Abstract
Given the increasing demand for biocompatible implant materials in regenerative engineering, novel surface modification techniques are essential to enhance tissue integration, durability, and corrosion resistance. This study investigates the application of plasma electrolytic oxidation (PEO), a high-voltage anodic oxidation technique, for the surface modification of magnesium (Mg) implants. The research emphasizes both functionality enhancement and process sustainability, adhering to green chemistry principles. A comprehensive analysis was conducted to evaluate the physicochemical and biological properties of the modified surfaces. The chemical structure of the coatings was characterized using Fourier-transform infrared spectroscopy (FT-IR) and atomic absorption spectroscopy (ASA). Surface morphology and composition were examined via scanning electron microscopy (SEM), while wettability was assessed through contact angle measurements. Additionally, biodegradation and biocorrosion studies were performed to evaluate stability, and cytotoxicity was tested using MG-63 human osteosarcoma cells. Results demonstrated that carefully optimized PEO process parameters, combined with appropriate electrolyte compositions, enabled the formation of MgO coatings with significantly enhanced stability, reduced biocorrosion, and improved biocompatibility. These findings indicate the potential of surface-modified magnesium implants for advanced biomedical applications.
1. Introduction
Tissue engineering and regenerative medicine have witnessed remarkable advancements in recent years, fueled by the increasing demand for innovative and sustainable materials that can improve patient outcomes. These advancements are especially evident in the development of implant materials designed to repair or replace damaged bone tissue. The ideal implant material should exhibit excellent biocompatibility, mechanical strength, controlled biodegradation, and bioactivity to support tissue integration and regeneration. However, the complex physiological environment poses significant challenges for the development of such materials, particularly for long-term biomedical applications. Surface modification, therefore, plays a crucial role in enhancing implant functionality and durability by improving tissue integration, corrosion resistance, and bioactivity. In this context, the adoption of green chemistry principles is essential to ensure the sustainability and safety of biomedical materials throughout their lifecycle. Bone implants are indispensable in modern orthopedic, maxillofacial, and dental surgery for repairing fractures, filling bone defects, and replacing missing or damaged bone structures. They serve as scaffolds or mechanical supports to restore function and promote the healing of bone tissue. Implant materials can be broadly categorized into metallic, polymeric, and ceramic types, each with its unique advantages and limitations [1,2,3,4,5,6].
Polymeric materials, such as polylactic acid (PLA), polycaprolactone (PCL), and polyetheretherketone (PEEK), have been extensively studied for biomedical applications. They offer benefits such as tunable biodegradation rates, flexibility, and ease of processing. However, their inherent low mechanical strength and poor load-bearing capacity limit their use in critical applications such as dental and orthopedic implants. Additionally, polymeric materials often lack the bioactivity required to support direct bone bonding, necessitating surface modification or the incorporation of bioactive fillers.
Ceramic materials, including hydroxyapatite (HA) and calcium phosphate (CaP) ceramics, are widely used due to their excellent bioactivity and osteoconductivity, which promote bone regeneration. However, ceramics are inherently brittle and prone to fracture, making them unsuitable for load-bearing applications. Their stiffness can also lead to stress shielding, a phenomenon where the implant bears most of the load, causing bone resorption and weakening over time [1,2,3,4,5,6].
In contrast, metallic materials offer superior mechanical properties, making them ideal for load-bearing applications. Metals such as titanium (Ti), cobalt–chromium (Co-Cr) alloys, and stainless steel have high tensile strength, ductility, and fracture resistance, enabling them to withstand the mechanical stresses encountered in the musculoskeletal system. Titanium and its alloys are particularly favored due to their excellent biocompatibility, corrosion resistance, and favorable mechanical properties. Despite these advantages, traditional metallic implants are not without limitations. Their high stiffness compared to natural bone can cause stress shielding, and their permanent nature can lead to complications such as inflammation and implant-related infections [7,8,9,10,11,12,13,14].
To overcome these challenges, biodegradable metallic materials have gained attention as promising alternatives to traditional permanent implants. Magnesium (Mg) and its alloys have emerged as attractive candidates due to their unique combination of properties. Magnesium is biocompatible, has mechanical properties closely resembling those of natural bone, and is gradually absorbed by the body over time, eliminating the need for secondary removal surgeries. Furthermore, magnesium ions (Mg2+) released during degradation play a vital role in bone metabolism, stimulating osteoblast activity and promoting mineralization. This makes magnesium particularly attractive for applications in orthopedic and dental implants. Despite these advantages, the practical application of magnesium implants is hindered by their poor corrosion resistance in physiological environments. Rapid degradation can lead to the formation of hydrogen gas pockets and adverse tissue reactions, compromising the implant’s mechanical stability and biocompatibility. To address these challenges, surface modification techniques are essential for controlling degradation rates and enhancing the bioactivity of magnesium implants. Surface modification techniques play a critical role in enhancing the performance of metallic implants by creating protective coatings that improve corrosion resistance, bioactivity, and mechanical stability. Among these techniques, plasma electrolytic oxidation (PEO) has gained significant attention due to its ability to produce thick, porous oxide coatings on metal surfaces. PEO is an electrochemical process conducted under high-voltage conditions, generating microdischarges that facilitate the formation of ceramic-like oxide layers. These coatings provide a barrier against corrosion, improve mechanical properties, and serve as a platform for incorporating bioactive agents to promote tissue integration [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33].
PEO has been extensively studied and optimized for titanium implants, where it has demonstrated significant improvements in corrosion resistance, osseointegration, and antibacterial properties. Researchers have developed bioactive coatings enriched with calcium phosphate, hydroxyapatite, and antimicrobial nanoparticles to enhance the performance of titanium implants. However, despite the growing interest in biodegradable magnesium implants, research on PEO modification of magnesium remains limited. The high reactivity of magnesium poses unique challenges for PEO processing, including the formation of non-uniform coatings and instability during the oxidation process. These challenges necessitate tailored approaches to electrolyte composition, process parameters, and coating design [34,35,36,37,38,39,40,41,42,43].
The current state of research in the field of PEO modification predominantly focuses on titanium-based implants, reflecting the long-standing reliance on titanium in biomedical applications. While significant progress has been made in optimizing PEO processes for titanium, there is a noticeable gap in the literature regarding the application of PEO to magnesium implants. Magnesium’s potential as a biodegradable and bioactive implant material remains underexplored, particularly in the context of dental applications where controlled degradation and enhanced bioactivity are critical [44,45,46,47,48,49,50,51,52,53].
This study aims to address this research gap by investigating the PEO surface modification of magnesium implants, with a focus on achieving uniform, stable, and bioactive coatings that enhance corrosion resistance and biocompatibility. The process was designed in accordance with green chemistry principles, emphasizing sustainability and safety at each stage. Comprehensive analyses of the physicochemical and biological properties of the modified magnesium surfaces were conducted, including chemical structure analysis via Fourier-transform infrared spectroscopy (FTIR) and atomic absorption spectroscopy (AAS), surface morphology assessment using scanning electron microscopy (SEM), biodegradation and biocorrosion studies, and cytotoxicity evaluation using MG-63 osteoblast-like cells.
The novelty of this research lies in its focus on magnesium as an implant material, leveraging PEO technology to create bioactive and corrosion-resistant coatings. By addressing the challenges associated with magnesium degradation and investigating sustainable modification methods, this study contributes valuable insights to the field of dental and orthopedic implantology. The findings pave the way for the development of next-generation biodegradable implants, offering a promising alternative to traditional titanium-based solutions and aligning with the principles of sustainable and regenerative medicine.
2. Materials and Methods
2.1. Materials
For the experiments, disks of pure magnesium were prepared from magnesium rod (99.99% purity) purchased from Oxymet, Kraków, Poland. The discs had a diameter of 1.8 cm and thickness of 4 mm. Before the experiments, all disks were purified using an ultrasonic bath and polished using sandpaper. All of the reagents, including Na2SiO3, KOH, NaOH, and NH4F, were purchased from Warchem, Zakręt, Poland. Lysine and L-aspartic acid were purchased from PolAura, Dywity, Poland. Simulated Body Fluid (SBF) in an amount of 1 L was prepared by dissolving 8.035 g of NaCl, 0.355 g of NaHCO3, 0.225 g of KCl, 0.231 g of KH2PO4, 0.311 g of MgCl2·6H2O, 0.292 g of CaCl2·2H2O, and 0.072 g of Na2SO4 in approximately 900 mL of distilled water. To stabilize the solution and maintain physiological conditions, 6.118 g of Tris(hydroxymethyl)aminomethane (TRIS) buffer was added, and the pH was adjusted to 7.4 using 1 M HCl. The solution was then diluted to a final volume of 1 L with distilled water and filtered through a 0.22 µm membrane to ensure sterility. A cell culture medium was prepared using Dulbecco’s Modified Eagle Medium (DMEM) with high glucose content and phenol red. To prevent microbial contamination, antybiotic/antimycotin solution was added (1% concentration in the final volume), as well as fetal bovine serum (FBS), to provide nutrients (10% final concentration). All reagents, together with DMEM without phenol red, XTT assay (Roche), phosphate buffer solution (PBS), and trypsin/EDTA solution, were purchased from SigmaAldrich, Lublin, Poland. The cytotoxicity study was carried out on an MG-63 osteosarcoma cell line purchased from SigmaAldrich, Lublin, Poland, dedicated to scientific research only, and obtained from the European Collection of Authenticated Cell Cultures (ECACC).
2.2. Methods
Biomaterials Preparation
At the beginning of the research study, anodization was performed using a magnesium alloy as the raw material. The aim of the anodization was to create a magnesium oxide layer on the surface of the alloy, intended to slow down biocorrosion and biodegradation processes, thereby enhancing the material’s suitability for implantology. The process was carried out using six different electrolytes, the compositions of which are presented in Table 1. The anodic oxidation process parameters are presented in Table 2. Due to the anodization process being too intensive, the processes in some cases were split into two stages, starting with a lower voltage and then increasing it.

Table 1.
Chemical composition and content of electrolytes used in anodic oxidation of magnesium.

Table 2.
Parameters for conducting anodic oxidation process.
2.3. Wettability Assessment
To evaluate the wettability of the prepared implants, a testing workstation was set up with a small table placed behind a backlit white screen. A volume of 2 µL of distilled water was carefully instilled (one drop) onto sample surfaces, and the wetting angle was measured using the Angle Meter application. The test was conducted on all samples at 21 °C temperature.
2.4. Structure and Morphology
The analysis of surface morphology and composition was carried out using the Apreo 2 S LoVac Scanning Electron Microscope (SEM) (Thermo Fisher Scientific, Waltham, MA, USA), which offers two primary operating modes: high vacuum and low vacuum. These modes allow the examination of both conductive materials, such as metals and alloys, as well as non-conductive materials, including organic substances, ceramics, and semiconductors. The samples were additionally coated with a thin layer of sputtered gold, and the studies were conducted using various accelerating voltage values.
2.5. Biocorrosion Studies
The corrosion resistance of the produced layers was evaluated using the potentiostatic method. The corrosive behavior of the samples was analyzed through electrochemical measurements in a simulated body fluid environment (SBF). The working electrode was made from a magnesium implant, the counter electrode was a platinum wire, and the reference electrode was a saturated calomel electrode. The experiment was conducted at room temperature, with potential scanning performed at a rate of 200 mV/min. To conduct the measurements, a dedicated setup was constructed, incorporating a cylindrical, funnel-shaped vessel with a foot and a sealing gasket pressed against the metallic sample of the magnesium implant. This design ensured identical measurement conditions for each sample. Based on the results, the corrosion potential and corrosion current were calculated using Tafel extrapolation, which relies on the tangent line method, utilizing linear values from the plot to estimate potentials (Figure 1).
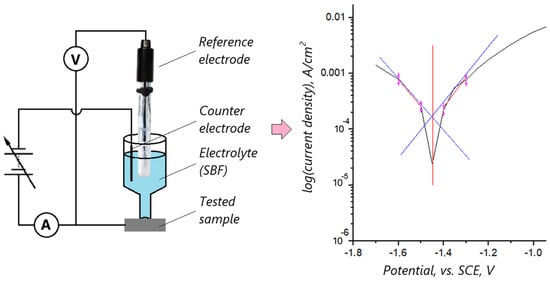
Figure 1.
A schematic diagram of the electrochemical setup for biocorrosion studies on implant samples prepared using the PEO method, and a graphical representation of Tafel parameter determination.
2.6. Cytotoxicity Assessment
Cytotoxicity was assessed using the human MG-63 cell line under direct contact conditions. Samples were sterilized using 70% ethanol and UV radiation and then placed in polystyrene Petri dishes for cell culture. The samples were then covered with the cell suspension and complete culture medium was added. After 48 h, a qualitative cytotoxicity assessment was performed. The quantitative cytotoxicity assessment was carried out using an XTT assay, which, due to the ability of viable cells to reduce tetrazolium salt to formazan, thanks to the activity of the mitochondrial dehydrogenase enzyme, helps to quantitatively estimate the percentage of metabolically active cells. A control standard cell culture was used. An XTT assay was performed using a Microplate reader RT-6900 purchased from DRG MedTek Sp. z o.o., Warsaw, Poland. The test was carried out according to producer protocol (Roche). Cell culture observations and qualitative assessments were performed using the inverted optical microscope DeltaOptics, Planeta Oczu, Zielona Góra, Poland, under 40× and 100× magnifications.
3. Results and Discussion
3.1. Analysis of Chemical Structure Using ASA and FT-IR Methods
Figure 2 reveals the main idea behind the paper.

Figure 2.
A diagram of the process of implant surface preparation using the PEO method, along with the compounds that constitute the electrolyte components.
The chemical analysis, performed using atomic absorption spectrometry (ASA), is summarized in Table 3 for the samples Mg_10, Mg_15, Mg_16, and Mg_17. The results reveal consistent findings across all the tested samples. A notable feature observed in the analyses was the presence of functional groups, including hydroxyl (-OH) groups, which exhibited a characteristic stretching vibration band at 3352 cm−1. Additionally, carbon–oxygen (C-O) bonds were identified with a band at 1019 cm−1, which is typical for the formation of oxide layers and suggests the presence of oxygenated species on the surface. Carbon–chlorine (C-Cl) bonds were also detected at 787 cm−1, indicating potential interactions between the magnesium surface and chlorine species from the electrolyte during the plasma electrolytic oxidation (PEO) process. These findings confirm that electrochemical modification led to the creation of new surface coatings on the magnesium samples.

Table 3.
Analysis results showing the metal content (%) in the alloy sample subjected to the PEO process, which was used as implant material.
Further confirmation of the surface modifications was obtained through Fourier-transform infrared (FT-IR) spectroscopy, as presented in Figure 3. The FT-IR spectra provided additional insights into the chemical composition and bonding structures of the modified magnesium surfaces, confirming the successful formation of protective coatings. The detected functional groups and their respective vibration bands suggest that the electrochemical treatment facilitated the formation of a robust oxide layer, enhancing the surface properties of the magnesium alloys. These modifications are critical for improving the corrosion resistance and biocompatibility of the implants, making them more suitable for use in biomedical applications such as bone and dental implants.
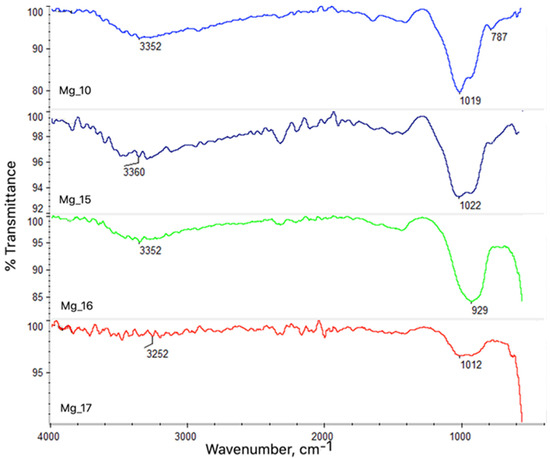
Figure 3.
The results of the chemical structure analysis using the FT-IR method.
3.2. Wettability Study
Due to the highly hydrophilic nature of the majority of the samples, it was not possible to measure the wetting angles accurately. However, the wetting angles of samples Mg_14, Mg_15, Mg_16, and Mg_17 could be determined, as these samples exhibited slightly lower wettability compared to the others (Figure 4). The results for these samples are presented in Table 4. The wetting angles obtained for Mg_14, Mg_15, Mg_16, and Mg_17 indicate a notable difference in surface characteristics compared to the highly hydrophilic samples, suggesting that these specific samples have a slightly reduced tendency to attract water molecules. This could be indicative of the formation of less porous or more compact surface coatings due to the electrochemical modification process. The variation in wettability among the samples is important for understanding their potential interactions with biological fluids and their overall suitability for applications where surface properties such as hydrophilicity are critical for cell adhesion and tissue integration.
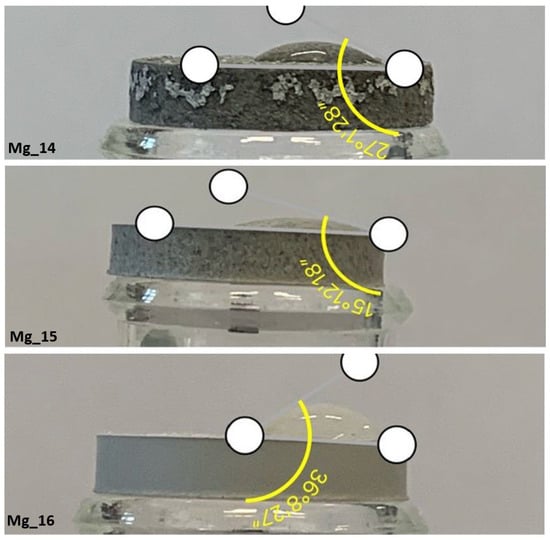
Figure 4.
Contact angle determination.

Table 4.
Wetting angle values and wettability ratings for individual samples.
3.3. Morphology Study
Figure 5, Figure 6, Figure 7 and Figure 8 display the oxide coatings formed through anodization, which are characterized by a porous structure conducive to cell adhesion and growth. The surfaces of these coatings are heterogeneous and exhibit well-developed characteristics. Notably, the pores present on these surfaces are numerous and small, which could have a significant impact on the longevity of the protective layer when exposed to aggressive environments. The observed structures, typical of plasma electrolytic oxidation (PEO) reactions, further confirm the successful surface modification of the magnesium samples. These findings suggest that the anodized oxide coatings created through PEO are not only structurally optimized for enhancing biocompatibility but also provide a suitable environment for long-term stability and protection against corrosion in physiological conditions. The detailed morphology of the coatings, with their well-distributed microstructural features, reinforces the idea that PEO treatment can be a promising technique for improving the durability and performance of magnesium-based biomaterials in biomedical applications.
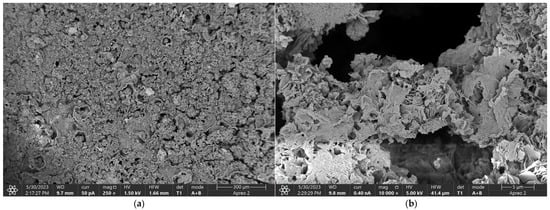
Figure 5.
The SEM—Oxide coating of the Mg_2 sample produced by anodic oxidation, at magnifications (a) 250×; (b) 10,000×.

Figure 6.
The SEM—Oxide coating of the Mg_10 sample produced by anodic oxidation, at magnifications (a) 20,000×; (b) 12,500×.

Figure 7.
The SEM—Oxide coating of the Mg_17 sample produced by anodic oxidation, at magnifications (a) 1000×; (b) 5000×.
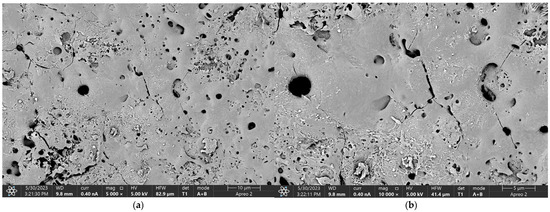
Figure 8.
The SEM—Oxide coating of the Mg_16 sample produced by anodic oxidation, at magnifications (a) 2500×; (b) 20,000×.
The chemical composition of the layers was analyzed using the Energy Dispersive X-ray Spectroscopy (EDS) method. This technique enabled the determination of the distribution of key elements such as nitrogen, oxygen, sodium, carbon, and magnesium across the coatings. Notably, the elemental composition observed in the coatings closely resembles that of natural bone, further supporting the potential of these modified magnesium implants for biomedical applications. The distribution of these elements suggests that the surface modifications made through plasma electrolytic oxidation (PEO) enhance the material’s bioactivity and biocompatibility. The detailed results of the EDS analysis, including the elemental profiles and their respective distributions, are presented in Figure 9, Figure 10 and Figure 11 and Table 5, Table 6 and Table 7. These findings underline the promising characteristics of the modified magnesium implants in terms of their structural and chemical similarity to bone, potentially improving their performance in clinical applications such as orthopedics and dentistry.
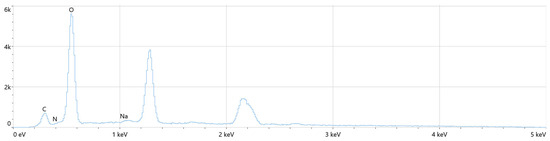
Figure 9.
The distribution of selected elements present in the composition of the Mg_2 sample.

Figure 10.
The distribution of selected elements present in the composition of the Mg_10 sample.
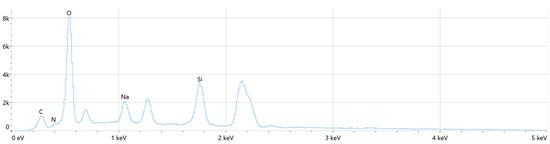
Figure 11.
The distribution of selected elements present in the composition of the Mg_17 sample.

Table 5.
The obtained results of the EDS analysis of the surface of the Mg_2 sample.

Table 6.
The obtained results of the EDS analysis of the surface of the Mg_10 sample.

Table 7.
The obtained results of the EDS analysis of the surface of the Mg_17 sample.
Based on the analysis of the obtained results presented in Figure 9 and Table 5, it can be concluded that oxygen and magnesium exhibit the highest content among the elements detected in the oxide coatings. The dominant presence of magnesium is indicative of the successful modification of the magnesium substrate through the anodization process. Additionally, the presence of sodium is attributed to its role as a key component of the electrolyte used during the anodization, further supporting the involvement of the electrolyte in the surface modification process. This elemental distribution reinforces the effectiveness of the anodization process in creating a robust and bioactive surface layer on the magnesium implants.
Based on the analysis of the obtained results presented in Figure 10 and Table 6, it is evident that oxygen and silicon are the elements with the highest concentrations in the oxide coatings. The presence of silicon and sodium can be attributed to their roles as key components of the electrolytes used during the anodization process. This elemental composition suggests that the incorporation of silicon, in particular, may contribute to enhancing the properties of the oxide layer, potentially improving its protective and bioactive characteristics.
The analysis of the obtained results, presented in the Figure 11 and Table 7, indicates that oxygen and silicon have the highest concentrations among the elements. The significant presence of silicon and sodium can be attributed to their roles as components of the electrolyte used in the anodic oxidation process.
3.4. Biocorrosion Study
The corrosion behavior of magnesium-based implants was investigated by analyzing corrosion potential, corrosion current, and biocorrosion after in vivo implementation. The electrochemical behavior of the samples was determined through corrosion potential and corrosion current measurements (Table 8). In particular, the effects of different anodization treatments (varying voltage levels and the incorporation of amino acids like lysine into the electrolytes) on the corrosion resistance of magnesium implants were analyzed (Figure 12 and Figure 13).

Table 8.
The obtained results of the corrosion potential and current values for the tested samples.

Figure 12.
The obtained values of corrosion potentials for each sample.

Figure 13.
The obtained values of corrosion current for individual samples.
The analysis of the corrosion potential values indicated that the application of low voltages during anodic oxidation (e.g., for samples Mg_7-Mg_11) did not yield satisfactory results. These samples showed less uniform surface coverage, suggesting that the anodizing process was not optimized at lower voltages. However, after increasing the voltage, uniform surface coverage was observed, significantly enhancing the corrosion resistance of the implants. This finding suggests that higher voltages result in more effective oxide layer formation, which is critical for improving the durability and performance of magnesium implants in aggressive in vivo environments.
Regarding the corrosion current, the best results were achieved for samples Mg_7, Mg_10-Mg_13, and Mg_17, where low corrosion current values were recorded, indicating slower degradation. This was consistent with the findings that higher voltage applications resulted in better corrosion protection. Conversely, low voltage applications led to higher corrosion current values, which corresponded to an increased degradation rate, a common concern when magnesium is used as a material for biodegradable implants. Additionally, the incorporation of lysine in the electrolytic bath further improved the corrosion resistance, with samples treated first with high voltages and then with lysine (e.g., Mg_14 and Mg_15) exhibiting reduced corrosion current values compared to untreated samples. The PEO-modified samples, particularly Mg_10 and Mg_11, demonstrated controlled degradation, which was crucial for their functionality as bone implants. A significant advantage of magnesium-based implants over traditional titanium-based implants lies in their biodegradability. Magnesium alloys are absorbed by the body over time, eliminating the need for secondary surgeries to remove the implants. In our study, PEO-modified magnesium implants exhibited minimal inflammatory responses and showed efficient bone integration. The oxide layer formed through PEO slowed down the release of hydrogen gas and buffered the increase in pH around the implant, thus mitigating potential adverse effects like tissue irritation and delayed healing.
3.5. Cytotoxicity Assessment
For samples Mg_10 and Mg_11, no cytotoxic effects were observed, with the cells forming clusters and maintaining normal morphology throughout the assessment period. This indicates that the surface modifications applied to these samples did not compromise cell viability or functionality. However, for the remaining samples, a reduction in cell viability was noted, which was attributed to an increase in the pH of the medium, which reached approximately 8.5. This pH increase correlated with a more pronounced release of hydrogen gas, a typical byproduct of magnesium corrosion. As the pH rose, it created a more alkaline environment, potentially altering cellular behavior and leading to a decrease in viability.
Figure 14 presents the results for all tested samples. A key observation is that nearly all of the samples did not show cytotoxicity, with cell viability exceeding the 70% threshold compared to the control culture, as outlined in ISO 10993-5 [54] for the evaluation of medical devices. This suggests that these materials can be classified as non-toxic under the specified conditions, thus reinforcing their suitability for biomedical applications.
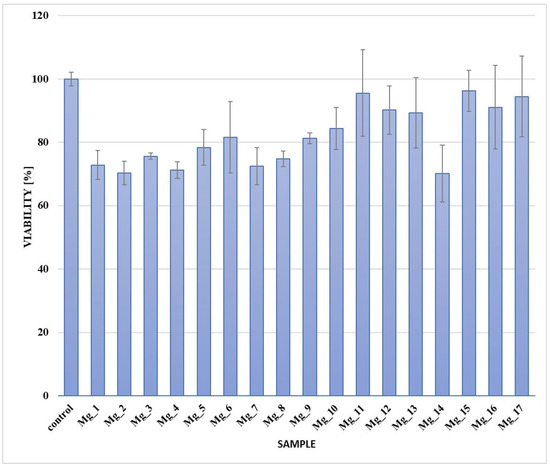
Figure 14.
XTT assay.
The formation of additional protective coatings on the magnesium surfaces effectively reduced biocorrosion and slowed the pH increase, likely due to the buffering properties inherent to the culture medium. The electrolyte composition, which included L-aspartic acid and lysine, could have further influenced the bioactivity of the magnesium implants. Both L-aspartic acid and lysine are known to enhance surface modification processes during plasma electrolytic oxidation (PEO), potentially improving the corrosion resistance and biocompatibility of magnesium alloys. L-aspartic acid, being an amino acid with a carboxyl group, may contribute to the formation of a stable oxide layer, while lysine, with its amino group, could provide additional interactions with the surface, influencing both the formation of the oxide coating and the material’s interaction with surrounding biological tissues. These components, when incorporated into the electrolyte, may also play a role in stabilizing the pH levels in the culture medium, further supporting the non-toxic nature of the samples.
Among all the samples, Mg_11 showed the most promising results, demonstrating a significant improvement in cell viability and overall biocompatibility, which makes it a suitable candidate for further development and clinical applications in biomedical fields, particularly in dental implantology and orthopedic applications.
4. Conclusions
This study demonstrates the potential of magnesium (Mg) as a sustainable and biocompatible alternative to traditional non-resorbable materials, such as titanium and its alloys, for biomedical implants. The use of plasma electrolytic oxidation (PEO) as an eco-friendly surface modification method significantly enhanced the properties of Mg implants by forming a protective oxide coating. The process, conducted under optimized conditions at 240 V, improved corrosion resistance and mechanical stability while maintaining biocompatibility, as confirmed by biodegradation, biocorrosion, and cytotoxicity analyses on MG-63 osteosarcoma cells. The findings underscore the potential of PEO-modified Mg implants as a promising solution for applications in orthopedics and dental implantology, offering a green and efficient alternative to current implant technologies.
Author Contributions
Conceptualization, Ł.J. and J.R.-P.; methodology, J.R.-P., N.R.-P. and Ł.J.; validation, A.S.-B., J.R.-P. and Ł.J.; investigation, K.L., A.S.-B. and J.R.-P.; data curation, Ł.J., K.K., J.R.-P. and N.R.-P.; writing—original draft preparation, K.K. and J.R.-P.; resources, Ł.J. and J.R.-P.; supervision, J.R.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, J.; Fan, H.; Li, H.; Hua, L.; Du, J.; He, Y.; Jin, Y. Recent Advancements in the Surface Modification of Additively Manufactured Metallic Bone Implants. Addit. Manuf. Front. 2025, 200195. [Google Scholar] [CrossRef]
- Chang, J.Z.-C.; Hsu, J.-T.; Li, M.-J.; Lin, H.-Y.; Sun, J.; Tsou, N.-T.; Sun, J.-S. Optimizing Dental Implant Design: Structure, Strength, and Bone Ingrowth. J. Dent. Sci. 2024, in press. [Google Scholar] [CrossRef]
- Elboraey, M.O.; Alqutaibi, A.Y.; Aboalrejal, A.N.; Borzangy, S.; Zafar, M.S.; Al-Gabri, R.; Alghauli, M.A.; Ramalingam, S. Regenerative Approaches in Alveolar Bone Augmentation for Dental Implant Placement: Techniques, Biomaterials, and Clinical Decision-Making: A Comprehensive Review. J. Dent. 2025, 154, 105612. [Google Scholar] [CrossRef]
- Ouqi, Y.; Wang, J.; Yang, X.; Man, Y. Factors Influencing Labial Bone Resorption After Implant Insertion with Simultaneous Guided Bone Regeneration: Retrospective Cone Beam Computed Tomography Study. Int. J. Oral Maxillofac. Surg. 2024, 54, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Chen, J.-J.; Chen, C.-S. Using Optimization Approach to Design Dental Implant in Three Types of Bone Quality—A Finite Element Analysis. J. Dent. Sci. 2025, 20, 126–136. [Google Scholar] [CrossRef]
- An, Y.; Zhang, H.; Zhang, S.-A.; Zhang, Y.; Zheng, L.; Chen, X.; Tong, W.; Xu, J.; Qin, L. Degradation Products of Magnesium Implant Synergistically Enhance Bone Regeneration: Unraveling the Roles of Hydrogen Gas and Alkaline Environment. Bioact. Mater. 2025, 46, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Malinovschi, V.; Marin, A.; Ducu, C.; Moga, S.; Craciun, V.; Lungu, C.P.; Cimpoesu, R.; Golgovici, F.; Cristea, D. Preparation and Characterization of Ti5Al16O34 PEO Ceramic Coatings Deposited on CP-Ti in Mixed Aluminate-Phosphate Electrolytes. Ceram. Int. 2024, 50, 40955–40975. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Kaseem, M. Corrosion Behavior Amelioration of Ti-Based Alloys by the Hybrid Plasma Electrolytic Oxidation (PEO)/Polymer Coatings: A Review. Hybrid Adv. 2024, 5, 100151. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Cardoso, G.C.; Rossi, M.C.; Afonso, C.R.M.; Grandini, C.R. Analyzing PEO Anodization Time to Monitor Coatings Phases, Composition, Morphology, Thickness, and Microhardness During the Growth of TiO2 Pores on the CP-Ti Surface. Mater. Lett. 2024, 363, 136226. [Google Scholar] [CrossRef]
- Nisar, S.S.; Choe, H.-C. Mechanical Hydroxyapatite Coatings on PEO-Treated Ti–6Al–4V Alloy for Enhancing Implant’s Surface Bioactivity. Ceram. Int. 2024, 50, 17703–17719. [Google Scholar] [CrossRef]
- Nadimi, M.; Dehghanian, C. Incorporation of ZnO–ZrO2 Nanoparticles into TiO2 Coatings Obtained by PEO on Ti–6Al–4V Substrate and Evaluation of Its Corrosion Behavior, Microstructural, and Antibacterial Effects Exposed to SBF Solution. Ceram. Int. 2021, 47, 33413–33425. [Google Scholar] [CrossRef]
- Lim, S.-G.; Choe, H.-C. Bioactive Apatite Formation on PEO-Treated Ti-6Al-4V Alloy After 3rd Anodic Titanium Oxidation. Appl. Surf. Sci. 2019, 484, 365–373. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, D.; Yang, Z.; Zhu, Z.; Lu, X.; Ou, B.; Zhang, J.; Jin, S.; Wang, Q.; Yu, K. Structural Characterization and Biological Compatibilities of PEO Coated Ti–Mg Metal Matrix Composites. J. Mater. Res. Technol. 2024, 30, 2911–2921. [Google Scholar] [CrossRef]
- Lim, B.-S.; Lim, S.-G.; Choe, H.-C. Precipitation of Bone-Like Apatite on Plasma Electrolytic Oxidized Ti-6Al-4V Alloy. Thin Solid Film. 2022, 746, 139136. [Google Scholar] [CrossRef]
- Gonzlez, S.; Pellicer, E.; Suriach, S.; Bar, M.D.; Sort, J. Biodegradation and Mechanical Integrity of Magnesium and Magnesium Alloys Suitable for Implants. In Biodegradation–Engineering and Technology; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-K.; Jang, Y.-S.; Lee, M.-H. Advantages of Electrochemically Deposited Bioceramic-Coating on Magnesium Implant for Anti-Corrosion and Bone Regeneration. Surf. Interfaces 2025, 59, 105936. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X. Surface Modification of Biodegradable Magnesium and Its Alloys for Biomedical Applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Wydawnictwo Politechniki Poznańskiej. Materiały Inżynierskie w Zastosowaniach Biomedycznych; Wydawnictwo Politechniki Poznańskiej: Poznań, Poland, 2011. [Google Scholar]
- Li, X.; Gao, P.; Wan, P.; Pei, Y.; Shi, L.; Fan, B.; Shen, C.; Xiao, X.; Yang, K.; Guo, Z. Novel Bio-functional Magnesium Coating on Porous Ti6Al4V Orthopaedic Implants: In Vitro and In Vivo Study. Sci. Rep. 2017, 7, 40755. [Google Scholar] [CrossRef]
- Park, J.Y.; Jung, Y.S.; Lee, K.K.; Park, H.J. Behavior of Osteoblast-Like Cells on a β-Tricalcium Phosphate Synthetic Scaffold Coated with Calcium Phosphate and Magnesium. J. Craniofac. Surg. 2016, 27, 898–903. [Google Scholar] [CrossRef]
- Witte, F. The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective Coatings on Magnesium and Its Alloys—A Critical Review. J. Alloys Compd. 2002, 336, 88–99. [Google Scholar] [CrossRef]
- Kainer, K.U.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Corrosion of Magnesium and Its Alloys; In Shreir’s Corrosion; Elsevier: Oxford, UK, 2010. [Google Scholar]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical Coatings on Magnesium Alloys—A Review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Saternus, M. Magnez—Technologia, Produkcja, Perspektywy; Rudy Metale: Sacramento, CA, USA, 2008. [Google Scholar]
- Karmańska, A. Magnez—Aktualny Stan Wiedzy. Bromatol. I Chem. Toksykol. Arch. 2015, 4, 677–689. [Google Scholar]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-Derived Magnesium Induces Local Neuronal Production of CGRP to Improve Bone-Fracture Healing in Rats. J. Mater. Sci. 2017, 52, 5277–5289. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An Update on Physiological, Clinical and Analytical Aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Tian, Q.; Lin, J.; Rivera-Castaneda, L.; Tsanhani, A.; Dunn, Z.S.; Rodriguez, A.; Aslani, A.; Liu, H. Nano-to-Submicron Hydroxyapatite Coatings for Magnesium-Based Bioresorbable Implants—Deposition, Characterization, Degradation, Mechanical Properties, and Cytocompatibility. Acta Biomater. 2016, 43, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma Electrolytic Oxidation of Magnesium and Its Alloys: Mechanism, Properties, and Applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Lucas, R.R.; Silva, E.R.R.; Marques, L.F.B.; da Silva, F.J.G.; Abrahão, A.B.R.M.; Vieira, M.d.O.L.; Hein, L.R.d.O.; Botelho, E.C.; Mota, R.P.; Sales-Contini, R.d.C.M. Analysis of Plasma Electrolytic Oxidation Process Parameters for Optimizing Adhesion in Aluminum–Composite Hybrid Structures. Appl. Sci. 2024, 14, 7972. [Google Scholar] [CrossRef]
- Ji, R.; Wang, S.; Zhao, X.; Zou, Y.; Zhang, T.; Qian, X.; Chen, G.; Wang, Y.; Ouyang, J.; Jia, D.; et al. Enhanced Wear Resistance, Corrosion Behavior, and Thermal Management in Magnesium Alloys with PEO Coatings. Surf. Coat. Technol. 2024, 494, 131438. [Google Scholar] [CrossRef]
- Dong, H.; Wang, S.; Xie, D.; Li, Q.; Zhao, D.; Guo, H.; Wu, J.; Jiang, W.; Meng, X.; An, L. Enhancing Understanding of Cooperation Mechanism of Electrolyte Composition on PEO Coating Growth for Magnesium Alloys via Load Characteristics. Surf. Coat. Technol. 2024, 494, 131307. [Google Scholar] [CrossRef]
- Kojouri Naftchali, N.; Mehdinavaz Aghdam, R.; Najjari, A.; Dehghanian, C. Investigating a Nanocomposite Coating of Cerium Oxide/Merwinite via PEO/EPD for Enhanced Biocorrosion Resistance, Bioactivity and Antibacterial Activity of Magnesium-Based Implants. Ceram. Int. 2024, 50, 42766–42779. [Google Scholar] [CrossRef]
- Wu, G.; Li, L.; Chen, X.; Zhu, L.; Wang, Y.; Wen, C.; Yao, J. The Growth Mechanism and Corrosion Resistance of Laser-Assisted Plasma Electrolytic Oxidation (PEO) Composite Coating on AZ31B Magnesium Alloy. J. Magnes. Alloys 2024, in press. [Google Scholar] [CrossRef]
- Nachtsheim, J.; Ma, S.; Burja, J.; Markert, B. In Vitro Evaluation of Stress Corrosion Cracking Susceptibility of PEO-Coated Rare-Earth Magnesium Alloy WE43. Surf. Coat. Technol. 2024, 477, 130391. [Google Scholar] [CrossRef]
- Khalili, V.; Ghaleh, H.; Namdar Asl, H.; Ege, D.; Dikici, B.; Kaseem, M.; Breisch, M.; Frenzel, J.; Eggeler, G. Assessing the Properties of Biodegradable Magnesium Alloy AZ31 Protected by a Polymer Layer on a Plasma Electrolytic Oxidized (PEO) Surface. Surf. Coat. Technol. 2024, 487, 131002. [Google Scholar] [CrossRef]
- Nachtsheim, J.; Ma, S.; Burja, J.; Šetina Batič, B.; Markert, B. Tuning the Long-Term Corrosion Behaviour of Biodegradable WE43 Magnesium Alloy by PEO Coating. Surf. Coat. Technol. 2023, 474, 130115. [Google Scholar] [CrossRef]
- Knap, V.; Blawert, C.; Serdechnova, M.; Pastorek, F.; Kajánek, D.; Obertová, V.; Hadzima, B. Use of NaAlO2 Additions to Extend the Corrosion Resistance of PEO Layer on EV31 Magnesium Alloy. J. Mater. Res. Technol. 2024, 29, 2083–2096. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Nomerovskii, A.D.; Filonina, V.S.; Ustinov, A.Y.; Gnedenkov, S.V. Design of Self-Healing PEO-Based Protective Layers Containing In-Situ Grown LDH Loaded with Inhibitor on the MA8 Magnesium Alloy. J. Magnes. Alloys 2023, 11, 3688–3709. [Google Scholar] [CrossRef]
- Bai, L.-j.; Gao, X.; Luo, Y.; Chen, G.; Wu, X.; Sun, X. In-Situ Preparation of (Ti, Al) Codoped Blue PEO Ceramic Coating on Magnesium Alloy and Chromogenic Mechanism. Surf. Coat. Technol. 2023, 470, 129829. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable Biomaterials Based on Magnesium Corrosion. Corros. Sci. 2007, 49, 2781–2793. [Google Scholar] [CrossRef]
- Dobrzański, L. Metaloznawstwo Opisowe Stopów Metali Nieżelaznych; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2008. [Google Scholar]
- Przybyłowicz, P.J. Repetytorium z Materiałoznawstwa, Część VI, Metale i Stopy Nieżelazne; Politechnika Świętokrzyska: Kielce, Poland, 1997. [Google Scholar]
- Atrens, A.L.; Liu, M.; Zainal Abidin, N.I. Corrosion Mechanism Applicable to Biodegradable Magnesium Implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Song, G. Control of Biodegradation of Biocompatible Magnesium Alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Saji, V.S. Organic Conversion Coatings for Magnesium and Its Alloys. J. Ind. Eng. Chem. 2019, 75, 20–37. [Google Scholar] [CrossRef]
- Wierzchoń, B.J. Ćwiczenia Laboratoryjne z Inżynierii Powierzchni; OWPW: Warszawa, Poland, 1996. [Google Scholar]
- Chang, L. Growth Regularity of Ceramic Coating on Magnesium Alloy by Plasma Electrolytic Oxidation. J. Alloys Compd. 2009, 468, 462–465. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Surf. Coat. Technol. 2011, 205, 1500–1509. [Google Scholar] [CrossRef]
- Vertaľ, J.; Kajánek, D.; Kubásek, J.; Minárik, P. Improving Biodegradable Mg-Zn(-Ca) Alloys by Surface Treatment via Plasma Electrolytic Oxidation. Materials 2025, 18, 747. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Wang, B.; Xia, J.; Fu, K.; Han, J. The Incorporation of Nano-MoSi2 Particles into a Black PEO Coating on Ti Alloy and Its Corrosion Performance. Coatings 2025, 15, 145. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).