The Influence of Mineral Matter on X-Ray Photoelectron Spectroscopy Characterization of Surface Oxides on Carbon
Abstract
1. Introduction
- Carboxen has been selected as an essentially ash-free reference carbon. It is an ideal model carbon to ensure the reliable peak assignment of XPS carbon–oxygen functionalities, ruling out interference due to the presence of inorganics.
- Char from a sub-bituminous coal, characterized by a large content of both inertinite and vitrinite macerals, has been investigated as a sample of an ash-bearing carbon.
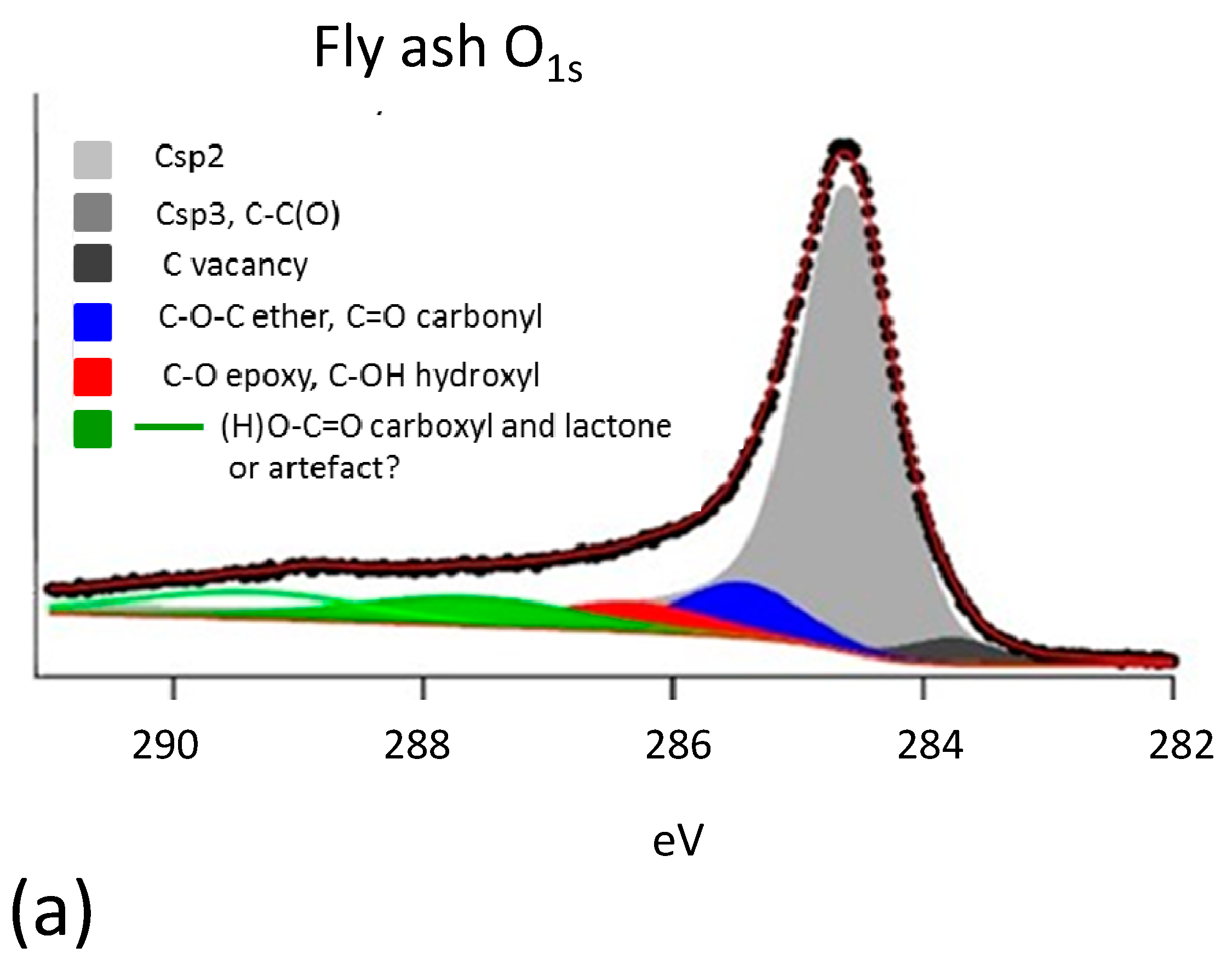
- Fly ash from entrained flow combustion of the same sub-bituminous coal has been selected as an ideal sample of ash-enriched carbon. The sample contains 68.0% carbon and 15.7% ash. The latter is predominantly constituted by an amorphous alumina silica glass phase (62.1%) and by two crystalline phases, mullite (31.8%) and quartz (6.1%).
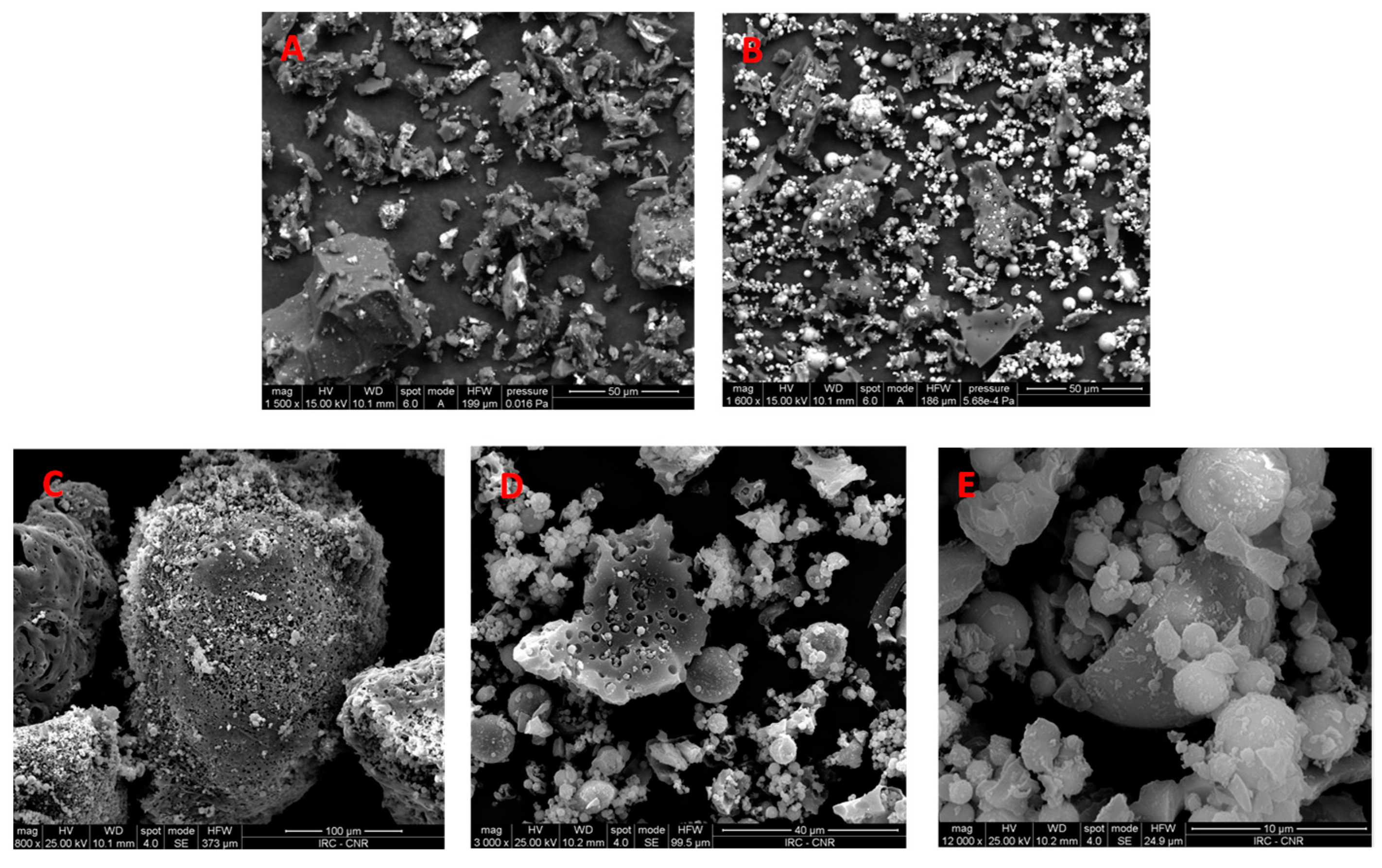
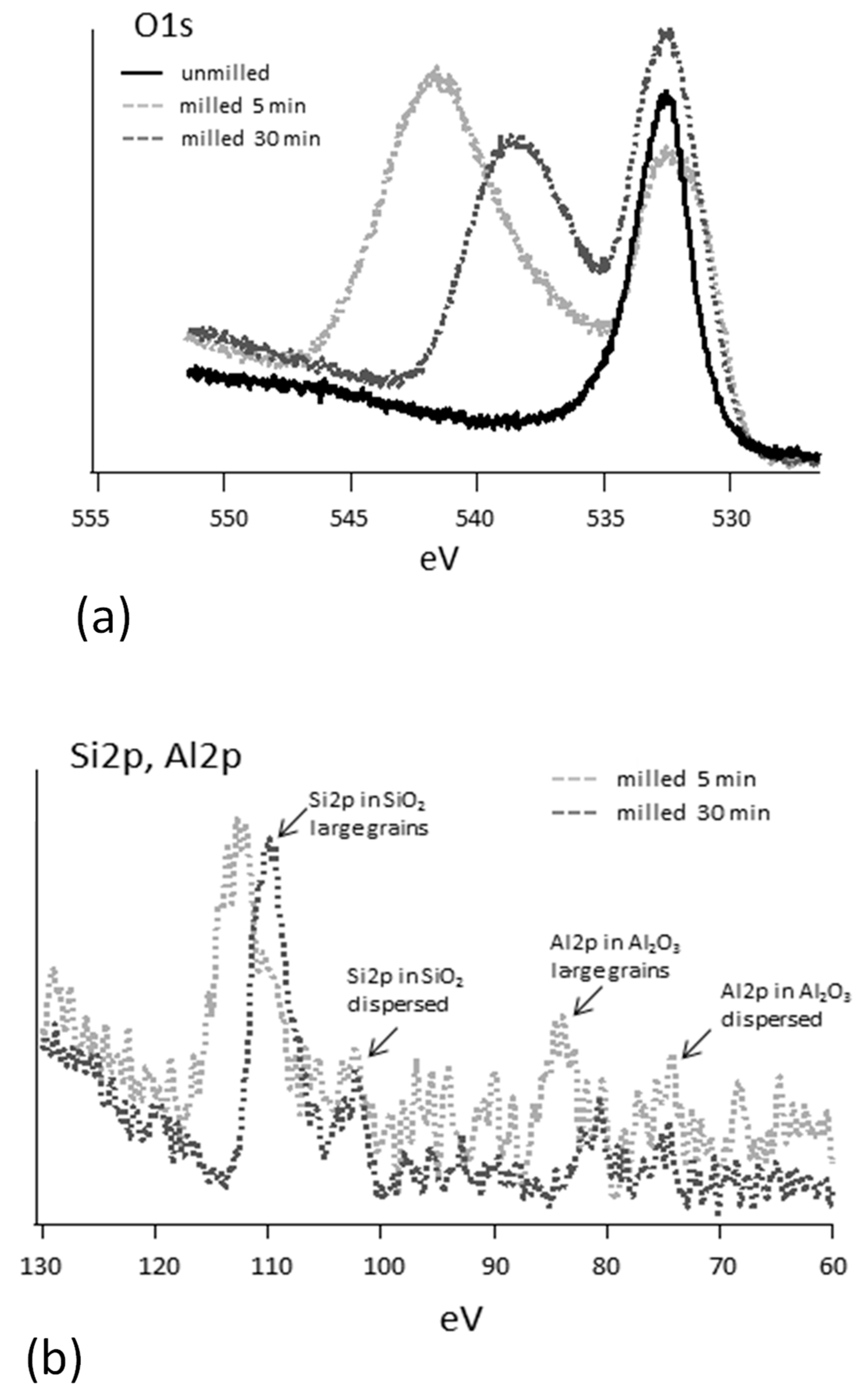
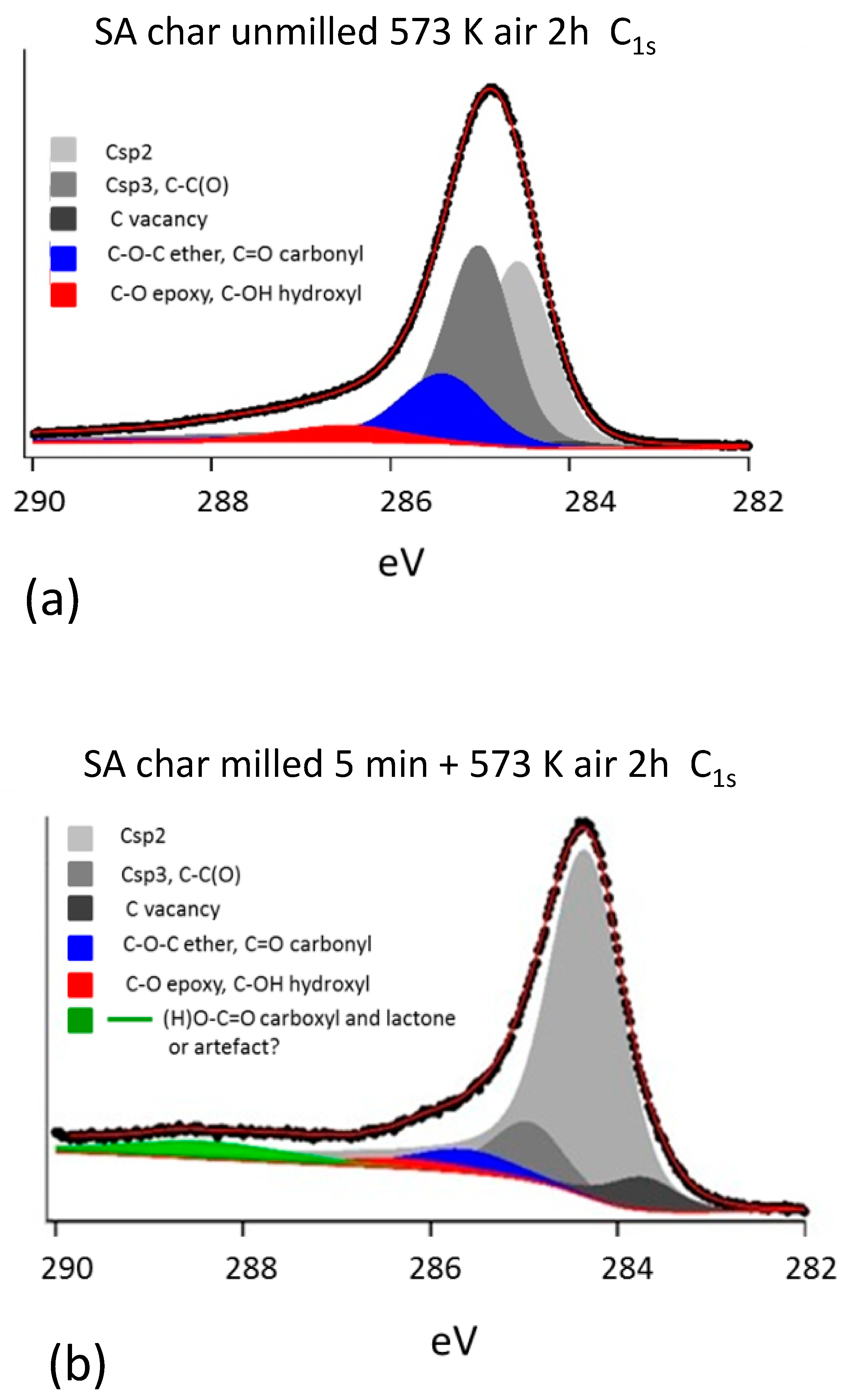
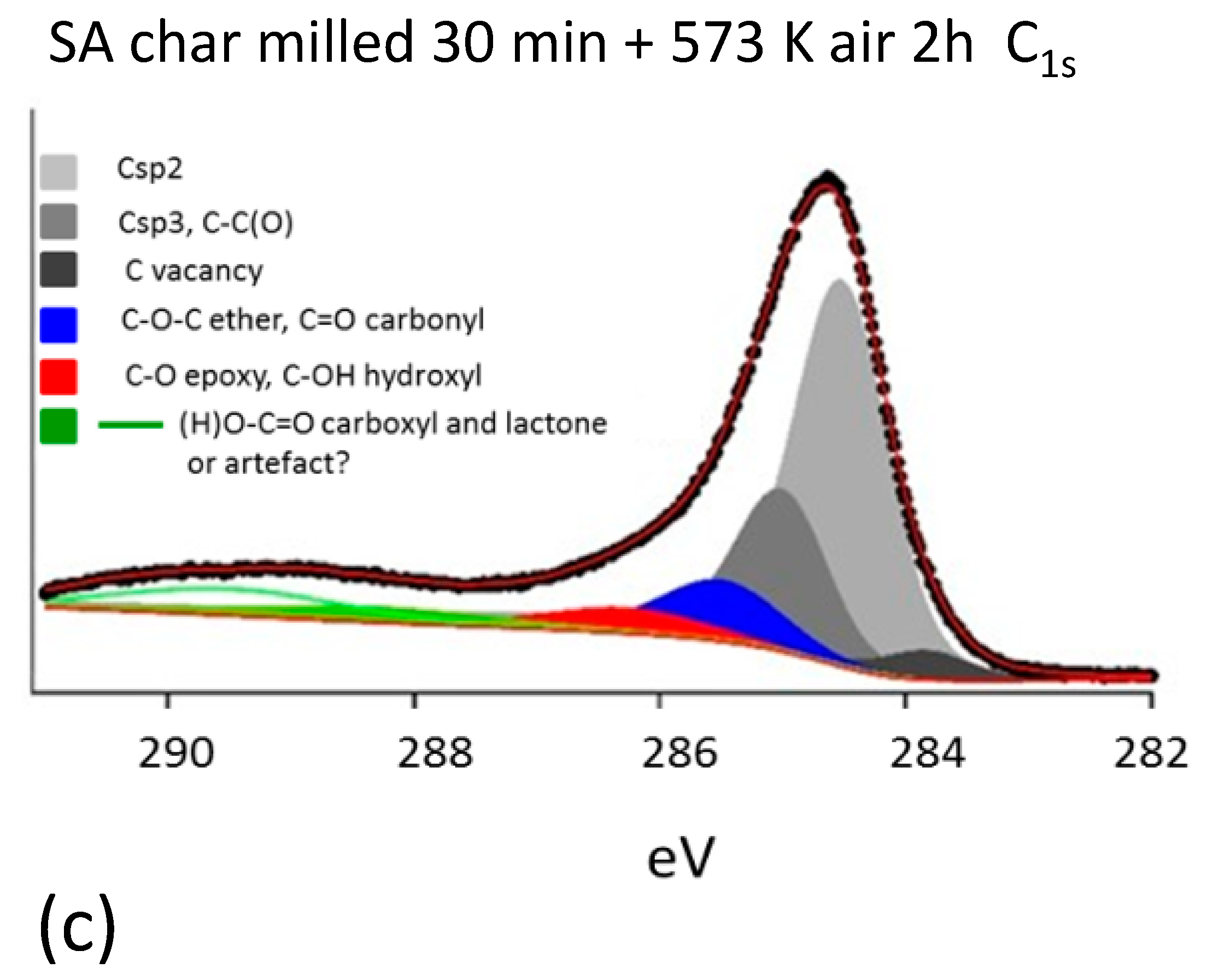
- Milled char samples have been investigated within the scope of elucidating how the exposure of metal oxides from the bulk to the surface of carbon, which is indeed enhanced by grinding, affects the characterization of the sample surfaces by XPS.
2. Materials and Methods
3. Results
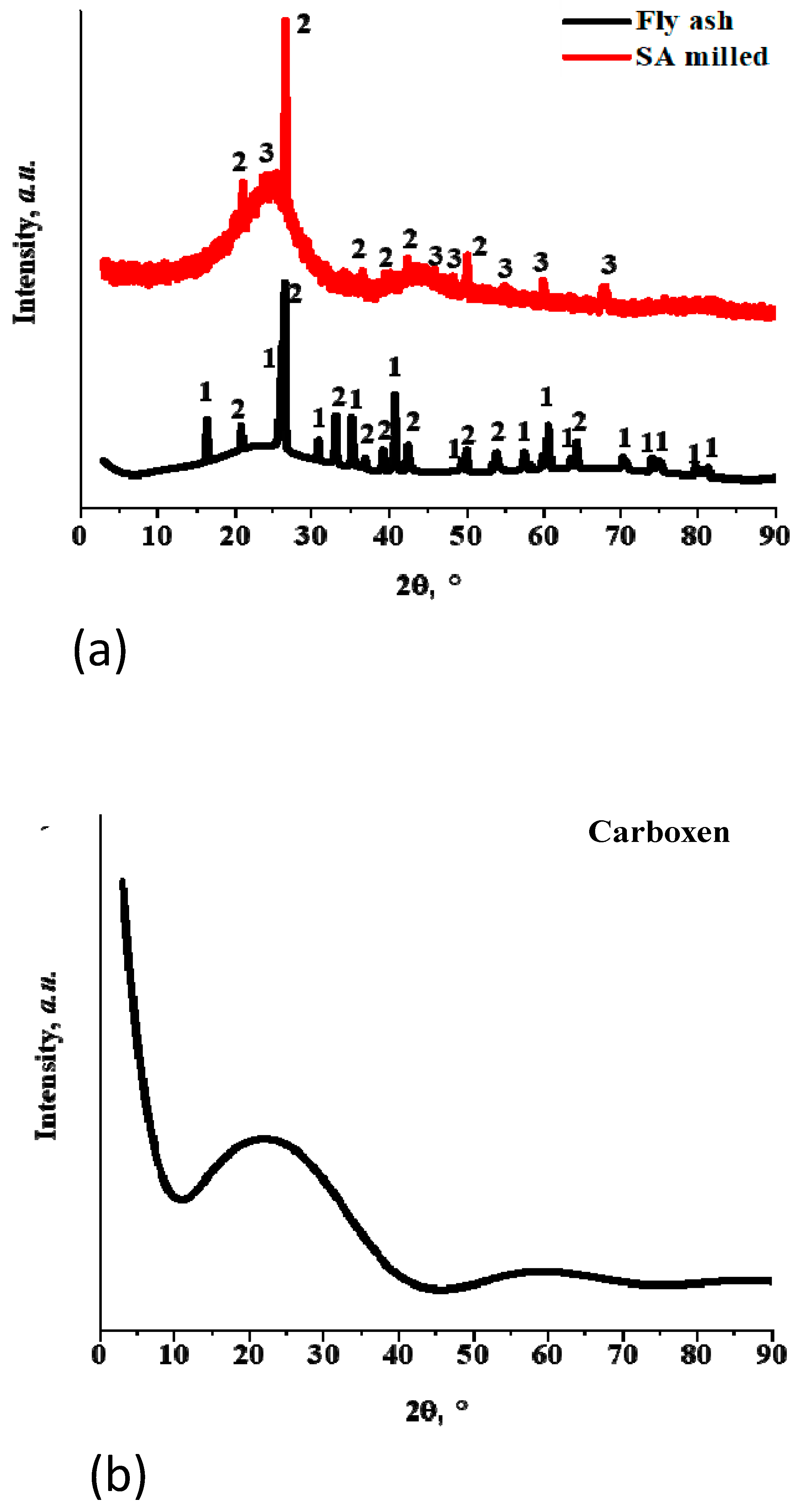
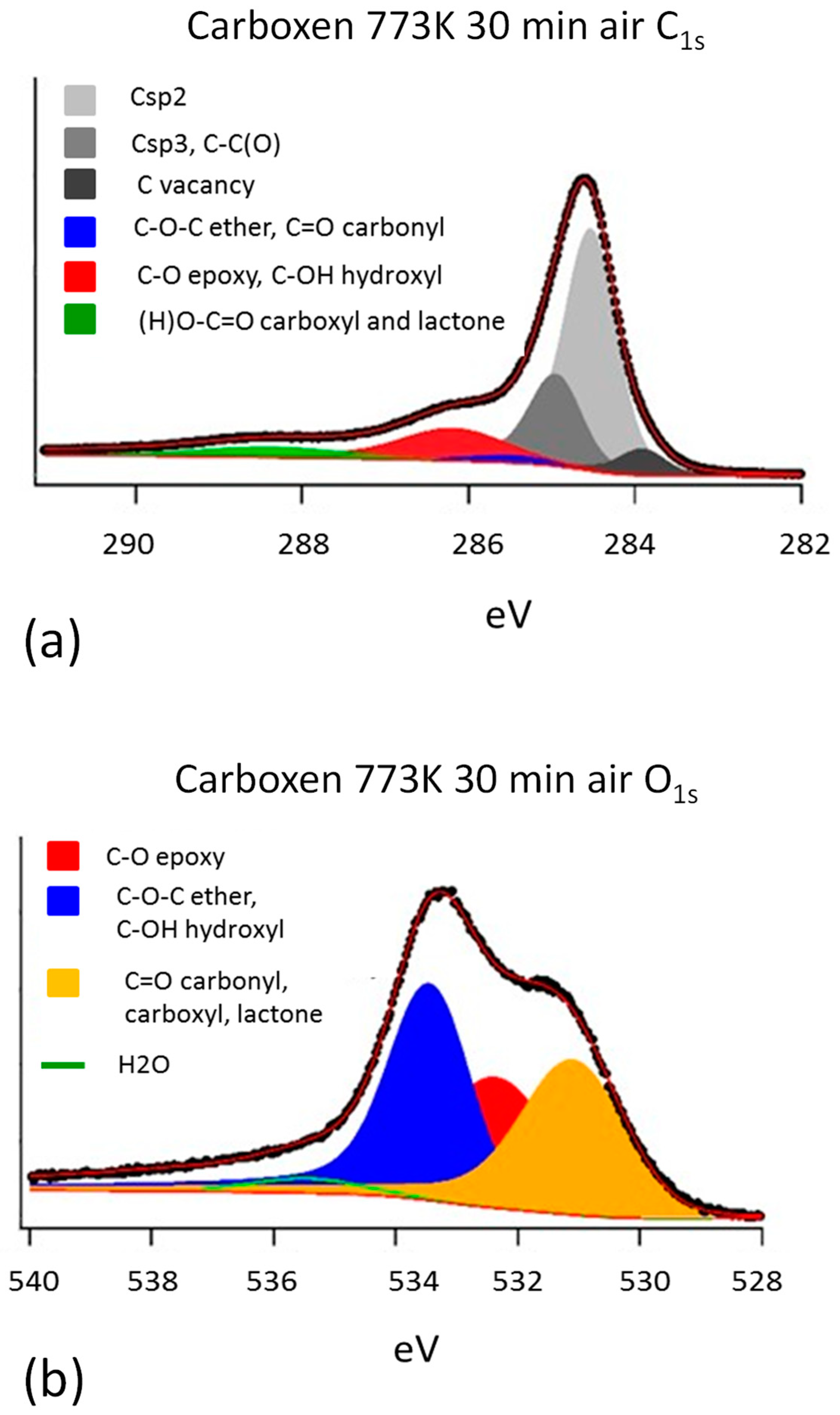
3.1. Characterization of Carboxen
3.2. Characterization of Milled and Unmilled Oxidized Coal Char Samples
- A weaker intensity at the binding energy at which the signals from silica and alumina are expected (about 103–104 eV for Si2p and about 74–75 eV for Al2p [57]) that can be assigned to small mineral particles dispersed within the organic carbonaceous matrix that experiences weak or no charging effects.
- A more intense signal shifted by 6–10 eV towards higher binding energy that can be due to larger inorganic grains where photo-induced charging effects are stronger.
3.3. Characterization of Residual Carbon in Fly Ash
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| EDX Analysis | Char Sample | Char Oxidized and Milled | Ash | Ash Milled (Char Particles) | Ash Milled, Ash Particles |
|---|---|---|---|---|---|
| wt% | |||||
| C | 91.1 | 74 | 61.60 | 91.34 | 17.38 |
| O | 7.8 | 20.7 | 21.09 | 5.63 | 42.41 |
| Na | n.d. | 0.3 | 0.54 | 0.32 | 1.92 |
| Al | 0.3 | 1.3 | 4.41 | 0.90 | 13.19 |
| Si | 0.3 | 2.1 | 5.12 | 0.82 | 17.45 |
| K | n.d. | 0.2 | 0.93 | 0.17 | 2.04 |
| Ca | 0.1 | 0.2 | 1.42 | 0.15 | 0.71 |
| Fe | n.d. | 0.6 | 4.5 | 0.59 | 4.3 |
Appendix B
| Sample | Heat Treatment Air | C sp2 | C sp3, C-C(O) | C Vacancy | |||
|---|---|---|---|---|---|---|---|
| Intensity (a.u.) | BE (eV) | Intensity (a.u.) | BE (eV) | Intensity (a.u.) | BE (eV) | ||
| Fly ash | 2554.42 | 284.57 | 152.62 | 284.99 | 130.76 | 283.72 | |
| Carboxen | 773 K 30 min | 3191.41 | 284.51 | 1221.02 | 284.93 | 317.31 | 283.89 |
| SA unmilled | 573 K 2 h | 2267.22 | 284.54 | 2508.19 | 284.98 | 52.87 | 283.94 |
| SA milled 5 min | 573 K 2 h | 1884.81 | 284.31 | 305.76 | 284.92 | 166.67 | 283.69 |
| SA milled 30 min | 573 K 2 h | 2114.01 | 284.49 | 867.34 | 284.98 | 147.69 | 283.8 |
| Sample | Heat Treatment Air | Ether, Carbonyl | Epoxy, Hydroxyl | Carboxyl, Lacton | Artefact | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity (a.u.) | BE (eV) | Intensity (a.u.) | Intensity (a.u.) | Intensity (a.u.) | BE (eV) | Intensity (a.u.) | BE (eV) | ||
| Carboxen | 129.94 | 285.44 | 778.71 | 286.12 | 305.86 | 288.29 | |||
| Fly ash | 773 K 30 min | 362.05 | 285.41 | 250.86 | 286.28 | 338.49 | 288.14 | 316.32 | 289.27 |
| SA unmilled | 573 K 2 h | 452.16 | 285.46 | 319.06 | 286.26 | ||||
| SA milled 5 min | 573 K 2 h | 160.21 | 285.61 | 72.199 | 286.19 | 184.97 | 288.31 | ||
| SA milled 30 min | 573 K 2 h | 414.18 | 285.46 | 211.44 | 286.21 | 153.32 | 288.19 | 317.58 | 289.39 |
| Sample | Heat Treatment Air | Epoxy | Ether, Hydroxyl | Carbonyl, Carboxyl, Lacton | Water Adsorb | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity (a.u.) | BE (eV) | Intensity (a.u.) | BE (eV) | Intensity (a.u.) | BE (eV) | Intensity (a.u.) | BE (eV) | ||
| Carboxen | 773 K 30 min | 1137.61 | 532.28 | 1435.52 | 533.39 | 1256.81 | 531.02 | 129.98 | 535.37 |
References
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energ. Rev. 2018, 82, 1394–1414. [Google Scholar] [CrossRef]
- Faraji, S.; Nasir Ani, F. The development supercapacitor from activated carbon by electroless plating—A review. Renew. Sustain. Energ. Rev. 2015, 42, 823–834. [Google Scholar] [CrossRef]
- Najafabadi, A.T. Emerging applications of graphene and its derivatives in carbon capture and conversion: Current status and future prospects. Renew. Sustain. Energ. Rev. 2015, 41, 1515–1545. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energ. Rev. 2018, 82, 713–733. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Huang, H.; Xuan, J.; Wang, H.; Leung, D.Y.C. Graphene materials in green energy applications: Recent development and future perspective. Renew. Sustain. Energ. Rev. 2020, 120, 109656. [Google Scholar] [CrossRef]
- Levi, G.; Causà, M.; Lacovig, P.; Salatino, P.; Senneca, O. Mechanism and thermochemistry of coal char oxidation and desorption of surface oxides. Energy Fuels 2017, 31, 2308–2316. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. 1972, 5, 4709. [Google Scholar] [CrossRef]
- Barinov, A.; Malcioglu, O.B.; Fabris, S.; Sun, T.; Gregoratti, L.; Dalmiglio, M.; Kiskinova, M. Initial Stages of Oxidation on Graphitic Surfaces: Photoemission Study and Density Functional Theory Calculations. J. Phys. Chem. 2009, 113, 9009–9013. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. Investigation of changes in surface properties of bituminous coal during natural weathering processes by XPS and SEM. Appl. Surf. Sci. 2014, 293, 293–298. [Google Scholar] [CrossRef]
- Gonga, B.; Pigramb, P.J.; Lamba, R.N. Surface studies of low-temperature oxidation of bituminous coal vitrain bands using XPS and SIMS. Fuel 1998, 77, 1081–1087. [Google Scholar] [CrossRef]
- Yamada, Y.; Yasuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T.; Sato, S. Analysis of heat-treated graphite oxide by X-ray photoelectron spectroscopy. J. Mater. Sci. 2013, 48, 8171–8198. [Google Scholar] [CrossRef]
- Zhang, W.; Carravetta, V.; Li, Z.; Luo, Y.; Yang, J. Oxidation States of Graphene: Insights from Computational Spectroscopy. J. Chem. Phys. 2009, 131, 244505. [Google Scholar] [CrossRef]
- Kim, S.; Zhou, S.; Hu, Y.; Acik, M.; Chabal, Y.J.; Berger, C.; de Heer, W.; Bongiorno, A.; Riedo, E. Room-temperature metastability of multilayer graphene oxide films. Nat. Mater. 2012, 11, 544. [Google Scholar] [CrossRef]
- Levi, G.; Senneca, O.; Causà, M.; Salatino, P.; Lacovig, P.; Lizzit, S. Probing the chemical nature of surface oxides during coal char oxidation by high-resolution XPS. Carbon 2015, 90, 181–196. [Google Scholar] [CrossRef]
- Doniach, S.; Šunjić, M. Many-electron singularity in X-ray photoemission and X-ray line spectra from metals. J. Phys. C Solid State Phys. 1970, 3, 285–291. [Google Scholar] [CrossRef]
- Cheung, T.T.P. X-ray photoemission of carbon: Lineshape analysis and application to studies of coals. J. Appl. Phys. 1982, 53, 6857–6862. [Google Scholar] [CrossRef]
- Haerle, R.; Riedo, E.; Pasquarello, A.; Baldereschi, A. Sp2/Sp3 hybridization ratio in amorphous carbon from C 1s core-level shifts: X-ray photoelectron spectroscopy and first-principles calculation. Phys. Rev. 2001, 65, 045101. [Google Scholar] [CrossRef]
- Cloke, M.; Gilfillan, A.; Lester, E.H. The characterization of coals and density separated coal fractions using FTIR and manual and automated petrographic analysis. Fuel 1997, 76, 1289–1296. [Google Scholar] [CrossRef]
- Gilfillan, A.; Lester, E.H.; Cloke, M.; Snape, C.E. The structure and reactivity of density separated coal fractions. Fuel 1999, 78, 1639–1644. [Google Scholar] [CrossRef]
- Manoj, B.; Narayanan, P. Study of Changes to the Organic Functional Groups of a High Volatile Bituminous Coal during Organic Acid Treatment Process by FTIR Spectroscopy. J. Min. Mater. Charact. Eng. 2013, 1, 39–43. [Google Scholar] [CrossRef]
- Christoph Schneider, C.; Sonia Rincón Prat, S.; Thomas Kolb, T. Determination of active sites during gasification of biomass char with CO2 using temperature-programmed desorption. Part 1: Methodology & desorption spectra. Fuel 2020, 267, 116726. [Google Scholar]
- Radovic, L.R. Active Sites in Graphene and the Mechanism of CO2 Formation in Carbon Oxidation. J. Am. Chem. Soc. 2009, 131, 17166–17175. [Google Scholar] [CrossRef] [PubMed]
- Rincón Prat, S.; Schneider, C.; Kolb, T. Determination of active sites during gasification of biomass char with CO2 using temperature-programmed desorption. Part 2: Influence of ash components. Fuel 2020, 267, 117179. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Mondragon, F. Role of the Epoxy Group in the Heterogeneous CO2 Evolution in Carbon Oxidation Reactions. J. Phys. Chem. C 2007, 111, 612–617. [Google Scholar] [CrossRef]
- Hall, P.J.; Calo, J.M. Secondary interactions upon thermal desorption of surface oxides from coal chars. Energy Fuels 1989, 3, 370–376. [Google Scholar] [CrossRef]
- Zhuang, Q.; Kyotani, T.; Tomita, A. Dynamics of Surface Oxygen Complexes during Carbon Gasification with Oxygen. Energy Fuels 1995, 9, 630–634. [Google Scholar] [CrossRef]
- Montoya, A.; Truong, T.T.T.; Mondragon, F.; Truong, T.N. CO Desorption from Oxygen Species on Carbonaceous Surface: 1. Effects of the Local Structure of the Active Site and the Surface Coverage. J. Phys. Chem. A 2001, 105, 6757–6764. [Google Scholar] [CrossRef]
- Senneca, O.; Salatino, P.; Sorrenti, R. Beneficiation of coal fly ash by oxygen chemisorption. Exp. Therm. Fluid Sci. 2012, 43, 76–81. [Google Scholar] [CrossRef]
- Orrego-Ruiz, J.A.; Hernandez, R.C.; Mejia-Ospino, E. Structural Study of Colombian Coal by Fourier Transform Infrared Spectroscopy Coupled to Attenuated Total Reflectance (FTIR-ATR). Rev. Mex. Física 2010, 56, 251–254. [Google Scholar]
- Mielczarski, J.A.; Deńca, A.; Strojek, J.W. Application of Attenuated Total Reflection Infrared Spectroscopy to the Characterization of Coal. Appl. Spectrosc. 1986, 40, 998–1004. [Google Scholar] [CrossRef]
- Danlami Musa, D.; Zhonghua, T.; Ibrahim, A.O.; Habib, M. China’s energy status: A critical look at fossils and renewable options. Renew. Sustain. Energ. Rev. 2018, 81, 2281–2290. [Google Scholar] [CrossRef]
- Cerciello, F.; Senneca, O.; Coppola, A.; Lacovig, P.; Forgione, A.; Salatino, P. The influence of temperature on the nature and stability of surface-oxides formed by oxidation of char. Renew. Sustain. Energ. Rev. 2021, 137, 110595. [Google Scholar] [CrossRef]
- Cerciello, F.; Coppola, A.; Lacovig, P.; Senneca, O.; Salatino, P. Characterization of surface-oxides on char under periodically changing oxidation/desorption conditions. Renew. Sustain. Energy Rev. 2021, 137, 110453. [Google Scholar] [CrossRef]
- Cros, A. Charging effects in X-ray photoelectron spectroscopy. J. Electron Spectros. Relat. Phenom. 1992, 59, 1–14. [Google Scholar] [CrossRef]
- Vereecke, G.; Paul, G.R. Surface Charging of Insulating Samples in X-ray Photoelectron Spectroscopy. Surf. Interface Anal. 1998, 26, 490–497. [Google Scholar] [CrossRef]
- Cabaniss, G.E.; Linton, R.W. Characterization of Surface Species on Coal Combustion Particles by X-ray Photoelectron Spectroscopy in Concert with Ion Sputtering and Thermal Desorption. Environ. Sci. Technol. 1984, 18, 271–275. [Google Scholar] [CrossRef]
- Sezen, H.; Suzer, S. XPS for chemical- and charge-sensitive analyses. Thin Solid Film. 2013, 534, 1–11. [Google Scholar] [CrossRef]
- Metson, J.B. Charge compensation and binding energy referencing in XPS analysis. Surf. Interface Anal. 1999, 27, 1069–1072. [Google Scholar] [CrossRef]
- Baer, D.R.; Engelhard, M.H.; Gaspar, D.J.; Lea, S.; Windisch, C.F. Use and limitations of electron flood gun control of surface potential during XPS: Two non-homogeneous sample types. Surf. Interface Anal. 2002, 33, 781–790. [Google Scholar] [CrossRef]
- Wagner, C.D. Studies of the charging of insulators in ESCA. J. Electron Spectros. Relat. Phenom. 1980, 18, 345–349. [Google Scholar] [CrossRef]
- Cazaux, J. About the charge compensation of insulating samples in XPS. J. Electron Spectros. Relat. Phenom. 2000, 113, 15–33. [Google Scholar] [CrossRef]
- Greczynski, G.; Lars Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Kelly, M.A. Analysing Insulators with XPS and AES. In Surface Analysis by Auger and X-Ray Photoelectron Spectroscopy; Briggs, D., Grant, J.T., Eds.; IM Publications: Chichester, UK; SurfaceSpectra: Manchester, UK, 2003; pp. 191–210. [Google Scholar]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Li, N.; Rao, F.; He, L.; Yang, S.; Bao, Y.; Huang, C.; Bao, M.; Chen, Y. Evaluation of biochar properties exposing to solar radiation: A promotion on surface activities. Chem. Eng. J. 2020, 384, 123353. [Google Scholar] [CrossRef]
- Baer, D.R.; Artyushkova, K.; Cohen, H.; Easton, C.D.; Engelhard, M.; Gengenbach, T.R.; Greczynski, G.; Mack, P.; Morgan, D.J.; Roberts, A. XPS guide: Charge neutralization and binding energy referencing for insulating samples. J. Vac. Sci. Technol. A 2020, 38, 031204. [Google Scholar] [CrossRef]
- Gorham, J.M.; Osborn, W.A.; Woodcock, J.W.; Scott, K.C.K.; Heddleston, J.M.; Walker, A.R.; Gilman, J.W. Detecting Carbon in Carbon: Exploiting Differential Charging to Obtain Information on the Chemical Identity and Spatial Location of Carbon Nanotube Aggregates in Composites by Imaging X-ray Photoelectron Spectroscopy. Carbon 2016, 96, 1208–1216. [Google Scholar] [CrossRef][Green Version]
- Greczynski, G.; Hultman, L. Binding energy referencing in X-ray photoelectron spectroscopy. Nat. Rev. Mater. 2025, 10, 62–78. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Referencing to adventitious carbon in X-ray photoelectron spectroscopy: Can differential charging explain C 1s peak shifts? Appl. Surf. Sci. 2022, 606, 154855. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The same chemical state of carbon gives rise to two peaks in X ray photoelectron spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef] [PubMed]
- Levi, G.; Causà, M.; Cortese, L.; Salatino, P.; Senneca, O. On how mild oxidation affects the structure of carbons: Comparative analysis by different techniques. Appl. Energy Combust. Sci. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Senneca, O.; Salatino, P.; Chirone, R.; Cortese, L.; Solimene, R. Mechanochemical activation of high-carbon fly ash for enhanced carbon reburning. Proc. Comb. Inst. 2011, 33, 2743–2753. [Google Scholar] [CrossRef]
- Fabozzi, A.; Cerciello, F.; Senneca, O. Direct reduction of iron ore using biomass biochar: Reduction rate, microstructural and morphological analysis. Fuel 2025, 383, 133976. [Google Scholar] [CrossRef]
- Jitianu, A.; Cacciaguerra, T.; Benoit, R.; Delpeux, S.; Beguin, F.; Bonnamy, S. Synthesis and characterization of carbon nanotubes–TiO2 nanocomposites. Carbon 2004, 42, 1147–1151. [Google Scholar] [CrossRef]
- Available online: https://srdata.nist.gov/xps/ (accessed on 12 March 2023).


| Ash a | Volatiles a | C a | H a | N a |
|---|---|---|---|---|
| (wt%) | (wt%) | (wt%) | (wt%) | (wt%) |
| 3.0 | 5.0 | 92.0 | 0.7 | 0.1 |
| Ash a | Volatiles a | Cfix a | C a | H a | N a | S a | Vitrinite Rr | |
|---|---|---|---|---|---|---|---|---|
| (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (wt%) | (%) | |
| Coal | 15.7 | 23.1 | 61.2 | 68.0 | 3.8 | 1.2 | 0.6 | 0.72 |
| Char | 20.4 | 4.6 | 75.0 | 75.4 | 1.2 | 1.8 | n.d. | n.d. |
| Ash | SiO2 | 44.1 | Al2O2 | 34.0 | CaO | 8.1 | MgO | 2.2 |
| K2O | 0.62 | Na2O | 0.15 | FeO | 1.53 | MnO | 0.01 | |
| TiO2 | 1.41 | P2O5 | 2.35 | SO3 | 2.08 | Others | 3.45 |
| Sample | Heat Treatment Air |
|---|---|
| Fly ash | None |
| Carboxen | 773 K 30 min |
| SA unmilled | 573 K 2 h |
| SA milled 5 min | 573 K 2 h |
| SA milled 30 min | 573 K 2 h |
| C1s | C Sp2 | C Sp3 C-C(O) a | C Vacancy b | Ether, Carbonyl | Epoxy, Hydroxyl | Carboxyl, Lacton |
|---|---|---|---|---|---|---|
| 284.3–284.5 | 284.9–285.0 | 283.8–284.7 | 285.7–285.6 | 286.3–286.0 | 287.7–288.2 | |
| O1s | epoxy | ether, hydroxyl | carbonyl, carboxyl, lacton | |||
| 532.6–532.0 | 533.5–533.2 | 531.1–530.7 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerciello, F.; Forgione, A.; Lacovig, P.; Lizzit, S.; Fabozzi, A.; Salatino, P.; Senneca, O. The Influence of Mineral Matter on X-Ray Photoelectron Spectroscopy Characterization of Surface Oxides on Carbon. Appl. Sci. 2025, 15, 2993. https://doi.org/10.3390/app15062993
Cerciello F, Forgione A, Lacovig P, Lizzit S, Fabozzi A, Salatino P, Senneca O. The Influence of Mineral Matter on X-Ray Photoelectron Spectroscopy Characterization of Surface Oxides on Carbon. Applied Sciences. 2025; 15(6):2993. https://doi.org/10.3390/app15062993
Chicago/Turabian StyleCerciello, Francesca, Annunziata Forgione, Paolo Lacovig, Silvano Lizzit, Antonio Fabozzi, Piero Salatino, and Osvalda Senneca. 2025. "The Influence of Mineral Matter on X-Ray Photoelectron Spectroscopy Characterization of Surface Oxides on Carbon" Applied Sciences 15, no. 6: 2993. https://doi.org/10.3390/app15062993
APA StyleCerciello, F., Forgione, A., Lacovig, P., Lizzit, S., Fabozzi, A., Salatino, P., & Senneca, O. (2025). The Influence of Mineral Matter on X-Ray Photoelectron Spectroscopy Characterization of Surface Oxides on Carbon. Applied Sciences, 15(6), 2993. https://doi.org/10.3390/app15062993








