Probiotics and Prebiotics in the Aspect of Health Benefits and the Development of Novel Plant-Based Functional Food
Abstract
1. Introduction
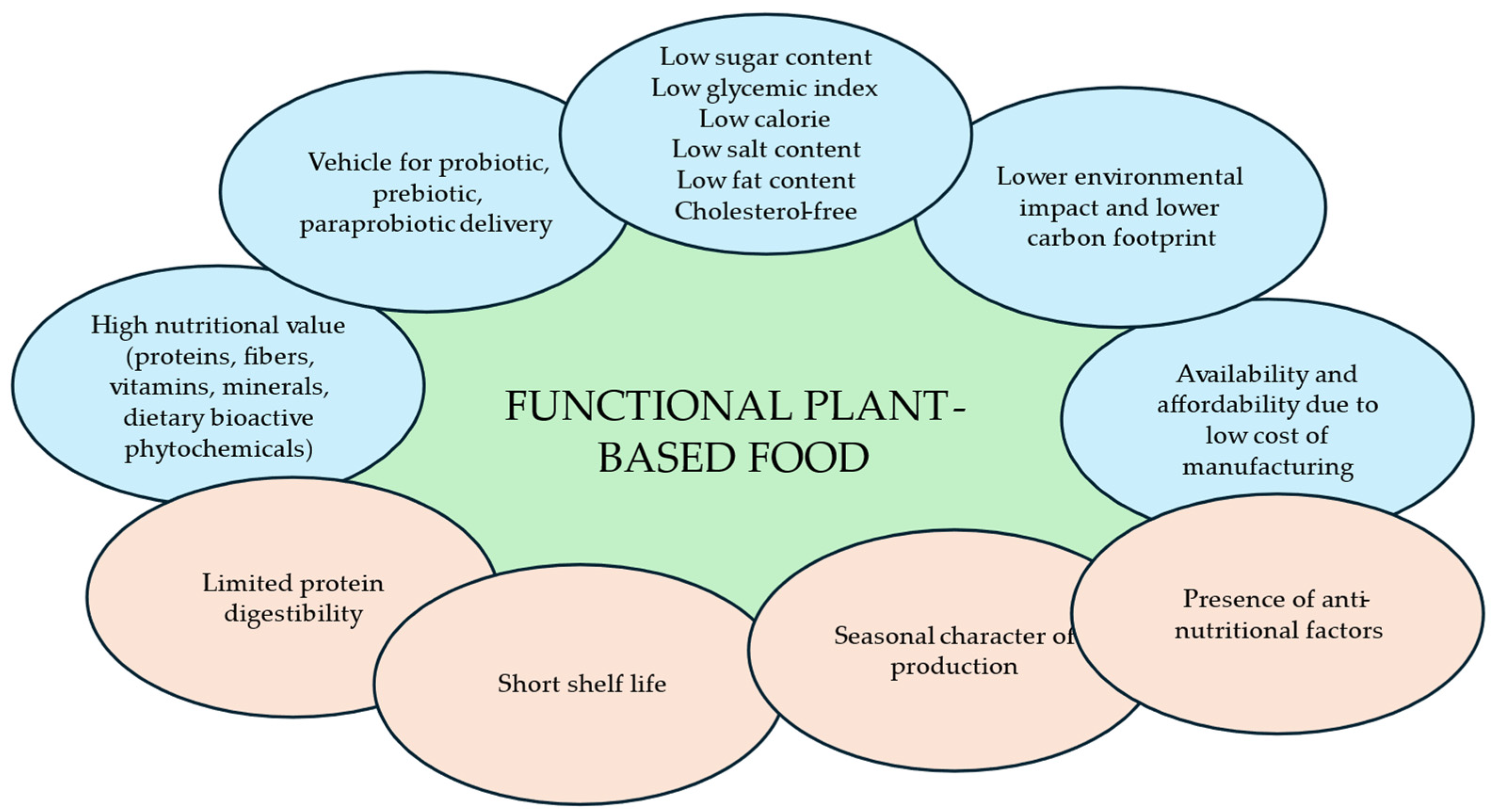
2. Characteristics of Plant-Based Food Matrices as Carriers of Probiotics
2.1. Plant Food Probiotic Microorganisms
2.2. Main Prebiotics of Plant Food
2.3. Plant-Based Matrix Structure in Relation to Probiotic Incorporation
2.4. Survival of Probiotics in Plant-Food Products
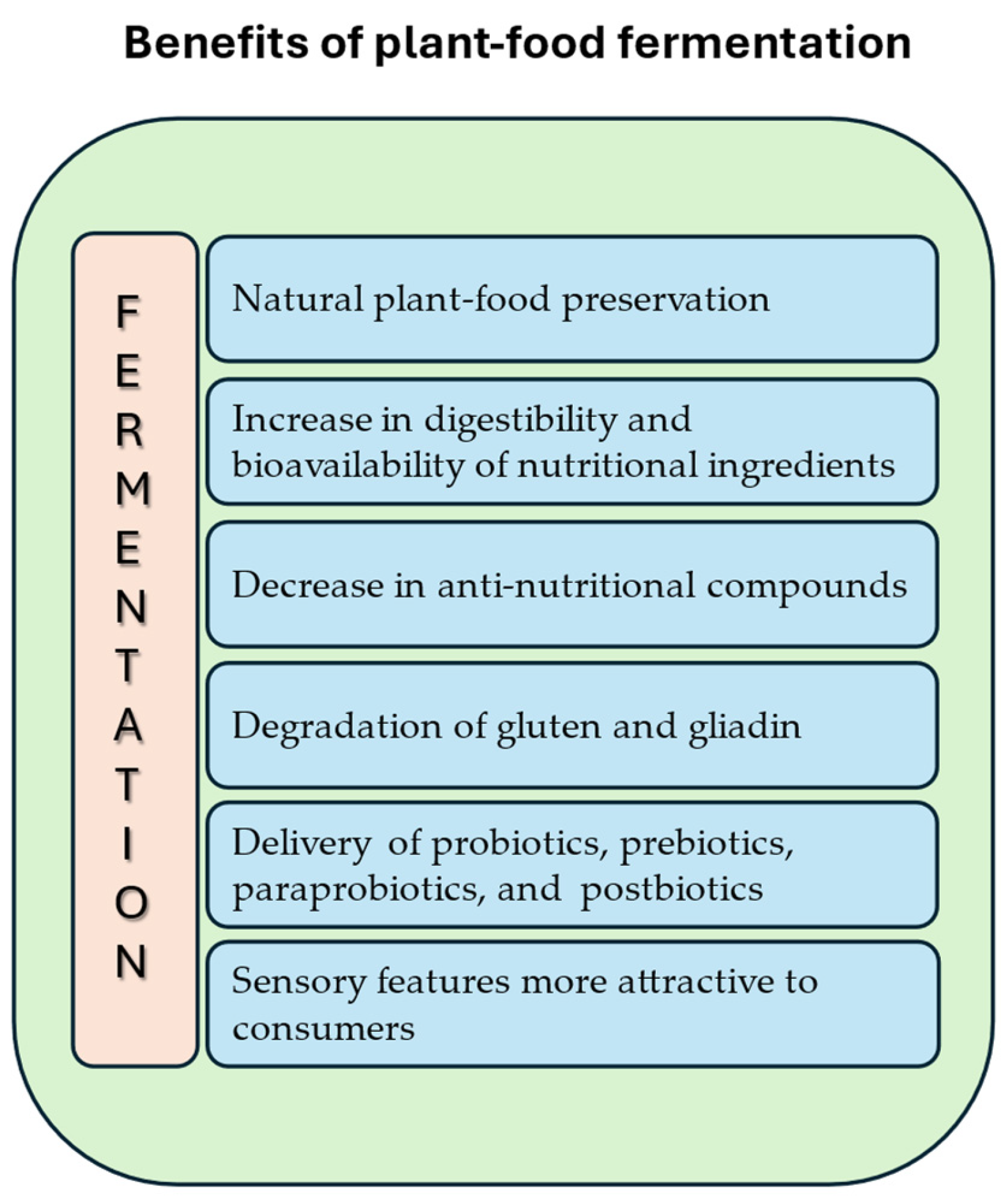
3. Fermentation as the Method of Food Preservation Increasing the Health Benefits of Plant Food
4. The Process Control Using System Tools
- -
- -
- Control of critical parameters of the fermentation process, such as temperature, pH value, and fermentation time, to ensure an appropriate number of live bacteria in the final products and selection and use of a suitable strain with probiotic properties, taking into account the source of isolation of this strain. This issue was addressed in many previous works [106,107,108,109], where the impact of the strain used in plant products on the quality of the final product was demonstrated.
5. Organic Plant-Based Food Production
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crowe, F.L.; Appleby, P.N.; Travis, R.C.; Key, T.J. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: Results from the EPIC-Oxford cohort study. Am. J. Clin. Nutr. 2013, 97, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J. Am. Heart Assoc. 2019, 8, 012865. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet quality as assessed by the Healthy Eating Index, the Alternative Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and health outcomes: An updated systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2018, 118, 74–100.e11. [Google Scholar] [CrossRef]
- Arai, S. Studies on functional foods in Japan–state of the art. Biosci. Biotechnol. Biochem. 1996, 60, 9–15. [Google Scholar] [CrossRef]
- Ashaolu, T.J. A review on selection of fermentative microorganisms for functional foods and beverages: The production and future perspectives. Food Sci. Technol. 2019, 54, 2511–2519. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Guidelines for the Evaluation of Probiotics in Food; Joint FAO/WHO Working Group Meeting: London, Canada, 2002. Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report (FAO/WHO). Available online: https://isappscience.org/wp-content/uploads/2019/04/probiotic_guidelines.pdf (accessed on 21 December 2024).
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; Robertson, L.; et al. Update of the list of QPS—recommended biological agents intentionally added to food or feed as notified to EFSA 6: Suitability of taxonomic units notified to EFSA until March 2017. EFSA J. 2017, 15, 4884. [Google Scholar]
- Braiek, O.B.; Smaoui, S. Enterococci: Between Emerging Pathogens and potential probiotics. Biomed. Res. Int. 2019, 2019, 5938210. [Google Scholar] [CrossRef]
- Suvorov, A. What is wrong with enterococcal probiotics? Probiotics Antimicrob. Proteins 2020, 12, 1–4. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; van den Broek, M.F.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef]
- Bernal-Castro, C.; Espinosa-Poveda, E.; Gutiérrez-Cortés, C.; Díaz-Moreno, C. Vegetable substrates as an alternative for the inclusion of lactic acid bacteria with probiotic potential in food matrices. Food Sci. Technol. 2024, 61, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Naseem, Z.; Mir, S.A.; Wani, S.M.; Rouf, M.A.; Bashir, I.; Zehra, A. Probiotic-fortified fruit juices: Health benefits, challenges, and future perspective. Nutrition 2023, 115, 112154. [Google Scholar] [CrossRef] [PubMed]
- Flach, J.; van der Waal, M.B.; van den Nieuwboer, M.; Claassen, E.; Larsen, O.F.A. The underexposed role of food matrices in probiotic products: Reviewing the relationship between carrier matrices and product parameters. Crit. Rev. Food Sci. Nutr. 2018, 58, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Market Intelligence Report—Global Forecast to 2027. Available online: https://www.marketresearch.com/Think-Market-Intelligence-v4247/eLearning-Intelligence-Global-Forecast-31991364/ (accessed on 1 November 2024).
- Chaudhary, A. Probiotic Fruit and Vegetable Juices: Approach Towards a Healthy Gut. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1265–1279. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Roy, N.C.; José Frutos, M.; Anderson, C.R. Influence of the Fruit Juice Carriers on the Ability of Lactobacillus plantarum DSM20205 to Improve in Vitro Intestinal Barrier Integrity and Its Probiotic Properties. J. Agric. Food Chem. 2017, 65, 5632–5638. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, R.; Liu, M.; Chen, S.; He, L.; Ao, X.; Zou, L.; Liu, S.; Zhou, K. Study of the probiotic properties of lactic acid bacteria isolated from chinese traditional fermented pickles. J. Food Process. Preserv. 2017, 41, e12954. [Google Scholar] [CrossRef]
- Tokatli, M.; Gulgor, G.; Bagder Elmaci, S.; Arslankoz Isleyen, N.; Ozcelik, F. In vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. Biomed. Res. Int. 2015, 2015, 315819. [Google Scholar] [CrossRef]
- Zhang, L.; Lou, Y.; Schutyser, M.A.I. 3D printing of cereal-based food structures containing probiotics. Food Struct. 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Zhang, M. Incorporation of probiotics (Bifidobacterium animalis subsp. lactis) into 3D printed mashed potatoes: Effects of variables on the viability. Food Res. Int. 2020, 128, 108795. [Google Scholar]
- Obayomi, O.V.; Olaniran, A.F.; Owa, S.O. Unveiling the role of functional foods with emphasis on prebiotics and probiotics in human health: A review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Koley, H.; Howlader, D.R.; Bhaumik, U. New Look to Phytomedicine: Advancements in Herbal Products as Novel Drug Leads; Mohd, S.A.K., Iqbal, A., Debprasad, C., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 563–580. [Google Scholar]
- Gustaw, K.; Niedzwiedź, I.; Rachwał, K.; Polak-Berecka, M. New Insight into Bacterial Interaction with the Matrix of Plant-Based Fermented Foods. Foods 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Más, D.; Betancur, C.; Gebeyew, K.; Adebowale, T.; Hussain, T.; Lan, W.; Ding, X. Role of the phytochemical compounds like modulators in gut microbiota and oxidative stress. Curr. Pharm. Des. 2020, 26, 2642–2656. [Google Scholar] [CrossRef] [PubMed]
- ISAAP. Available online: https://isappscience.org/for-scientists/resources/prebiotics/ (accessed on 4 March 2025).
- Ferreira, V.C.; Barroso, T.L.C.T.; Castro, L.E.N.; da Rosa, R.G.; de Siqueira Oliveira, L. An overview of prebiotics and their applications in the food industry. Eur. Food Res. Technol. 2023, 249, 2957–2976. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- D’Almeida, A.P.; Neta, A.A.I.; de Andrade-Lima, M. Tiago Lima de Albuquerque Plant-based probiotic foods: Current state and future trends. Food Sci. Biotechnol. 2024, 33, 3401–3422. [Google Scholar] [CrossRef]
- Farid, M.S.; Anjum, R.; Yang, Y.; Tu, M.; Zhang, T.; Pan, D.; Sun, Y.; Wu, Z. Recent Trends in Fermented Plant-Based Analogues and Products, Bioactive Peptides, and Novel Technologies-Assisted Fermentation. Trends Food Sci. Technol. 2024, 149, 104529. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Costa, W.K.A.D.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.C.; Koh, W.Y.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H. Overview of fermentation process: Structure-function relationship on protein quality and non-nutritive compounds of plant-based proteins and carbohydrates. Crit. Rev. Food Sci. Nutr. 2022, 63, 7677–7691. [Google Scholar] [CrossRef]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Peres, C.; Peres, C.; Hernandez-Mendoza, A.; Malcata, F. Review on fermented plant material as carriers and sources of potentially probiotic lactic acid bacteria—With an emphasis on table olives. Trends Food Sci. Technol. 2012, 26, 31–42. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Sionek, B.; Szydłowska, A.; Küçükgöz, K.; Kołożyn-Krajewska, D. Traditional and New Microorganisms in Lactic Acid Fermentation of Food. Fermentation 2023, 9, 1019. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Trząskowska, M.; Kołożyn-Krajewska, D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic Fermented Fruit or Vegetable Juices: Past, Present and Future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Beganović, J.; Pavunc, A.L.; Gjuračić, K.; Špoljarec, M.; Šušković, J.; Kos, B. Improved Sauerkraut Production with Probiotic Strain Lactobacillus plantarum L4 and Leuconostoc mesenteroides LMG 7954. J. Food Sci. 2011, 76, M124–M129. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Rajendram, R.; Caggia, C. Lactic Acid Bacteria in Table Olive Fermentations. In Olives and Olive Oil in Health and Disease; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Singapore, 2010; pp. 369–376. [Google Scholar]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Liu, X.; Su, S.; Yao, J.; Zhang, X.; Wu, Z.; Jia, L.; Liu, L.; Hou, Y.; Faragb, M.A.; Liu, L. Research advance about plant polysaccharide prebiotics, benefit for probiotics on gut homeostasis modulation. Food Biosci. 2024, 59, 103831. [Google Scholar] [CrossRef]
- De Bellis, P.; Sisto, A.; Lavermicocca, P. Probiotic bacteria and plant-based matrices: An association with improved health-promoting features. J. Funct. Foods 2021, 87, 104821. [Google Scholar] [CrossRef]
- Collins, S.; Reid, G. Distant Site Effects of Ingested Prebiotics. Nutrients 2016, 8, 523. [Google Scholar] [CrossRef]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic oligosaccharides: Special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Kang, H.R.; Cho, S.K. Changes over the fermentation period in phenolic compounds and antioxidant and anticancer activities of blueberries fermented by Lactobacillus plantarum. J. Food Sci. 2019, 84, 2347–2356. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, J.; Fan, L.; Qin, Z.; Chen, Q.; Zhao, L. Antioxidant properties of a vegetable-fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotechnol. 2018, 27, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J. The Food Matrix: Implications in Processing, Nutrition and Health. Crit. Rev. Food Sci. Nutr. 2018, 59, 1–43. [Google Scholar] [CrossRef]
- Konstankiewicz, K. Microstructure of Plant Tissue. In Encyclopedia of Agrophysics; Encyclopedia of Earth Sciences Series; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 476–481. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E. Bioavailability and biocon version of carotenoids. Annual Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef]
- Oliveira, M.A.; de Souza, V.M.; Bergamini, A.M.M.; de Martinis, E.C.P. Microbiological quality of ready-to-eat minimally processed vegetables consumed in Brazil. Food Control 2011, 22, 1400–1403. [Google Scholar] [CrossRef]
- Dufour, C.; Loonis, M.; Delosiere, M.; Buffiere, C.; Hafnaoui, N.; Sante-Lhoutellier, V.; Remond, D. The matrix of fruit & vegetables modulates the gastrointestinal bioaccessibility of polyphenols and their impact on dietary protein digestibility. Food Chem. 2018, 240, 314–322. [Google Scholar] [CrossRef]
- Szydłowska, A.; Kołożyn-Krajewska, D. Development of potentially probiotic and synbiotic pumpkin frozen desserts. CyTA-J. Food 2019, 17, 251–259. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Song, B.; Tian, J.; Gao, N.; Zhang, Y.; Shu, C. Protective effects of fermented blueberry juice with probiotics on alcohol-induced stomach mucosa injury in rats. Food Biosci. 2023, 55, 102974. [Google Scholar] [CrossRef]
- Jagelavičiutė, J.; Čižeikienė, D.; Bašinskienė, L. Enzymatic Modification of Apple Pomace and Its Application in Conjunction with Probiotics for Jelly Candy Production. Appl. Sci. 2025, 15, 599. [Google Scholar] [CrossRef]
- Jo, Y.M.; Kim, G.Y.; Kim, S.A.; Cheon, S.W.; Kang, C.H.; Han, N.S. Limosilactobacillus fermentum MG7011: An Amylase and Phytase Producing Starter for the Preparation of Rice-Based Probiotic Beverages. Front Microbiol. 2021, 12, 745952. [Google Scholar] [CrossRef] [PubMed]
- Deziderio, M.A.; de Souza, H.F.; Kamimura, E.S.; Petrus, R.R. Plant-Based Fermented Beverages: Development and Characterization. Foods 2023, 12, 4128. [Google Scholar] [CrossRef] [PubMed]
- Akalın, H.; Kınık, Ö.; Şatır, G. Manufacturing plant-based non-dairy and probiotic frozen desserts and their impact on physicochemical, sensory and functional aspects. J. Food Sci. Technol. 2024, 61, 1874–1883. [Google Scholar] [CrossRef]
- de Souza, R.C.; Magnani, M.; de Medeiros, V.P.B.; Marcolino, V.A.; Klososki, S.J.; dos Santos, L.M.; Pimentel, T.C. Lacticaseibacillus casei improves textural, functional, and sensory properties and phenolics’ bioaccessibility of frozen desserts prepared using water-soluble extract of rice by-product and Spirulina platensis. LWT 2023, 183, 114794. [Google Scholar] [CrossRef]
- Sheehan, V.M.; Ross, P.; Fitzgerald, G.F. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices Innovative. Food Sci. Emerg. Technol. 2007, 8, 279–284. [Google Scholar] [CrossRef]
- Neveling, D.P.; Endo, A.; Dicks, L.M.T. Fructophilic Lactobacillus kunkeei and Lactobacillus brevis isolated from fresh flowers, bees and bee-hives. Curr. Microbiol. 2012, 65, 507–515. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Vegetable and Fruit Fermentation by Lactic Acid Bacteria. In Biotechnology of Lactic Acid Bacteria: Novel Applications, 2nd ed.; Mozzi, F.M., Raya, R.R., Graciela, M.V., Eds.; Willey Blackwell: New York, NY, USA, 2015; pp. 216–230. [Google Scholar] [CrossRef]
- Espirito-Santo, A.P.; Carlin, F.; Renard, C.M.G.C. Apple, grape or orange juice: Which one offers the best substrate for lactobacilli growth? A screening study on bacteria viability, superoxide dismutase activity, folates production and hedonic characteristics. Food. Res. Int. 2015, 78, 352–353. [Google Scholar] [CrossRef]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R. Fermented Foods: Fermented Vegetable Products. In Encyclopedia of Food Microbiology; Batt, C.A., Tortorello, M.L., Eds.; Elsevier Academic Press: London, UK, 2014; Volume 1, pp. 875–883. [Google Scholar]
- Rodrigues, S.; Silva, L.C.A.; Mulet, A.; Cárcel, J.A.; Fernandes, F.A.N. Development of dried probiotic apple cubes incorporated with Lactobacillus casei NRRL B-442. J. Funct. Foods 2018, 41, 48–54. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, Y.; Kong, B.; Huang, Y.; Yan, D.; Tan, H.; Shang, L. Dual-core prebiotic microcapsule encapsulating probiotics for metabolic syndrome. ACS Appl. Mater Interfaces 2020, 12, 42586–42594. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Microencapsulating polymers for probiotics delivery systems: Preparation, characterization, and applications. Food Hydrocoll 2021, 120, 106882. [Google Scholar] [CrossRef]
- Corona-Hernandez, R.I.; Álvarez-Parrilla, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; De la Rosa, L.A.; Wall Medrano, A. Structural stability and viability of microencapsulated probiotic bacteria: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Villeda, B.E.; Falfan Cortes, R.N.; Rangel Vargas, E.; Santos Lopez, E.M.; Gomez Aldapa, C.A.; Torres Vitela, M.R.; Villarruel Lopez, A.; Castro Rosas, J. Synbiotic encapsulation: A trend towards increasing viability and probiotic effect. J. Food Process. Preserv. 2023, 2023, 7057462. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Double layer co- encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization. LWT-Food Sci. Technol. 2019, 110, 102. [Google Scholar] [CrossRef]
- Yoha, K.; Anukiruthika, T.; Anila, W.; Moses, J.; Anandharamakrishnan, C. 3D printing of encapsulated probiotics: Effect of different post-processing methods on the stability of Lactiplantibacillus plantarum (NCIM 2083) under static in vitro digestion condition. LWT 2021, 146, 111461. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667. [Google Scholar] [CrossRef]
- Untersmayr, E.; Jensen-Jarolim, E. The role of protein digestibility and antacids on food allergy outcomes. J. Allergy Clin. Immunol. 2008, 121, 1301–1308. [Google Scholar] [CrossRef]
- Kadiri, O. A Review on the Status of the Phenolic Compounds and Antioxidant Capacity of the Flour: Effects of Cereal Processing. Int. J. Food Prop. 2017, 20, S798–S809. [Google Scholar] [CrossRef]
- Melini, F.; Melin, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Hole, A.S.; Rud, I.; Grimmer, S.; Sigl, S.; Narvhus, J.; Sahlstrøm, S. Improved Bioavailability of Dietary Phenolic Acids in Whole Grain Barley and Oat Groat Following Fermentation with Probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 2012, 60, 6369–6375. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Polo, A.; Rizzello, C.G. The sourdough fermentation is the powerful process to exploit the potential of legumes, pseudo-cereals and milling by-products in baking industry. Crit. Rev. Food. Sci. Nutr. 2020, 60, 2158–2173. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sour dough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef]
- Manini, F.; Casiraghi, M.C.; Poutanen, K.; Brasca, M.; Erba, D.; Plumed-Ferrer, C. Characterization of lactic acid bacteria isolated from wheat bran sourdough. LWT-Food Sci. Technol. 2016, 66, 275–283. [Google Scholar] [CrossRef]
- De Angelis, M.; Coda, R.; Silano, M.; Minervini, F.; Rizzello, C.G.; Di Cagno, R.; Vicentini, O.; De Vincenzi, M.; Gobbetti, M. Fermentation by selected sourdough lactic acid bacteria to decrease coeliac intolerance to rye flour. J. Cereal Sci. 2006, 43, 301–314. [Google Scholar] [CrossRef]
- Abdelshafy, A.; Mustafa, M.; Mohamed, H.; Al-Asmari, F. Probiotic-fermentation of oat: Safety, strategies for improving quality, potential food applications and biological activities. Trends Food Sci. Technol. 2024, 151, 104640. [Google Scholar] [CrossRef]
- Nascimento, S.; Passos, T.; de Sousa Júnior, F.C. Probiotics in plant-based food matrices: A review of their health benefits. PharmaNutrition. 2024, 28, 100390. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 121667. [Google Scholar] [CrossRef]
- Di Cagno, R.; Surico, R.F.; Siragusa, S.; De Angelis, M.; Paradiso, A.; Minervini, F.; De Gara, L.; Gobbetti, M. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, french beans or marrows. Int. J. Food Microbiol. 2008, 127, 220–228. [Google Scholar] [CrossRef]
- Wolkers-Rooijackers, J.C.M.; Endika, M.F.; Smid, E.J. Enhancing Vitamin B12 in Lupin Tempeh by in situ Fortification. LWT 2018, 96, 513–518. [Google Scholar] [CrossRef]
- Sionek, B.; Gantner, M. Probiotics and Paraprobiotics—New Proposal for Functional Food. Appl. Sci. 2025, 15, 366. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.F.; Sohail, A.; Ghazanfar, S.; Ahmad, A.; Riaz, A.; Abbasi, K.S.; Ibrahim, M.S.; Uzair, M.; Arshad, M. Recent Innovations in Non-dairy Prebiotics and Probiotics: Physiological Potential, Applications, and Characterization. Probiotics Antimicrob. Proteins 2023, 15, 239–263. [Google Scholar] [CrossRef]
- Chan, M.; Shao-Quan, L. Coffee brews as food matrices for delivering probiotics: Opportunities, challenges, and potential health benefits. Trends Food Sci. Technol. 2022, 119, 227–242. [Google Scholar] [CrossRef]
- Mureşan, C.C.; Marc, R.A.; Jimborean, M.; Rusu, I.; Mureşan, A.; Nistor, A.; Cozma, A.; Suharoschi, R. Food Safety System (HACCP) as Quality Checkpoints in a Spin-Off Small-Scale Yogurt Processing Plant. Sustainability 2020, 12, 9472. [Google Scholar] [CrossRef]
- ISO 22000:2018; Food Safety Management Systems—Requirements for Any Organization in the Food Chain. International Organization for Standardization with Technical Committee: Geneva, Switzerland, 2018.
- Panghal, A.; Chhikara, N.; Sindhu, N.; Jaglan, S. Role of Food Safety Management Systems in safe food production: A review. J. Food Saf. 2018, 38, e12464. [Google Scholar] [CrossRef]
- Sebnem, O.A. Model for implementation of HACCP system for prevention and control of mycotoxins during the production of red dried chili pepper. Food Sci. Technol. 2017, 37, 124–129. [Google Scholar]
- Dey, T.K.; Lindahl, J.F.; Sanjukta, R.; Arun Prince Milton, A.; Das, S.; Kannan, P.; Lundkvist, Å.; Sen, A.; Ghatak, S. Characterization of Lactic Acid Bacteria and Pathogens Isolated from Traditionally Fermented Foods, in Relation to Food Safety and Antimicrobial Resistance in Tribal Hill Areas of Northeast India. J. Food Qual. 2023, 2023, 6687015. [Google Scholar] [CrossRef]
- Awuchi, C.G. HACCP, Quality, and Food Safety Management in Food and Agricultural Systems. Cogent Food Agric. 2023, 9, 2176280. [Google Scholar] [CrossRef]
- Azizi, N.; Rajah Kumar, M.; Yeap, S.K.; Ong Abdullah, J.; Khalid, M.; Omar, A.; Osman, M.; Syed, S.; Alitheen, N. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Aslani, R.; Mazaheri, Y.; Jafari, M.; Sadighara, P.; Molaee-aghaee, E.; Özçakmak, S.; Reshadat, Z. Implementation of Hazard Analysis and Critical Control Point (HACCP) in Yogurt Production. J. Dairy Res. 2024, 91, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska, A.; Zielińska, D.; Kołożyn-Krajewska, D. Effect of Pumpkin Cultivar on the Selected Quality Parameters of Functional Non-Dairy Frozen Desserts. Appl. Sci. 2022, 12, 8063. [Google Scholar] [CrossRef]
- Multari, S.; Carafa, I.; Barp, L.; Caruso, M.; Licciardello, C.; Larcher, R.; Tuohy, K.; Martens, S. Effects of Lactobacillus spp. on the phytochemical composition of juices from two varieties of Citrus sinensis L. Osbeck: ‘Tarocco’ and ‘Washington navel’. LWT 2020, 125, 109205. [Google Scholar]
- Waithera Kuria, M.; Wafula Matofarim, J.; Masani Nduko, J. Physicochemical, antioxidant, and sensory properties of functional mango (Mangifera indica L.) leather fermented by lactic acid bacteria. J. Agric. Food Res. 2021, 6, 100206. [Google Scholar]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołożyn-Krajewska, D. Comparison of Antibacterial Activity of Lactobacillus plantarum Strains Isolated from Two Different Kinds of Regional Cheeses from Poland: Oscypek and Korycinski Cheese. BioMed Res. Int. 2017, 2017, 6820369. [Google Scholar] [CrossRef]
- Zielińska, D.; Kolożyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. BioMed Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Selected probiotic lactic acid bacteria isolated from fermented foods for functional milk production: Lower cholesterol with more beneficial compounds. LWT 2020, 135, 110061. [Google Scholar] [CrossRef]
- El-Razik, M.M.A.; Gadallah, M.G.E.; Hassan, M.F.Y. Application of Hazard Analysis Critical Control Points System (HACCP) During Production of Tarhana. Curr. J. Appl. Sci. Technol. 2018, 29, 1–13. [Google Scholar] [CrossRef]
- El-Razik, M.M.A.; Hassan, M.F.Y.; Gadallah, M.G.E. Implementation of HACCP Plan for the Production of Egyptian Kishk (A Traditional Fermented Cereal-Milk Mixture). Food Nutr. Sci. 2016, 7, 1262–1275. [Google Scholar] [CrossRef][Green Version]
- Rodrigues Junior, U.J.; Dziedzic, M. The water footprint of beef cattle in the amazon region, Brazil. Ciênc. Rural 2021, 51, 20190294. [Google Scholar] [CrossRef]
- Polyak, E.; Breitenbach, Z.; Frank, E.; Mate, O.; Figler, M.; Zsalig, D.; Simon, K.; Szijarto, M.; Szabo, Z. Food and sustainability: Is it a matter of choice? Sustainability 2023, 15, 7191. [Google Scholar] [CrossRef]
- Company, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Silva, L.R.; Rivera, L.P.; Marcos-García, M.; García-Fraile, P.; Martínez-Molina, E.; Mateos, P.F.; Velázquez, E.; Andrade, P.; Rivas, R. Plants Probiotics as a Tool to Produce Highly Functional Fruits: The Case of Phyllobacterium and Vitamin C in Strawberries. PLoS ONE 2015, 10, e0122281. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; Celador-Lera, L.; Fradejas-Bayón, M.; Rivas, R. Plant probiotic bacteria enhance the quality of fruit and horticultural crops. AIMS Microbiol. 2017, 19, 483–501. [Google Scholar] [CrossRef]
- Background|Technical Platform on the Measurement and Reduction of Food Loss and Waste|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/platform-food-loss-waste/background/en (accessed on 20 January 2025).
| Product | Applied Probiotic | Applied Prebiotic | Research Issue | Reference |
|---|---|---|---|---|
| Pumpkin frozen desserts | L. rhamnosus Lock 0900 | Inulin | Pumpkin and pumpkin–pineapple sorbets with potential probiotic strains and/or prebiotics demonstrated encouraging results in the case of functional product development data | [54] |
| Blueberry juice | L. plantarum and L. casei | - | Positive effect of lactic acid fermentation on phenolic content | [55] |
| Jelly candies with enzymatically modified apple pomace | Bifidobacterium animalis DSM 20105 | - | Evaluation of the applicational possibilities of enzymatically modified apple pomace in jelly candies with probiotics | [56] |
| Rice-based beverages | L. fermentum MG7011 | - | Choice of probiotic starter culture data | [57] |
| Fermented beverages prepared with almonds (Prunus dulcis), rice (Oryza sativa L.), oats (Avena sativa L.), Brazil nuts (Bertholletia excelsa H.B.K), and soybean (Glycine max L.) extracts | Streptococcus thermophilus, L. acidophilus LA-5®, and Bifidobacterium BB-12 | - | Possibilities complete substitution of dairy ingredients with water-soluble plant extract | [58] |
| Frozen dessert containing plant-based milk (almond, hazelnut, lupine) | Lb. acidophilus | - | Positive impact of plant-based milk on probiotics; viability due to the high phenolic components and antioxidant capacity | [59] |
| Frozen desserts processed with water-soluble extract of rice by-product and S. platensis | Lb. casei | Polydextrose | Probiotic and/or synbiotic products have an impact on final functional products such as the chemical composition, phenolic compounds’ bioaccessibility, selected parameters of texture, improved sensory quality, ⍺-amylase, and ⍺-glucosidase inhibitory activities | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sionek, B.; Szydłowska, A. Probiotics and Prebiotics in the Aspect of Health Benefits and the Development of Novel Plant-Based Functional Food. Appl. Sci. 2025, 15, 3137. https://doi.org/10.3390/app15063137
Sionek B, Szydłowska A. Probiotics and Prebiotics in the Aspect of Health Benefits and the Development of Novel Plant-Based Functional Food. Applied Sciences. 2025; 15(6):3137. https://doi.org/10.3390/app15063137
Chicago/Turabian StyleSionek, Barbara, and Aleksandra Szydłowska. 2025. "Probiotics and Prebiotics in the Aspect of Health Benefits and the Development of Novel Plant-Based Functional Food" Applied Sciences 15, no. 6: 3137. https://doi.org/10.3390/app15063137
APA StyleSionek, B., & Szydłowska, A. (2025). Probiotics and Prebiotics in the Aspect of Health Benefits and the Development of Novel Plant-Based Functional Food. Applied Sciences, 15(6), 3137. https://doi.org/10.3390/app15063137







