analyze and draw figures. Table 1 demonstrates the process for allocating relevancy scores to each article.
The study included articles that provided detailed technical insights into computer science methods and algorithms while specifically addressing asthma. The selection process strictly followed the relevancy score criteria outlined in Table 1. The relevancy table was designed in accordance with our review methodology and research focus. If a reviewed article focused on pediatric asthma analyzed using ML or DL models, it was assigned a score of 2. If the study also incorporated environmental or respiratory data, the score increased to 3. Similarly, an article analyzing adult asthma along with environmental factors and EHR data using ML or DL algorithms received a score of 4, and so on.
Since the authors of this review have expertise in both asthma and ML/DL methodologies, we carefully extracted relevant terms and maintained a checklist in an Excel sheet to systematically align with the scoring table, ensuring an efficient article screening process. However, one limitation exists: if a study, for example, focused on pediatric asthma while also incorporating environmental and EHR data analyzed through ML or DL methods, we determined the most appropriate score based on keyword relevance. In such cases, a score of 5 was considered, as the keywords strongly aligned with environmental and EHR data analyzed via ML/DL methods (relevant), while pediatric asthma (less relevant) was also included. Depending on the study’s quality and alignment with our criteria, specifically, asthma, environment, and EHR data with ML/DL techniques, a score of 4 or 4.5 was assigned. Emphasis was placed on incorporating recent publications for a comprehensive review. Particular attention was given to studies that discussed pre-processing techniques and data sources. Additionally, research highlighting ChatGPT’s role in asthma diagnosis, treatment, and management was specifically sought and included. Duplicate articles were removed, and ultimately, (n = 45) of the most relevant studies were selected. Papers with a relevancy score (<3) were excluded, as they lacked essential details on either asthma or AI modeling. This initial screening was conducted by reviewing abstracts to ensure the selected studies focused on both asthma and AI modeling. The key terms used in preparing search queries for database interactions are illustrated in Figure 4. Some of the research questions (provided in Section 7) were formulated to guide the investigation, focusing on the effectiveness, challenges, and advancements in ML, DL, and hybrid models.

Figure 4.
The cloud words depicting the key words that were used in preparation of the queries while searching relevant articles from the scientific databases.
The remainder of this article is structured as follows: Section 2 provides an overview of the historical events of the AI models in analyzing asthma and environment data. Section 3 describes the data types and benchmark databases in asthma prediction. In Section 4, pre-processing techniques and a description of ML/DL models along with their performance are discussed. Section 5 demonstrates the effectiveness of ChatGPT’s response for asthma management. Section 6 presents the challenges and opportunities existing in asthma predictive modeling. Section 7 addresses the key research questions explored in this review, leading to the overall conclusions of the study.
2. History of Artificial Intelligence in Asthma
AI is a broader field that includes ML, and DL serves as a specialized subset. ML models require hand-crafted features to classify the object into a particular class, often termed a classification problem. On the other hand, DL is an advanced approach that does not require manual feature crafting. The capability of both models always depends upon the nature of the task. With a classification model, the resemblance of features within a single class (intra-class) should be high, and between the classes (inter-class) it should be low. This results in an increase in classification performance.
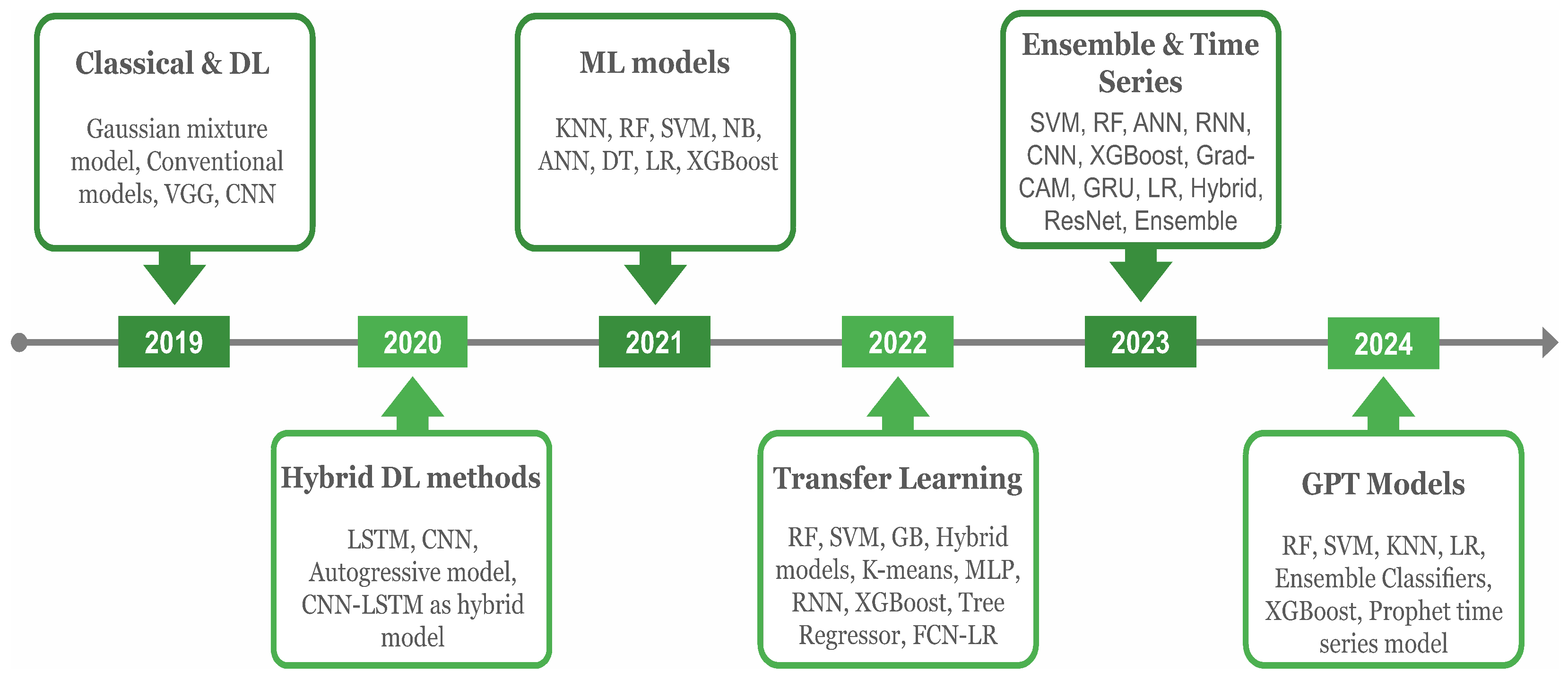
This review looked into various ML and DL models for predicting asthma triggers and encloses the year-wise progress as depicted in Figure 5. In 2019, commonly used DL models in asthma prediction included a pre-trained ‘visual geometry group’ (VGG) and a computer vision model famous in image analysis, the ‘convolutional neural network’ (CNN) along with conventional models. The CNN model, together with VGG-16 and a capsule network, were used to classify asthma in addition to other chest diseases, i.e., chronic obstructive pulmonary disease (COPD), tuberculosis, and pneumothorax from the chest X-ray images instead of EHR data in [19]. Since the input data consisted of X-ray radiographs, and DL is particularly effective for image-based data, the focus was placed on using DL models.
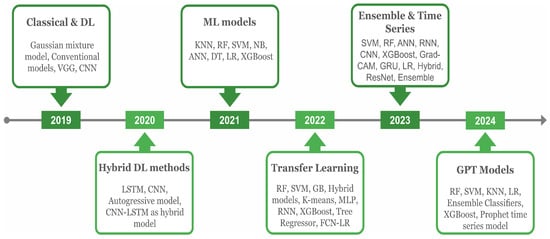
Figure 5.
The historical overview of AI models, including ML and DL algorithms, in analyzing asthma and environmental data. Emerging concepts in each year are depicted in the headings.
Environmental determinants play a crucial role in predicting asthma triggers, such as the intensity of particulate matter with a diameter of 2.5 μm or less , , , temp, and humidity. Therefore, these aspects were considered in asthma prediction in the study of [20]. In 2020, various models, including Long Short-Term Memory (LSTM), which predicts sequences and retains information over time, the computer vision-based CNN model, and the autoregressive model that makes predictions based on past time series data, were applied in [20,21]. The hybrid concept combines two or more models for better predictions, such as one for feature extraction and a second for classification. This technique is proven more efficient than the single standalone in various tasks [22,23]. The same hybrid models, including the CNN and LSTM, were implemented in asthma classification, showing adequate performance in forecasting.
In 2021, the EHR data consisted of clinical variables, and additional environmental factors were considered in asthma prediction. Since ML models are efficient in such data, various authors have applied them extensively. These models including K-nearest neighbors (KNN) that classify data points based on closest neighbors, random forest (RF), which generates multiple DTs and combines their predictions, and SVM were applied in [24,25,26,27,28]. Likewise, LR, DT, and NB models were used in [24,27]. Additionally, extreme gradient boosting (XGBoost), which combines ensemble learning and gradient-boosting techniques to enhance performance, the Artificial Neural Network (ANN), a model inspired by the human brain’s functioning, and the Deep Neural Network (DNN), a type of ANN with multiple layers, were applied in the studies of [24,25,26,28,29,30].
Overall, the XGBoost performed more efficiently than the others. The transfer learning (TL) concept, which refers to re-using the trained model from one data to another for better performance, was implemented in 2022 for asthma prediction in [31]. Similarly, other relevant ML models, including gradient boosting (GB), an ensemble method that builds models sequentially to improve predictions [32], multi-layer perceptron (MLP), a neural network with at least three layers [33], Tree Regressor, which uses decision tree principles to predict continuous values [32], K-means, a clustering algorithm that partitions data based on feature similarity [34], and hybrid models [35], were also implemented.
In 2023, the ‘Prophet’ forecasting model, developed by Facebook and known for its efficiency in time series forecasting, was used for traffic-related air pollution forecasting, incorporating environmental factors like , particulate matter with a diameter of 10 micrometer or less , and to predict asthma triggers [36]. In addition, the ensemble classifier that pertains to combining predictions of multiple models for better outcomes was implemented for and and total suspended particulate concentration projection [37]. Further, DL models, such as the ANN, CNN, pre-trained residual networks [38], and hybrid models, were also implemented for similar task classification. Bio-signal symptoms data collected using the smart device were also utilized in [39]. In general, a key achievement was the deployment of the highly effective Prophet model and ensemble classifier for environmental prediction, enhancing asthma trigger event forecasting. With the rising popularity of GPT models, GPT and transformer-based models are expected to rise and be implemented in the near future to predict asthma through clinical and environmental features.
3. Asthma Data Sources
Two types of data were brought to our attention during the review in the prediction of asthma: the first included environmental factors, i.e., , , temp, , , , humidity, and the second included medical data, i.e., EHRs, which involve patient characteristics and clinical aspects. The details about data types and sources are given in Section 3.1 and Section 3.2.
3.1. Data Types
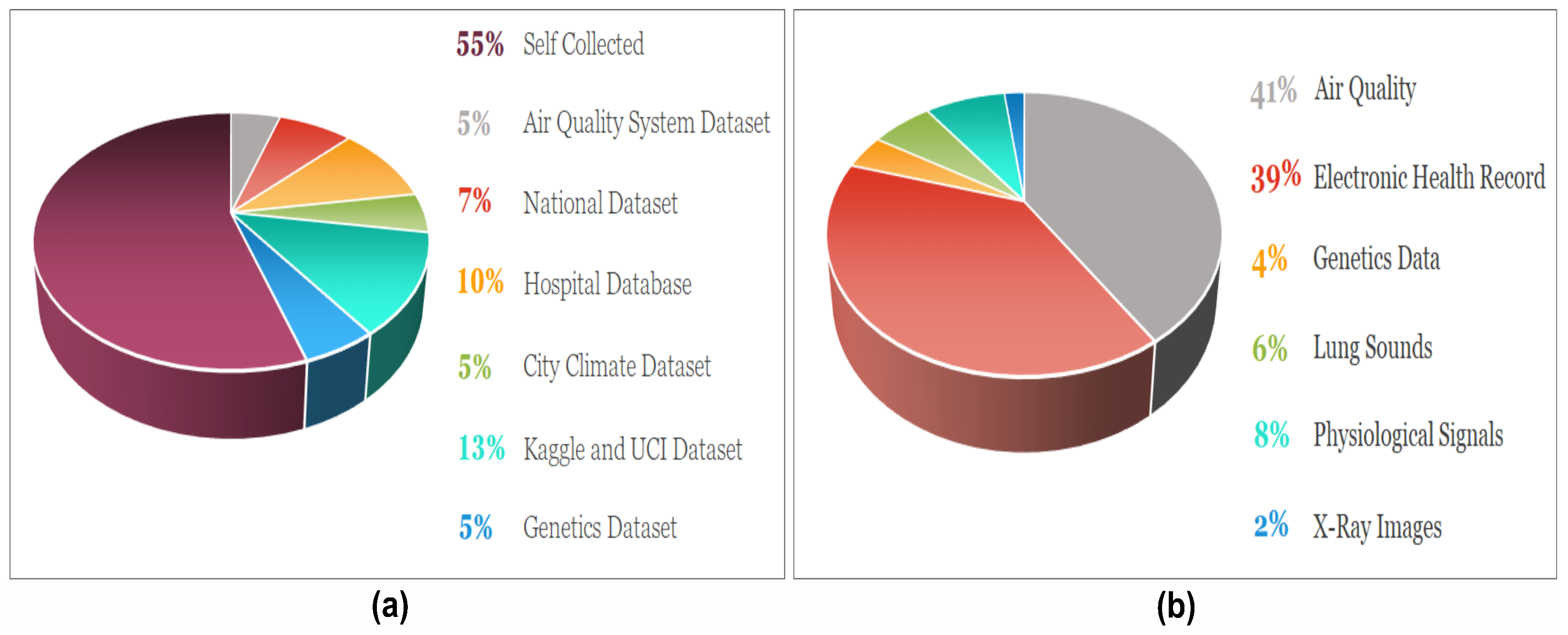
Understanding the data type is essential before applying AI models, as certain models, like CNN, perform better with imaging data. Meanwhile, ML models such as SVM, XGBoost, and RF work well with textual data. Therefore, knowledge about data helps in choosing a suitable model, which ultimately enhances performance. The proportion of datasets along with data types uncovered in this review are depicted in Figure 6a and Figure 6b, respectively.
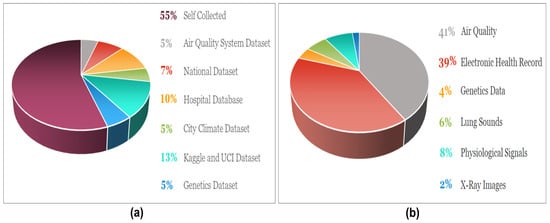
Figure 6.
(a) The ratio of the data sources outline in the review work. (b) The distribution of the data types used in the selected articles for asthma prediction.
3.1.1. Electronic Health Record
The EHRs are the digitalized version of clinical data that included patients’ demographics, i.e., gender, age, weight, height, medical history, diagnosis, and various clinical aspects consisting of symptoms and lab parameters [40]. Such data are available in an organized form and can be interpreted in a textual format. They can be extracted directly from the hospital database using a computer programming-oriented structured query language that facilitates interaction with the databases. The extraction process is comparatively straightforward, with a substantial amount of resulting data, and helps in training ML and DL models. Bhattacharjee S. et al. [33] collected the spirometry EHR data from 1314 patients to classify COPD, asthma, and non-obstructive disease. Similarly, Yao T. et al. [28] also collected EHR data, health insurance claims, and patient surveys from the hospital database to classify asthma disease, consisting of 31,724 patient records visited between 2011–2018. Likewise, in the studies of [26,34,41,42], self-collection of EHR data was applied for asthma prediction. Additionally, lung sounds [35,38,43], X-ray radiographs [19], and phenotypes data [44] were also regarded for asthma classification.
3.1.2. Environmental and Weather Data
The allergens, harsh weather, dust, environmental factors, and irritants trigger asthma. Weather and environmental data that play a significant role in asthma are detectable through the AI models. The environment and weather data, including concentration levels of , , humidity, temp, , and , are used with EHR data for enhanced asthma prediction. Various sensors are available for collecting indoor and outdoor data, including air quality sensing subsystem, and sensors, atmospheric temperature, air pressure sensors, illumination sensors, and sensors. The importance of such data increases when we need to predict safe route recommendations for asthmatic patients to avoid an asthma attack. The safe route recommendation by Eman A. et al. [39] utilized environmental data, EHR, and bio-signals to prevent trigger events.
3.2. Benchmark Datasets
Some datasets consisting of EHR and environment data are available publicly, which helps classify asthma using ML and DL models. This paper highlighted some common datasets during the review process, the details of which are given in the following subsections.
3.2.1. Diseases Symptoms
The EHR disease symptoms dataset, publicly available on the Kaggle platform, contains 40 diseases with 130 symptoms and is expanded to around 5000 rows. Four files are embedded, including the main dataset, symptoms description, precautions, and severity. In the primary dataset, i.e., the ‘comma-separated value’ file, one column is dedicated to the disease, i.e., bronchial asthma, with 17 other columns corresponding to various symptoms. The description file has detailed information about different symptoms. The precaution included one disease column and four precautions columns, i.e., bronchial asthma has precautions of ‘switch to loose clothing’, ‘take deep breaths’, ‘get away from trigger site’, and ‘seek help’. The severity of the symptoms is presented on a scale of 1 to 7. In the primary dataset, the single disease has several symptoms expanded over multiple rows. However, it is worth mentioning that no patient data are available in this dataset. The AI model can be trained on this dataset, allowing symptoms collected from the patient to help identify the potential disease. The study by Sridhar A. et al. [45] developed a mobile application that collects the symptoms from patients, and then information is passed to a trained AI model on the Kaggle disease dataset, which performs the classification.
3.2.2. Weather Data
The weather data, often called OpenWeather dataset [46], are available publicly with several features, including humidity, temperature, rainfall, snow, air pressure, wind speed, and percentage of clouds. The dataset also has geo-location information, which includes the city name, longitude, latitude, and time zone code. The weather is also labeled according to certain conditions, such as smoke, dust, thunderstorms, etc. One can select the city and period to extract the data. The information is quite helpful in predicting a safe or recommended route for asthma patients, similarly to the work in [39]. The data can be fetched through the application programmable interface with 1000 calls free of cost. Ari Yair et al. [32] realized these data for five cities in the UK between 2000 and 2020 in rainfall forecasting since climate change influences asthma triggers.
3.2.3. Pollution Data
The dataset in [47] is maintained by the central pollution control board, India, to collect the weather pollution factors, including temperature, humidity, , , CO, , , , etc. They began recording in February 2019 on a quarterly schedule, capturing hourly information. The preliminary purpose of this dataset is to locate the air quality index score supporting the information related to pollution factors. One can set the period and location to extract the required data. Mohsin I. et al. [48] collected such data for Rabindra and Victoria locations to predict the air quality index into categories: good, satisfactory, poor, and severe. The final score can also be utilized in route awareness for asthma patients.
3.2.4. Hospital Databases
The review revealed several authors sourced EHR data for asthma classification from hospital management information systems. The datasets are quite valuable, with a bulk of information and limited public availability. Jayamini W. et al. [49] collected an admission dataset for asthma individuals from the Auckland District Health Board, New Zealand, disseminated from 2017 to 2021 for four Auckland regions. The dataset contains 11,414 records for children and adults, featuring demographics and diagnostic variables for each patient. Yao T. et al. [28] collected a cohort dataset of asthma patients between 2011 and 2018 from the University of Washington Medicine data warehouse that holds patients’ EHR and administrative data. This retained 36,781 records of the individuals, including 5000 records of the children. The aim was to predict the continuity of care for the asthma outdoor department patients by integrating EHR and administrative data. Bo Lan et al. [41] collected a dataset that included 14,250 patient records with asthma or related conditions. They attempted to find the association between asthma and diverse factors, including race, particulate exposure, obesity, and gender variables. They tested their developed system, the Integrated Clinical and Environmental Exposures Service, which enables open data exposure and exploratory analysis of clinical information.
3.2.5. Lung X-Ray Radiographs
Asthma is categorized as a lung disease, with various diagnostic tests available for detection. One such method is a chest X-ray, which captures the lung’s health; hence, such images are also helpful in predicting asthma. The dataset contains X-ray images of the lungs, available publicly on the Kaggle platform [50] under the title of NIH chest-Xray. It has 112,120 images alongside disease labels from about 30,805 individuals. The dataset owner team reported that the labels are corrected with an accuracy exceeding 90%. The train and test list of the available images for the AI model evaluation is also available in a separate file. Additionally, ‘comma-separated value’ files are also given along with a dataset containing information about the bounding box list and data entries of the given radiographs. The complete dataset has 14 common categories presenting lung-related diseases from chest examinations. Anuradha D. et al. [19] utilized this dataset to perform binary classification between lung disease and normal. When implementing highly efficient DL models such as the CNN, VGG, and ResNet, the dataset is worth considering.
3.2.6. Cough Sounds
Coughing is the body’s natural way of expelling irritants from the lungs. Individuals with lung abnormalities may have diverse sounds, i.e., whistling or wheezing, compared to healthy people. Therefore, such lung sounds can be helpful in the detection of asthma disease. One such challenging dataset with about 18,000 general-purpose audio sounds is freely available on the Kaggle platform [51]. The dataset has 41 categories, one named ‘cough’, and could be practical in the study context. Rohan T. et al. [35] converted these sounds into a time-frequency domain using the spectrogram method and then annotated with ‘cough’ or ‘not cough’. In addition, these can also be differentiated from normal sounds using the time-frequency method. For example, Ihsan T. et al. [38] self-collected lung sounds using a Littmann stethoscope with 4 kHz frequency and converted them into a time-frequency domain using Mel-spectrograms to detect asthma. Alqudaihi K. et al. [52] reviewed the diagnosis of respiratory diseases using cough sounds and highlighted the challenges and opportunities of the AI models.
The summary with pros and cons of the given datasets is described in Table 2. Other utilizations of self-collected environmental data via various sensors are reported in studies such as [36,37,53]. Additionally, the combination of EHRs and environmental factors for asthma classification is commonly observed in research works like [24,25,30,31,54].

Table 2.
Summary of the databases useful in asthma and how it concerns weather prediction.
4. Pre-Processing and Classification
AI methods enclosed a variety of tasks, such as classification, regression, and clustering. The classification is about categorizing objects into a particular class. Regression is concerned with forecasting future values, whereas clustering organizes similar items into one group and differentiates them from others. In any given task, applying the pre-processing techniques facilitates better performance before implementing ML and DL algorithms.
4.1. Pre-Processing
The dataset can be refined using multiple pre-processing techniques, including data augmentation, removing null values, eliminating outliers, and selecting key features. The application of such techniques varies according to the need and performance. However, these techniques have proven beneficial in achieving superior performance when implementing ML and DL methods. The presented review also investigated some of the pre-processing techniques adopted by several authors in asthma and environmental condition prediction. Data enhancement using the Synthetic Minority Oversampling Technique, which produces synthetic samples for the minority classes to address class imbalance, was exploited in the work by [25,45]. Zne-Jung L. et al. [54] employed a generative adversarial network, which is an ML framework with two pertinent components, including a generator and discriminator, and is famous for data augmentation purposes.
Similarly, audio data enhancement using the PyDub library for respiratory sounds was considered by Rohan T. et al. [35]. In converting audio sounds to spectrograms for the DL methods using the CNN, Ihsan T. [38] considered the Mel-transformation technique to realize the time-frequency analysis. Since the textual dataset, i.e., EHRs and environment are commonly influenced by various limitations, the most familiar techniques are outlier removal and null values handling [25,39,48,55,56,57].
Reducing irrelevant features and selecting important ones is a common pre-processing step, which can be achieved using various libraries. Ari Yair et al. [32] used a correlation matrix for selecting key features. Sudipto B. et al. [33] applied the ‘analysis of variance’ test to assess the statistical significance of attributes. Yifeng L. et al. [58] employed Kernel principal component analysis, a non-linear dimensionality reduction technique, for feature selection. Zineb J. et al. [27] utilized the Chi-squared test for effective feature selection. Taofeek D. et al. [36] have separated the features into categorical and continuous values and Arias JC et al. [59] performed data aggregation by integrating multiple data sources. Finally, model hyper-parameter tuning, crucial for optimizing performance, was carried out by Katsuyuki T. et al. [42] using the Bayesian optimization method.
4.2. Classification and Regression Models
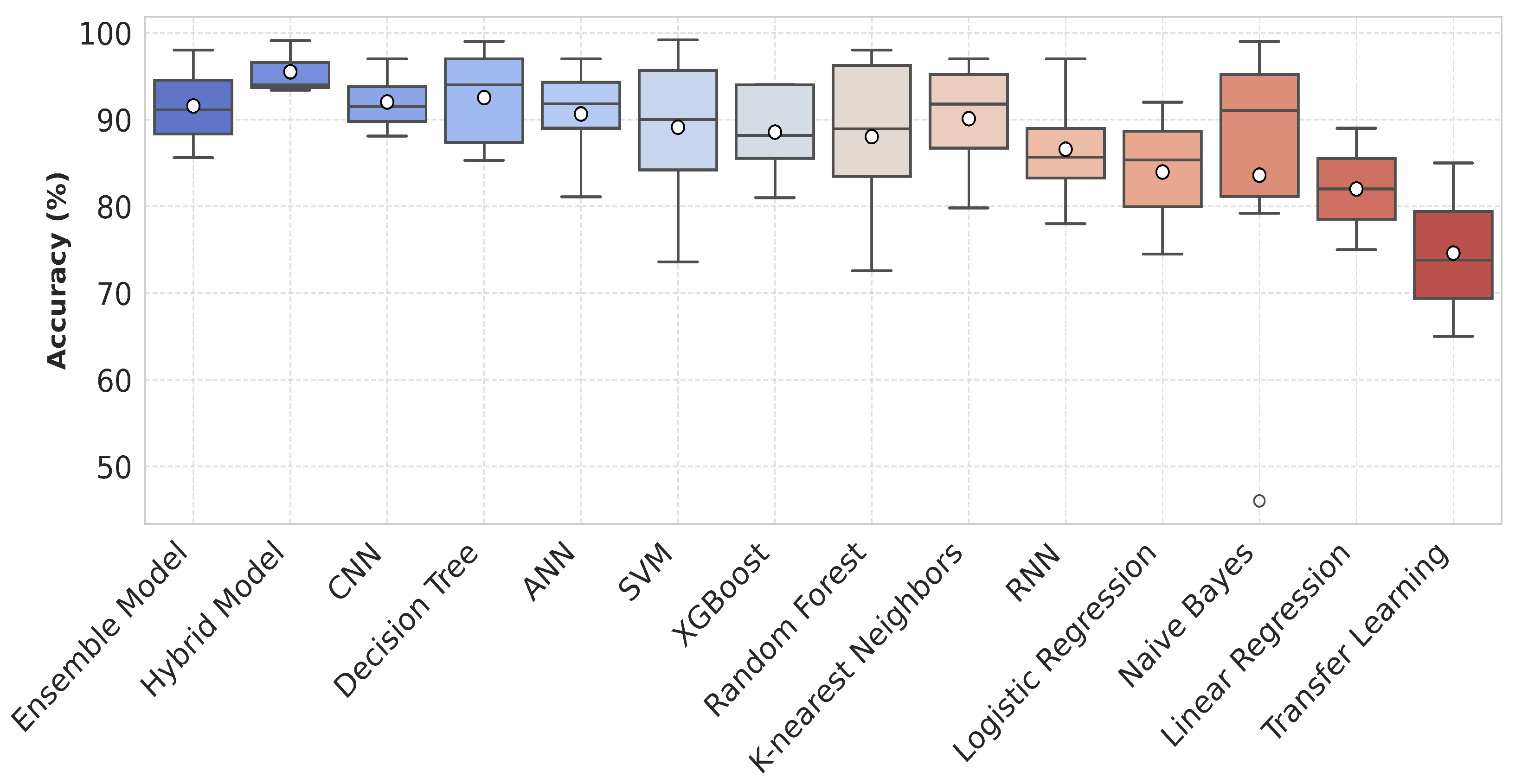
The primary goal of AI methods is to develop machine intelligence comparable to human cognition, achieved through advanced mathematical concepts and algorithms. As evident today, the advancements in these methods were not as pronounced in previous decades. Instead, these became highly efficient occasionally, with contributions from several scientists worldwide. Some models, such as the CNN, LSTM, and RNN perform well with the downside of high memory consumption, making functionality extremely hard with limited sources of central processing units. ML methods like SVM, RF, and NB can run on simple processors instead of graphics processing units and are efficient when handling specific data. The advantages and disadvantages of the models proven efficient in asthma classification (shown in Figure 7) are described in Table 3.
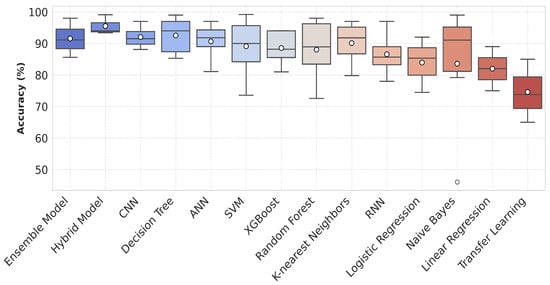
Figure 7.
Box plot showing the accuracy distribution of various machine learning and deep learning models including ensemble classifiers and hybrid models. Each box represents the spread of available accuracy data on y-axis for the respective model.

Table 3.
Popular models in asthma classification with their benefits and drawbacks.
ML and DL methods are commonly utilized in asthma analysis through predictions and classification. The choice and application of these methods also depend upon the nature of data, such as ML, which is chosen widely when considering EHR and environment-oriented textual data. Meanwhile, DL methods, i.e., the CNN and LSTM, are exercised when the data are more about imaging and sounds. The bagging technique refers to the average of the output of several classifiers with respect to stable results and was considered by Anirudh S. et al. [45] using KNN, SVM, DT, RF, NB, and ANN methods that attained 98.84% accuracy. A review work by Yadav P. et al. [60] explored the importance of the SVM algorithm in CT chest scan classification. Zne-Jung et al. [54] applied the XGBoost classifier on EHR and environmental data by obtaining 94.03% accuracy with 0.929 area under the curve (AUC) score. Zineb J. et al. [27] assessed LR, DT, SVM, and RF classifiers on allergens and EHR data, of which RF outperformed others with 87.8% accuracy, 87% sensitivity, 88% specificity, and 0.875 score. Ruchi S. et al. [44] adopted a new approach to asthma identification by analyzing volatile organic compounds (VOCs) from phenotype data in 79 exhaled breath samples using a portable gas chromatography device. Biomarker selection was performed using linear discriminant analysis, principal component analysis, and statistical methods. Results revealed that VOC profiling can be effective in diagnosing asthma and phenotyping. Eman A. [39] utilized EHR, bio-signals symptoms, and environmental data using XGBoost at two layers. First, the model predicted asthma attacks, followed by the recommended route, achieving 94% accuracy, with 95.2% recall on 5-fold cross-validation.
The Mean Squared Error (MSE) quantifies the average squared differences between actual and predicted values, while the Root Mean Squared Error (RMSE), as the square root of the MSE, represents the average magnitude of prediction errors, and the Mean Absolute Error (MAE) calculates the average absolute differences. Widana J. et al. [49] assessed the RF classifier on EHR data, reporting an RMSE of 2.48, MAE of 1.67, and MSE of 6.15, where the lowest error depicts the better performance. Abeda B. et al. [55] applied decision tree methods, including RF, random tree, the J48 model, and optimized forest on EHR data. The results revealed the least RMSE of 0.07 by the J48 model. Raj M. et al. [56] applied LR, SVM, DT, KNN, and RF classifiers to EHR data, with RF achieving the highest performance at 98% accuracy, 98% precision, 95% recall, and an score of 0.95. The air quality index classification problem was determined by Mohsin I. et al. [48] on air quality data using SVM and RF models.
In the study by Pyingkodiet M. et al. [61], a binary classification was applied based on the environmental data to classify safe and risky environments for asthma patients using SVM, KNN, and CNN models. The results showed that SVM attained 97.98% while RF showed 93.29% accuracy on two different location datasets. Jothi E. et al. [62] compared the performance of SVM, RF, and KNN that supports RF with the highest accuracy of 96%, specificity of 95%, sensitivity of 94%, and 0.93 score. Roghaye K. et al. [25] evaluated SVM, tree-based model, multinomial LR, KNN, Gaussian NB, XGBoost, and RF classifiers on EHR and environmental data. They followed an ensemble classifier method achieving 92.15% accuracy, 87.3% sensitivity, 94.8% specificity, 89.5% precision, and 0.878 AUC. Figure 7 illustrates the performance of various AI models in asthma management for improved decision-making. The given box plot compares the accuracy distributions of various ML and DL models. Each model is represented along the x-axis, while the y-axis shows accuracy values as percentages. The plot includes median values and interquartile ranges. Models like RF, XGBoost, and the CNN show higher accuracies, with fewer outliers, while Naive Bayes and KNN display a wider spread of results. The mean accuracy is highlighted by a black marker for each model.
Chien-Hua H. et al. [63] considered EHRs that included personal characteristics, physiological data, and impulse oscillometry system profile and applied LR, RF, KNN, SVM, and multi-layer perceptron. The results revealed that the multi-layer perceptron achieved the highest accuracy of 78.60%. Rohan T. et al. [35] utilized respiratory sounds and environmental data in the evaluation of CNN, RNN, and STAIN (a hybrid form of the CNN and RNN) classifiers and reported that the STAIN acquired the highest accuracy of 93.4%, 92.7% sensitivity, and 94.2% specificity. Hwan Ing H. et al. [43] utilized cough audio sounds in asthma prediction using the Gaussian mixture model by achieving an accuracy of 83.76%, 82.81% sensitivity, and 84.76% specificity. Wan D. et al. [31] considered LR with transfer learning as a fully connected neural network and quantile regression method in asthma prediction on patients’ demographics and environment data. The classification results showed performance with 73.8% accuracy, 72.7% sensitivity, 75.7% specificity, 68.70% precision, and 0.689 score. Xie F. et al. [64] conducted a review in analyzing temporal EHR data with the help of the RNN, CNN, LSTM, Autoencoder, GAN, and Transformer model. Likewise, Raj S. et al. [65] reviewed the existing literature on the detection of chronic disease with a focus on several ML and DL models.
Amani Y. et al. [26] evaluated DNN and KNN performance on EHR data, which found KNN to be the best model with 95% accuracy, 100% sensitivity, and 92.7% specificity. Abirami S. et al. [20] assessed convolutional LSTM and a hybrid form of CNN-LSTM on environmental data. The results demonstrated that their proposed hybrid model obtained the lowest MAE of 4.16 and R squared of 0.94. Gautam S. et al. [30] compared the performance of the ANN, DNN, fully connected neural network, and CNN on EHR and environmental data. The outcomes revealed that the CNN outperformed others with the least MAE of 2.12 on overall data, 1.09 on individual patients, RMSE of 2.42 on overall data, and 1.36 on individual patients. Ari Yair et al. [32] examined the performance of LSTM, XGBoost, gradient boosting regressor, SVM, and extra trees regressor on environmental data and reported LSTM with the lowest MSE and MAE. In the review article by Shuting Xu. et al. [66], the authors expressed that the CNN is the most common DL model while SVM is the most common ML model. The spirometry EHR data were classified by Sudipto B. et al. [33] using SVM, RF, NB, and MLP models, which indicated that MLP outperformed others with 85.7% test accuracy on five-fold cross-validation.
Taofeek D. et al. [36] examined the RNN, FastAI tabular model with Prophet time series algorithm, and multioutput regressor for predictions on environmental data. Their proposed model, DL with categorical embedding, achieved the lowest an RMSE of 8.31 in predictions, 5.34 in , and 5.44 in prediction. Yigui F. et al. [67] evaluated LSTM, GRU, RNN, CNN, sample convolution and interaction network (SCINet), and a proposed hybrid model, SCINet-CRINet. The results showed that SCINet-CRINet achieved the lowest error, with RMSE of 28.41 and MAE of 24.94 on the Beijing dataset for pollution prediction. Yifeng L. et al. [58] applied a temporal convolutional network with multi-head self-attention and a combination of light gradient boosting machines on the environmental dataset. Study results revealed that their proposed model obtained the lowest RMSE of 60.2 and 4.6 MAE.
Table 4 provides a comparative analysis of AI models used for asthma and lung disease prediction, highlighting their performance based on dataset type and pre-processing techniques. Hybrid models combining CNN, RNN, and traditional classifiers generally achieved higher accuracy. For example, the ResNet-18 and SVM hybrid model reached 99.1%, while the bagging ensemble (KNN, SVM, DT, RF, NB, ANN) performed similarly at 98.84%. The CNN-RNN hybrid model also demonstrated strong predictive performance with an accuracy of 93.4%. Traditional ML models, such as decision trees (RMSE: 0.07) and SVM (absolute error reduction of 15%), exhibited competitive results. XGBoost models maintained stable accuracy of about 94%, whereas the Gaussian mixture model had the lowest accuracy among the listed approaches (83.76%).

Table 4.
Comparison of AI models for asthma/lung disease prediction.
Table 4 also highlights performance variations based on data types and pre-processing techniques. Models trained on both environmental and EHR data generally achieved higher accuracy, particularly when combined with data augmentation, i.e., SMOTE or advanced data handling approaches including outlier removal, feature selections, and missing values handling. For instance, the XGBoost model utilizing both data augmentation and data handling reached 94% accuracy, while the hybrid TCN–gradient boosting model, trained entirely on environmental data, showed elevated error values (RMSE: 60.2, MAE: 4.36). Models incorporating comprehensive pre-processing steps, such as data handling and augmentation, tended to outperform those relying on limited or no pre-processing. Overall, DL and hybrid approaches demonstrate superior predictive performance, with ensemble models and boosting techniques maintaining strong accuracy across different datasets.
5. ChatGPT in Asthma Care
Large language models (LLMs), built on natural language processing and advanced transformer-based neural networks, commonly known as ‘GPT models’, are becoming increasingly popular and proving effective in daily tasks. One of the common models in such a way is ‘ChatGPT’, which has gained popularity across multiple issues, including healthcare. The performance of such methods closely aligns with human perception and has opened new opportunities for demonstrating the success of AI algorithms. Therefore, the authors of this review included recent works from 2023–2024 on ChatGPT models in asthma assessment to highlight the progress made and future directions. Random scientific databases, such as Elsevier, Nature, etc., were explored to ensure a broad perspective, given that ChatGPT models are relatively new, and to avoid missing valuable insights by focusing on a single database.
Simon H. et al. [68] evaluated ChatGPT for the 26 asthma questions under a scale of 1–5 that were related to asthma summary, symptoms, risk factors, complications diagnosis, treatment, and prognosis under the observation of respiratory diseases professionals. The study results depicted that 21 responses scored ≥4 or higher, while the remaining five scored between 3 to 4. The mean observed score was 4, which shows the reliability of ChatGPT in asthma analysis. Alabdulmohsen D. et al. [69] collected 30 frequently asked questions (FAQs) from the ‘Global initiative for asthma’ source and asked ChatGPT for the answers through two different users. These responses were assessed by the five internal medicine physicians concerning reliability and acceptability and to see whether there was a difference in the twice-response theme. The results showed that feedback on 56 questions was reliable, with an average rating of 3.65 out of 4. They also considered various metrics, including the Flesch Reading Ease Scale showing ‘33.50 ± 14.37’, the Flesch–Kincaid Grade Level as ‘12.79 ± 2.89’, and ‘13.47 ± 2.38’ as a Simple Measure of Gobbledygook. A similar study by Kernitskyi V. et al. [70] was conducted over 32 FAQs and translated into the Ukrainian language with the aim of ChatGPT reliability assessment. The focus was to evaluate the accuracy of medical terminologies, information reliability, and comprehensiveness. The input from personal computers and smartphones was also compared under the supervision of seven respiratory physicians who considered the Likert scale of 0–5. The average scores for the answers delivered for computer and smartphone devices were 4.3 and 4.1, respectively.
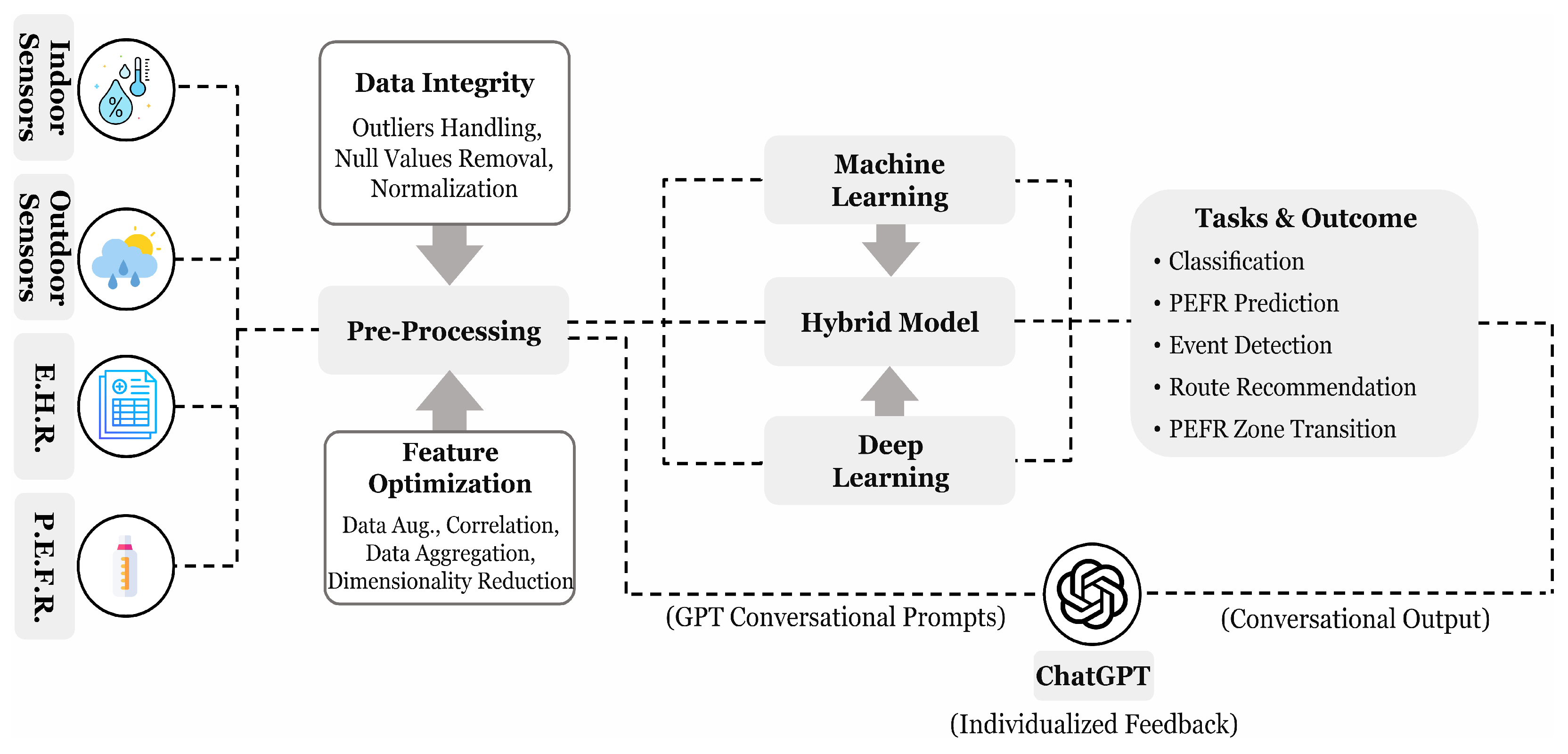
Luo H. et al. [71] presented 63 questions to ChatGPT concerning its effectiveness in respiratory medicine under basic knowledge, clinical diagnosis and presentation, and treatment and management. The outcome shows that 90% answers were correct for basic knowledge, 60% completely, and 20% partially correct for clinical diagnosis, and 39% completely and 27% partially correct for treatment and diagnosis category. Hajar A. et al. [72] presented a wearable device design that collects the physiological signals and environmental factors from individuals, analyzes them using the ML models, i.e., SVM, RF, and generates the timely alerts with respect to an asthma event trigger. They realized that the integration of ChatGPT with the model could serve a more interactive design with individualized advice and educational content. Loredana C. et al. [73] compared LLM-based models, including ChatGPT, GoogleBard, and GPTNeo, on the medical conditions and reported the limitations of traditional ML and natural language processing methods. They have also proposed a new chatbot that relies on existing model performance and experimented on two different medical datasets. The critical analysis of the ChatGPT model in asthma monitoring reveals promising results, demonstrating its potential to integrate with ML and DL models to provide enhanced patient feedback. In our observation, clinical symptoms and environmental factors can be transformed into a linguistic format suitable for GPT prompts, potentially leading to improved outcomes using LLM-based models. This approach can help explain asthma conditions and event triggers to patients more effectively. Furthermore, we emphasize the development of a more targeted and ethical GPT model, as suggested by [73], which could yield more accurate insights into respiratory diseases, including asthma. Figure 8 provides an overview of the process, including asthma-focused data pre-processing, classification, and delivering personalized feedback using a GPT model to enhance AI performance.
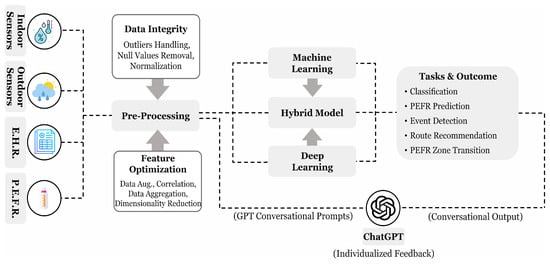
Figure 8.
A proposed process flow for asthma analysis illustrating commonly used data collection methods, pre-processing techniques with AI algorithms, and potential applications. Conversational prompts, derived from clean data and model outputs, provides natural language-based personalized feedback through ChatGPT.
6. Challenges and Opportunities
Pre-processing techniques and various AI models were emphasized for their role in achieving advanced performance in predicting asthma triggers and environmental predictions. However, some gaps in the research continue to exist and should be addressed. The current review highlighted these shortcomings and suggested new ways to conduct novel research within the presented area.
- Classifying cough sounds into binary classes, as performed in the study by [35] using time-frequency analysis, can be extended to recommend relatively safer travel routes. Similarly, the binary classification of asthma vs. normal samples [43] uses relatively small datasets, which may impact model generalization.This review suggests that leveraging a high-volume dataset, such as the Kaggle 2018 free sound dataset with around 18,000 samples across 41 categories (including ‘Cough’), could provide significant benefits. It could be useful for pre-training and transfer learning, for example, by training a base model on the entire dataset and then fine-tuning it with ‘cough’ samples. Additionally, analyzing samples using efficient pre-processing techniques like Fourier or wavelet transforms could further enhance model performance.
- Since ML and DL algorithms are often viewed as black-box models for classification and regression tasks, analyzing the results can be challenging, making it difficult to identify strong and weak performance. Explainable AI techniques like ‘Shapley Additive Explanations’ can improve understanding of the decision-making processes of ML and DL models in asthma analysis, especially for regression and forecasting tasks. These methods can enhance model reliability by revealing how clinical and environmental factors influence predictions, helping to better predict asthma outcomes.
- AI models typically focus on maximizing intra-class similarity and minimizing inter-class similarity to improve performance. However, individual-based asthma classification presents a challenge, as it involves variation in classification labels, such as healthy vs. sick, or categorizing into the three asthma zones—green, yellow, and red for each individual dataset—requiring a more efficient approach to deal with the issue.This review suggests a step-by-step methodology to improve model generalization and performance: first, develop a generalized model that predicts asthma zones based on population-level data rather than individual cases. Then, perform reliable zone transitions to help enhance outcomes when addressing individual-based asthma conditions. This approach could lead to more accurate and consistent predictions for individuals.
- Asthma classification using respiratory sounds collected from stethoscopes or smartphone devices is a relatively new area, in contrast to the more established use of EHR data. Weather and environmental factors also play a crucial role in predicting asthma triggers. Therefore, combining respiratory sounds with weather and environmental data could enhance prediction accuracy, mainly through bagging and ensemble classifier approaches. Interpreting the results by ChatGPT can further improve patient feedback.
- The review highlighted the use of standalone ML, DL, and hybrid models to achieve satisfactory performance. However, transformer models, particularly in NLP, such as ChatGPT-based models, are gaining popularity. Therefore, evaluating GPT prompts with linguistic forms of EHR, pollution, and weather data for LLMs is worth considering.
7. Addressing Research Questions
This study examines various techniques designed to address key research questions related to asthma prediction and AI-based analysis. The following questions have been formulated to guide the investigation, focusing on the effectiveness, challenges, and advancements in ML, DL, and hybrid models. These questions aim to explore the role of diverse data sources, model performance, and future research directions in asthma assessment and management.
- How effectively can machine learning and deep learning models diagnose asthma compared to traditional diagnostic methods?The application of ML has demonstrated significant improvements in asthma prediction compared to both DL and traditional statistical methods. Notably, ensemble and hybrid ML models, along with DT, SVM, RF, and KNN, have shown predictive performance well when compared to DL approaches such as the RNN and CNN, as illustrated in Figure 7. Additionally, asthma analysis primarily relies on air quality and EHR data, as depicted in Figure 6b. Since these datasets are often presented in numerical form, ML techniques are particularly well suited for handling and analyzing them with greater precision.
- How do different feature selection and data pre-processing techniques impact the performance of AI-based asthma prediction models?In numerical data analysis, pre-processing and feature selection techniques play a crucial role in enhancing asthma model performance, as discussed in Section 4.1 and illustrated in Figure 8. Eliminating null values and identifying outliers, which can introduce distortions in predictive analysis, are essential steps to prevent performance decline in AI models. Moreover, techniques such as correlation matrix analysis and principal component analysis help identify key features while reducing the influence of irrelevant variables. Additionally, data augmentation strategies like SMOTE contribute to improving model generalization by addressing class imbalance, ultimately leading to more reliable predictions.
- How can multi-modal data integration (EHR, environmental, and physiological data) improve asthma forecasting and early intervention strategies?The review findings indicate that most studies have focused either on EHR and environmental data or separately on physiological signals. However, integrating these diverse data sources into a multi-modal framework can offer a more comprehensive understanding of asthma triggers. Combining environmental factors, patient medical history, and real-time physiological signals enhances feature representation, providing a more broad view of asthma progression. These enriched data merging can lead to improved predictive accuracy, enabling early intervention strategies and more personalized asthma management.
- How can large language models (LLMs) like ChatGPT enhance asthma management, patient education, and decision support for physicians?With the growing prominence of LLMs in processing natural language and extracting meaningful insights, their integration into asthma care presents significant advantages. By transforming the outputs of traditional ML or DL models into conversational prompts, ChatGPT can provide personalized feedback, offering patients a clearer understanding of their health status, as illustrated in Figure 8. Additionally, preliminary studies, discussed in Section 5, highlight the potential of ChatGPT in assisting physicians with decision support and improving patient education, hence enhancing asthma management strategies.
8. Conclusions
This review highlights the use of AI algorithms in asthma prediction across diverse data sources with discussion on year-wise improvement and the development of new methods. The novelty of this work emphasizes the transition from classical to advanced models, including ML, DL, and ChatGPT, on manifold data in asthma analysis while outlining challenges and future directions. Support vector machine and random forest stand out as the most considered ML models, achieving an average classification accuracy of around 90%. Similarly, ensemble classifiers, decision trees, hybrid models, and deep learning approaches have demonstrated effectiveness, achieving average performance of 90–95%. Pre-processing techniques such as handling outliers, data enhancement, normalization, and essential feature extraction, are proven more effective. ML models demonstrated superior application compared to DL in terms of performance. ChatGPT has shown reliability and effectiveness in asthma management, particularly in diagnosis and treatment. Challenges and opportunities addressed during the review are summarized and elaborated, unlocking potential research directions in asthma prediction. In the future, the scope of the review will be extended covering integration of ML and DL with the large language models for more targeted feedback to the patients.
Study Limitations
The following constraints can be observed for the presented review:
- Most of the articles in the review work were extracted from the Elsevier database rather than MDPI and IEEExplore due to the highly matched relevancy criteria described in Table 1.
- The reported accuracies in Figure 7 are based on the models’ appearances across various studies rather than their actual performance in identical conditions. These accuracies may be influenced by variations in datasets and methodologies used in each study.
Author Contributions
Conceptualization, S.A. and M.F.S.; methodology, M.F.S.; validation, S.A, M.F.S. and P.P.; formal analysis, M.F.S.; investigation, S.A.; resources, P.P.; data curation, S.A. and M.F.S.; writing—original draft preparation, S.A. and M.F.S.; writing—review and editing, S.A. and M.F.S.; visualization, N.A.A.A.; supervision, S.A.; project administration, S.A.; funding acquisition, S.A. and N.A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by United Arab Emirates University, UAE grant number G00004281.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| DL | Deep Learning |
| ML | Machine Learning |
| PM | Particulate Material |
| GPT | Generative Pre-trained Transformer |
| EHR | Electronic Health Records |
| LR | Linear Regression |
| SVM | Support Vector Machine |
| COPD | Chronic Obstructive Pulmonary Disease |
| CNN | Convolutional Neural Network |
| RF | Random Forest |
| NB | Naive Bayes |
| RMSE | Root Mean Squared Error |
| MSE | Mean Squared Error |
| MAE | Mean Absolute Error |
| SMOTE | Synthetic Minority Oversampling Technique |
References
- Exarchos, K.P.; Beltsiou, M.; Votti, C.A.; Kostikas, K. Artificial intelligence techniques in asthma: A systematic review and critical appraisal of the existing literature. Eur. Respir. J. 2020, 56, 2000521. [Google Scholar] [CrossRef] [PubMed]
- Gater, A.; Nelsen, L.; Fleming, S.; Lundy, J.J.; Bonner, N.; Hall, R.; Marshall, C.; Staunton, H.; Krishnan, J.A.; Stoloff, S.; et al. Assessing asthma symptoms in adolescents and adults: Qualitative research supporting development of the asthma daily symptom diary. Value Health 2016, 19, 440–450. [Google Scholar] [CrossRef]
- Yu, G.; Li, Z.; Li, S.; Liu, J.; Sun, M.; Liu, X.; Sun, F.; Zheng, J.; Li, Y.; Yu, Y.; et al. The role of artificial intelligence in identifying asthma in pediatric inpatient setting. Ann. Transl. Med. 2020, 8, 1367. [Google Scholar] [CrossRef]
- Kharaba, Z.; Feghali, E.; El Husseini, F.; Sacre, H.; Abou Selwan, C.; Saadeh, S.; Hallit, S.; Jirjees, F.; AlObaidi, H.; Salameh, P.; et al. An assessment of quality of life in patients with asthma through physical, emotional, social, and occupational aspects. A cross-sectional study. Front. Public Health 2022, 10, 883784. [Google Scholar] [CrossRef] [PubMed]
- Borna, E.; Nwaru, B.I.; Bjerg, A.; Mincheva, R.; Rådinger, M.; Lundbäck, B.; Ekerljung, L. Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy 2019, 74, 1703–1715. [Google Scholar] [CrossRef]
- Hafke-Dys, H.; Kuźnar-Kamińska, B.; Grzywalski, T.; Maciaszek, A.; Szarzyński, K.; Kociński, J. Artificial intelligence approach to the monitoring of respiratory sounds in asthmatic patients. Front. Physiol. 2021, 12, 745635. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A review of the role of artificial intelligence in healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- Meedeniya, D.; Kumarasinghe, H.; Kolonne, S.; Fernando, C.; De la Torre Díez, I.; Marques, G. Chest X-ray analysis empowered with deep learning: A systematic review. Appl. Soft Comput. 2022, 126, 109319. [Google Scholar] [CrossRef]
- Gassenmaier, S.; Küstner, T.; Nickel, D.; Herrmann, J.; Hoffmann, R.; Almansour, H.; Afat, S.; Nikolaou, K.; Othman, A.E. Deep learning applications in magnetic resonance imaging: Has the future become present? Diagnostics 2021, 11, 2181. [Google Scholar] [CrossRef]
- Safdar, M.F.; Alkobaisi, S.S.; Zahra, F.T. A comparative analysis of data augmentation approaches for magnetic resonance imaging (MRI) scan images of brain tumor. Acta Inform. Medica 2020, 28, 29. [Google Scholar] [CrossRef] [PubMed]
- Snider, E.J.; Hernandez-Torres, S.I.; Boice, E.N. An image classification deep-learning algorithm for shrapnel detection from ultrasound images. Sci. Rep. 2022, 12, 8427. [Google Scholar] [CrossRef]
- Ong, M.S.; Sordillo, J.E.; Dahlin, A.; McGeachie, M.; Tantisira, K.; Wang, A.L.; Lasky-Su, J.; Brilliant, M.; Kitchner, T.; Roden, D.M.; et al. Machine Learning Prediction of Treatment Response to Inhaled Corticosteroids in Asthma. J. Pers. Med. 2024, 14, 246. [Google Scholar] [CrossRef]
- Thafar, M.A.; Olayan, R.S.; Ashoor, H.; Albaradei, S.; Bajic, V.B.; Gao, X.; Gojobori, T.; Essack, M. DTiGEMS+: Drug–target interaction prediction using graph embedding, graph mining, and similarity-based techniques. J. Cheminformatics 2020, 12, 44. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Uesawa, Y. Prediction model with high-performance constitutive androstane receptor (car) using deepsnap-deep learning approach from the Tox21 10K compound library. Int. J. Mol. Sci. 2019, 20, 4855. [Google Scholar] [CrossRef] [PubMed]
- Asaad, C.; Ghogho, M. AsthmaKGxE: An asthma–environment interaction knowledge graph leveraging public databases and scientific literature. Comput. Biol. Med. 2022, 148, 105933. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Dhar, M. Use of ChatGPT by physicians to build rehabilitation plans for the elderly: A mini-review of case studies. J. Indian Acad. Geriatr. 2023, 19, 86–93. [Google Scholar] [CrossRef]
- Al Meslamani, A.Z. How AI is advancing asthma management? Insights into economic and clinical aspects. J. Med. Econ. 2023, 26, 1489–1494. [Google Scholar] [CrossRef]
- Gunasinghe, A.D.; Aponso, A.C.; Thirimanna, H. Early prediction of lung diseases. In Proceedings of the 2019 IEEE 5th International Conference for Convergence in Technology (I2CT), Bombay, India, 29–31 March 2019; pp. 1227–1230. [Google Scholar]
- Abirami, S.; Chitra, P.; Madhumitha, R.; Kesavan, S.R. Hybrid spatio-temporal deep learning framework for particulate matter (pm 2.5) concentration forecasting. In Proceedings of the 2020 International Conference on Innovative Trends in Information Technology (ICITIIT), Kottayam, India, 13–14 February 2020; pp. 11–16. [Google Scholar]
- Kim, M.S.; Lee, J.H.; Jang, Y.J.; Lee, C.H.; Choi, J.H.; Sung, T.E. Hybrid deep learning algorithm with open innovation perspective: A prediction model of asthmatic occurrence. Sustainability 2020, 12, 6143. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Çamur, H.; Savaş, M.A.; Sabo, A.K.; Mustapha, M.; Abba, S.I. Hybrid artificial intelligence models with multi objective optimization for prediction of tribological behavior of polytetrafluoroethylene matrix composites. Appl. Sci. 2022, 12, 8671. [Google Scholar] [CrossRef]
- Li, M.; Waheed, R.; Kirikkaleli, D.; Aziz, G. Relevance of hybrid artificial intelligence for improving the forecasting accuracy of natural resource prices. Geosci. Front. 2024, 15, 101670. [Google Scholar] [CrossRef]
- Manocha, A.; Bhatia, M.; Kumar, G. Dew computing-inspired health-meteorological factor analysis for early prediction of bronchial asthma. J. Netw. Comput. Appl. 2021, 179, 102995. [Google Scholar] [CrossRef]
- Khasha, R.; Sepehri, M.M.; Taherkhani, N. Detecting asthma control level using feature-based time series classification. Appl. Soft Comput. 2021, 111, 107694. [Google Scholar] [CrossRef]
- Yahyaoui, A.; Yumuşak, N. Deep and machine learning towards pneumonia and asthma detection. In Proceedings of the 2021 International Conference on Innovation and Intelligence for Informatics, Computing, and Technologies (3ICT), Virtual, 29–30 September 2021; pp. 494–497. [Google Scholar]
- Jeddi, Z.; Gryech, I.; Ghogho, M.; El Hammoumi, M.; Mahraoui, C. Machine learning for predicting the risk for childhood asthma using prenatal, perinatal, postnatal and environmental factors. Healthcare 2021, 9, 1464. [Google Scholar] [CrossRef]
- Tong, Y.; Lin, B.; Chen, G.; Zhang, Z. Predicting continuity of asthma care using a machine learning model: Retrospective cohort study. Int. J. Environ. Res. Public Health 2022, 19, 1237. [Google Scholar] [CrossRef]
- Alharbi, E.; Nadeem, F.; Cherif, A. Smart healthcare framework for asthma attack prediction and prevention. In Proceedings of the 2021 National Computing Colleges Conference (NCCC), Taif, Saudi Arabia, 27–28 March 2021; pp. 1–6. [Google Scholar]
- Bhat, G.S.; Shankar, N.; Kim, D.; Song, D.J.; Seo, S.; Panahi, I.M.; Tamil, L. Machine learning-based asthma risk prediction using IoT and smartphone applications. IEEE Access 2021, 9, 118708–118715. [Google Scholar] [CrossRef]
- Bae, W.D.; Alkobaisi, S.; Horak, M.; Park, C.S.; Kim, S.; Davidson, J. Predicting health risks of adult asthmatics susceptible to indoor air quality using improved logistic and quantile regression models. Life 2022, 12, 1631. [Google Scholar] [CrossRef]
- Barrera-Animas, A.Y.; Oyedele, L.O.; Bilal, M.; Akinosho, T.D.; Delgado, J.M.D.; Akanbi, L.A. Rainfall prediction: A comparative analysis of modern machine learning algorithms for time-series forecasting. Mach. Learn. Appl. 2022, 7, 100204. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Saha, B.; Bhattacharyya, P.; Saha, S. Classification of obstructive and non-obstructive pulmonary diseases on the basis of spirometry using machine learning techniques. J. Comput. Sci. 2022, 63, 101768. [Google Scholar] [CrossRef]
- Azim, A.; Rezwan, F.I.; Barber, C.; Harvey, M.; Kurukulaaratchy, R.J.; Holloway, J.W.; Howarth, P.H. Measurement of exhaled volatile organic compounds as a biomarker for personalised medicine: Assessment of short-term repeatability in severe asthma. J. Pers. Med. 2022, 12, 1635. [Google Scholar] [CrossRef]
- Bhowmik, R.T.; Most, S.P. A personalized respiratory disease exacerbation prediction technique based on a novel spatio-temporal machine learning architecture and local environmental sensor networks. Electronics 2022, 11, 2562. [Google Scholar] [CrossRef]
- Akinosho, T.D.; Bilal, M.; Hayes, E.T.; Ajayi, A.; Ahmed, A.; Khan, Z. Deep learning-based multi-target regression for traffic-related air pollution forecasting. Mach. Learn. Appl. 2023, 12, 100474. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, W.; Ly, H.B.; Qi, C.; Nguyen, T.A.; Nguyen, M.H.; Huang, J.; Pham, B.T. Optimization of neural network parameters in improvement of particulate matter concentration prediction of open-pit mining. Appl. Soft Comput. 2023, 147, 110769. [Google Scholar] [CrossRef]
- Topaloglu, I.; Barua, P.D.; Yildiz, A.M.; Keles, T.; Dogan, S.; Baygin, M.; Gul, H.F.; Tuncer, T.; Tan, R.S.; Acharya, U.R. Explainable attention ResNet18-based model for asthma detection using stethoscope lung sounds. Eng. Appl. Artif. Intell. 2023, 126, 106887. [Google Scholar] [CrossRef]
- Alharbi, E.; Cherif, A.; Nadeem, F. Adaptive Smart eHealth Framework for Personalized Asthma Attack Prediction and Safe Route Recommendation. Smart Cities 2023, 6, 2910–2931. [Google Scholar] [CrossRef]
- Nasarudin, N.A.; Al Jasmi, F.; Sinnott, R.O.; Zaki, N.; Al Ashwal, H.; Mohamed, E.A.; Mohamad, M.S. A review of deep learning models and online healthcare databases for electronic health records and their use for health prediction. Artif. Intell. Rev. 2024, 57, 249. [Google Scholar] [CrossRef]
- Lan, B.; Haaland, P.; Krishnamurthy, A.; Peden, D.B.; Schmitt, P.L.; Sharma, P.; Sinha, M.; Xu, H.; Fecho, K. Open application of statistical and machine learning models to explore the impact of environmental exposures on health and disease: An asthma use case. Int. J. Environ. Res. Public Health 2021, 18, 11398. [Google Scholar] [CrossRef]
- Tomita, K.; Yamasaki, A.; Katou, R.; Ikeuchi, T.; Touge, H.; Sano, H.; Tohda, Y. Construction of a Diagnostic Algorithm for Diagnosis of Adult Asthma Using Machine Learning with Random Forest and XGBoost. Diagnostics 2023, 13, 3069. [Google Scholar] [CrossRef]
- Hee, H.I.; Balamurali, B.; Karunakaran, A.; Herremans, D.; Teoh, O.H.; Lee, K.P.; Teng, S.S.; Lui, S.; Chen, J.M. Development of machine learning for asthmatic and healthy voluntary cough sounds: A proof of concept study. Appl. Sci. 2019, 9, 2833. [Google Scholar] [CrossRef]
- Sharma, R.; Zang, W.; Zhou, M.; Schafer, N.; Begley, L.A.; Huang, Y.J.; Fan, X. Real time breath analysis using portable gas chromatography for adult asthma phenotypes. Metabolites 2021, 11, 265. [Google Scholar] [CrossRef]
- Sridhar, A.; Mawia, A.; Amutha, A. Mobile application development for disease diagnosis based on symptoms using machine learning techniques. Procedia Comput. Sci. 2023, 218, 2594–2603. [Google Scholar] [CrossRef]
- OpenWeather. Open Weather API Dataset. 2024. Available online: https://openweathermap.org/ (accessed on 15 August 2024).
- CPCB. Weather Pollution Dataset. 2024. Available online: https://cpcb.nic.in/ (accessed on 15 August 2024).
- Imam, M.; Adam, S.; Dev, S.; Nesa, N. Air quality monitoring using statistical learning models for sustainable environment. Intell. Syst. Appl. 2024, 22, 200333. [Google Scholar] [CrossRef]
- Jayamini, W.K.D.; Mirza, F.; Naeem, M.A.; Chan, A.H.Y. State of asthma-related hospital admissions in New Zealand and predicting length of stay using machine learning. Appl. Sci. 2022, 12, 9890. [Google Scholar] [CrossRef]
- Kaggle-NIH. NIH Chest X-Ray Images. 2024. Available online: https://www.kaggle.com/datasets/nih-chest-xrays/data (accessed on 15 August 2024).
- Kaggle-Audio. Audio Sounds Dataset. 2024. Available online: https://www.kaggle.com/c/freesound-audio-tagging (accessed on 15 August 2024).
- Alqudaihi, K.S.; Aslam, N.; Khan, I.U.; Almuhaideb, A.M.; Alsunaidi, S.J.; Ibrahim, N.M.A.R.; Alhaidari, F.A.; Shaikh, F.S.; Alsenbel, Y.M.; Alalharith, D.M.; et al. Cough sound detection and diagnosis using artificial intelligence techniques: Challenges and opportunities. IEEE Access 2021, 9, 102327–102344. [Google Scholar] [CrossRef]
- Majdi, A.; Alrubaie, A.J.; Al-Wardy, A.H.; Baili, J.; Panchal, H. A novel method for Indoor Air Quality Control of Smart Homes using a Machine learning model. Adv. Eng. Softw. 2022, 173, 103253. [Google Scholar] [CrossRef]
- Lee, Z.J.; Yang, M.R.; Hwang, B.J. A Sustainable Approach to Asthma Diagnosis: Classification with Data Augmentation, Feature Selection, and Boosting Algorithm. Diagnostics 2024, 14, 723. [Google Scholar] [CrossRef]
- Mahammad, A.B.; Kumar, R. Machine Learning Approach to Predict Asthma Prevalence with Decision Trees. In Proceedings of the 2022 2nd International Conference on Technological Advancements in Computational Sciences (ICTACS), Tashkent, Uzbekistan, 10–12 October 2022; pp. 263–267. [Google Scholar]
- Raj, M.K.; Malardhas, J.P.; Devapriya, I. Machine Learning Approach To Predict Multiple Diseases Based On Symptoms. In Proceedings of the 2024 10th International Conference on Communication and Signal Processing (ICCSP), Melmaruvathur, India, 12–14 April 2024; pp. 1195–1199. [Google Scholar]
- Vyas, N.; Das, P.; Mahajan, Y. The Impact of Air Pollution on Respiratory Health Results: An Analysis of Asthma and COPD in a Population Study. In Proceedings of the 2023 International Conference on Computing, Communication, and Intelligent Systems (ICCCIS), Greater Noida, India, 3–4 November 2023; pp. 141–146. [Google Scholar]
- Lu, Y.; Wang, J.; Wang, D.; Yoo, C.; Liu, H. Incorporating temporal multi-head self-attention convolutional networks and LightGBM for indoor air quality prediction. Appl. Soft Comput. 2024, 157, 111569. [Google Scholar] [CrossRef]
- Arias, J.; Ramos, M.I.; Cubillas, J.J. Predicting emergency health care demands due to respiratory diseases. Int. J. Med. Inform. 2023, 177, 105163. [Google Scholar] [CrossRef]
- Yadav, P.; Rastogi, V.; Yadav, A.; Parashar, P. Artificial Intelligence: A Promising Tool in Diagnosis of Respiratory Diseases. Intell. Pharm. 2024, 2, 784–791. [Google Scholar] [CrossRef]
- Pyingkodi, M.; Thenmozhi, K.; NR, W.B.; Selvaraj, P.; Kumar, K.; Aadarsh, V. Asthma Disease Risk Prediction Using Machine Learning Techniques. In Proceedings of the 2023 International Conference on Computer Communication and Informatics (ICCCI), Coimbatore, India, 23–25 January 2023; pp. 1766–1771. [Google Scholar]
- Jothi, E.S.J.; Justin, J.; Vanithamani, R.; Varsha, R. On-mask sensor network for lung disease monitoring. Biomed. Signal Process. Control 2023, 83, 104655. [Google Scholar]
- Huang, C.H.; Chou, K.T.; Perng, D.W.; Hsiao, Y.H.; Huang, C.W. Using Machine Learning with Impulse Oscillometry Data to Develop a Predictive Model for Chronic Obstructive Pulmonary Disease and Asthma. J. Pers. Med. 2024, 14, 398. [Google Scholar] [CrossRef]
- Xie, F.; Yuan, H.; Ning, Y.; Ong, M.E.H.; Feng, M.; Hsu, W.; Chakraborty, B.; Liu, N. Deep learning for temporal data representation in electronic health records: A systematic review of challenges and methodologies. J. Biomed. Inform. 2022, 126, 103980. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.S.; Kusuma, M. A Comprehensive Analysis of Chronic Health Diseases using Big Data. In Proceedings of the 2023 International Conference on Evolutionary Algorithms and Soft Computing Techniques (EASCT), Bengaluru, India, 20–21 October 2023; pp. 1–5. [Google Scholar]
- Xu, S.; Deo, R.C.; Soar, J.; Barua, P.D.; Faust, O.; Homaira, N.; Jaffe, A.; Kabir, A.L.; Acharya, U.R. Automated detection of airflow obstructive diseases: A systematic review of the last decade (2013–2022). Comput. Methods Programs Biomed. 2023, 241, 107746. [Google Scholar] [CrossRef]
- Feng, Y.; Qin, Y.; Zhao, S. Correlation-split and Recombination-sort Interaction Networks for air quality forecasting. Appl. Soft Comput. 2023, 145, 110544. [Google Scholar] [CrossRef]
- Høj, S.; Thomsen, S.F.; Ulrik, C.S.; Meteran, H.; Sigsgaard, T.; Meteran, H. Evaluating the scientific reliability of ChatGPT as a source of information on asthma. J. Allergy Clin. Immunol. Glob. 2024, 3, 100330. [Google Scholar] [CrossRef]
- Alabdulmohsen, D.M.; Almahmudi, M.A.; Alhashim, J.N.; Almahdi, M.H.; Alkishy, E.F.; Almossabeh, M.J.; Alkhalifah, S.A. Is ChatGPT a Reliable Source of Patient Information on Asthma? Cureus 2024, 16, e64114. [Google Scholar] [CrossRef]
- Kernitskyi, V.; Poberezhets, V.; Demchuk, A.; Slepchenko, N.; Konstantynovych, T.; Viltsaniuk, O.; Ovcharuk, M.; Dovhan, A. Assessing the potential of using ChatGPT by patients with asthma. Eur. Respir. J. 2024, 68, PA4377. [Google Scholar]
- Luo, H.; Yan, J.; Zhou, X. Evaluating artificial intelligence responses to respiratory medicine questions. Respirology 2024, 29, 640–643. [Google Scholar] [CrossRef]
- Almuhanna, H.; Alenezi, M.; Abualhasan, M.; Alajmi, S.; Alfadhli, R.; Karar, A.S. AI Asthma Guard: Predictive Wearable Technology for Asthma Management in Vulnerable Populations. Appl. Syst. Innov. 2024, 7, 78. [Google Scholar] [CrossRef]
- Caruccio, L.; Cirillo, S.; Polese, G.; Solimando, G.; Sundaramurthy, S.; Tortora, G. Can ChatGPT provide intelligent diagnoses? A comparative study between predictive models and ChatGPT to define a new medical diagnostic bot. Expert Syst. Appl. 2024, 235, 121186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).