Featured Application
The findings of this study on the cytotoxic and antioxidant properties of kale and lupine sprouts, supplemented with γ-polyglutamic acid, suggest their promising applications in functional food development and agricultural practices. The bioactive properties observed, particularly the enhanced antioxidant activity in kale sprouts and the change in the content and composition of polyphenols in both species, highlight the potential of γ-PGA as a modifier of the health-promoting properties of sprouts. γ-PGA supplementation demonstrated its ability to influence sprout growth and metabolism, suggesting its role as an environmentally friendly additive (green agriculture) to enhance plant resilience and reduce the need for chemical inputs.
Abstract
γ-Polyglutamic acid (γ-PGA) is a biodegradable and non-toxic biopolymer with numerous potential applications in agriculture, food, and health sciences due to its antioxidant and antimicrobial properties. This study evaluated the cytotoxic and antioxidant properties of kale (Brassica oleracea var. sabellica) and lupine (Lupinus luteus) sprouts supplemented with different concentrations of γ-PGA. The sprouts were cultivated for various durations (6–10 days), and their bioactive compound profiles were analyzed using HPLC with a PDA 100 UV-VIS detector. Antioxidant activity was assessed via DPPH and FRAP assays, while cytotoxicity was tested against cancer and normal colon cell lines. Results demonstrated that kale sprouts had significantly higher antioxidant activity compared to lupine, with the highest levels observed in kale sprouts supplemented with 0.01% γ-PGA on day 8 (DPPH: 63.6 ± 0.4 μM TEAC/g dw; FRAP: 181.8 ± 2.1 μM/Fe2+/g dw). In contrast, supplementation of lupine sprouts with γ-PGA showed mixed effects, with antioxidant activity depending on concentration and cultivation duration (DPPH in the range 6.5 ± 0.2 ÷ 12.4 ± 0.2 μM TEAC/g dw; FRAP in the range 14.3 ± 0.4 ÷ 25.2 ± 0.9 μM/Fe2+/g dw). Cytotoxicity assays revealed that neither kale nor lupine extracts were toxic to normal colon cells (approx. 100% of alive cells), suggesting selectivity in their action, but fortification with γ-PGA resulted in a weaker or even unfavorable effect on the cytotoxic activity of the examined sprouts. The findings highlight the potential of γ-PGA to enhance the bioactive properties of sprouts, although its effects are influenced by species and cultivation conditions. These results provide a foundation for developing functional foods and sustainable agricultural practices.
Keywords:
sprouts; kale; lupine; γ-polyglutamic acid; phenolic compounds; cytotoxicity; antioxidant activity 1. Introduction
In recent years, interest in γ-polyglutamic acid (γ-PGA) has increased due to its specific biochemical properties and potential for use in various fields [1]. γ-PGA is a biopolymer composed of D- and L-glutamic acid monomers, which are connected by amide bonds between α-amino and γ-carboxyl groups [2]. The γ bond between glutamate units gives it resistance to proteases that break down α-amine bonds. γ-PGA may exist in two forms: (i) soluble in water as a salt with cations or (ii) insoluble as a free acid [3]. Due to advantages such as the low cost of substrates for its synthesis, mild process conditions, and low impact on environmental pollution, the main method of producing γ-PGA is microbial fermentation [1,4]. Bacteria producing γ-PGA are categorized into two groups based on the dependence of their metabolism on glutamic acid. The first group includes strains that require the presence of glutamic acid in the medium, so it should be supplemented with glutamate. The second group consists of glutamate-independent strains that use, for example, citric acid, appropriate amino acids, or glucose. By cultivating bacteria in appropriate combinations, it is possible to supervise the process and obtain γ-PGA with different molecular weights [2,5]. The molecular weight of γ-PGA synthesized by microorganisms is in a wide range of 1–8000 kDa [4]. Apart from the type of bacteria, other factors, such as aeration of the solution in which this compound is synthesized, pH value, and process time, also influence the molecular weight of γ-PGA [6]. The different molecular weight values of γ-PGA influence the physicochemical properties of it and, consequently, its wide range of applications in various systems [1]. Numerous biochemical properties of γ-PGA, such as solubility in water, lack of toxicity and immunogenicity, edibility, water retention capacity, and biodegradability, as well as antioxidant and antimicrobial activity, determine its uniqueness [1,2,6,7]. These properties make it possible to be used in the food, agricultural, cosmetic, and pharmaceutical industries and even in environmental engineering [2,4].
Several studies indicate the multifunctional role of γ-PGA in both vegetative growth and reproductive success in plants, improving also the plant–microbe relationships. In rice cultivars, γ-PGA can enhance seed germination and sprouting by improving various physiological processes [8,9,10]. It may enhance enzymatic activity and enhance water absorption in seeds, leading to improved germination rates [11]. γ-PGA is known to improve root and shoot development, stimulating the production of growth hormones, such as auxins and cytokinins, which play crucial roles during the vegetative stage. Research highlights γ-PGA’s role in improving plants’ resistance to abiotic stresses such as drought and salinity [12,13]. It has been found to regulate osmotic pressure and promote the accumulation of stress-related metabolites [13]. Its use has been linked to increased production of phytoalexins and other defense compounds, improving resistance against pathogens [14]. Some studies have reported increased fruit and flower yields when y-PGA is applied during the reproductive phase [15]. This can be attributed to its effects on nutrient uptake and enhancement of floral development, significantly increasing plant height, chlorophyll content, above-ground biomass, and underground biomass [16].
γ-PGA has garnered attention for its diverse applications in functional food systems, offering numerous benefits that enhance both the quality and nutritional value of food products [17]. One of its most notable attributes is its ability to improve the bioavailability of nutrients and bioactive compounds. Many essential compounds, such as polyphenols and flavonoids, suffer from poor solubility and stability, limiting their absorption in the human body. Research has demonstrated that γ-PGA can significantly enhance their solubility, thereby increasing their functional efficacy in food formulations [18]. γ-PGA has also been used as an additive to food products with a high content of mineral components in order to accelerate their absorption in the small intestine [19]. Beyond its nutritional benefits, γ-PGA plays a crucial role in improving the texture and shelf life of functional foods. It can be used to prevent ageing of starch-based bakery products and pasta, and to improve their texture as well as to enhance the rheological and thermal properties of wheat dough [20]. Its high water retention capacity and gel-forming properties allow it to also function as a stabilizer and texture modifier of other food products, helping maintain their consistency and structural integrity. This property makes it particularly valuable in the formulation of plant-based and processed foods, where moisture balance and stability are critical factors [21]. Another promising application of γ-PGA lies in its ability to reduce off-flavors and mask the extraneous taste of plant-based foods [22]. Many plant-derived protein products and frozen foods often have undesirable bitter notes, which can affect consumer acceptance. γ-PGA has been found to effectively mask these off-flavors, making plant-based alternatives more palatable without the need for artificial additives [7].
Due to biologically and environmentally beneficial features of γ-PGA, such as increasing the resistance of plants to salt or drought stress (by remarkably increasing the activity of plant antioxidant enzymes), the product is currently applied to many plant cultures to reduce the use of chemical fertilizers and to reduce the overall effects of agricultural pollution. Thus, γ-PGA seems to be an attractive agent in the development of green agriculture [23,24].
Overall, γ-PGA presents a multifunctional approach to improving functional food systems. Whether through enhancing bioavailability, optimizing texture and stability, masking undesirable flavors, acting as a natural preservative, or supporting agricultural sustainability, its applications highlight its potential as a valuable ingredient in the evolving landscape of functional and health-oriented foods. As far as we know, our experiment is the first ever to describe the use of γ-PGA in the cultivation of kale and lupin sprouts.
The increasing popularity of sprouts from the Brassicaceae family is caused by their nutritional and medicinal properties resulting from the content of compounds such as glucosinolates (GLS) and phenolic compounds. They have cytotoxic, antimicrobial, and antioxidant properties [25,26]. For this reason, sprouts are classified as functional foods [27]. Factors that influence the concentrations of bioactive compounds in sprouts include a specific variety, development conditions, and exposure to environmental stress [26]. Phytochemicals such as glucosinolates and different polyphenolic compounds occur in the highest doses in the initial days of the germination process, in quantities much higher than in mature plants [26]. One of the representatives of the Brassicaceae family that is the source of the above-mentioned compounds is kale—Brassica oleracea var. sabellica. It belongs to the group of dark green vegetables, headless cabbages, and is characterized by the presence of leaves along the stem. Kale is a valued plant in agriculture due to its simple cultivation, low costs, and resistance to unfavorable environmental conditions [28]. Kale is distinguished by its rich GLS content and, therefore, the various health properties described above. Moreover, it has antimicrobial activity against, among others, Enterococcus faecalis and Staphylococcus aureus [29]. Kale sprout extract has also been found to have neuroprotective activity in vitro by reducing inflammatory markers [30].
Lupin (genus Lupinus) is a leguminous plant recognized for its significant nutritional and health benefits. It has been known for a long time and is widely used as an ingredient of animal feed. However, it is now also becoming more and more popular in the human diet. The seeds of lupin are notably high in protein, with content reaching up to 40%, and are also rich in dietary fiber, essential amino acids, and bioactive compounds such as polyphenols, carotenoids, phytosterols, and tocopherols. These components contribute to its antioxidant, antimicrobial, and anti-inflammatory properties [31]. Regular consumption of lupin has been associated with various health benefits, including improved satiety, better glycemic control, and reduced blood pressure. Studies have shown that whole lupin intake can lead to beneficial changes in these health parameters [32]. Additionally, lupin seeds are a good source of macro- and microelements, including calcium, potassium, sodium, magnesium, iron, zinc, and manganese, as well as vitamins such as thiamine, niacin, and riboflavin. The presence of these nutrients, along with bioactive compounds like carotenoids, tocopherols, and phenolic compounds, enhances the antioxidant capacity of lupin seeds [33]. Due to its rich nutrient profile and health-promoting properties, including an estrogen-like effect, lupin is gaining popularity as a functional food ingredient that has great potential in the prevention and treatment of various diseases, such as cardiovascular diseases and diabetes [34]. Because of these interesting properties, we chose its seeds for sprouting.
The aim of the study was to investigate the antioxidant and cytotoxic properties of kale and lupine sprouts, which were additionally supplemented with γ-polyglutamic acid. Our goal was also to detect active compounds belonging to phenolic acids in both kinds of sprouts and isoflavones in lupine and to determine their profile.
2. Materials and Methods
2.1. Plant Material and Growth Conditions for Sprouts
Kale seeds (Brassica oleracea L. var. sabellica L.) were purchased in W. Legutko Breeding and Seed Company LLC Poland. Lupine seeds (Lupinus luteus L. var. MISTER) were purchased in Małopolska Hodowla Roślin (Kraków, Poland). Both evaluated seeds were stored in the Department of Food Chemistry and Nutrition seeds collection, with appropriate voucher numbers: BOS/PP/PL1045 and LL/PP/PL1048, respectively.
Kale and lupin seeds were immersed in commercially available mineral water (controls) or in such water with two different γ-PGA concentrations (0.01% and 0.05% for kale; 0.1% and 0.5% for lupin) for 30 min. Then, they were evenly distributed in the gutters of three EQMM Easy Green Microfarm sprouters, each with five gutters (i.e., five biological replicates). The chamber of the first germinator was filled with water, the second with lower concentrated solutions of γ-polyglutamic acid (0.1% for lupin; 0.01% for kale), and the third one with a solution of this acid at higher concentrations (0.5% for lupin; 0.05% for kale). The cultivation of lupine and kale sprouts was carried out independently. The kale sprouts were grown for 6, 7, or 8 days after seeding, at a temperature of 24 ± 2 °C, in natural light conditions. They were watered 3 times per day with the same solutions. The procedure for lupine sprouts was analogous, with the difference being that the sprouts were harvested on days 6, 8, and 10. The selection of both γ-PGA concentrations and sprouting duration was based on estimated calculations [12] and prior optimization studies, focusing primarily on the appearance, growth, drying, and rotting of the sprouts. The γ-PGA concentrations were chosen based on their observed effects on seedling development, ensuring that they supported normal growth without causing excessive sprout elongation or structural abnormalities. The germination time was carefully selected to prevent overgrowth, which could lead to the sprouts becoming weaker, drying out, or becoming more susceptible to rotting. Extended sprouting periods can also create microgreens and favorable conditions for microbial contamination, which could compromise the quality and safety of the sprouts. Therefore, the chosen conditions were optimized to balance healthy sprout growth while minimizing potential negative effects such as desiccation or decay. For each of the treatments, three replicates were taken for analysis [35].
The preparation of appropriate γ-PGA solutions is described briefly below. Bacillus licheniformis was isolated from organic soils and maintained in sterile glycerol (50%, w/v) at −80 °C. The strain was confirmed using MS MALDI-TOF spectrometry, by the direct transfer method. The medium used for culture growth and γ-polyglutamic production contained (per liter) glucose—60 g, monosodium glutamate—25 g, citric acid—5 g, hydrated MgSO4—0.5 g, hydrated MnSO4—0.1 g, K2HPO4—1.0 g, hydrated CaCl2—0.2 g, and hydrated FeCl3—0.02 g [36]. The initial pH was adjusted to pH 6.8 ± 0.1 with 1 M NaOH in 500 mL baffled flasks and incubated aerobically at 150 rpm and 37 °C for 48 h. The presence of γ-PGA in the culture broth was estimated by the most specific chemical precipitation method with saturated CuSO4. To obtain the pure fraction, we used a triple micro-ultra and nanofiltration method, using a preliminary 0.25 µm filter, then 100 kDa and 200 Da filters [37]. The fraction used in the experiment consisted of a 4% mixture of γ-PGA polymers in the 100 kDa to 200 Da range.
2.2. Extracts Preparation
The sprouts underwent Soxhlet extraction using methanol for a duration of three hours. Following the extraction, the resulting methanol extracts were separated, subjected to centrifugation, and subsequently stored at −20 °C in a freezer until they were analyzed for total polyphenol content, antioxidant activity, and HPLC examination. A portion of the methanol extracts was further concentrated through evaporation, with the remaining dry residues dissolved in DMSO for use in evaluating cytotoxic potential [35].
2.3. HPLC Determination of Phenolic Acids and Isoflavones in Sprouts
The analysis was performed according to [38], on a Dionex HPLC system, with a PDA 100 UV-VIS detector. Analysis was performed on a Hypersil Gold (C-18) column (5 μm, 250 × 4.6 mm, Thermo EC). The mobile phase consisted of a 1% water solution of formic acid (A) and acetonitrile (B), with 5–60% B, in a 60 min gradient (1 mL/min flow). The compounds were identified by their retention times and UV spectra (254 nm and 285 nm), as referred to in the standards (biochanin, daidzin, genistein, genistin, glycitein, chlorogenic acid, protocatechuic acid, synapic acid). The amounts of the identified compounds were determined according to the standard curves (obtained from the reference standards in the concentration range 0.0625–1 mg/mL). All analyses were performed in triplicate, and the results are presented as mean values (mg/100 g d.w.).
2.4. Cytotoxicity and Viability Assay
The experiment was conducted on three human cancer cell lines and one normal. All cancer cell lines belonged to the gastrointestinal panel: DLD-1 colon adenocarcinoma (ATCC CCL-221), HCT116 colon carcinoma (ATCC CCL-247), and HT29 colorectal adenocarcinoma (ATCC HTB-38). The normal cell line was the CCD 841 CoN normal human colon epithelial line (CRL-1790). Cells were grown at 37 °C in a 5% CO2 atmosphere, with relative humidity, using as culture medium McCoy’s (HT29), DMEM high glucose (HCT116 and DLD-1), or MEM (CCD 841 CoN), with the addition of 10% fetal bovine serum and 1% antibiotics solution. All cell lines, cultures media, and supplements were obtained from Merck (Darmstadt, Germany). The cells were seeded in 96-well plates (1.5 × 104 cells/well) for 24 h. Then, the culture medium was replaced with the same medium containing different concentrations of the tested substances from 0 to 200 µg/mL and incubated for 24 h. The concentration range used in our study is the standard approach, consisting of at least five concentrations. We used the range from 0 µg/mL (control, untreated cells) to 200 µg/mL. We did not use higher concentrations as this would indicate only weak activity, if any. Cell viability was measured with an MTT assay, as described previously [38]. DSMO diluted accordingly was also tested, to exclude the false cytotoxic effect. Doxorubicin was used as a reference standard. The absorbance (570 nm) was measured on a microplate reader (BioTek Instruments Inc., Winooski, VT, USA). Cell viability was calculated as % of untreated, control cells, and IC50 values if possible. Each experiment was performed in triplicate.
2.5. Determination of Antioxidant Activity and Total Reducing Capacity
The antioxidant activity of the tested materials was assessed using two different assays. The DPPH radical scavenging assay was performed based on the method described by Brand-Williams et al. [39], with certain modifications [40]. Similarly, the ferric reducing antioxidant power (FRAP) assay was carried out following the procedure established by Benzie and Strain [41], also with modifications [40]. The total extractable polyphenol content, which is also referred to as the Total Reducing Capacity (TRC), was determined using the Folin–Ciocalteu method, incorporating specific adjustments [40]. All analyses, including DPPH, FRAP, and TRC assays, were conducted using a Synergy-2 reader (BioTek, USA) equipped with syringe-based rapid dispensers.
2.6. Statistical Analysis
For parameters measured several times, the following descriptive statistics were calculated: mean ± standard deviation, median, minimum, and maximum. For parameters measured two or three times, only the mean ± standard deviation was calculated. In order to assess the significance of differences between mean values on different days of cultivation, in the case of the normal distribution of parameters and homogeneous variances, the analysis of variance in the repeated-measures scheme was used, with Tukey’s multiple comparison test. If the above-mentioned conditions were not met, the non-parametric Friedman test was used—for sprouts of the same plant, in the same culture supplementation scheme (repeated measurements scheme), and the non-parametric Kruskal–Wallis test—when comparing sprouts of plants in different culture supplementation schemes, but on the same day (independent measurements scheme). The results obtained by these tests, for the same plants, were verified by the Dunn multiple comparison test, and for different plants, in different culture supplementation schemes, by the linear forward multiple testing procedure of Benjamini, Krieger, and Yekutieli (BKY procedure). The critical level of significance was p = 0.05. The statistical partial least squares (PLS) model was applied to check the existence of the correlation structure between predictive parameters (concentrations of phenolic acids and isoflavones in sprouts, DPPH, FRAP, TRC) and response parameters (cell viability tested in selected cell lines). Statistical analysis was performed using GraphPad Prism 8.0.1. (GraphPad Software Company, Boston, MA, USA) and SIMCA-P v.9 software (Umetrics, Umeå, Sweden). The STATISTICA v. 13.3. package (TIBCO Software Inc., Palo Alto, CA, USA) was used for graphic representation of the PLS model.
3. Results and Discussion
The results regarding antioxidant activities in lupine and kale sprouts as well as their bioactive compounds are summarized in Table 1 and Table 2, respectively, while the cytotoxic activity is presented in Figure 1 and Figure 2.

Table 1.
The bioactive compounds in lupine and kale sprouts (mean value ± SD; n = 3; results within each column marked with the same lowercase letter indicate significant differences for sprouts of the same plant and in the same culture supplementation scheme, while results marked with the same uppercase letter indicate significant differences when comparing sprouts of plants in different culture supplementation schemes, but on the same day); abbreviations: L1—lupine sprouts soaked with water, on day 6; L2—lupine sprouts soaked with water, on day 8; L3—lupine sprouts soaked with water, on day 10; L4—lupine sprouts soaked with 0.1% PGA solution, on day 6; L5—lupine sprouts soaked with 0.1% PGA solution, on day 8; L6—lupine sprouts soaked with 0.1% PGA solution, on day 10; L7—lupine sprouts soaked with 0.5% PGA solution, on day 6; L8—lupine sprouts soaked with 0.5% PGA solution, on day 8; L9—lupine sprouts soaked with 0.5% PGA solution, on day 10; K1—kale sprouts soaked with water, on day 6; K2—kale sprouts soaked with water, on day 7; K3—kale sprouts soaked with water, on day 8; K4—kale sprouts soaked with 0.01% PGA solution, on day 6; K5—kale sprouts soaked with 0.01% PGA solution, on day 7; K6—kale sprouts soaked with 0.01% PGA solution, on day 8; K7—kale sprouts soaked with 0.05% PGA solution, on day 6; K8—kale sprouts soaked with 0.05% PGA solution, on day 7; K9—kale sprouts soaked with 0.05% PGA solution, on day 8; ND—not detected).

Table 2.
The antioxidant capacities in lupine and kale sprouts (mean value ± SD; n = 3; results within each column marked with the same lowercase letter indicate significant differences for sprouts of the same plant and in the same culture supplementation scheme, while results marked with the same uppercase letter indicate significant differences when comparing sprouts of plants in different culture supplementation schemes, but on the same day); abbreviations: L1—lupine sprouts soaked with water, on day 6; L2—lupine sprouts soaked with water, on day 8; L3—lupine sprouts soaked with water, on day 10; L4—lupine sprouts soaked with 0.1% PGA solution, on day 6; L5—lupine sprouts soaked with 0.1% PGA solution, on day 8; L6—lupine sprouts soaked with 0.1% PGA solution, on day 10; L7—lupine sprouts soaked with 0.5% PGA solution, on day 6; L8—lupine sprouts soaked with 0.5% PGA solution, on day 8; L9—lupine sprouts soaked with 0.5% PGA solution, on day 10; K1—kale sprouts soaked with water, on day 6; K2—kale sprouts soaked with water, on day 7; K3—kale sprouts soaked with water, on day 8; K4—kale sprouts soaked with 0.01% PGA solution, on day 6; K5—kale sprouts soaked with 0.01% PGA solution, on day 7; K6—kale sprouts soaked with 0.01% PGA solution, on day 8; K7—kale sprouts soaked with 0.05% PGA solution, on day 6; K8—kale sprouts soaked with 0.05% PGA solution, on day 7; K9—kale sprouts soaked with 0.05% PGA solution, on day 8; DPPH—1,1-diphenyl-2-picrylhydrazyl; FRAP—ferric reducing antioxidant power; TEAC—Trolox equivalent antioxidant capacity; TRC—total reducing capacity; ND—not detected).
3.1. Active Compounds
Qualitative analysis of kale sprouts using HPLC, carried out in the work by Paśko et al. [42], showed the presence of the following phenolic acids: chlorogenic acid, protocatechuic acid, and sinapic acid. The results of the quantitative analysis of these compounds in that study were, respectively, 18 ± 2 µg/100 g d.w., 10 ± 0 µg/100 g d.w., and 40 ± 10 µg/100 g d.w., which means that they were one order of magnitude lower than in our study, in sprouts watered with water on days 6, 7, and 8 of cultivation (Table 1), which can be explained by different conditions of plant cultivation.
The results of the qualitative analysis of lupine sprouts in this study indicate the presence of the following isoflavones: biochanin, daidzin, genistein, genistin, and glycitein (Table 1). However, the results of the quantitative analysis indicate an unfavorable effect of supplementing lupine sprouts with a 0.1% PGA solution on the content of biochanin, daidzin, and glycitein compared to the content of these compounds in sprouts watered with water (Table 1). Additionally, the concentration of genistin and biochanin was almost twice as high in lupine sprouts watered with 0.5% PGA solution on day 8 of culture compared to sprouts watered with water on that day.
3.2. Cytotoxic Activity
Several studies have explored kale sprouts’ effects on cell viability, apoptosis induction, and metabolic activity. A study on selenium-fortified kale and kohlrabi sprouts found that while selenium enrichment enhanced nutritional value, it did not significantly change the cytotoxic activity of the sprouts [43]. Selenium-enriched Chinese kale seedlings exhibited cytotoxic effects on Caco-2 (colon), MCF-7 (breast), and HepG2 (liver) cancer cells, inducing apoptosis and reducing cell viability [44]. Additionally, kale and lupine sprouts showed protective effects against hepatotoxicity in rats exposed to CCl₄, likely due to their antioxidant and anti-inflammatory properties, suggesting a potential detoxifying role [45].
As sprouts are edible elements of the human diet, we decided to direct our study on the panel of human colon cells, comprising cancer cells, differing in their metastatic potential, and also normal epithelial colon cells (CCD 841 CoN). This was to give the answer to whether the γ-PGA-treated sprouts of kale and lupin can reveal chemopreventive potential towards colon cancer, and can be safe for normal colon cells. The colon cancer cells used in the study included highly aggressive HCT116 colon carcinoma cells; Duke’s type C adenocarcinoma DLD-1 cells, with the potential to lymph node metastasis; and colorectal adenocarcinoma HT29 cells, originating from a primary tumor. The most noticeable difference in cytotoxicity was seen in 6-day-old kale sprouts. For DLD-1 cells, the addition of γ-PGA led to a notable increase in cell viability by 42.1% in KS6_0.01%PGA and by 43.9% in KS6_0.05%PGA, indicating a substantial reduction in cytotoxic activity. A similar trend was observed in HCT-116 cells, where viability increased by 21.3 in KS6_0.01%PGA and by 25.8% in KS6_0.05%PGA, suggesting weakened cytotoxicity. For HT-29 cells, the changes were less pronounced. In general, kale sprouts exerted a more profound cytotoxic effect (Figure 1) to the examined cancer cell lines, as compared to lupine sprouts (Figure 2), although the latter were treated with a higher concentration of PGA. This may suggest that PGA treatment is not beneficial in terms of the enhancement of the cytotoxic effect. Interestingly, a different cellular response was noted for the tested sprouts, as in the case of kale sprouts, where HT29 cells were most resistant, while for lupine sprouts, the weakest effect was observed for DLD-1 cells. The obtained effects were significantly weaker, as compared to doxorubicin, used as a reference standard, with IC50 in a range from 1 to 4.1 µg/mL. Kale sprouts caused an increase in DLD-1 cells viability, and the effect was dependent on the used γ-PGA concentration, regardless of the harvesting day. A similar increase was observed for HCT116 cells, but only for 6-day-old sprouts. For the 7- and 8-day-old sprouts, such an effect was noted only for the treatment with 0.05% γ-PGA. Conversely, the cytotoxic effect on HT29 cells was higher only in the case of the 8-day-old sprouts treated with 0.01% γ-PGA, as compared to the other treatments. In lupine sprouts, stimulation with γ-PGA in most cases decreased the cytotoxic effect observed in the untreated sprouts. This was especially observed in 8-day-old sprouts, where the treatment with γ-PGA led to a clear reduction in cytotoxicity. Cell viability of the DLD-1 cell line increased by 14.4% in LS8_0.1%PGA and by 20.2% in LS8_0.5%PGA. An even more pronounced effect was observed in HCT-116, where γ-PGA greatly reduced the cytotoxic effect at the lower concentration but had little impact at the higher concentration, and cell viability returned to the values observed in control sprouts.
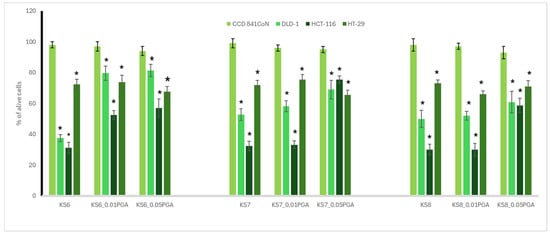
Figure 1.
Cytotoxic potential in the most concentrated extract of control kale sprouts (KS) and those supplemented with γ-PGA (KS_0.01%PGA and KS_0.05%PGA), harvested after 6, 7, and 8 days. An asterisk (*) indicates a significant difference (* p < 0.05) between normal and cancerous cell lines in each set.
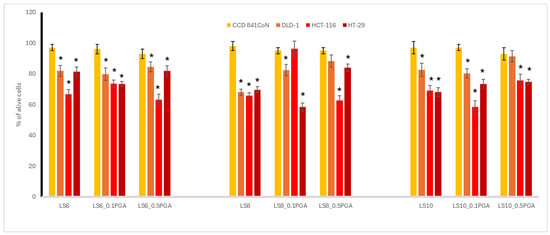
Figure 2.
Cytotoxic potential in the most concentrated extract of control lupine sprouts (LS) and those supplemented with γ-PGA (LS_0.1%PGA and LS_0.5%PGA), harvested after 6, 8, and 10 days. An asterisk (*) indicates a significant difference (* p < 0.05) between normal and cancerous cell lines in each set.
The opposite trend was noted for 10-day-old lupine sprouts, where the cytotoxic effect on HCT116 cells was enhanced in the case of the treatment with 0.1% γ-PGA. Such an enhancement was also observed for HT29 cells, treated with the extracts from 6- and 8-day-old sprouts with 0.1% but not 0.5% γ-PGA. What is most important is that none of the tested extracts were toxic to non-cancerous colon epithelial CCD 841 CoN cells, which implicates their safety and selectivity of action, as compared to doxorubicin toxicity (IC50 1.7 µg/mL). To sum up, both kale and lupine sprouts may be important in the context of chemoprevention of colon cancer, with the predominance of the former, as a varied cytotoxic effect was noted on cancer cells differing in their malignancy. Among them, highly aggressive HCT116 colon cancer cells were significantly affected by kale sprouts (IC50 in range 130.1–153.4 μg/mL), which should be underlined.
As to our best knowledge, this is the first ever attempt to fortify sprouts with γ-PGA, and we can only compare the obtained results with some other fortification approaches in terms of the effect on cytotoxic activity. In our previous experiments, we reported on the significant enhancement of the cytotoxic effect of selenium-enriched kale sprouts for colon cancer HT29 cells, with a weaker effect on colon cancer Caco-2 cells [42], and also colon carcinoma SW480 and SW620 cells [43], in a dose-dependent manner. A similar effect was also noted by Luang-In et al. for selenium-enriched Chinese kale seedlings on Caco-2 cells [44]. A significant decrease in colon cancer CT26 cells’ viability was observed for cabbage and broccoli sprouts enriched with sulfur salts [46]. Only scarce data exist on the cytotoxic activity of lupine sprouts. One study reported on selenium-enriched lupine sprouts, with no enhancement in cytotoxic activity observed towards human leukemia HL60 cells [47]. Our previous study indicated a significant improvement in the cytotoxic effect on breast cancer MCF7 cells of lupine sprouts, stimulated to grow with different LED lights, and the effect was dose and time dependent [35]. Solid-state fermentation of lupin with Bifidobacterium species exhibited cytotoxic effects against two cancer cell lines, along with antioxidant, antihypertensive, and antidiabetic properties [48]. Another study explored the effect of LED lights on isoflavone content in lupin sprouts and their impact on normal and cancerous breast and prostate cells. Certain LED light conditions enhanced cytotoxic activity against cancer cells while remaining safe for non-cancerous cells [35]. These findings suggest that while unprocessed and selenium-enriched lupin sprouts do not exhibit cytotoxicity, fermented and LED-stimulated lupin sprouts may have potential anticancer effects, warranting further research in different cancer models.
3.3. Antioxidant Potential
This study examined the effect of γ-polyglutamic acid supplementation at concentrations of 0.1% and 0.5% (lupine sprouts) and 0.01% and 0.05% (kale sprouts) on the content of phenolic compounds and the antioxidant activity in the mentioned sprouts. The results obtained using both the DPPH and FRAP methods (Table 2) indicate a much higher antioxidant activity of kale sprouts compared to lupine sprouts. This is probably related to the higher content of phenolic compounds in kale sprouts than in lupine sprouts, as was determined using the Folin–Ciocalteu method. The highest value of antioxidant activity in the FRAP method was achieved by kale sprouts watered with a 0.01% PGA solution on day 8 of cultivation, while the lowest value among kale sprouts was achieved by those watered with a 0.05% PGA solution on the seventh day of cultivation. In turn, in the group of lupine sprouts, the highest value was achieved by sprouts watered with a 0.5% PGA solution on day 8. The highest value of antioxidant activity in the group of lupine sprouts was almost three times lower than the lowest value of antioxidant activity in kale sprouts, which confirms the higher antioxidant activity of the second plant (Table 2).
Lupine sprouts watered with a 0.1% PGA solution achieved the highest result on day 6 of cultivation. Supplementation of lupine sprouts with a 0.5% PGA solution on days 6 and 8 of culture had a positive effect on obtaining higher results compared to sprouts watered with water or a 0.1% PGA solution on the same days. However, on the 10th day of cultivation, both groups of sprouts watered with a 0.1% or 0.5% PGA solution obtained lower results of antioxidant activity compared to sprouts watered with water on that day (Table 2). This is related to the lower total concentrations of phenolic compounds in these groups of sprouts compared to sprouts watered with water on day 10.
The highest value of antioxidant activity determined by the DPPH method was demonstrated, as for the FRAP method, for kale sprouts watered with a 0.01% PGA solution on day 8 of culture, which is related also with the highest polyphenol content determined in these sprouts. The lowest value of antioxidant activity for kale sprouts was determined using the DPPH method for sprouts watered with a 0.05% PGA solution on day 7. It is also associated with the lowest total polyphenol content in this group of sprouts. This result proves the unfavorable effect of supplementation with a 0.05% PGA solution compared to sprouts watered with water on the same day of cultivation, for which this value was almost twice as high. In the group of lupine sprouts, the highest value of the antioxidant activity measured by the DPPH method was determined for sprouts watered with water on the 10th day of cultivation. Moreover, in this case, it can be assumed that it was caused by a higher total concentration of polyphenols. However, the highest value for lupine sprouts was 2.5 times lower than the lowest value for kale sprouts, which again confirms the higher antioxidant activity of kale sprouts (Table 2).
In a similar work, Paśko et al. [42] examined how selenium compounds affect the antioxidant properties of kale sprouts. In the control group of sprouts, which was watered with water, the result of the FRAP method was 91.0 ± 2.4 µg Fe2+/g d.w., which is lower compared to the results obtained in this study for kale sprouts watered with water on days 6, 7, and 8, which were equal to, respectively, 102.2 ± 0.7 µM Fe2+/g d.w., 151.8 ± 0.6 µM Fe2+/g d.w., 157.7 ± 0.9 µM Fe2+/g d.w. In turn, the result of the antioxidant activity in the same work [42], using the DPPH method for the control group of kale sprouts, was 285 ± 13 µM Trolox/g d.w. In the case of kale sprouts watered with water on days 6, 7, and 8 in the present study, this method gave the following values: 38.0 ± 0.5 µM Trolox/g d.w., 61.1 ± 0.2 µM Trolox/g d.w., and 54.3 ± 0.3 µM Trolox/g d.w. The discrepancy in results may be caused by different sprout cultivation conditions. Kale sprouts in the work by Paśko et al. [42] were grown in a greenhouse, in different type of sprouters, using a different method of irrigation and a different composition of the irrigation fluid. These sprouts were additionally illuminated for 10 h, which significantly influenced their growth. In the present study, sprouts were grown in a room with reduced air humidity, no additional lighting was used, and the cultivation took place only in natural light. The culture conditions in this case could therefore significantly reduce the values of the DPPH parameter.
3.4. Multivariate Analysis of Studied Parameters
It was assumed that the parameters with large absolute values of their coordinates (>0.3) on the first two latent components of the PLS model determine the axes of the new coordinate system to the greatest extent. For pairs of such parameters, the correlation coefficients between them were calculated as cosines of the corresponding angles in order to express the strength of the bivariate associations. The “corresponding angle” is defined here as the angle subtended by the two lines connecting the origin with the coordinates of both parameters in the PLS loadings diagram. Two significant latent components of the PLS model were constructed with eigenvalues higher than 1 (equaled 8.56 and 1.27, respectively). They explained 89.3% of the variance in the predictive parameters, and 49.3% of response parameter variance. Figure 3 shows the loadings for the first two latent components in the PLS model.
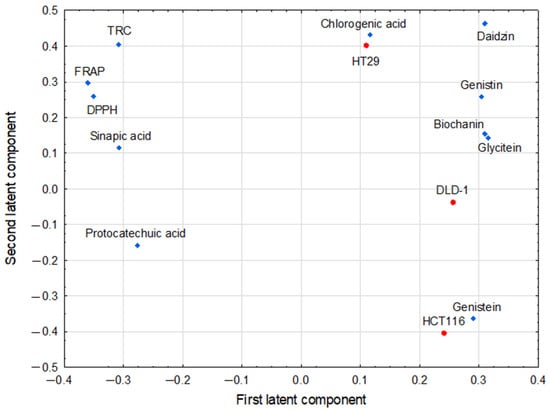
Figure 3.
Partial least squares model loadings (first and second latent components in PLS). The model correlated the predictive parameters (biochanin, chlorogenic acid, daidzin, genistein, genistin, glycitein, protocatechuic acid, synapinic acid, DPPH, FRAP, TRC; depicted as blue diamonds) and response ones (cell viability tested in cell lines: DLD-1, HCT116, HT29; depicted as red dots).
The first latent component in this model had positive weights predominantly for glycitein, biochanin, daidzin, and genistin, which formed the cluster of strongly correlated parameters (Table 3). The same latent component was loaded negatively by the group of antioxidant parameters: FRAP, DPPH, sinapic acid, and TRC, all also being strongly positively correlated (Table 2). The second latent component was loaded positively mainly by daidzin, chlorogenic acid, TRC, and HT29, while was negatively loaded by HCT116 and genistein (HT29, HCT116—response parameters). The last pair of parameters was negatively correlated with the antioxidant parameters mentioned above, predominantly with TRC (Table 3).

Table 3.
Correlation coefficients for the pairs of parameters with largest absolute values of their coordinates on the first two latent components of partial least squares model (correlation coefficients below absolute value 0.700 were not shown).
The score scatterplot of the PLS model, which points various groups of kale and lupine sprouts in the space determined by the first two latent components, is shown in Figure 4.
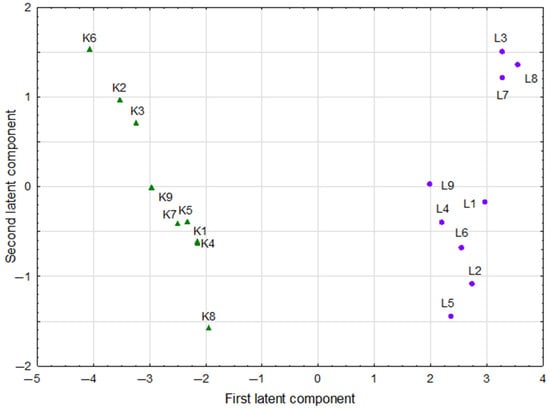
Figure 4.
The projection of kale (K1…K8) and lupine (L1…L8) sprouts into the space defined by first two latent components of partial least squares model.
The horizontal division, reflecting two different species of sprouts, is very clear. Compared to the plot of loadings of latent components in the PLS model, this confirms that both species differed mainly in the values of parameters such as glycitein, biochanin, daidzin, genistin (all not detected in kale sprouts), FRAP (p = 0.000), DPPH (p = 0.000), sinapic acid (not detected in lupine sprouts), and TRC (p = 0.001).
The comparison of two botanically unrelated plants in our study was intentional, as we selected species from different botanical families with significant relevance in global food production. This preliminary study serves as a starting point for further research, allowing for a broader perspective on how γ-PGA interacts with bioactive compounds across diverse plant species. By choosing Brassicaceae (kale) and Fabaceae (lupine), we aimed to explore the effects of γ-PGA on two nutritionally valuable plant groups—one rich in glucosinolates and polyphenols, the other abundant in isoflavones and proteins. Investigating whether γ-PGA modulates secondary metabolite production differently in brassica and legumins plants will provide valuable insights into its potential applications in our future study. Additionally, this approach allows for a better understanding of how γ-PGA influences plant metabolism across different functional groups, potentially leading to new strategies for improving food quality, nutritional value, and agricultural sustainability. The findings from this study lay the groundwork for further analyses and the development of optimized applications of γ-PGA in various plant-based food systems. However, at this stage of research, any mechanistic explanations would be mere speculation.
3.5. Limitations of This Work
The methods used did not allow for a complete determination of the polyphenol profile in the tested sprout samples. Only extractable polyphenols were taken into account, whereas sprouts contain both bound polyphenols and non-extractable polymeric polyphenols, which were not included in the analysis.
The PLS plot in our study did not include replicates. When using mean values in PLS, the scatter of results is reduced, which means that fewer latent components are needed to describe the data; however, the model robustness may decrease.
The relevance of statistical correlations revealed in the PLS model is not clear since this does not directly translate into biological relationships, but it sheds light on the issue and provides a basis for further research.
4. Conclusions
Kale sprouts were characterized by higher antioxidant activity than lupine sprouts, and the highest antioxidant activity coexisted with the highest total polyphenol content in this group of sprouts. The presence of several phenolic compounds—various isoflavones and chlorogenic acid—has been identified in lupine sprouts. The addition of γ-PGA did not cause a clear and consistent increase in the antioxidant properties of the tested sprouts; a greater influence on these properties was found in the case of plant species or day of cultivation. The cytotoxicity results suggest that fortification with PGA results in a weaker or even unfavorable effect on the cytotoxic activity of the examined sprouts, which is more profound in kale sprouts; however, further studies are needed to clarify this issue. A chemometric approach revealed a correlation structure between original parameters and confirmed a clear difference between the two sprout species. Our research is the first attempt to use γ-PGA in the cultivation of sprouts, and the results obtained are preliminary and observational. Continuing, we will try to explain the observed effect on the cytotoxic activity of sprouts supplemented with γ-PGA; in particular, the effect of γ-PGA on some metabolic pathways related to the synthesis of polyphenols and intracellular signaling.
Author Contributions
Conceptualization, A.G., P.K., P.P., T.S. and P.Z.; methodology, A.G., E.P., P.P. and T.S.; software, R.P.; validation, P.K., R.P. and T.S.; formal analysis, A.G. and P.P.; investigation, A.G., P.K., E.P. and P.P.; resources, A.G., P.P. and P.Z.; data curation, A.G., E.P. and P.Z; writing—original draft preparation, P.K. and P.Z.; writing—review and editing, A.G., P.P., R.P. and P.Z.; visualization, P.P. and P.Z.; supervision, E.P., R.P. and T.S.; project administration, P.P. and P.Z.; funding acquisition, P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
On request.
Acknowledgments
This study was partly supported by internal grant N42/DBS/000321.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, L.; Chen, S.; Yu, B. Poly-γ-glutamic acid: Recent achievements, diverse applications and future perspectives. Trends Food Sci. Technol. 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Wojtowicz, K.; Steliga, T.; Kapusta, P.; Brzeszcz, J.; Skalski, T. Evaluation of the effectiveness of the biopreparation in combination with the polymer γ-PGA for the biodegradation of petroleum contaminants in soil. Materials 2022, 15, 400. [Google Scholar] [CrossRef]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels Bioprod. 2016, 9, 134. [Google Scholar] [CrossRef]
- Feng, J.; Gu, Y.; Quan, Y.; Cao, M.; Gao, W.; Zhang, W. Improved poly-γ-glutamic acid production in Bacillus amyloliquefaciens by modular pathway engineering. Metab. Eng. 2015, 32, 106–115. [Google Scholar] [CrossRef]
- Nabi, I.; Das, S. Poly-glutamic acid (PGA)—Structure, synthesis, genomic organization and its application: A review. Int. J. Pharm. Sci. Res. 2015, 6, 2258–2280. [Google Scholar]
- Li, D.; Hou, L.; Gao, Y.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Recent advances in microbial synthesis of poly-γ-glutamic acid: A review. Foods 2022, 11, 739. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Feng, X.; Xu, X.; Xu, H.; Yang, H.; Tang, W. Effect of poly(γ-glutamic acid) on microbial community and nitrogen pools of soil. Acta Agr. Scand. 2013, 63, 657–668. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, C.; Xu, X.; Feng, X.; Xu, H. Effect of poly (γ-glutamic acid) on wheat productivity, nitrogen use efficiency and soil microbes. J. Soil. Sci. Plant Nut. 2013, 13, 744–755. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Pang, X.; Li, H.; Feng, X.; Xu, H. Exogenous application of poly-γ-glutamic acid enhances stress defense in Brassica napus L. seedlings by inducing crosstalks between Ca2+, H2O2, brassinolide, and jasmonic acid in leaves. Plant Physiol. Biochem. 2017, 118, 460–470. [Google Scholar] [CrossRef]
- Ngearnpat, N.; Chunhachart, O.; Kotabin, N.; Issakul, K. Comparative assessment of gamma-polyglutamic acid and Bacillus subtilis cells as biostimulants to improve rice growth and soil quality. J. Ecol. Eng. 2023, 24, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Skalski, T.; Zając, E.; Jędrszczyk, E.; Papaj, K.; Kohyt, J.; Góra, A.; Kasprzycka, A.; Shytum, D.; Skowera, B.; Ziernicka-Wojtaszek, A. Effects of γ-polyglutamic acid on grassland sandy soil properties and plant functional traits exposed to drought stress. Sci. Rep. 2024, 14, 3769. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, J.; Lei, P.; Wang, Q.; Feng, X.; Xu, H. Poly-γ-glutamic acid induces system tolerance to drought stress by promoting abscisic acid accumulation in Brassica napus L. Sci. Rep. 2020, 10, 252. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Mi, D.; Luo, Y.; Guo, J. Poly-γ-glutamic acid productivity of Bacillus subtilis BsE1 has positive function in motility and biocontrol against Fusarium graminearum. J. Microbiol. 2017, 55, 554–560. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Feng, X.; Xu, X.; Liang, J.; Chi, B.; Xu, H. Calcium involved in the poly(γ-glutamic acid)-mediated promotion of Chinese cabbage nitrogen metabolism. Plant Physiol. Biochem. 2014, 80, 144–152. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, J.; Chen, T.; Zhang, H.; Hou, X.; Li, J. Effects of γ-polyglutamic acid supplementation on alfalfa growth and rhizosphere soil microorganisms in sandy soil. Sci. Rep. 2024, 14, 6440. [Google Scholar] [CrossRef]
- Tanimoto, H. Food applications of poly-gamma-glutamic acid. In Amino-Acid Homopolymers Occurring in Nature; Hamano, Y., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 155–168. [Google Scholar]
- Jeon, Y.O.; Lee, J.S.; Lee, H.G. Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and γ-poly (glutamic acid). Colloids Surf. B Biointerfaces 2016, 147, 224–233. [Google Scholar] [CrossRef]
- Tanimoto, H.; Fox, T.; Eagles, J.; Satoh, H.; Nozawa, H.; Okiyama, A.; Morinaga, Y.; Susan, J.; Fairweather-Tait, S.J. Acute effect of poly-glutamic acid on calcium absorption in post-menopausal women. J. Am. Coll. Nutr. 2007, 26, 645–649. [Google Scholar] [CrossRef]
- Shyu, Y.S.; Hwang, J.Y.; Hsu, C.K. Improving the rheological and thermal properties of wheat dough by the addition of c-polyglutamic acid. LWT Food Sci. Technol. 2008, 41, 982–987. [Google Scholar] [CrossRef]
- Elbanna, K.; Alsulami, F.S.; Neyaz, L.A.; Abulreesh, H.H. Poly (γ) glutamic acid: A unique microbial biopolymer with diverse commercial applicability. Front. Microbiol. 2024, 15, 1348411. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Q.; Yang, Y.M.; Lee, S.U.; Han, J.S.; Jang, K.J.; Tong, T. Applications and func-tions of γ-poly-glutamic acid and its derivatives in medicine. Curr. Pharm. Biotechnol. 2021, 22, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Xu, Z.; Liang, J.; Luo, X.; Zhang, Y.; Feng, X. Poly(γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul. 2016, 78, 233–241. [Google Scholar] [CrossRef]
- Quan, J.; Zheng, W.; Tan, J.; Li, Z.; Wu, M.; Hong, S.B. Glutamic acid and poly-γ-glutamic acid enhanced the heat resistance of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) by improving carotenoid biosynthesis, photosynthesis, and ROS signaling. Int. J. Mol. Sci. 2022, 23, 11671. [Google Scholar]
- Kapusta-Duch, J.; Kusznierewicz, B. Young shoots of white and red headed cabbages like novel sources of glucosinolates as well as antioxidative substances. Antioxidants 2021, 10, 1277. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive compounds and bioactivities of Brassica oleracea L. Var. Italica sprouts and microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. Acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of glucosinolates and their breakdown products: Impact of processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Antunes-Ricardo, M.; Jacobo-Velázquez, D.A. Improving the health-benefits of kales (Brassica oleracea L. var. acephala DC) through the application of controlled abiotic stresses: A review. Plants 2021, 10, 2629. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Bryant, L.; Rangan, A.; Grafenauer, S. Lupins and health outcomes: A systematic literature review. Nutrients 2022, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Estivi, L.; Brandolini, A.; Gasparini, A.; Hidalgo, A. Lupin as a source of bioactive antioxidant compounds for food products. Molecules 2023, 28, 7529. [Google Scholar] [CrossRef] [PubMed]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. Nutritional, health, and technological functionality of lupin flour addition to bread and other baked products: Benefits and challenges. Crit. Rev. Food Sci. Nutr. 2015, 56, 835–857. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Zagrodzki, P.; Miret, M.; Paśko, P. Chickpea and lupin sprouts, stimulated by different led lights, as novel examples of isoflavones-rich functional food, and their impact on breast and prostate cells. Molecules 2022, 27, 9030. [Google Scholar] [CrossRef]
- Mahaboob Ali, A.A.; Momin, B.; Ghogare, P. Isolation of a novel poly-γ-glutamic acid-producing Bacillus licheniformis A14 strain and optimization of fermentation conditions for high-level production. Prep. Biochem. Biotech. 2020, 50, 445–452. [Google Scholar] [CrossRef]
- Yao, J.; Jing, J.; Xu, H.; Liang, J.; Wu, Q.; Feng, X.; Ouyang, P. Investigation on enzymatic degradation of γ-polyglutamic acid from Bacillus subtilis NX-J. Mol. Catal. B Enzym. 2009, 56, 158–164. [Google Scholar] [CrossRef]
- Galanty, A.; Juncewicz, P.; Podolak, I.; Grabowska, K.; Służały, P.; Paśko, P. Comparative analysis of polyphenolic profile and chemopreventive potential of hemp sprouts, leaves, and flowers of the Sofia variety. Plants 2024, 13, 2023. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pasko, P.; Gdula-Argasinska, J.; Podporska-Carroll, J.; Quilty, B.; Wietecha-Posluszny, R.; Tyszka-Czochara, M.; Zagrodzki, P. Influence of selenium supplementation on fatty acids profile and biological activity of four edible amaranth sprouts as new kind of functional food. J. Food Sci. Technol. 2015, 52, 4724–4736. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Żmudzki, P.; Bieniek, U.; Prochownik, E. Varied effect of fortification of kale sprouts with novel organic selenium compounds on the synthesis of sulphur and phenolic compounds in relations to cytotoxic, antioxidant and anti-inflammatory activity. Microchem. J. 2022, 179, 107509. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Paśko, P.; Galanty, A.; Tyszka-Czochara, M.; Wietecha-Posłuszny, R.; Salvans Rubio, P.; Bartoń, H.; Prochownik, E.; Muszyńska, B.; Sułkowska-Ziaja, K.; et al. Does selenium fortification of kale and kohlrabi sprouts change significantly their biochemical and cytotoxic properties. J. Trace Elem. Med. Biol. 2020, 59, 126466. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Saengha, W.; Buranrat, B.; Chantiratikul, A.; Ma, N.L. Cytotoxicity of selenium-enriched Chinese kale (Brassica oleracea var. alboglabra L.) seedlings against Caco-2, MCF-7 and HepG2 cancer cells. Pharmacogn. J. 2020, 12, 674–681. [Google Scholar] [CrossRef]
- EL-Nahas, F.G.; M El Gezery, H.M. Effect of kale, lupine and its mixture sprouts on the biochemical parameters of hepatotoxicity rats with CCl4. Med. Environ. Sci. 2023, 18, 1957–1973. [Google Scholar] [CrossRef]
- Kestwal, R.M.; Lin, C.J.; Bagal-Kestwal, D.; Chiang, B.H. Glucosinolates fortification of cruciferous sprouts by sulphur supplementation during cultivation to enhance anti-cancer activity. Food Chem. 2011, 126, 1164–1171. [Google Scholar] [CrossRef]
- Frias, J.; Gulewicz, P.; Martínez-Villaluenga, C.; Pilarski, R.; Blazquez, E.; Jiménez, B.; Gulewicz, K.; Vidal-Valverde, C. Influence of germination with different selenium solutions on nutritional value and cytotoxicity of lupin seeds. J. Agric. Food Chem. 2009, 57, 1319–1325. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.; Liu, S.; Al-Mheiri, A.; Abushelaibi, A. Cytotoxicity, antihypertensive, antidiabetic and antioxidant activities of solid-state fermented lupin, quinoa and wheat by Bifidobacterium species: In-vitro investigations. LWT 2018, 95, 295–302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).