Characterization of Non-Polar and Polar Bioactive Compounds Obtained by Pressurized Biobased Solvents from Different Arctium lappa L. Root Ecotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples and Sample Preparation
2.3. Optimizing the Mixture Solvent Composition and Temperature to Obtain Phenolic Compounds by PLE
2.4. Total Phenolic Content (TPC)
2.5. Antioxidant Capacity Determination
2.5.1. DPPH Radical Scavenging Assay
2.5.2. Oxygen Radical Absorbance Capacity (ORAC)
2.6. Anticholinergic Capacity Determination
2.7. Characterization of Non-Polar Fraction by GC-MS
2.8. Phenolic Characterization by HPLC-DAD-IT-MS
2.9. Statistical Analysis
3. Results
3.1. Optimization of PLE Extraction by a Simplex-Centroid Experimental Design
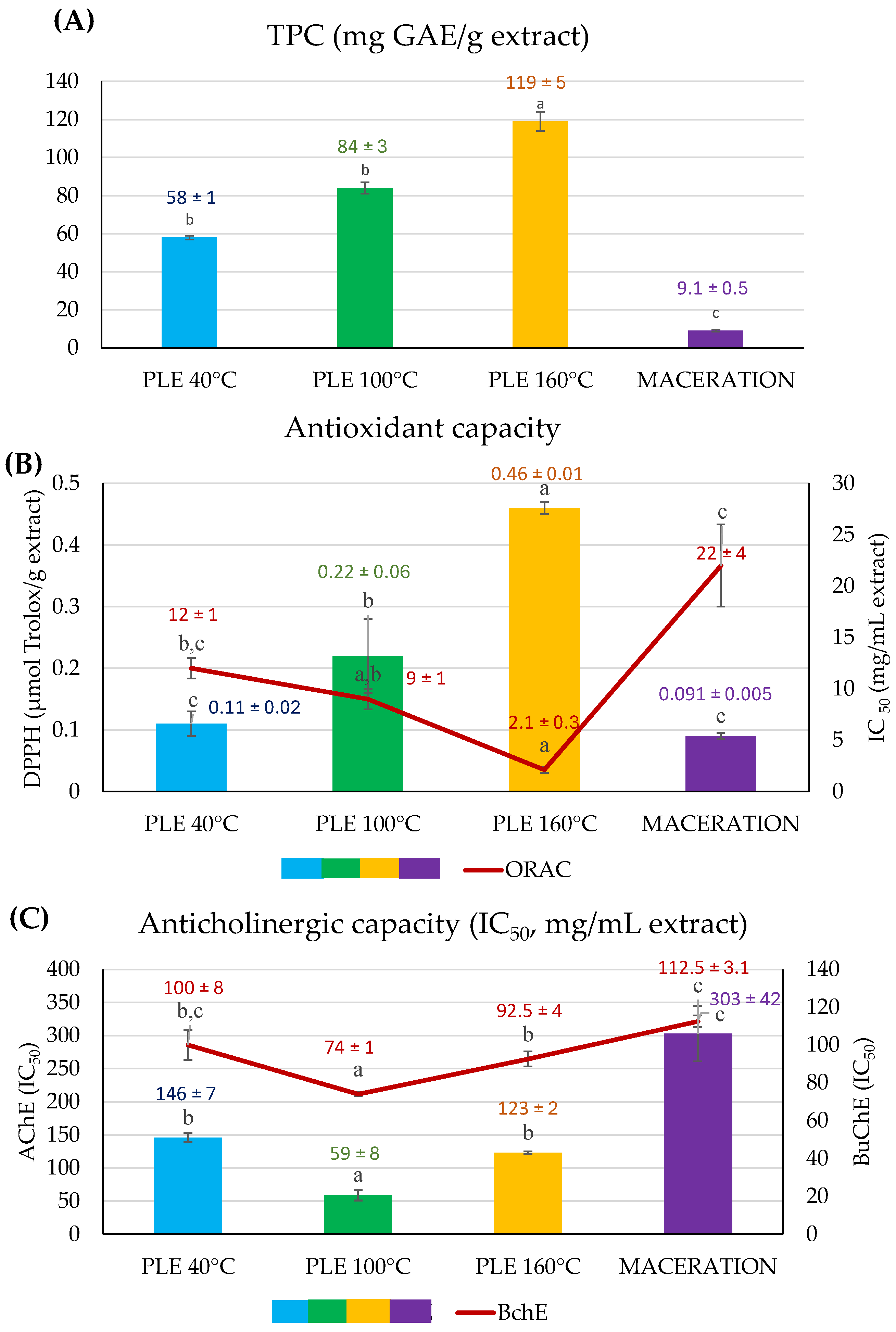
3.2. Optimizing the PLE Temperature According to the Recovery of Antioxidant and Anticholinergic Phenolic Compounds
3.3. Influence of the Growing Conditions on the Bioactivity of Burdock Roots
3.4. Characterization of Non-Polar Compounds in Different Ecotypes of Burdock Roots by GC-MS
3.5. Phenolic Characterization of Different Ecotypes of Burdock Roots by HPLC-IT-MS
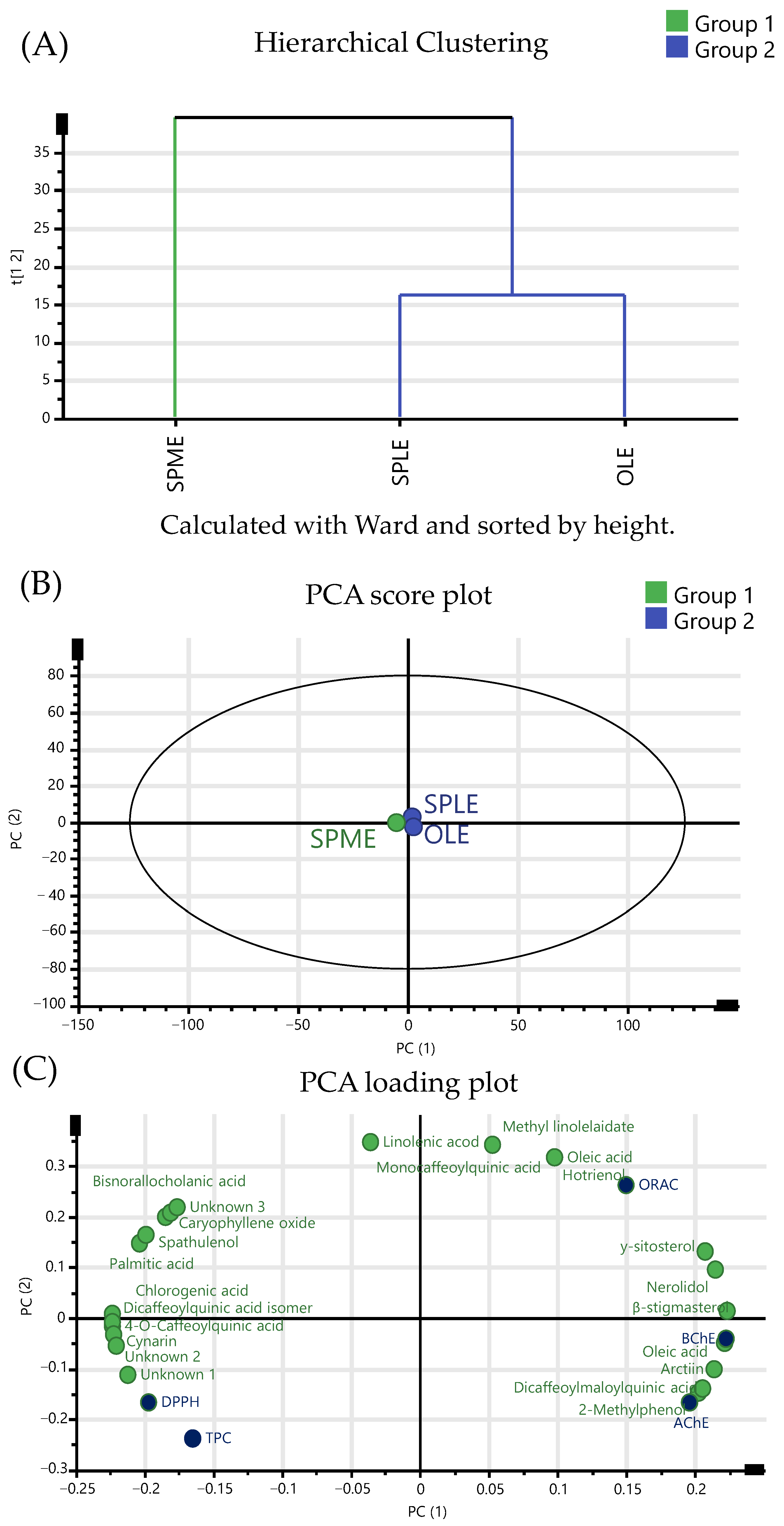
3.6. Evaluation of the Role of Growing Conditions of Burdock Roots in Their Bioactivity, Non-Polar, and Polar Composition
4. Discussion
4.1. Influence of Biobased Solvent Composition and Extraction Temperature on the Recovery of Phenolic Compounds from Burdock Roots
4.2. Bioactive, Terpenoid, and Phenolic Profile Comparison Among Different Ecotypes of Burdock Roots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radha; Kumari, N.; Prakash, S.; Sharma, N.; Puri, S.; Thakur, M.; Singh, J.; Kumar, M. Medicinal and Aromatic Plants as Potential Sources of Bioactives Along with Health-Promoting Activities. Curr. Food Sci. Technol. Rep. 2024, 2, 359–376. [Google Scholar] [CrossRef]
- De Souza, A.R.C.; De Oliveira, T.L.; Fontana, P.D.; Carneiro, M.C.; Corazza, M.L.; De Messias Reason, I.J.; Bavia, L. Phytochemicals and Biological Activities of Burdock (Arctium lappa L.) Extracts: A Review. Chem. Biodivers. 2022, 19, e202200615. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-C.; Weng, H.-K.; Hsu, Y.-S.; Huang, P.-J.; Wang, Y.-K. Aqueous Extract of Arctium lappa L. Root (Burdock) Enhances Chondrogenesis in Human Bone Marrow-Derived Mesenchymal Stem Cells. BMC Complement. Med. Ther. 2020, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Beta, T.; Chai, Z.; Zhang, X.; Li, Y.; Huang, W. Effect of in Vitro Gastro-Intestinal Digestion on the Phenolic Composition and Antioxidant Capacity of Burdock Roots at Different Harvest Time. Food Chem. 2021, 358, 129897. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Valdés, A.; Gallego, R.; Suárez-Montenegro, Z.J.; Alarcón, M.; Ibañez, E.; Alvarez-Rivera, G.; Cifuentes, A. Blood–Brain Barrier Permeability Study of Potential Neuroprotective Compounds Recovered From Plants and Agri-Food by-Products. Front. Nutr. 2022, 9, 924596. [Google Scholar] [CrossRef]
- Yousaf, M.; Hammond, N.L.; Peng, J.; Wahyuono, S.; McIntosh, K.A.; Charman, W.N.; Mayer, A.M.S.; Hamann, M.T. New Manzamine Alkaloids from an Indo-Pacific Sponge. Pharmacokinetics, Oral Availability, and the Significant Activity of Several Manzamines against HIV-I, AIDS Opportunistic Infections, and Inflammatory Diseases. J. Med. Chem. 2004, 47, 3512–3517. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Amador-Luna, V.M.; Benešová, K.; Pernica, M.; Parada-Alfonso, F.; Ibáñez, E. Biorefinery Approach with Green Solvents for the Valorization of Citrus Reticulata Leaves to Obtain Antioxidant and Anticholinergic Extracts. Food Chem. 2024, 456, 140034. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.D.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Trujillo-Roldán, M.A. Factors That Influence the Extraction Methods of Terpenes from Natural Sources. Chem. Pap. 2024, 78, 2783–2810. [Google Scholar] [CrossRef]
- Zarate-Vilet, N.; Gué, E.; Delalonde, M.; Wisniewski, C. Valorization of Grapefruit (Citrus × Paradisi) Processing Wastes. In Mediterranean Fruits Bio-Wastes; Ramadan, M.F., Farag, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 179–220. ISBN 978-3-030-84435-6. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Silva, S.S.; Justi, M.; Chagnoleau, J.-B.; Papaiconomou, N.; Fernandez, X.; Santos, S.A.O.; Passos, H.; Ferreira, A.M.; Coutinho, J.A.P. Using Biobased Solvents for the Extraction of Phenolic Compounds from Kiwifruit Industry Waste. Sep. Purif. Technol. 2023, 304, 122344. [Google Scholar] [CrossRef]
- Del Pilar Sánchez-Camargo, A.; Pleite, N.; Herrero, M.; Cifuentes, A.; Ibáñez, E.; Gilbert-López, B. New Approaches for the Selective Extraction of Bioactive Compounds Employing Bio-Based Solvents and Pressurized Green Processes. J. Supercrit. Fluids 2017, 128, 112–120. [Google Scholar] [CrossRef]
- Herrero, M. Towards Green Extraction of Bioactive Natural Compounds. Anal. Bioanal. Chem. 2024, 416, 2039–2047. [Google Scholar] [CrossRef]
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange Peel Waste Valorisation through Limonene Extraction Using Bio-Based Solvents. Biochem. Eng. J. 2019, 151, 107298. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.-E.; Ranga, F.; Roman, I.; Khaoula, H.; Boudaya, E.; Ltaief, A.B.; Aouani, W.; Thiamrat, M.; Vodnar, D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules 2022, 27, 3771. [Google Scholar] [CrossRef]
- Reyes-Vaquero, L.; Álvarez-Rivera, G.; Mendiola, J.A.; Del Villar-Martínez, A.A.; Ibáñez, E.; Bueno, M. Utilizing Green Solvents in Compressed Fluids Technologies for Extracting Bioactive Compounds from Ruta graveolens L. Ind. Crops Prod. 2024, 216, 118717. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.D.R.; Sosa Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant Growth Promoting Rhizobacteria Improve the Antioxidant Status in Mentha Piperita Grown under Drought Stress Leading to an Enhancement of Plant Growth and Total Phenolic Content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Farhadi, N.; Ghassemi-Golezani, K. Physiological Changes of Mentha Pulegium in Response to Exogenous Salicylic Acid under Salinity. Sci. Hortic. 2020, 267, 109325. [Google Scholar] [CrossRef]
- Brahmi, F.; Lounis, N.; Mebarakou, S.; Guendouze, N.; Yalaoui-Guellal, D.; Madani, K.; Boulekbache-Makhlouf, L.; Duez, P. Impact of Growth Sites on the Phenolic Contents and Antioxidant Activities of Three Algerian Mentha Species (M. pulegium L., M. rotundifolia (L.) Huds., and M. spicata L.). Front. Pharmacol. 2022, 13, 886337. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Sgrò, F.; Raimondo, F.M.; Ingegneri, M.; Mastracci, L.; D’Angelo, V.; Germanò, M.P.; Trombetta, D.; Smeriglio, A. Pedoclimatic Conditions Influence the Morphological, Phytochemical and Biological Features of Mentha pulegium L. Plants 2022, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Delfine, S.; Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Menichini, F.; Senatore, F. Variation of Malva Sylvestris Essential Oil Yield, Chemical Composition and Biological Activity in Response to Different Environments across Southern Italy. Ind. Crops Prod. 2017, 98, 29–37. [Google Scholar] [CrossRef]
- Aboukhalid, K.; Al Faiz, C.; Douaik, A.; Bakha, M.; Kursa, K.; Agacka-Mołdoch, M.; Machon, N.; Tomi, F.; Lamiri, A. Influence of Environmental Factors on Essential Oil Variability in Origanum compactum Benth. Growing Wild in Morocco. Chem. Biodivers. 2017, 14, e1700158. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and Supercritical Fluid Extraction of Bioactive Compounds from Plants, Food-by-Products, Seaweeds and Microalgae—An Update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Plaza, M.; Oliveira, D.; Nilsson, A.; Turner, C. Green and Efficient Extraction Method to Determine Polyphenols in Cocoa and Cocoa Products. Food Anal. Methods 2017, 10, 2677–2691. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In Vitro Neuroprotective Potential of Terpenes from Industrial Orange Juice by-Products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Fales, H.M.; Spande, T.F.; Basile, A.S. A Gas Chromatographic–Mass Spectral Assay for the Quantitative Determination of Oleamide in Biological Fluids. Anal. Biochem. 1999, 270, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, Y.; Zhang, Y.; Liu, F.; Chen, H.; Liu, J.; Handberg, E.S.; Chagovets, V.V.; Chingin, K. Molecular Analysis of Semen-like Odor Emitted by Chestnut Flowers Using Neutral Desorption Extractive Atmospheric Pressure Chemical Ionization Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.M.; Hameed, I.H.; Ibraheem, O.A. Antimicrobial Activity and Spectral Chemical Analysis of MethanolicLeaves Extract of Adiantum Capillus-Veneris Using GC-MS and FT-IR Spectroscopy. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 369–385. [Google Scholar]
- Maietta, M.; Colombo, R.; Lavecchia, R.; Sorrenti, M.; Zuorro, A.; Papetti, A. Artichoke (Cynara cardunculus L. var. Scolymus) Waste as a Natural Source of Carbonyl Trapping and Antiglycative Agents. Food Res. Int. 2017, 100, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Pistón, M.; Machado, I.; Branco, C.S.; Cesio, V.; Heinzen, H.; Ribeiro, D.; Fernandes, E.; Chisté, R.C.; Freitas, M. Infusion, Decoction and Hydroalcoholic Extracts of Leaves from Artichoke (Cynara cardunculus L. subsp. cardunculus) Are Effective Scavengers of Physiologically Relevant ROS and RNS. Food Res. Int. 2014, 64, 150–156. [Google Scholar] [CrossRef]
- Skowrońska, W.; Granica, S.; Dziedzic, M.; Kurkowiak, J.; Ziaja, M.; Bazylko, A. Arctium lappa and Arctium Tomentosum, Sources of Arctii Radix: Comparison of Anti-Lipoxygenase and Antioxidant Activity as Well as the Chemical Composition of Extracts from Aerial Parts and from Roots. Plants 2021, 10, 78. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Li, M.; Yuan, Y.; Cui, S.; Wang, G.; Li, R. An Integrated Strategy for Revealing the Pharmacological Changes Based on Metabolites Profiling and Network Pharmacology: Arctiin as an Example. J. Chromatogr. B 2020, 1157, 122270. [Google Scholar] [CrossRef]
- Zhang, X.; Herrera-Balandrano, D.D.; Huang, W.; Chai, Z.; Beta, T.; Wang, J.; Feng, J.; Li, Y. Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China. Foods 2021, 10, 2095. [Google Scholar] [CrossRef]
- Su, J.-Y.; Chen, Y.; Yang, C.-Y. Impact of Nutrient from Aqueous Extract of Burdock Roots and Ultrasonic Stress on the Growth and β-Glucosidase Activity of Lactiplantibacillus Plantarum FEL112. LWT 2023, 175, 114495. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Yang, C.-Y. Assessment of Ethanolic Extraction of Chlorogenic Acid, Cynarin, and Polyphenols from Burdock (Arctium lappa L.) Roots Under Ultrasound. Molecules 2024, 29, 5115. [Google Scholar] [CrossRef]
- Petkova, N.; Ivanov, I.; Mihaylova, D.; Lante, A. Effect of pressure liquid extraction and ultrasonic irradiation frequency on inulin, phenolic content and antioxidant activity in burdock (Arctium lappa L.) ROOTS. Acta Sci. Pol. Hortorum Cultus 2020, 19, 125–133. [Google Scholar] [CrossRef]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Rapinel, V.; Tomao, V.; Stathopoulos, P.; Skaltsounis, A.L.; Tabasso, S.; Jacques, L.; Chemat, F. Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent. Foods 2022, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Sáenz De Miera, B.; Cañadas, R.; Santiago, R.; Díaz, I.; González-Miquel, M.; González, E.J. A Pathway to Improve Detoxification Processes by Selective Extraction of Phenols and Sugars from Aqueous Media Using Sustainable Solvents. Sep. Purif. Technol. 2022, 299, 121675. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Evaluation of Bio-Based Solvents for Phenolic Acids Extraction from Aqueous Matrices. J. Mol. Liq. 2021, 338, 116930. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard Reaction Chemistry in Formation of Critical Intermediates and Flavour Compounds and Their Antioxidant Properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. ISBN 978-0-12-816911-7. [Google Scholar]
- Pérez-Ochoa, M.L.; Vera-Guzmán, A.M.; Mondragón-Chaparro, D.M.; Sandoval-Torres, S.; Carrillo-Rodríguez, J.C.; Mayek-Pérez, N.; Chávez-Servia, J.L. Effects of Annual Growth Conditions on Phenolic Compounds and Antioxidant Activity in the Roots of Eryngium Montanum. Plants 2023, 12, 3192. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, S.; Liu, J.; Tian, Y.; Zheng, X.; Wang, S.; Xie, Y.; Li, Y.; Jia, P. Planting Conditions Can Enhance the Bioactivity of Mulberry by Affecting Its Composition. Front. Plant Sci. 2023, 14, 1133062. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Wani, A.W.; Bakshi, M.; Dhiman, Z. Exploring Phenolic Compounds as Natural Stress Alleviators in Plants- a Comprehensive Review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Rodriguez. Zunino Optimización de Ensayos de Inhibición de Acetilcolinesterasa En Insectos. Rev. Fac. Cienc. Exactas Físicas Nat. 2018, 5, 2362–2539. [Google Scholar]
- Pfeifer, M.T.; Koepke, P.; Reuder, J. Effects of Altitude and Aerosol on UV Radiation. J. Geophys. Res. 2006, 111, 2005JD006444. [Google Scholar] [CrossRef]
- Zidorn, C. Altitudinal Variation of Secondary Metabolites in Flowering Heads of the Asteraceae: Trends and Causes. Phytochem. Rev. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Suleiman, M.H.A.; ALaerjani, W.M.A.; Mohammed, M.E.A. Influence of Altitudinal Variation on the Total Phenolic and Flavonoid Content of Acacia and Ziziphus Honey. Int. J. Food Prop. 2020, 23, 2077–2086. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Lu, C.-W.; Lin, T.-Y.; Hsieh, P.; Chiu, K.-M.; Lee, M.-Y.; Wang, S.-J. Cynarin, a Caffeoylquinic Acid Derivative in Artichoke, Inhibits Exocytotic Glutamate Release from Rat Cortical Nerve Terminals (Synaptosomes). Neurochem. Int. 2023, 167, 105537. [Google Scholar] [CrossRef]
- Carlotto, J.; Da Silva, L.M.; Dartora, N.; Maria-Ferreira, D.; Sabry, D.D.A.; Filho, A.P.S.; De Paula Werner, M.F.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M.; et al. Identification of a Dicaffeoylquinic Acid Isomer from Arctium lappa with a Potent Anti-Ulcer Activity. Talanta 2015, 135, 50–57. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Liu, P.; Li, M.; Dong, H.; Qiao, X. Semi-Preparative Separation of 10 Caffeoylquinic Acid Derivatives Using High Speed Counter-Current Chromatogaphy Combined with Semi-Preparative HPLC from the Roots of Burdock (Arctium lappa L.). Molecules 2018, 23, 429. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, M.; Zuo, Z. Overview of the Anti-Inflammatory Effects, Pharmacokinetic Properties and Clinical Efficacies of Arctigenin and Arctiin from Arctium lappa L. Acta Pharmacol. Sin. 2018, 39, 787–801. [Google Scholar] [CrossRef]
- Cha, H.J.; Lee, G.T.; Lee, K.S.; Lee, K.K.; Hong, J.T.; Lee, N.K.; Kim, S.-Y.; Lee, B.M.; An, I.-S.; Hahn, H.J.; et al. Photoprotective Effect of Arctiin against Ultraviolet B-Induced Damage in HaCaT Keratinocytes Is Mediated by microRNA Expression Changes. Mol. Med. Rep. 2014, 10, 1363–1370. [Google Scholar] [CrossRef]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A Systematic Review on the Neuroprotective Perspectives of Beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; De Carvalho, J.E.; et al. Antioxidant, Anti-Inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium Guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Manjima, R.B.; Ramya, S.; Kavithaa, K.; Paulpandi, M.; Saranya, T.; Harysh Winster, S.B.; Balachandar, V.; Arul, N. Spathulenol Attenuates 6-Hydroxydopamine Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Gene Rep. 2021, 25, 101396. [Google Scholar] [CrossRef]
- Golbaz, F.; Zarei, S.; Garakani, F. The Essential Oil Composition of Arctium lappa Root and Leaf. Iran. J. Pharm. Sci. 2018, 14, 1–6. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicine, 3rd ed.; Pharmaceutical Press: London, UK, 2007; ISBN 9780853696230. [Google Scholar]
- Ghedira, K.; Goetz, P. Arctium lappa L. (Asteraceae): Bardane. Phytothérapie 2013, 11, 376–380. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic Profile of the Bioactive Compounds of Burdock (Arctium lappa) Seeds, Roots and Leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Moro, T.M.A.; Clerici, M.T. Burdock (Arctium lappa L.) Roots as a Source of Inulin-Type Fructans and Other Bioactive Compounds: Current Knowledge and Future Perspectives for Food and Non-Food Applications. Food Res. Int. 2021, 141, 109889. [Google Scholar] [CrossRef]
- Fideles, S.O.M.; De Cássia Ortiz, A.; Buchaim, D.V.; De Souza Bastos Mazuqueli Pereira, E.; Parreira, M.J.B.M.; De Oliveira Rossi, J.; Da Cunha, M.R.; De Souza, A.T.; Soares, W.C.; Buchaim, R.L. Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System. Antioxidants 2023, 12, 149. [Google Scholar] [CrossRef]
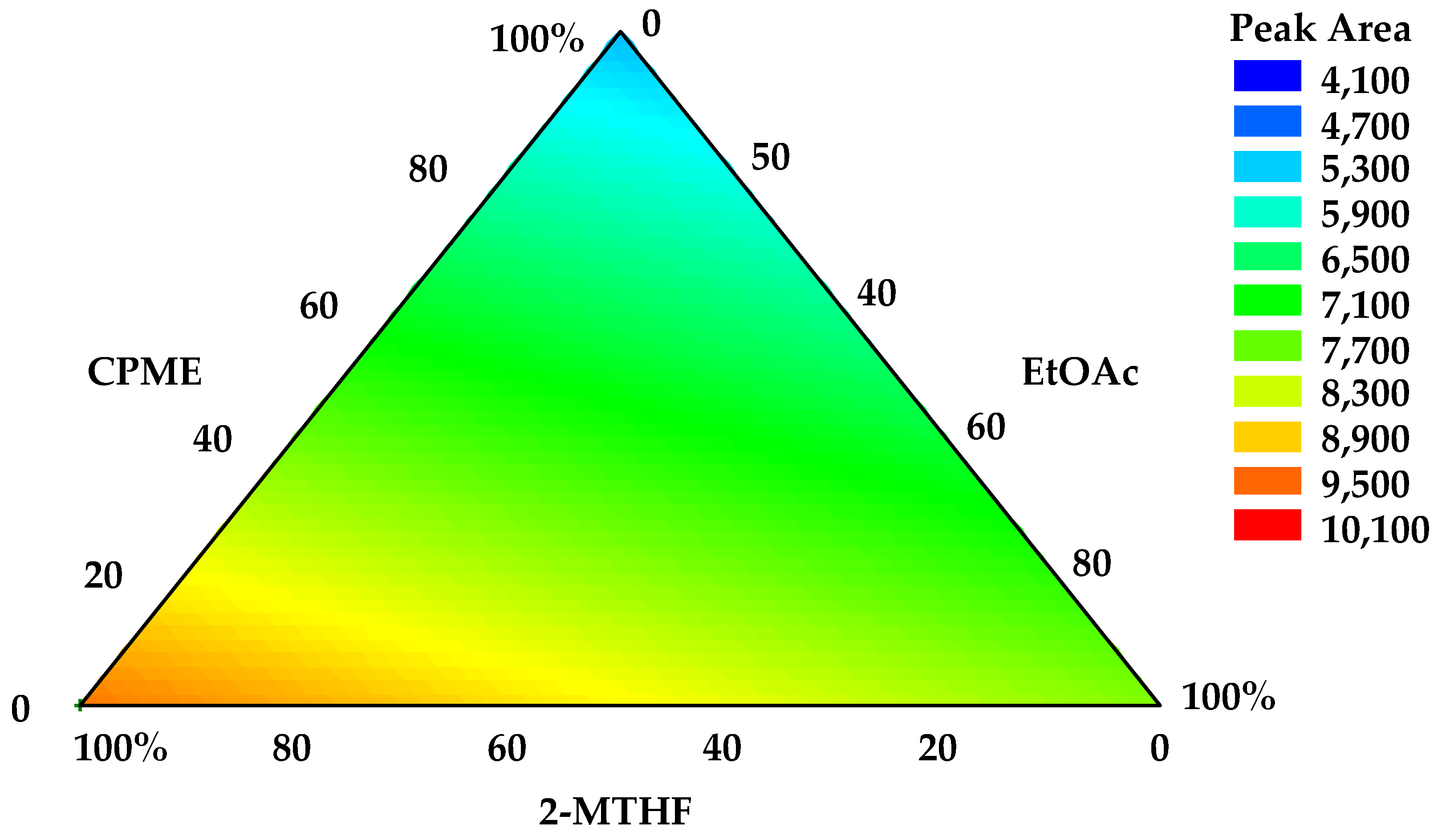
| Independent Variables | Dependent Variable | |||
|---|---|---|---|---|
| Mixture (% solvent, v/v) | ||||
| Run | CPME | 2-MTHF | EtOAc | Total Peak Area at 280 nm |
| 1 | 50 | 0 | 50 | 4181 ± 389 e |
| 2 | 33 | 33 | 33 | 6843 ± 93 c |
| 3 | 100 | 0 | 0 | 5998 ± 15 d |
| 4 | 50 | 50 | 0 | 8308 ± 258 b |
| 5 | 0 | 0 | 100 | 9484 ± 17 a |
| 6 | 0 | 100 | 0 | 9363 ± 135 a |
| 7 | 0 | 50 | 50 | 8062 ± 306 b |
| Method | SPLE | OLE | SPME |
|---|---|---|---|
| TPC (mg gallic acid/g extract) | 22.9 ± 0.9 c | 48.8 ± 0.2 b | 64 ± 5 a |
| DPPH• (uM Trolox/g extract) | 0.21 ± 0.01 c | 0.43 ± 0.03 b | 0.739 ± 0.005 a |
| ORAC (IC50, mg/mL extract) | 9 ± 1 b | 4.8 ± 0.3 a | 3.1 ± 0.3 a |
| AChE (IC50, mg/mL extract) | 59 ± 8 b | 94 ± 2 c | 29 ± 2 a |
| BChE (IC50, mg/mL extract) | 75 ± 2 b | 83 ± 2 b | 43 ± 3 a |
| ID | Proposed Compound | Rt (min) | Molecular Formula | Measured Mass | Main Fragments Ions (m/z) | SPLE | OLE | SPME |
|---|---|---|---|---|---|---|---|---|
| 1 | 4-Methyloctanoic acid | 7.468 | C9H18O2 | 158 | 129, 101, 99, 83, 73, 60, 57, 55, 45, 43, 41 | 359 ± 13 | 384 ± 26 | 401 ± 16 |
| 2 | 2-Methylphenol | 7.502 | C7H8O | 108 | 108, 107, 90, 89, 79, 77, 63, 53, 51, 44 | 740 ± 21 | 863 ± 40 | 609 ± 67 |
| 3 | Oleic acid | 7.758 | C18H34O2 | 282 | 137, 125, 111, 101, 98, 97, 83, 81, 73, 69, 67, 60, 55, 43, 41 | 33 0± 51 | 390 ± 14 | 113 ± 17 |
| 4 | Palmitic acid | 13.706 | C16H32O2 | 256 | 256, 213, 129, 97, 87, 85, 83, 73, 71, 69, 60, 57, 55, 45, 43, 41 | 675 ± 73 | 609 ± 34 | 746 ± 11 |
| 5 | Methyl linolelaidate | 18.267 | C19H34O2 | 294 | 149, 135, 109, 95, 82, 81, 79, 69, 67, 55, 54, 43, 41 | 2598 ± 278 | 1371 ± 47 | 1645 ± 26 |
| 6 | Linolenic acid | 18.454 | C18H30O2 | 278 | 278, 149, 108, 95, 93, 79, 67, 55, 41 | 748 ± 80 | 579 ± 5 | 677 ± 37 |
| 7 | Oleic acid amide | 27.121 | C18H35NO | 281 | 114, 100, 86, 72, 69, 67, 60, 59, 55, 44, 43, 41 | 2749 ± 140 | - | - |
| 8 | Hotrienol | 31.878 | C10H16O | 152 | 119, 105, 91, 82, 79, 71, 67, 55, 51, 43, 41 | 1076 ± 31 | - | - |
| 9 | Nerolidol | 44.181 | C15H26O | 222 | 207, 161, 136, 119, 107, 97, 93, 81, 79, 71, 69, 57, 55, 44, 43, 41 | 294 ± 1 | 258 ± 26 | 124 ± 19 |
| 10 | β-stigmasterol | 44.609 | C29H48O | 412 | 412, 351, 300, 255, 159, 133, 119, 105, 97, 91, 83, 81, 79, 69, 57, 55, 43 | 1884 ± 163 | 1919 ± 40 | 867 ± 9 |
| 11 | γ-sitosterol | 45.419 | C29H50O | 414 | 414, 396, 329, 303, 255, 213, 173, 161, 159, 147, 145, 133, 131, 121, 118, 107, 97, 95, 85, 81, 69, 67, 57, 55, 43, 41 | 4093 ± 367 | 3321 ± 4 | 1623 ± 161 |
| 12 | Caryophyllene oxide | 48.163 | C15H24O | 220 | 177, 161, 147, 133, 121, 109, 96, 93, 81, 79, 69, 67, 55, 53, 43, 41 | 271 ± 13 | 97 ± 1 | 375 ± 23 |
| 13 | Spathulenol | 49.576 | C15H26O | 222 | 222, 207, 189, 133, 121, 109, 107, 95, 81, 79, 69, 67, 55, 43 | 162 ± 3 | 115 ± 78 | 205 ± 3 |
| 14 | Bisnorallocholanic acid | 49.767 | C22H36O2 | 332 | 217, 215, 161, 149, 147, 135, 133, 124, 121, 119, 109, 108, 107, 105, 97, 95, 93, 91, 81, 79, 77, 73, 69, 67, 57, 55, 53, 45, 44, 43 | 313 ± 15 | 101 ± 11 | 426 ± 21 |
| No. | Compound | Rt (min) | [M-H]− | MS2 Ions | SPLE PLE | OLE PLE | SPME PLE |
|---|---|---|---|---|---|---|---|
| 1 | Chlorogenic acid | 8.8 | 374.9 [M-H + Na]− | 353, 200.8, 190.9, 178.8, 172.9, 160.9, 135 | 452 ± 79 | 264 ± 18 | 2069 ± 75 |
| 2 | 4-O-Caffeoylquinic acid | 13.3 | 353.0 | 190.8, 178.8, 172.8, 135, 127 | 167 ± 43 | 121 ± 10 | 1681 ± 4 |
| 3 | Dicaffeoylquinic acid (Cynarin) | 20.7 | 515.2 | 379, 353, 334.9, 190.9, 178.9 | 1392 ± 81 | 658 ± 84 | 14,039 ± 56 |
| 4 | Dicaffeoylquinic acid isomer | 21.8 | 537 [M-H + Na]− | 515.1, 375, 353, 335, 264.9, 190.9, 178.9, 172.9 | 60 ± 6 | 181 ± 21 | 4121 ± 214 |
| 5 | Arctiin | 28.9 | 533 | 515, 371, 355, 289, 249, 151, 136, 121 | 61 ± 14 | 95 ± 6 | - |
| 6 | Dicaffeoylmaloylquinic acid | 29.7 | 631 | 352.8, 325, 250.9, 190.9, 172.9, 160.8, 135 | 214 ± 23 | 395 ± 12 | - |
| 7 | Monocaffeoylquinic acid | 53.3 | 353.1 | 333, 394.9, 232.8, 192.9, 179 | 236 ± 21 | - | - |
| 8 | Unknown 1 | 54.1 | 441.3 | 429, 422.2, 399.4, 383.4, 340.9, 327.2, 325, 304, 182.8, 124.9 | 286 ± 7 | 336 ± 5 | 479 ± 5 |
| 9 | Unknown 2 | 54.5 | 471.4 | 451.3, 441.2, 407.2, 367.3, 337, 218.9, 187 | 298 ± 9 | 380 ± 26 | 1137 ± 16 |
| 10 | Unknown 3 | 58.4 | 383.4 | 365.2, 337.2 | 3115 ± 60 | - | 4540 ± 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, E.; Domínguez-Rodríguez, G.; Mannina, L.; Cifuentes, A.; Ibáñez, E. Characterization of Non-Polar and Polar Bioactive Compounds Obtained by Pressurized Biobased Solvents from Different Arctium lappa L. Root Ecotypes. Appl. Sci. 2025, 15, 2491. https://doi.org/10.3390/app15052491
Romano E, Domínguez-Rodríguez G, Mannina L, Cifuentes A, Ibáñez E. Characterization of Non-Polar and Polar Bioactive Compounds Obtained by Pressurized Biobased Solvents from Different Arctium lappa L. Root Ecotypes. Applied Sciences. 2025; 15(5):2491. https://doi.org/10.3390/app15052491
Chicago/Turabian StyleRomano, Enrico, Gloria Domínguez-Rodríguez, Luisa Mannina, Alejandro Cifuentes, and Elena Ibáñez. 2025. "Characterization of Non-Polar and Polar Bioactive Compounds Obtained by Pressurized Biobased Solvents from Different Arctium lappa L. Root Ecotypes" Applied Sciences 15, no. 5: 2491. https://doi.org/10.3390/app15052491
APA StyleRomano, E., Domínguez-Rodríguez, G., Mannina, L., Cifuentes, A., & Ibáñez, E. (2025). Characterization of Non-Polar and Polar Bioactive Compounds Obtained by Pressurized Biobased Solvents from Different Arctium lappa L. Root Ecotypes. Applied Sciences, 15(5), 2491. https://doi.org/10.3390/app15052491










