Investigation of Aluminium White Dross for Hydrogen Generation Hydrolysis in Low-Concentration Alkali
Abstract
1. Introduction
2. Materials and Methods
- SOW: dross from pure aluminium (expected approx. 80% aluminium);
- HDC: dross from 7% aluminium alloys (expected approx. 40% aluminium and approx. impurity content: 7% Si, 2% Mg, 0.5% Ti, and 1% Sr);
- RM: dross from 1xxx series aluminium (approx. 40% aluminium) (approx. other impurity content: 1% B, 2% Ti, and 5% V).
3. Results
3.1. Elemental and Structural Analysis
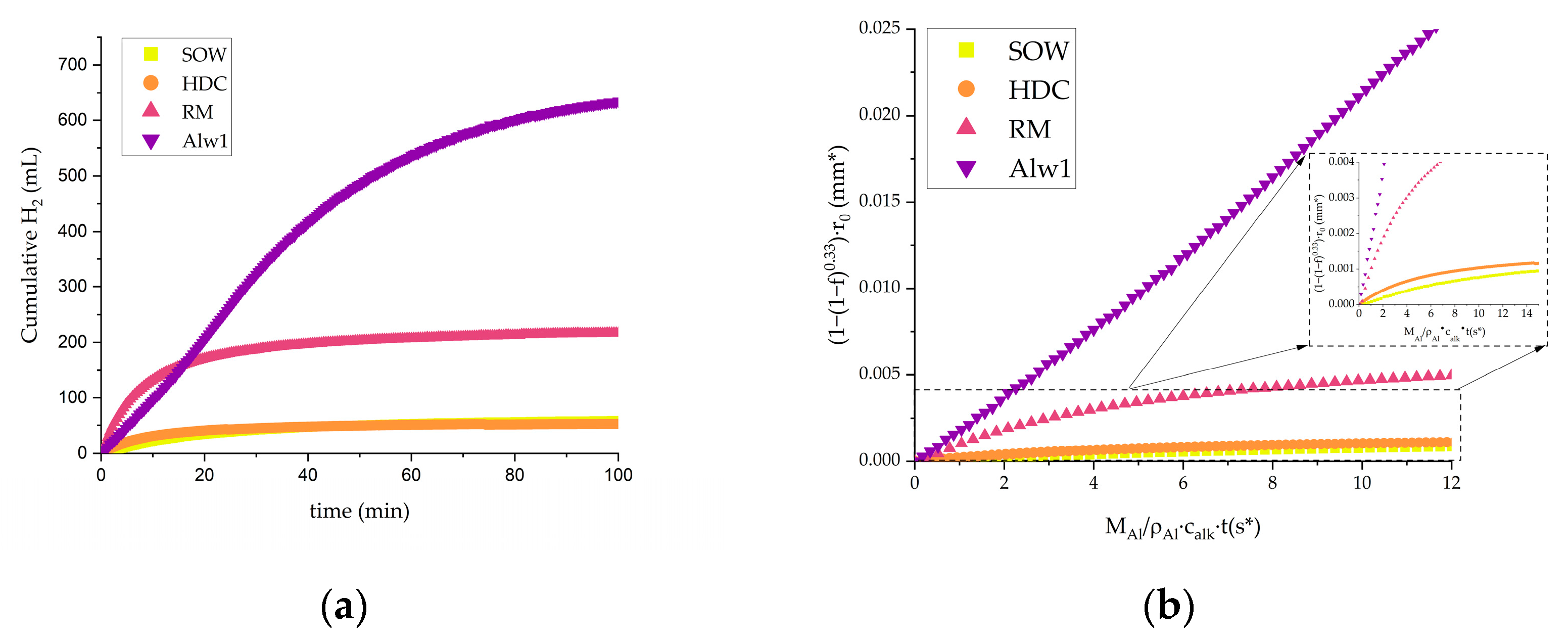
3.2. Hydrogen Production via Hydrolysis
3.3. Comparison of Hydrogen Generation and Reaction Efficiency
3.4. Power Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Supporting, S.; Resource, M.U.S. Geological Survey Mineral Resources Program—Program. 2007. Available online: https://www.usgs.gov/programs/mineral-resources-program (accessed on 15 November 2024).
- EN12258; Aluminium and aluminium alloys—Terms and definitions—Part 1: General terms. European Standard: Avenue Marnix 17, B-1000 Brussels, 2012.
- ISO/IEC 80079-34:2018; Potentially explosive atmospheres—Application of quality systems. European Standard: Avenue Marnix 17, B-1000 Brussels, 2018.
- Capuzzi, S.; Timelli, G. Preparation and melting of scrap in aluminum recycling: A review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Blomberg, J.; Söderholm, P. The economics of secondary aluminium supply: An econometric analysis based on European data. Resour. Conserv. Recycl. 2009, 53, 455–463. [Google Scholar] [CrossRef]
- Green John, A.S. Aluminum Recycling and Processing for Energy Conservation and Sustainability; ASM International: Materials Park, Ohio, USA, 2007. [Google Scholar]
- Padamata, S.K.; Yasinskiy, A.; Polyakov, P. A Review of Secondary Aluminum Production and Its Byproducts. JOM 2021, 73, 2603–2614. [Google Scholar] [CrossRef]
- Bell, S.; Davis, B.; Javaid, A.; Essadiqi, E. Final Report on Refining Technologies of Aluminum Enhanced Recycling, Action Plan 2000 on Climate Change, Minerals and Metals Program-The Government of Canada Action Plan 2000 on Climate Change Minerals and Metals Program, Managed by the Minerals and Meta. 2003. Available online: https://ressources-naturelles.canada.ca/sites/www.nrcan.gc.ca/files/mineralsmetals/pdf/mms-smm/busi-indu/rad-rad/pdf/2003-21(cf)cc-eng.pdf (accessed on 12 February 2025).
- Smith, Y.R.; Nagel, J.R.; Rajamani, R.K. Eddy current separation for recovery of non-ferrous metallic particles: A comprehensive review. Miner. Eng. 2019, 133, 149–159. [Google Scholar] [CrossRef]
- Schlesinger, M.E. Aluminum Recycling; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Mesina, M.B.; De Jong, T.P.R.; Dalmijn, W.L. Improvements in separation of non-ferrous scrap metals using an electromagnetic sensor. Phys. Sep. Sci. Eng. 2003, 12, 87–101. [Google Scholar] [CrossRef]
- Coates, G.; Rahimifard, S. Modelling of post-fragmentation waste stream processing within UK shredder facilities. Waste Manag. 2009, 29, 44–53. [Google Scholar] [CrossRef]
- Venkoba Rao, B.; Kapur, P.C.; Konnur, R. Modeling the size-density partition surface of dense-medium separators. Int. J. Miner. Process. 2003, 72, 443–453. [Google Scholar] [CrossRef]
- Hiraki, T.; Takeuchi, M.; Hisa, M.; Akiyama, T. Hydrogen production from waste aluminum at different temperatures, with LCA. Mater. Trans. 2005, 46, 1052–1057. [Google Scholar] [CrossRef]
- Mezulis, A.; Richter, C.; Lesnicenoks, P.; Knoks, A.; Varnagiris, S.; Urbonavicius, M.; Milcius, D.; Kleperis, J. Studies on Water–Aluminum Scrap Reaction Kinetics in Two Steps and the Efficiency of Green Hydrogen Production. Energies 2023, 16, 5554. [Google Scholar] [CrossRef]
- Urbonavicius, M.; Varnagiris, S.; Mezulis, A.; Lesnicenoks, P.; Knoks, A.; Richter, C.; Milcius, D.; Meirbekova, R.; Gunnarsson, G.; Kleperis, J. Hydrogen from industrial aluminium scraps: Hydrolysis under various conditions, modelling of pH behaviour and analysis of reaction by-product. Int. J. Hydrogen Energy 2024, 50, 431–446. [Google Scholar] [CrossRef]
- Urbonavicius, M.; Varnagiris, S.; Knoks, A.; Mezulis, A.; Kleperis, J.; Richter, C.; Meirbekova, R.; Gunnarsson, G.; Milcius, D. Enhanced Hydrogen Generation through Low-Temperature Plasma Treatment of Waste Aluminum for Hydrolysis Reaction. Materials 2024, 17, 2637. [Google Scholar] [CrossRef]
- Ingason, H.T.; Sigfusson, T.I. Processing of Aluminum Dross: The Birth of a Closed Industrial Process. JOM 2014, 66, 2235–2242. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.S.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Hong, J.P.; Jun, W.; Chen, H.Y.; Sun, B.D.; Li, J.J.; Chong, C. Process of aluminum dross recycling and life cycle assessment for Al-Si alloys and brown fused alumina. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2010, 20, 2155–2161. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Hydrolysis of aluminum dross material to achieve zero hazardous waste. J. Hazard. Mater. 2012, 209–210, 501–509. [Google Scholar] [CrossRef]
- Olivares-Ramirez, J.M.; de Jesus, A.M.; Jimenez-Sandoval, O.; Pless, R.C. Hydrogen Generation by Treatment of Aluminium Metal with Aqueous Solutions: Procedures and Uses. In Hydrogen Energy-Challenges and Perspectives; IntechOpen: London, UK, 2012. [Google Scholar]
- Zhao, Z.; Chen, X.; Hao, M. Hydrogen generation by splitting water with Al-Ca alloy. Energy 2011, 36, 2782–2787. [Google Scholar] [CrossRef]
- Küp Aylikci, N.; Mert, S.O.; Aylikci, V.; Bahceci, E.; Depci, T.; Oruç, Ö. Microhydrogen production with water splitting from daily used waste aluminum. Int. J. Hydrogen Energy 2021, 46, 28912–28924. [Google Scholar] [CrossRef]
- Alviani, V.N.; Hirano, N.; Watanabe, N.; Oba, M.; Uno, M.; Tsuchiya, N. Local initiative hydrogen production by utilization of aluminum waste materials and natural acidic hot-spring water. Appl. Energy 2021, 293, 116909. [Google Scholar] [CrossRef]
- Elsarrag, E.; Elhoweris, A.; Alhorr, Y. The production of hydrogen as an alternative energy carrier from aluminium waste. Energy Sustain. Soc. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Uggowitzer, P.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making sustainable aluminum by recycling scrap: The science of “dirty” alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar]
- Dangtungee, R.; Vatcharakajon, P.; Techawinyutham, L. Aluminium dross neutralization and its application as plant fertilizer In Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; Volume 52, pp. 2420–2426. [Google Scholar]
- Kleperis, J.; Boss, D.; Mezulis, A.; Zemite, L.; Lesnicenoks, P.; Knoks, A.; Dimanta, I. Analysis of the Role of the Latvian Natural Gas Network for the use of Future Energy Systems: Hydrogen from Res. Latv. J. Phys. Tech. Sci. 2021, 58, 214–226. [Google Scholar] [CrossRef]
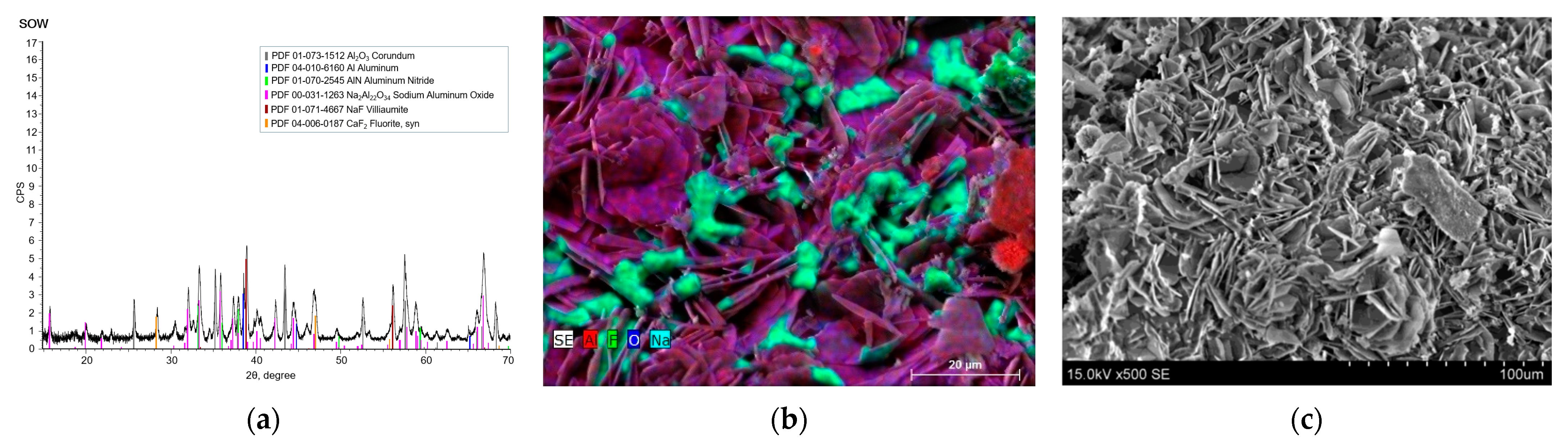
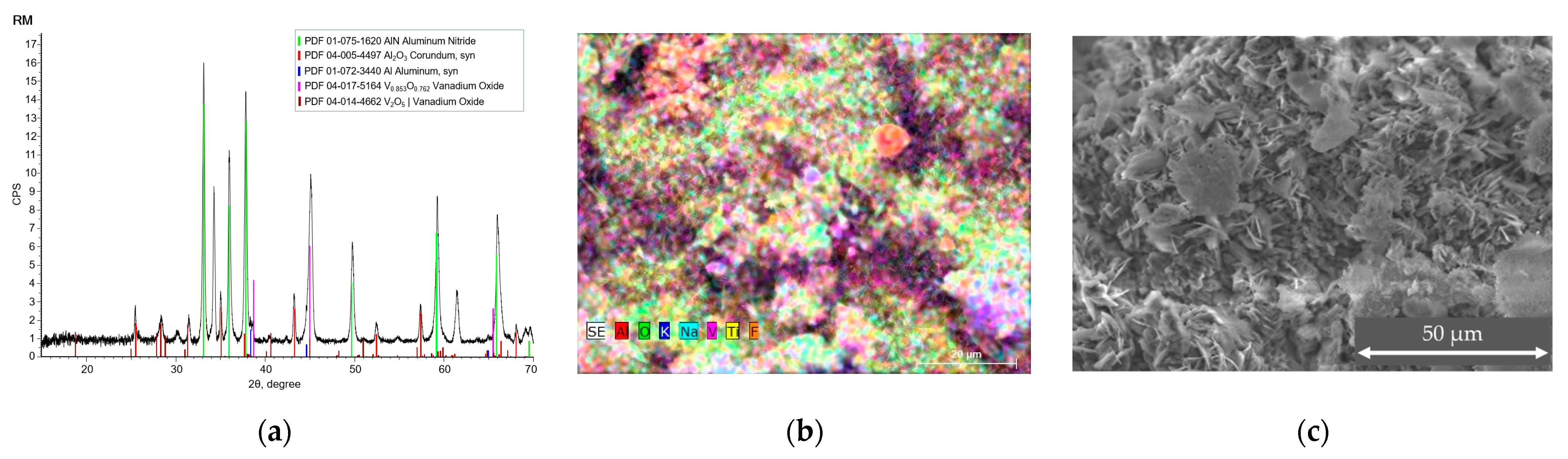


| SOW | RM | HDC | ||||
|---|---|---|---|---|---|---|
| Element | Start | End | Start | End | Start | End |
| Argon | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Carbon Dioxide | 0.1 | 0.0 | 0.2 | 0.2 | 0.2 | 0.2 |
| Hydrogen | 97.4 | 99.6 | 98.3 | 97.2 | 98.3 | 92.5 |
| Methane | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.4 |
| Nitrogen | 1.2 | 0.0 | 0.4 | 0.5 | 0.4 | 5.2 |
| Oxygen | 0.3 | 0.0 | 0.1 | 0.2 | 0.1 | 1.0 |
| Water | 0.7 | 0.1 | 1.9 | 1.9 | 0.9 | 0.5 |
| Element | SOW | RM | HDC | Alw1 |
|---|---|---|---|---|
| Argon | 0.5 | 0.0 | 0.0 | 0.0 |
| Carbon Dioxide | 0.1 | 0.1 | 0.0 | 0.1 |
| Ethane | 0.0 | 0.0 | 0.0 | 0.0 |
| Hydrogen | 68.3 | 94.4 | 99.3 | 98.2 |
| Methane | 0.0 | 0.0 | 0.2 | 0.0 |
| Nitrogen | 24.8 | 4.21 | 0.3 | 0.4 |
| Oxygen | 6.1 | 0.1 | 0.1 | 0.1 |
| Water | 0.2 | 0.2 | 0.1 | 1.1 |
| Sample | Yield of the Theoretical Value (%) | Reaction Rate Constant ks (mm·s−1) |
|---|---|---|
| RM | 65.7 | 0.001 |
| SOW | 24.9 | 0.000107 |
| HDC | 12.7 | 0.000229 |
| Alw1 | 99.9 | 0.0125 |
| Source | Al | Cr | Fe | Ca | Si | Na | K | Cu | Zn | Ni | Mg | Ti | Pb | Sn | Mn | B | C | F | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [21] | 43.3 | 0.088 | 4.32 | 0.45 | 10.9 | 0.8 | 0.21 | 1.17 | 0.9 | 0.87 | 1.85 | 0.27 | 0.053 | – | 0.2 | – | |||
| [20] | 73.05 | – | 0.91 | 2.79 | 7.13 | 5.78 | 9.26 | 1.08 | |||||||||||
| [25] | 56 | 12.7 | 0.000229 | ||||||||||||||||
| [26] | 11.65 | 0.18 | 1.51 | 0.08 | 17.96 | 0.05 | – | – | – | 0.02 | |||||||||
| [26] | 40.02 | 0.08 | 0.34 | 0.07 | 0.48 | 0.02 | – | – | – | 0.86 | |||||||||
| Alw1 [16] | 94.3 | – | – | 0.6 | 5.0 | 0.1 | |||||||||||||
| RM | 29.19–32.46 | – | – | – | – | – | Trace–0.71 | Trace–0.1 | – | – | – | Trace–0.28 | – | – | – | – | Trace–5.70 | Trace–1.98 | 13.19–57.76 |
| SOW | 19.11–48.20 | – | Trace–0.29 | Trace–0.29 | – | 4.75–18.96 | – | – | – | – | – | – | – | – | 19.03–53.76 | 7.58–27.74 | |||
| HDC | 58.81 | trace | 0.24 | 29.20 | trace | 1.66 | trace | – | – | – | Trace–28.32 | 8.42–16.60 | 1.67–4.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoks, A.; Mezulis, A.; Richter, C.; Varnagiris, S.; Urbonavicius, M.; Milcius, D.; Meirbekova, R.; Gunnarsson, G.; Jurjevs, D.; Sika, R.; et al. Investigation of Aluminium White Dross for Hydrogen Generation Hydrolysis in Low-Concentration Alkali. Appl. Sci. 2025, 15, 2640. https://doi.org/10.3390/app15052640
Knoks A, Mezulis A, Richter C, Varnagiris S, Urbonavicius M, Milcius D, Meirbekova R, Gunnarsson G, Jurjevs D, Sika R, et al. Investigation of Aluminium White Dross for Hydrogen Generation Hydrolysis in Low-Concentration Alkali. Applied Sciences. 2025; 15(5):2640. https://doi.org/10.3390/app15052640
Chicago/Turabian StyleKnoks, Ainars, Ansis Mezulis, Christiaan Richter, Sarunas Varnagiris, Marius Urbonavicius, Darius Milcius, Rauan Meirbekova, Gudmundur Gunnarsson, Daniels Jurjevs, Raitis Sika, and et al. 2025. "Investigation of Aluminium White Dross for Hydrogen Generation Hydrolysis in Low-Concentration Alkali" Applied Sciences 15, no. 5: 2640. https://doi.org/10.3390/app15052640
APA StyleKnoks, A., Mezulis, A., Richter, C., Varnagiris, S., Urbonavicius, M., Milcius, D., Meirbekova, R., Gunnarsson, G., Jurjevs, D., Sika, R., & Kleperis, J. (2025). Investigation of Aluminium White Dross for Hydrogen Generation Hydrolysis in Low-Concentration Alkali. Applied Sciences, 15(5), 2640. https://doi.org/10.3390/app15052640









