Featured Application
This scoping review provides an overall assessment of the application of normalization on electrocardiogram-derived cardiac risk indices, aiming to clarify the rationale behind its application and the consistency and comparability of these normalized indices in the literature.
Abstract
Changes in cardiac function and morphology are reflected in variations in the electrocardiogram (ECG) and, in turn, in the cardiac risk indices derived from it. These variations have led to the introduction of normalization as a step to compensate for possible biasing factors responsible for inter- and intra-subject differences, which can affect the accuracy of ECG-derived risk indices in assessing cardiac risk. The aim of this work is to perform a scoping review to provide a comprehensive collection of open-access published research that examines normalized ECG-derived parameters used as markers of cardiac anomalies or instabilities. The literature search was conducted from February to July 2024 in the major global electronic bibliographic repositories. Overall, 39 studies were selected. Results suggest extensive use of normalization on heart rate variability-related indices (49% of included studies), QT-related indices (18% of included studies), and T-wave alternans (5% of included studies), underscoring their recognized importance and suggesting that normalization may enhance their role as clinically useful risk markers. However, the primary objective of the included studies was not to evaluate the effect of normalization itself; thus, further research is needed to definitively assess the impact and advantages of normalization across various ECG-derived parameters.
1. Introduction
Cardiovascular risk assessment plays a crucial role in preventive cardiology, as it helps identify individuals at high risk for adverse cardiovascular events, such as myocardial infarction, stroke, or malignant ventricular arrhythmias, which can lead to sudden cardiac death [1]. Besides considering common risk factors, the cardiovascular risk assessment typically involves the evaluation of cardiac risk indices [2]. Cardiac risk indices are measures or parameters able to identify subjects at higher risk for adverse cardiovascular events. Most cardiac risk indices are derived from analysis of the electrocardiogram (ECG), which is particularly notable for its non-invasiveness, cost-effectiveness, and widespread accessibility [3,4]. Moreover, they are mainly based on cardiac repolarization, and the QT interval measure is an archetypal example [3,5]. However, the limitations of QT in predicting arrhythmogenicity have led to intense research to develop other non-invasive risk indices, such as QT dispersion, heart rate variability (HRV), T-wave peak–T-wave end interval, and microvolt T-wave alternans (TWA) [6,7]. Specifically, the review published by Gimeno-Blanes et al. discussed in detail the role of HRV, heart rate turbulence (HRT), and TWA on sudden cardiac death risk stratification [3].
Age, sex, life habits, body composition and weight, environmental conditions, and ethnic origins are all factors that can affect cardiac characteristics, leading to differences among individuals. Aging is associated with many cardiac changes, such as aortic stiffening and ventricular hypertrophy, that are known to contribute to age-related increases in cardiovascular risk [8]. Cardiac function and morphology are known to differ between male and female populations, as well as among different ethnic groups. As an example, it has been shown that T-wave height differs with both ethnic origin and sex [8,9,10]. Moreover, life habits, including diet, physical activity, smoking, and stress management, are also known to affect heart function. To illustrate, an unhealthy diet can lead to electrolyte imbalances that can affect the electrical activity of the heart [11]. There is evidence that environmental conditions, such as high altitude, can also lead to cardiovascular changes, including ventricular hypertrophy and T-wave inversion [12,13]. Last but not least, studies have demonstrated that changes in body composition and weight, measured in terms of bioelectrical impedance, body fat percentage, and/or body mass index, can have an impact on cardiac electrical activity [14,15] as well. All these changes in cardiac function and morphology are inevitably reflected in variations in the ECG and, in turn, in the risk indices derived from it. Indeed, there is a large variety in the morphology, amplitude, and polarity of ECG waves among different individuals, as well as in serial ECGs taken at different times from the same individual [8,9,10,11,12,13,14,15].
The variability and non-reproducibility of ECG waves have led to the introduction of normalization as a step to compensate for inter- and intra-subject differences. Specifically, it can be considered as a preprocessing step, usually applied to the ECG signal directly, or as a corrective step, usually applied to ECG-derived measures (and thus on ECG-derived risk indices). As a preprocessing step, the normalization is intended to adjust and scale data before analysis, as well as to avoid redundancy by removing unnecessary information. It is typically used to bring all the variables to the same scale; thus, it usually includes amplitude scaling, which consists of adjusting the signal amplitudes so that they fall within a specific range (which is usually [−1, 1]). Moreover, normalization is an essential preprocessing step for machine learning applications, as highlighted by the systematic review conducted by Musa et al. in July 2022 [16]. Indeed, especially when features in the dataset have varying scales and ranges, the normalization ensures that different features contribute equally to model training. As a corrective step, the normalization ensures that the parameter is independent from any confounding factor that can influence the ECG pattern (such as risk factors) and that is standardized, allowing for consistent comparisons across various studies and populations. Without the proper adjustments provided by normalization, variations due to confounding factors can lead to misinterpretation of results. This approach to normalization is essential for maintaining the integrity and comparability of ECG data analysis.
While there has been significant work on ECG normalization as a preprocessing step, there is a lack of in-depth understanding of why normalization is applied to ECG-derived measures and how it affects their marker role, i.e., their accuracy in assessing the cardiac risk. In order to fill this gap, a scoping review was conducted. The aim of this review is to systematically map the research on this topic and provide a comprehensive collection of open-access published research that examines normalized ECG-derived parameters used as markers of cardiac anomalies or instabilities, where normalization is applied as a corrective step to compensate for potential biasing factors. More specifically, the objectives of this review are to provide answers to the following questions:
- Which ECG-derived features used as cardiac risk indices have been normalized in the literature?
- How was normalization performed?
- Why was normalization performed?
2. Materials and Methods
An extensive search was conducted to identify relevant scientific articles related to the normalization of ECG-derived risk indices. The methodology used followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, updated to 2020 [17], and the PRISMA extension for scoping reviews (PRISMA-ScR), updated to 2018 [18], the checklist of which is reported in Table A1 of Appendix A. This systematic search involved querying global bibliographic repositories to retrieve all open-access scientific studies relevant to the topic.
2.1. Literature Search Strategy
The literature search was conducted from February to July 2024 in three major global electronic bibliographic repositories: PubMed, Scopus, and Web of Science (WoS). To align with the topic and objectives of this review, the search query was designed so that it could identify relevant scientific articles focusing on three primary areas: (1) electrocardiogram; (2) normalization; and (3) risk indices serving as markers of cardiac anomalies or instabilities. The inclusion search terms or term roots (referred to as ‘keywords’ hereafter) based on these areas were as follows:
- ECG, EKG, electrocardiogra*;
- normaliz*;
- alternans, risk, threat*, arrhythmi*, instability, abnorma*, anomal*, irregular*, vulnerab*, susceptib*.
Specifically, the keywords ‘ECG’, ‘EKG’, and ‘electrocardiogra*’ were chosen to ensure the inclusion of all documents focusing on the ECG as the primary signal of interest. The keyword ‘normaliz*’ was chosen to capture all studies processing the ECG signal through any form of normalization. Lastly, the search query included the keywords ‘alternans’, ‘risk’, ‘threat*’, ‘arrhythmi*’, ‘instability’, ‘abnorma*’, ‘anomal*’, ‘irregular*’, ‘vulnerab*’, and ‘susceptib*’ to encompass documents related to conditions of cardiac risk. All keywords within each search area were combined using the Boolean operator OR. Subsequently, the three resulting keyword combinations were further combined using the Boolean operator AND to formulate the final search query for each bibliographic repository. The search was limited to title and abstract fields concurrently. Automatic filters for language and publication access modality were applied, specifically restricting the search to English language and open-access documents. Moreover, documents were manually filtered according to the type; specifically, reviews, book chapters, and corrections of previous articles were excluded. No filters were imposed on the publication year. The same search query was entered into the advanced search tools of all considered bibliographic repositories, with syntax adjustments made to meet the specific requirements of each database. The specific query forms are detailed in Appendix B.
2.2. Selection of Documents
The outcomes of the literature search resulting from the application of the query were imported into the Zotero reference management system to identify and remove duplicate entries. Subsequently, documents were manually screened, and those lacking an accessible abstract or full text were excluded from further consideration. Manual screening of the remaining documents was conducted according to the following criteria:
- Documents where the term ‘normalization’ is defined as the transformation process of a parameter consisting in dividing it by an intra-subject or inter-subject consistent factor.
- Documents where normalization is applied specifically to ECG-derived parameters.
- Documents where normalization is used to enhance the effectiveness or reliability of the parameter.
- Documents where the application modality of normalization is uniquely described.
- Documents where the application of normalization is adequately justified.
The established inclusion criteria have to be met concurrently by the included documents. As an implicit consequence, our exclusion criteria were:
- Documents in which the term ‘normalization’ is defined as the restoration of the physiological condition;
- Documents in which normalization is included in the standard definition of a parameter, not applied after the parameter extraction, or applied to parameters extracted from signals other than the ECG;
- Documents in which normalization was intended as a default preprocessing step for a machine learning application;
- Documents in which there was no adequate explanation of why and/or how normalization was applied.
A multi-tiered screening process was performed. Indeed, initially, documents were screened based on their titles and abstracts. If neither provided sufficient information for a proper screening evaluation, a full-text analysis was conducted. Any documents that met at least one of the exclusion criteria were progressively discarded. After verifying that they met all inclusion criteria, the remaining documents were screened according to whether they belonged to human medicine or veterinary medicine, and those related to veterinary medicine (i.e., animal studies) were further excluded. The last remaining documents were included in the review for further evaluation steps. To guarantee consistency with the established inclusion/exclusion criteria, the manual screening of documents by title, abstract, and full text where necessary, was carried out by two authors. In case of disagreements on the selection of studies, the evaluation of the documents was carried out by also including the other authors until a consensus was reached.
2.3. Data Charting
The authors jointly developed a data charting template to determine what information to gather from each document in order to answer the questions underlying the aim of this review. The two authors in charge of selecting the documents independently gathered the related information according to the established data charting template; then, they compared and discussed the results, iteratively updating the data charting template if necessary. Again, if disagreements arose, data charting was resolved through discussion and consensus with the other authors. Initially, all included documents were arranged chronologically by publication year, and their distribution over time was considered. Then, the full text of each document was examined in order to collect and report the main characteristics of the populations studied in the documents: specifically, the population was characterized in terms of size, age, sex, and health status. Among these characteristics, continuous features were expressed according to the reporting modality of the reference document and categorical features were reported as absolute number or percentage. If the information was not available or not pertinent, ‘UA’ (i.e., ‘unavailable’) or ‘NA’ (i.e., ‘not applicable’) were indicated, respectively. Regarding health status, in case of diseased populations, documents were grouped according to the affecting pathology, and the group sizes were calculated and expressed as a percentage of all included documents. Besides the population’s characteristics, the targets of the studies were collected and analyzed. Lastly, the normalized ECG-derived parameters were described in terms of the original feature normalized in the study, the domain of normalization, the modality of normalization (how), and the reason of normalization (why).
A clear definition of each item included in the final version of the data charting template is provided in Table A2 of Appendix C.
3. Results
The systematic literature search of the three bibliographic repositories produced 1458 documents. In detail, 443 documents resulted from the search on PubMed, 559 resulted from the search on Scopus, and 456 resulted from the search on WoS. Of these, 762 documents were automatically recognized as duplicates, so the remaining 696 were left for the next selection stage. After manually screening the documents according to document type, 668 remained. Of these, 4 documents did not have full text available. From the manual evaluation of title, abstract and full text, 621 documents were excluded according to the chosen exclusion criteria. Specifically, in 418 documents, the normalization was defined as the restoration of the physiological condition; in 93 documents, the normalization was included in the standard definition of a parameter/not applied after parameter extraction/applied to parameters not extracted from the ECG; in 33 documents, normalization was intended as a default preprocessing step for a machine learning application; and in 77 documents, there was no explanation of why and/or how normalization was applied. So, 43 documents met all the established inclusion criteria. Among them, 4 documents were animal studies and so were further excluded. Lastly, 39 documents were included in this review [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Using a flowchart, Figure 1 shows the entire process of the literature search, from the application of the query on each bibliographic repository to the manual screening leading to the selected documents.
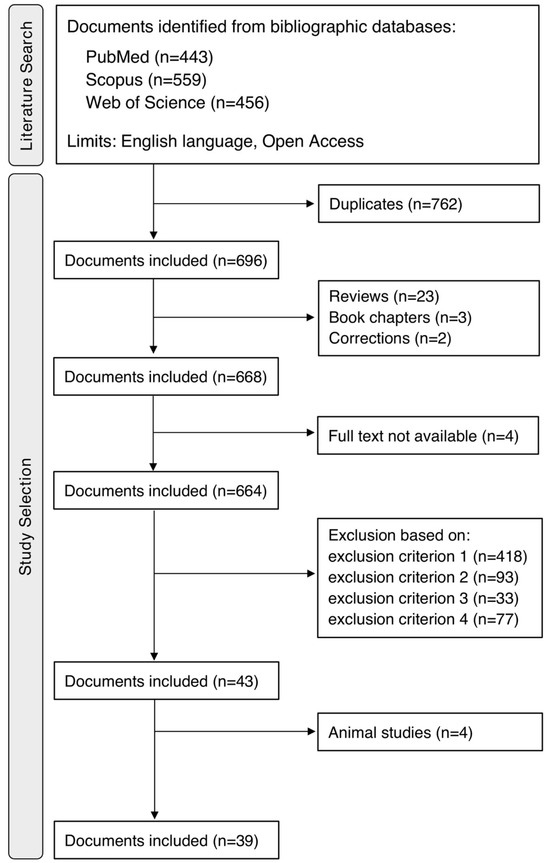
Figure 1.
Process of literature search and study selection.
The majority of the selected documents were original research articles [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,53,54,55,56], while only one document was a conference proceeding [52].
Overall, the included documents were published between 1986 and 2024, with more than half published in the last 10 years. The distribution of the document publication years is detailed in Figure 2.
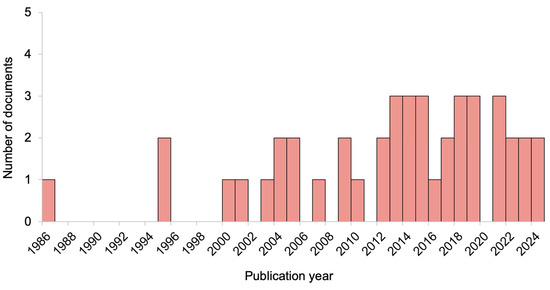
Figure 2.
Distribution of document publication years.
The general characteristics of the populations studied by the included documents are reported in Table 1. Specifically, the table details population size, sex (expressed as number of males and number of females), age (expressed as fetus, newborn, infant, child, adolescent, young adult, or adult), and clinical information, which includes health status (expressed as healthy or diseased) and details about the pathology, when present. Information about population sex was not available for 5 documents, while details about the pathology were not applicable when the studied populations were healthy.

Table 1.
Characteristics of the populations studied in the included documents.
Figure 3 further explores the health status of the studied populations. Specifically, panel A shows a pie chart representing whether the population was healthy or diseased, while panel B shows a pie chart representing the different pathologies affecting the diseased populations. Of the included documents, 7 studies enrolled healthy populations, while 15 studies considered patients with cardiac pathologies.
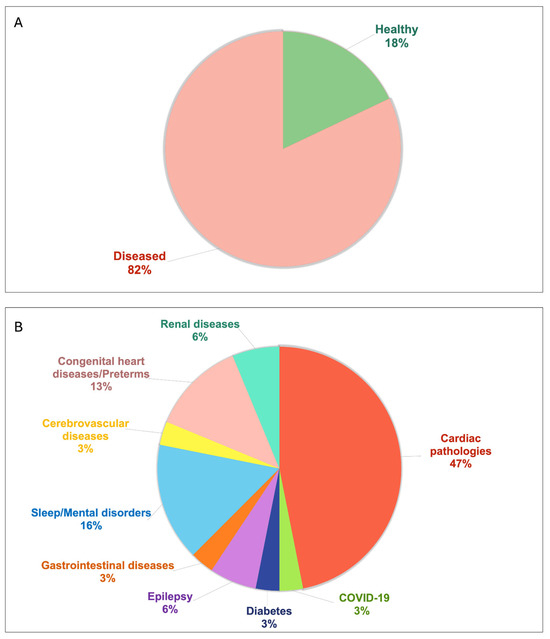
Figure 3.
Pie chart describing the health status of the populations studied in the included documents: (A) reports the percentage of studies involving healthy and diseased populations; (B) reports the percentage of studies involving diseased populations affected by various pathologies.
Table 2 outlines the targets of the studies described in the included documents, while Table 3 reports an overview of which ECG-derived parameters used as cardiac risk indices were normalized in the included documents, of how normalization was performed, and of the reason why normalization was applied. Among the included documents, 21 documents analyzed the ECG spectrum, with 19 (49% of all included documents) presenting normalized HRV indices derived from the frequency analysis of the tachogram. The remaining 18 documents analyzed the ECG in the time domain. Of these, 7 documents presented normalized measures for the analysis of QT interval variability, while 2 documents applied normalization to TWA measures.

Table 2.
Targets of the studies conducted in the included documents.

Table 3.
Overview of how and why the included documents apply normalization.
4. Discussion
This review systematically explored the open-access scientific literature about the normalization of ECG-derived parameters used as risk indices, with the aim of assessing the methodologies and the reasons behind its application in this context.
The literature search strategy was designed to cover a wide range of relevant open-access research, ensuring an initial overview on the topic addressed and providing results that can be considered representative of the free literature. Overall, 39 documents were included. The choice of considering only the open-access literature was prompted by the intent to ensure that all the reviewed literature is freely accessible, so that this study can be fully reproduced by any researcher in the global scientific community; instead, the exclusion of non-English documents was justified by the fact that the most widely used language in science is English. According to the document type, book chapters were excluded in order to focus only on research published in scientific journals, while reviews were excluded in order to provide a comprehensive analysis of primary research findings rather than secondary interpretations. The exclusion criteria were determined according to the purpose of our review, thus with the intent of leaving out studies that were not in line with the objective of understanding the use and impact of normalization on ECG data. No limits were imposed on the publication year since the trend in the use of normalization was one of the pieces of information we wanted to provide.
The chronological arrangement of document publication years that we performed (Figure 2) allowed to evaluate trends and shifts in research focus, as well as to understand the development of the field over the years. By analyzing the temporal distribution, we could identify a clear upward trend in research activity over time. From 1980 to 2000, the publication rate was relatively low, reflecting limited focus on this topic. From 2000 onward, the number of studies gradually increased, with a sharp rise after 2012. This period likely marks a significant intensification of research, probably due to advances in technology and growing attention to the topic, combined with researchers’ increasing interest in open-access publishing. Moreover, the upward trend continues in the most recent years, suggesting an ongoing research activity.
With regard to the general characteristics of the populations studied in the included documents, almost 80% of the studies considered adult populations. The median size of the studied populations was 53 subjects, with few studies (30%) involving more than 100 subjects, and very few (13%) involving more than 200 subjects. Excluding studies that did not specify the population sex, the studied populations were predominantly male. Moreover, most studies enrolled diseased subjects and, as expected, since the data analyzed were ECG data, the patients affected by cardiac pathologies were the most commonly studied. However, the pie chart (Figure 3) helps highlight that ECG-derived measures are computed to evaluate also populations not directly affected by cardiac pathologies, rather by epilepsy, diabetes, mental health issues, or even healthy populations.
The targets of the studies conducted in the included documents reported in Table 2 were provided to contextualize each study, and pointed out that, even if normalization was included in their methodologies (due to this review’s design), none of the studies had the primary purpose of assessing its effect. Indeed, most studies aimed at evaluating changes in HRV that reflect cardiac autonomic modulation, changes in QT interval variability that reflect ventricular repolarization lability, or the effects of some factors (such as drugs, age, physical activity, external stimulations) on them.
Among the included documents, 54% analyzed the ECG in the frequency domain, with almost all presenting normalized HRV indices, while the remaining 46% analyzed the ECG in the time domain, with half of them presenting normalized QT interval variability measures or normalized TWA measures. These results suggest that there is a significant trend towards the established use of normalization in the computation of HRV indices, likely indicating its recognized importance and effectiveness in this field. Moreover, it can be noticed that, among the ECG-derived features that have been normalized by the open-access literature, there were some features that referred to ventricular activity, such as QT-related indices, TWA, and total cosine R-to-T, while no features that referred specifically to atrial activity emerged from this search strategy, possibly suggesting that normalization has been poorly applied to P-wave indices, even if widely studied [58,59,60,61].
From the included documents, normalization was performed by dividing the original parameter to be normalized by itself computed in a different way, or by a different parameter. In the first case, in the time domain the original parameter can be divided by its mean/maximum during the acquisition protocol [19,31,48], or by its value in a condition of the acquisition protocol assumed as baseline [32,53,54,57]. In the frequency domain, normalization was performed by dividing a specific band of the spectrum by the entire spectrum [20,21,22,23,24,25,26,27,28,29,33,34,35,36,37,38,39,40,41,42,55]; indeed, for example, the high/low-frequency HRV spectral component was normalized by the entire tachogram spectrum. In the second case, the original parameter was divided by another parameter to compensate for its possible biasing effect, actually making a new index [43,44,45,46,47,49,50,51,52,56]. This normalization was applied on the basis of a possible interdependence between the two parameters. If the interdependence is known, the normalization is intended to prevent the influence of the biasing parameter from affecting the interpretation of the original parameter’s role; if the interdependence is only assumed, the normalization is intended to investigate mutual influences. In addition, one study characterized the original parameter as number of occurrences and divided it by the number of occurrences of an anomalous cardiac condition, investigating their interdependence [30].
The potential limitation of the application of normalization lies in the fact that it is based on an assumption. The assumption is the existence of an interdependence between the parameter to be normalized, which in our case is the ECG-derived risk index, and the normalizing parameter. This interdependence makes the latter a biasing factor that normalization is intended to neutralize for proper reading and interpretation of the risk index. As an implicit consequence, if this assumption no longer holds, the normalization loses significance and may even jeopardize the parameter’s role as a marker instead of strengthening it.
Studies presenting normalized QT interval measures mainly aimed at making QT interval variability independent from HRV, given their known influence [62]. In this context, it is proper to make a comment regarding the QT correction. Indeed, no studies presented the corrected QT (through the formula of Bazett, Fridericia, Framingham, etc.) as a form of normalization. This highlights that such a procedure is well established enough nowadays so as not to be considered a normalization.
Only two of the included documents applied normalization to TWA [49,57], for different purposes. Specifically, in the study conducted by Martín-Yebra et al. [49], TWA was normalized with the aim of compensating for possible changes in T-wave morphology, while in the study conducted by Pukropski et al. [57], TWA was detected one hour before (baseline), one hour during, and one hour after transcutaneous auricular vagus nerve stimulation, and normalization by dividing for the baseline TWA was applied to enhance the effect of stimulation. These results suggest that TWA normalization has been addressed by different research groups, but further investigation is needed to better define its benefit and modality.
Besides the obtained outcomes, another contribution of this review is the data charting template proposed and detailed in Appendix C. Indeed, it can represent a valid model to follow in future literature research on analogous topics. Moreover, this data charting template, which includes specific information not only on the normalized ECG parameters, but also on the modality and reason of normalization explicitly provided by the authors, allowed us to include only studies in which the normalization process was sufficiently clear and reproducible to be translated into formula, if not already provided by the article. As a result, we could make more specific considerations and evaluations of the different normalization forms, both in time and frequency domains. These allowed to better assess the consistency and comparability of these normalized indices in the literature.
Study Limitations
Although consideration of only the open-access literature is part of the core design of this study, we recognize that it could be interpreted as a limitation, as some scientific works may be overlooked. However, open-access publication is a growing trend, and in the case of our topic it may accurately reflect what emerged from the closed-access literature as well. Indeed, this scoping review achieved the goal of determining the coverage of the body of literature regarding the normalization of ECG-derived cardiac risk indices while also providing an overview of the main focuses and describing how the research was conducted. This approach represents a faithful picture of the literature that any researcher can access, not claiming a thorough analysis of the available evidence. Rather, it assesses its potential target and extent, opening the way to possible future systematic reviews, which are typically considered secondary studies with respect to scoping reviews. Indeed, as such, this scoping review is intended to serve as a precursor to systematic reviews on the normalization of specific ECG-derived indices, possibly including the open- and closed-access literature. Furthermore, our methodological approach, including a detailed data charting template, compensates for a limitation of this study, that is, the lack of quality assessment through a formal quality appraisal, although not expected in a scoping review. Lastly, although requiring a clear explanation of the rationale behind the normalization, no studies aimed at evaluating the effect of the procedure.
5. Conclusions
This review highlights the extensive use of normalization on ECG-derived risk indices. This applies especially for HRV-related and QT-related indices, suggesting that normalization may enhance their role as clinically useful markers of cardiac anomalies. On the other hand, the normalization of ECG-derived parameters that have been less studied, such as those related to the atria, should be investigated.
In conclusion, if evaluated after normalization, the risk index could be isolated from potential interfering factors, boosting its clinical evidence. Moreover, since none of the included studies aimed to evaluate the effect of normalization itself, this review points out the need for future research to fully assess the impact and advantages of normalization across various ECG-derived parameters.
Author Contributions
Conceptualization, E.I. and I.M.; methodology, E.I. and I.M.; software, E.I.; validation, A.S. and M.M.; formal analysis, E.I., A.S., and M.M.; investigation, E.I. and I.M.; data curation, E.I. and I.M.; writing—original draft preparation, E.I. and I.M.; writing—review and editing, A.S., M.M., and L.B.; visualization, E.I., I.M., and A.S.; supervision, L.B. and M.M.; project administration, L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
PRISMA-ScR checklist.
Table A1.
PRISMA-ScR checklist.
| SECTION | ITEM | PRISMA-ScR CHECKLIST ITEM | PAGE(S) |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 2 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 2 |
| MATERIALS and METHODS | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | NA |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 3 |
| Information sources | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 3 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | 15–16 |
| Selection of sources of evidence | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 3–4 |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 16–17 |
| Critical appraisal of individual sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | NA |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 4 |
| RESULTS | |||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | 4–5 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | 6–11 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | NA |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 7–11 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 9–11 |
| DISCUSSION | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 11–13 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 13 |
| CONCLUSIONS | |||
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 13–14 |
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 14 |
NA: not applicable. The grey background color highlights the different sections of this review.
Appendix B
Appendix B.1. Search Query Used on Scopus
(TITLE-ABS(ECG) OR TITLE-ABS(EKG) OR TITLE-ABS(electrocardiogra*)) AND TITLE-ABS(normaliz*) AND (TITLE-ABS(alternans) OR TITLE-ABS(risk) OR TITLE-ABS(threat*) OR TITLE-ABS(arrhythmi*) OR TITLE-ABS(instability) OR TITLE-ABS(abnorma*) OR TITLE-ABS(anomal*) OR TITLE-ABS(irregular*) OR TITLE-ABS(vulnerab*) OR TITLE-ABS(susceptib*)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (OA, “all”))
Appendix B.2. Search Query Used on PubMed
((ECG[Title/Abstract] OR EKG[Title/Abstract] OR electrocardiogra*[Title/Abstract]) AND (normaliz*[Title/Abstract])) AND (alternans[Title/Abstract] OR risk[Title/Abstract] OR threat*[Title/Abstract] OR arrhythmi*[Title/Abstract] OR instability[Title/Abstract] OR abnorma*[Title/Abstract] OR anomal*[Title/Abstract] OR irregular*[Title/Abstract] OR vulnerab*[Title/Abstract] OR susceptib*[Title/Abstract]) AND ((ffrft[Filter]) AND (english[Filter]))
Appendix B.3. Search Query Used on WoS
((TI=(ECG) OR TI=(EKG) OR TI=(electrocardiogra*) OR AB=(ECG) OR AB=(EKG) OR AB=(electrocardiogra*)) AND (TI=(normaliz*) OR AB=(normaliz*)) AND (TI=(alternans) OR TI=(risk) OR TI=(threat*) OR TI=(arrhythmi*) OR TI=(instability) OR TI=(abnorma*) OR TI=(anomal*) OR TI=(irregular*) OR TI=(vulnerab*) OR TI=(susceptib*) OR AB=(alternans) OR AB=(risk) OR AB=(threat*) OR AB=(arrhythmi*) OR AB=(instability) OR AB=(abnorma*) OR AB=(anomal*) OR AB=(irregular*) OR AB=(vulnerab*) OR AB=(susceptib*))) AND (LA==(“ENGLISH”) AND OA==(“OPEN ACCESS”))
Appendix C

Table A2.
Data charting template: items and their definition.
Table A2.
Data charting template: items and their definition.
| Item | Definition |
|---|---|
| Publication year | Year in which the document was officially published in an international scientific journal or conference |
| Population size | Total number of individuals within the population being studied |
| Sex | Total number of males and total number of females (biological sexes) within the population being studied |
| Age | Age range to which the population being studied belongs, considering the following:
|
| Clinical Information: Health status and Details | Definition of the health status of individuals within the population being studied, considering the following:
|
| Target of the study | Description of the rationale and intent of the study being described in the document (as it is reported in the document or uniquely derived from it) |
| Feature | Definition of the ECG-derived parameter being analyzed |
| Domain | Domain in which the feature was computed (time or frequency) |
| Normalization modality (how) | Description of how normalization was applied to the feature, expressed through a mathematical formula (as it is reported in the document or uniquely derived from it) |
| Normalization reason (why) | Description of why normalization was applied to the feature (as it is reported in the document or uniquely derived from it) |
References
- German, C.A.; Baum, S.J.; Ferdinand, K.C.; Gulati, M.; Polonsky, T.S.; Toth, P.P.; Shapiro, M.D. Defining preventive cardiology: A clinical practice statement from the American Society for Preventive Cardiology. Am. J. Prev. Cardiol. 2022, 12, 100432. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, D.; Xie, Z.; Huang, X.; Wang, Z.; Shangguan, H.; Zhu, W.; Wang, S. Assessment of Cardiovascular Risk Factors and Their Interactions in the Risk of Coronary Heart Disease in Patients with Type 2 Diabetes with Different Weight Levels, 2013–2018. Diabetes Metab. Syndr. Obes. 2021, 14, 4253–4262. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Blanes, F.J.; Blanco-Velasco, M.; Barquero-Pérez, Ó.; García-Alberola, A.; Rojo-Álvarez, J.L. Sudden Cardiac Risk Stratification with Electrocardiographic Indices—A Review on Computational Processing, Technology Transfer, and Scientific Evidence. Front. Physiol. 2016, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Akhtar, S.; Singh, S. The Clinical Relevance of ECG Parameters in the Prediction of Cardiac Mortality: A Comprehensive Review. Open Bioinform. J. 2024, 17, e18750362295563. [Google Scholar] [CrossRef]
- Straus, S.M.; Kors, J.A.; De Bruin, M.L.; van der Hooft, C.S.; Hofman, A.; Heeringa, J.; Deckers, J.W.; Kingma, J.H.; Sturkenboom, M.C.; Stricker, B.H.; et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J. Am. Coll. Cardiol. 2006, 47, 362–367. [Google Scholar] [CrossRef]
- Tse, G.; Yan, B.P. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. EP Eur. 2017, 19, 712–721. [Google Scholar] [CrossRef]
- Calò, L.; Lanza, O.; Crescenzi, C.; Parisi, C.; Panattoni, G.; Martino, A.; Rebecchi, M.; Tarzia, P.; Ciampi, P.; Romeo, F.; et al. The value of the 12-lead electrocardiogram in the prediction of sudden cardiac death. Eur. Heart J. Suppl. 2023, 25, C218–C226. [Google Scholar] [CrossRef]
- Carbone, V.; Guarnaccia, F.; Carbone, G.; Zito, G.B.; Oliviero, U.; Soreca, S.; Carbone, F. Gender differences in the 12-lead electrocardiogram: Clinical implications and prospects. Ital. J. Gend.-Specif. Med. 2020, 6, 126–141. [Google Scholar] [CrossRef]
- Mansi, I.A.; Nash, I.S. Ethnic differences in electrocardiographic amplitude measurements. Ann. Saudi Med. 2004, 24, 459–464. [Google Scholar] [CrossRef]
- Butt, J.H.; Claggett, B.L.; Miao, Z.M.; Jering, K.S.; Sim, D.; van der Meer, P.; Ntsekhe, M.; Amir, O.; Cho, M.C.; Carrillo-Calvillo, J.; et al. Geographic differences in patients with acute myocardial infarction in the PARADISE-MI trial. Eur. J. Heart Fail. 2023, 25, 1228–1242. [Google Scholar] [CrossRef]
- Alkhaqani, A. Electrocardiography Morphology of Electrolytes Disturbance. J. Clin. Nurs. 2023, 5, 1–6. [Google Scholar]
- Parodi, J.B.; Ramchandani, R.; Zhou, Z.; Chango, D.X.; Acunzo, R.; Liblik, K.; Farina, J.M.; Zaidel, E.J.; Ruiz-Mori, E.; Carreón, J.M.A.; et al. A systematic review of electrocardiographic changes in healthy high-altitude populations. Trends Cardiovasc. Med. 2023, 33, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, R.; Zhou, Z.; Parodi, J.B.; Farina, J.M.; Liblik, K.; Sotomayor, J.; Burak, C.; Herman, R.; Baranchuk, A. A Systematic Review of Electrocardiographic Changes in Populations Temporarily Ascending to High Altitudes. Curr. Probl. Cardiol. 2023, 48, 101630. [Google Scholar] [CrossRef] [PubMed]
- Hassing, G.J.; van der Wall, H.E.C.; van Westen, G.J.P.; Kemme, M.J.B.; Adiyaman, A.; Elvan, A.; Burggraaf, J.; Gal, P. Body mass index related electrocardiographic findings in healthy young individuals with a normal body mass index. Neth. Heart J. 2019, 27, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, E.I.; Ciucurel, C. The Relationship between Body Composition and ECG Ventricular Activity in Young Adults. Int. J. Environ. Res. Public Health 2022, 19, 11105. [Google Scholar] [CrossRef] [PubMed]
- Musa, N.; Gital, A.Y.; Aljojo, N.; Chiroma, H.; Adewole, K.S.; Mojeed, H.A.; Faruk, N.; Abdulkarim, A.; Emmanuel, I.; Folawiyo, Y.Y.; et al. A systematic review and Meta-data analysis on the applications of Deep Learning in Electrocardiogram. J. Ambient. Intell. Humaniz. Comput. 2023, 14, 9677–9750. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Bexton, R.S.; Vallin, H.O.; Camm, A.J. Diurnal variation of the QT interval—Influence of the autonomic nervous system. Br. Heart J. 1986, 55, 253–258. [Google Scholar] [CrossRef]
- Furlan, R.; Ardizzone, S.; Palazzolo, L.; Rimoldi, A.; Perego, F.; Barbic, F.; Bevilacqua, M.; Vago, L.; Bianchi Porro, G.; Malliani, A. Sympathetic overactivity in active ulcerative colitis: Effects of clonidine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 290, R224–R232. [Google Scholar] [CrossRef]
- Itagi, A.B.H.; Arora, D.; Patil, N.A.; Bailwad, S.A.; Yunus, G.Y.; Goel, A. Short-term acute effects of gutkha chewing on heart rate variability among young adults: A cross-sectional study. Int. J. Appl. Basic. Med. Res. 2016, 6, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Jarrin, D.C.; Ivers, H.; Lamy, M.; Chen, I.Y.; Harvey, A.G.; Morin, C.M. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J. Sleep. Res. 2018, 27, e12663. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.; Santos, M.D.B.; Silva, E.; Quitério, R.J.; Moreno, M.A.; Reis, M.S.; Verzola, I.A.; Oliveira, L.; Martins, L.E.; Gallo-Junior, L.; et al. Effects of age and physical activity on the autonomic control of heart rate in healthy men. Braz. J. Med. Biol. Res. 2005, 38, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Pecis, M.; Azevedo, M.J.; Moraes, R.S.; Ferlin, E.L.; Gross, J.L. Autonomic dysfunction and urinary albumin excretion rate are associated with an abnormal blood pressure pattern in normotensive normoalbuminuric type 1 diabetic patients. Diabetes Care. 2000, 23, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Tang, W.; Li, L.X.; Su, C.Y.; Wang, T. The study of spectral analysis of heart rate variability in different blood pressure types in euvolemic peritoneal dialysis patients. Ren. Fail. 2012, 34, 722–726. [Google Scholar] [CrossRef][Green Version]
- Poulikakos, D.; Banerjee, D.; Malik, M. Repolarisation descriptors and heart rate variability in hemodialysed patients. Physiol. Res. 2014, 64, 487–493. [Google Scholar] [CrossRef]
- Rauchenzauner, M.; Ernst, F.; Hintringer, F.; Ulmer, H.; Ebenbichler, C.F.; Kasseroler, M.T.; Joannidis, M. Arrhythmias and increased neuro-endocrine stress response during physicians’ night shifts: A randomized cross-over trial. Eur. Heart J. 2009, 30, 2606–2613. [Google Scholar] [CrossRef]
- Scavone, G.; Baril, A.A.; Montplaisir, J.; Carrier, J.; Desautels, A.; Zadra, A. Autonomic Modulation During Baseline and Recovery Sleep in Adult Sleepwalkers. Front. Neurol. 2021, 12, 680596. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.J.; Seok, J.H.; Kim, E.; Park, J.Y.; Kim, H.E.; Oh, J. Association and interaction between clinician-rated measures of depression and anxiety with heart rate variability in elderly patients with psychiatric disorders. Heliyon 2023, 9, e20740. [Google Scholar] [CrossRef]
- Chen, X.; Tereshchenko, L.G.; Berger, R.D.; Trayanova, N.A. Arrhythmia risk stratification based on QT interval instability: An intracardiac electrocardiogram study. Heart Rhythm. 2013, 10, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Fereniec, M.; Stix, G.; Kania, M.; Mroczka, T.; Maniewski, R. An analysis of the U-wave and its relation to the T-wave in body surface potential maps for healthy subjects and MI patients. Ann. Noninvasive Electrocardiol. 2013, 19, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Frimerman, A.; Meisel, S.; Shotan, A.; Blondheim, D.S. Enhancement of Standard ECGs by a New Method for Multi-Cycle Superimposition and Summation. Isr. Med. Assoc. J. 2018, 20, 14–19. [Google Scholar] [PubMed]
- Giuliani, C.; Agostinelli, A.; Fioretti, S.; Di Nardo, F.; Burattini, L. Abnormal repolarization in the acute myocardial infarction patients: A frequency-based characterization. Open Biomed. Eng. J. 2014, 8, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Swenne, C.A.; Man, S.; Agostinelli, A.; Fioretti, S.; Di Nardo, F.; Burattini, L. Predictive Power of f99 Repolarization Index for the Occurrence of Ventricular Arrhythmias. Ann. Noninvasive Electrocardiol. 2015, 21, 152–160. [Google Scholar] [CrossRef]
- Buchheit, M.; Simon, C.; Piquard, F.; Ehrhart, J.; Brandenberger, G. Effects of increased training load on vagal-related indexes of heart rate variability: A novel sleep approach. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2813–H2818. [Google Scholar] [CrossRef]
- Fei, L.; Slade, A.K.; Prasad, K.; Malik, M.; McKenna, W.J.; Camm, A.J. Is there increased sympathetic activity in patients with hypertrophic cardiomyopathy? J. Am. Coll. Cardiol. 1995, 26, 472–480. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Govindan, R.; Metzler, M.; Swisher, C.B.; Hitchings, L.; Wang, Y.; Baker, R.; Larry Maxwell, G.; Krishnan, A.; du Plessis, A.J. Heart rate variability is depressed in the early transitional period for newborns with complex congenital heart disease. Clin. Auton. Res. 2019, 30, 165–172. [Google Scholar] [CrossRef]
- Noben, L.; Verdurmen, K.M.J.; Warmerdam, G.J.J.; Vullings, R.; Oei, S.G.; van Laar, J.O.E.H. The fetal electrocardiogram to detect the effects of betamethasone on fetal heart rate variability. Early Hum. Dev. 2019, 130, 57–64. [Google Scholar] [CrossRef]
- Van Boven, A.J.; Brouwer, J.; Crijns, H.J.G.M.; Haaksma, J.; Lie, K.I. Differential autonomic mechanisms underlying early morning and daytime transient myocardial ischaemia in patients with stable coronary artery disease. Heart. 1995, 73, 134–138. [Google Scholar] [CrossRef][Green Version]
- Verdurmen, K.M.J.; Warmerdam, G.J.J.; Lempersz, C.; Hulsenboom, A.D.J.; Renckens, J.; Dieleman, J.P.; Vullings, R.; van Laar, J.O.E.H.; Oei, S.G. The influence of betamethasone on fetal heart rate variability, obtained by non-invasive fetal electrocardiogram recordings. Early Hum. Dev. 2018, 119, 8–14. [Google Scholar] [CrossRef]
- Yang, J.H.; Choi, S.H.; Lee, M.H.; Oh, S.M.; Choi, J.W.; Park, J.E.; Park, K.S.; Lee, Y.J. Association of heart rate variability with REM sleep without atonia in idiopathic REM sleep behavior disorder. J. Clin. Sleep. Med. 2021, 17, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Liviero, F.; Scapellato, M.L.; Volpin, A.; Battistella, M.; Fabris, L.; Brischigliaro, L.; Folino, F.; Moretto, A.; Mason, P.; Pavanello, S. Long term follow-up of heart rate variability in healthcare workers with mild COVID-19. Front. Neurol. 2024, 15, 1403551. [Google Scholar] [CrossRef] [PubMed]
- Haigney, M.C.; Zareba, W.; Gentlesk, P.J.; Goldstein, R.E.; Illovsky, M.; McNitt, S.; Andrews, M.L.; Moss, A.J.; Multicenter Automatic Defibrillator Implantation Trial II investigators. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J. Am. Coll. Cardiol. 2004, 44, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Pohl, R.K.; Yeragani, V. QT interval variability in panic disorder patients after isoproterenol infusions. Int. J. Neuropsychopharmacol. 2001, 4, 17–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takeuchi, Y.; Omeki, Y.; Horio, K.; Nishio, M.; Nagata, R.; Oikawa, S.; Mizutani, Y.; Nagatani, A.; Funamoto, Y.; Uchida, H.; et al. Relationship between QT and JT peak interval variability in prepubertal children. Ann. Noninvasive Electrocardiol. 2017, 22, e12444. [Google Scholar] [CrossRef]
- Kotidis, C.; Wertheim, D.; Weindling, M.; Rabe, H.; Turner, M.A. Assessing patent ductus arteriosus in preterm infants from standard neonatal intensive care monitoring. Eur. J. Pediatr. 2022, 181, 1117–1124. [Google Scholar] [CrossRef]
- Krahn, A.D.; Pickett, R.A.; Sakaguchi, S.; Shaik, N.; Cao, J.; Norman, H.S.; Guerrero, P. R-wave sensing in an implantable cardiac monitor without ECG-based preimplant mapping: Results from a multicenter clinical trial. Pacing Clin. Electrophysiol. 2013, 37, 505–511. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, C.M. Heartbeat classification using normalized RR intervals and morphological features. Math. Probl. Eng. 2014, 2014, 712474. [Google Scholar] [CrossRef]
- Martín-Yebra, A.; Caiani, E.G.; Monasterio, V.; Pellegrini, A.; Laguna, P.; Martínez, J.P. Evaluation of T-wave alternans activity under stress conditions after 5 d and 21 d of sedentary head-down bed rest. Physiol. Meas. 2015, 36, 2041–2055. [Google Scholar] [CrossRef]
- Piccirillo, G.; Magrì, D.; Matera, S.; Magnanti, M.; Torrini, A.; Pasquazzi, E.; Schifano, E.; Velitti, S.; Marigliano, V.; Quaglione, R.; et al. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: A prospective study. Eur. Heart J. 2007, 28, 1344–1350. [Google Scholar] [CrossRef]
- Toman, O.; Hnatkova, K.; Šišáková, M.; Smetana, P.; Huster, K.M.; Barthel, P.; Novotný, T.; Andršová, I.; Schmidt, G.; Malik, M. Short-Term Beat-to-Beat QT Variability Appears Influenced More Strongly by Recording Quality Than by Beat-to-Beat RR Variability. Front. Physiol. 2022, 13, 863873. [Google Scholar] [CrossRef] [PubMed]
- Tsipouras, M.G.; Fotiadis, D.I. An efficient system for the detection of arrhythmic segments in ECG recordings based on non-linear features of the RR interval signal. In Proceedings of the Computers in Cardiology, Thessaloniki, Greece, 21–24 September 2003; pp. 533–536. [Google Scholar] [CrossRef]
- Woodward, J.L.; Connolly, M.; Hennessy, P.W.; Holleran, C.L.; Mahtani, G.B.; Brazg, G.; Fahey, M.; Maganti, K.; Hornby, T.G. Cardiopulmonary Responses during Clinical and Laboratory Gait Assessments in People with Chronic Stroke. Phys. Ther. 2019, 99, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hamon, D.; Fang, Z.; Xu, Y.; Yang, B.; Ju, W.; Bradfield, J.; Shivkumar, K.; Chen, M.; Tung, R. Value of a Posterior Electrocardiographic Lead for Localization of Ventricular Outflow Tract Arrhythmias: The V4/V8 Ratio. JACC Clin. Electrophysiol. 2017, 3, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Dono, F.; Evangelista, G.; Consoli, S.; Venditti, R.; Russo, M.; De Angelis, M.V.; Faustino, M.; Di Iorio, A.; Vollono, C.; Anzellotti, F.; et al. Heart rate variability modifications in adult patients with early versus late-onset temporal lobe epilepsy: A comparative observational study. Neurophysiol. Clin. 2023, 53, 102852. [Google Scholar] [CrossRef]
- Kania, M.; Maniewski, R.; Kobylecka, M.; Zaczek, R.; Królicki, L.; Opolski, G.; Janusek, D. Prognostic value of the total cosine R to T measured in high resolution body surface potential mapping during exercise test. Biomed. Signal Process. Control 2015, 20, 135–141. [Google Scholar] [CrossRef]
- Pukropski, J.; Baumann, J.; Jordan, A.; Bausch, M.; von Wrede, R.; Surges, R. Short-term effects of transcutaneous auricular vagus nerve stimulation on T-wave alternans in people with focal epilepsy—An exploratory pilot study. Epilepsy Behav. Rep. 2024, 26, 100657. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Soliman, E.Z.; Alraies, M.C.; Adabag, S.; O’Neal, W.T.; Alonso, A.; Chen, L.Y. Relation of Prolonged P-Wave Duration to Risk of Sudden Cardiac Death in the General Population (from the Atherosclerosis Risk in Communities Study). Am. J. Cardiol. 2017, 119, 1302–1306. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Roetker, N.S.; Soliman, E.Z.; Koene, R.J.; Rooney, M.R.; O’Neal, W.T.; Shah, A.M.; Claggett, B.L.; Solomon, S.D.; et al. Refining Prediction of Atrial Fibrillation-Related Stroke Using the P2-CHA2DS2-VASc Score. Circulation 2019, 139, 180–191. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Soliman, E.Z.; Alonso, A.; Sotoodehnia, N.; Chen, L.Y. Association of P-Wave Abnormalities With Sudden Cardiac and Cardiovascular Death: The ARIC Study. Circulation. Arrhythmia Electrophysiol. 2021, 14, e009314. [Google Scholar] [CrossRef]
- Lehtonen, A.O.; Langén, V.L.; Puukka, P.J.; Kähönen, M.; Nieminen, M.S.; Jula, A.M.; Niiranen, T.J. Incidence rates, correlates, and prognosis of electrocardiographic P-wave abnormalities—A nationwide population-based study. J. Electrocardiol. 2017, 50, 925–932. [Google Scholar] [CrossRef]
- Andršová, I.; Hnatkova, K.; Šišáková, M.; Toman, O.; Smetana, P.; Huster, K.M.; Barthel, P.; Novotný, T.; Schmidt, G.; Malik, M. Heart Rate Influence on the QT Variability Risk Factors. Diagnostics 2020, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).