Characterization of NiCrAlY Layers Deposited on 310H Alloy Using the EB-PVD Method After Oxidation in Water at High Temperature and Pressure
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Material
2.2. Coating Method
2.3. Testing Method
2.3.1. Structural and Morphological Characterization
2.3.2. Electrochemical Tests
3. Results and Discussion
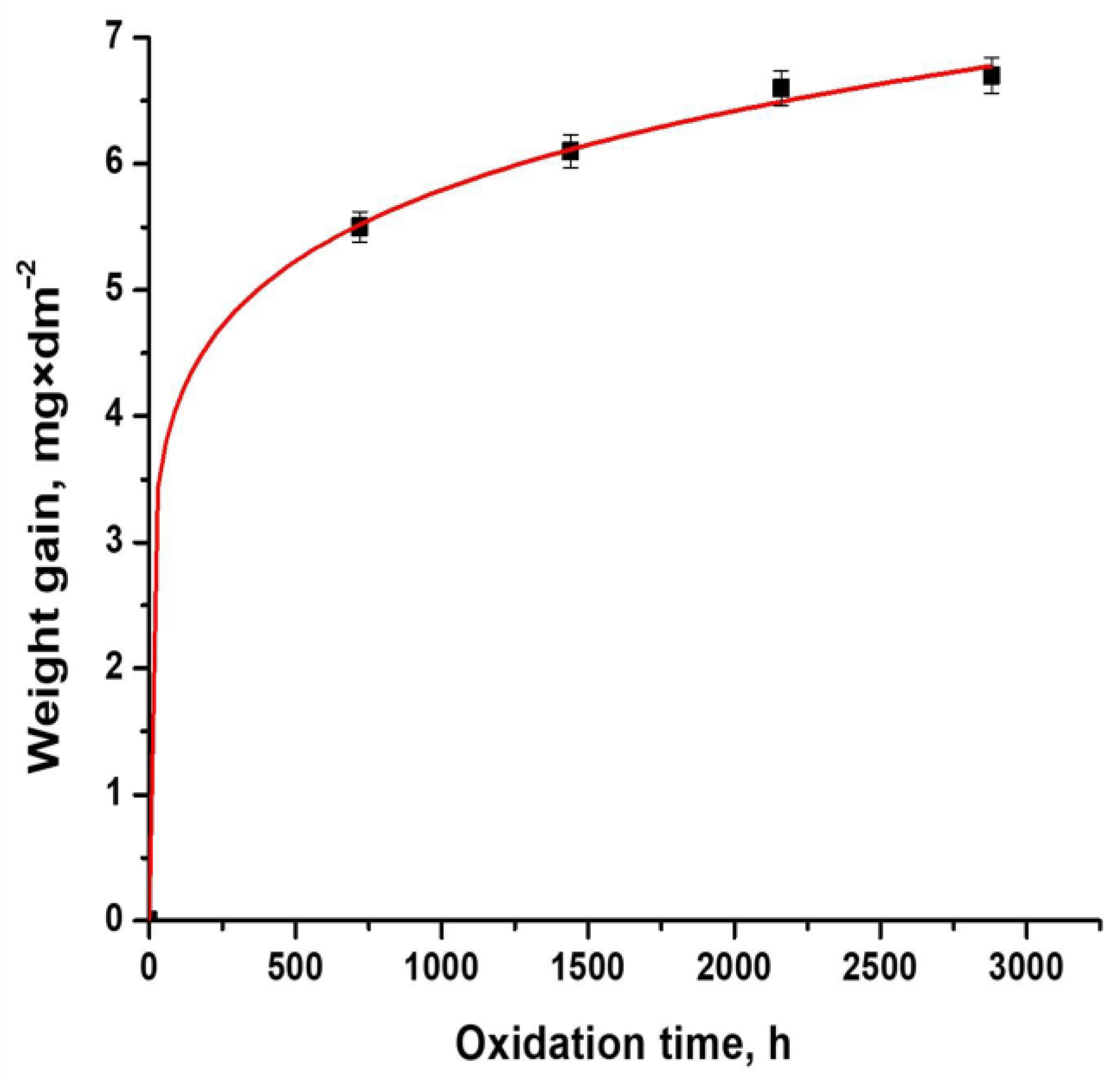
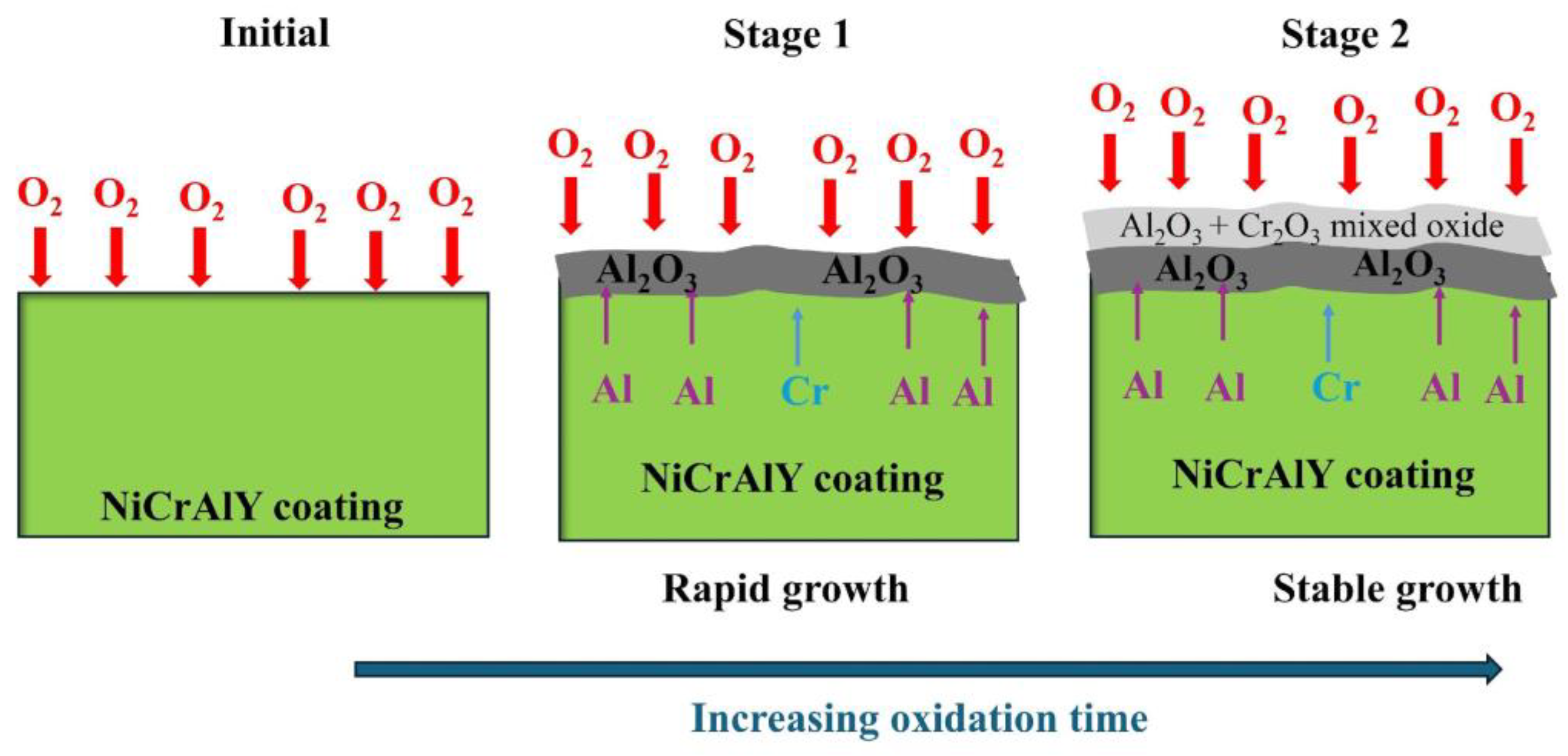
3.1. Oxidation Kinetics
3.2. Morphological and Structural Characterization of the Oxides Formed Following Oxidation
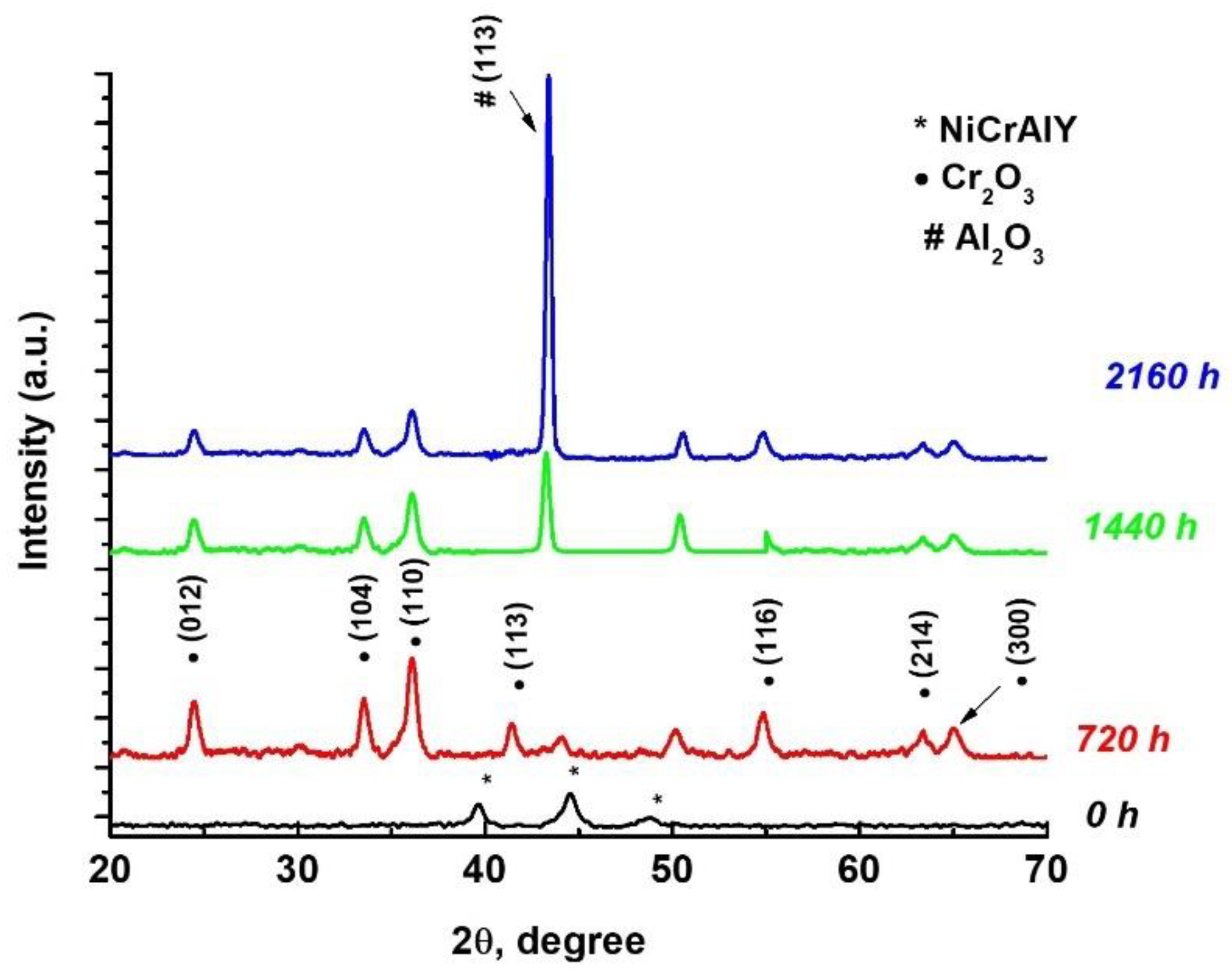
3.2.1. Grazing Incidence X-Ray Diffraction (GIXRD)
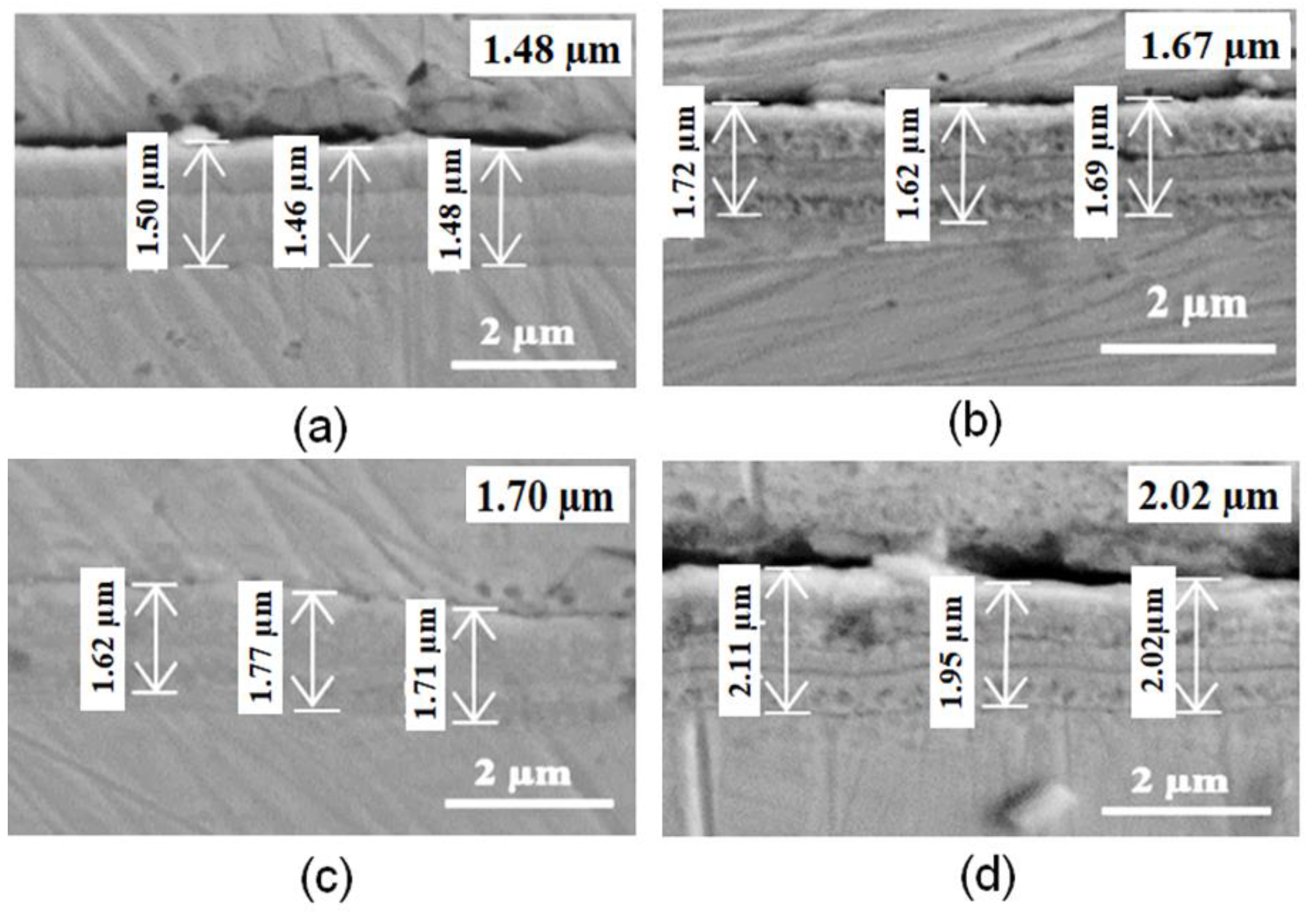
3.2.2. Scanning Electron Microscopy (SEM)
3.3. Corrosion Susceptibility Assessment Tests
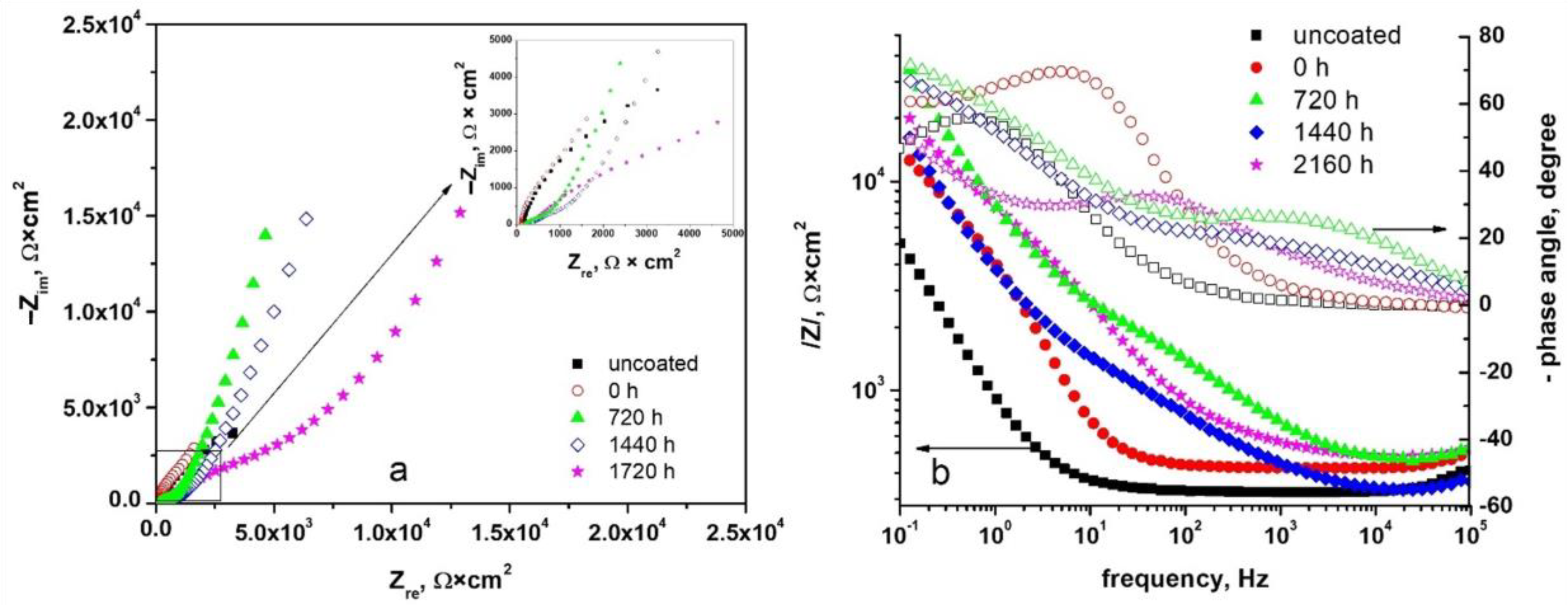
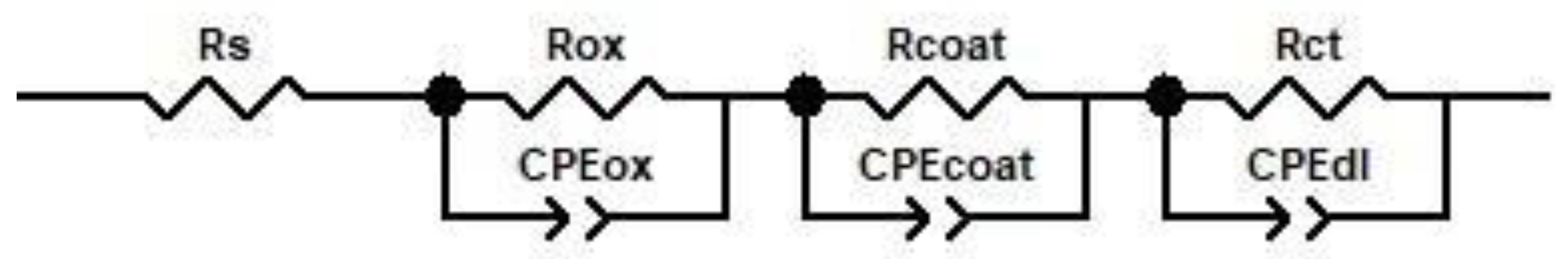
3.3.1. Electrochemical Impedance Spectroscopy (EIS)
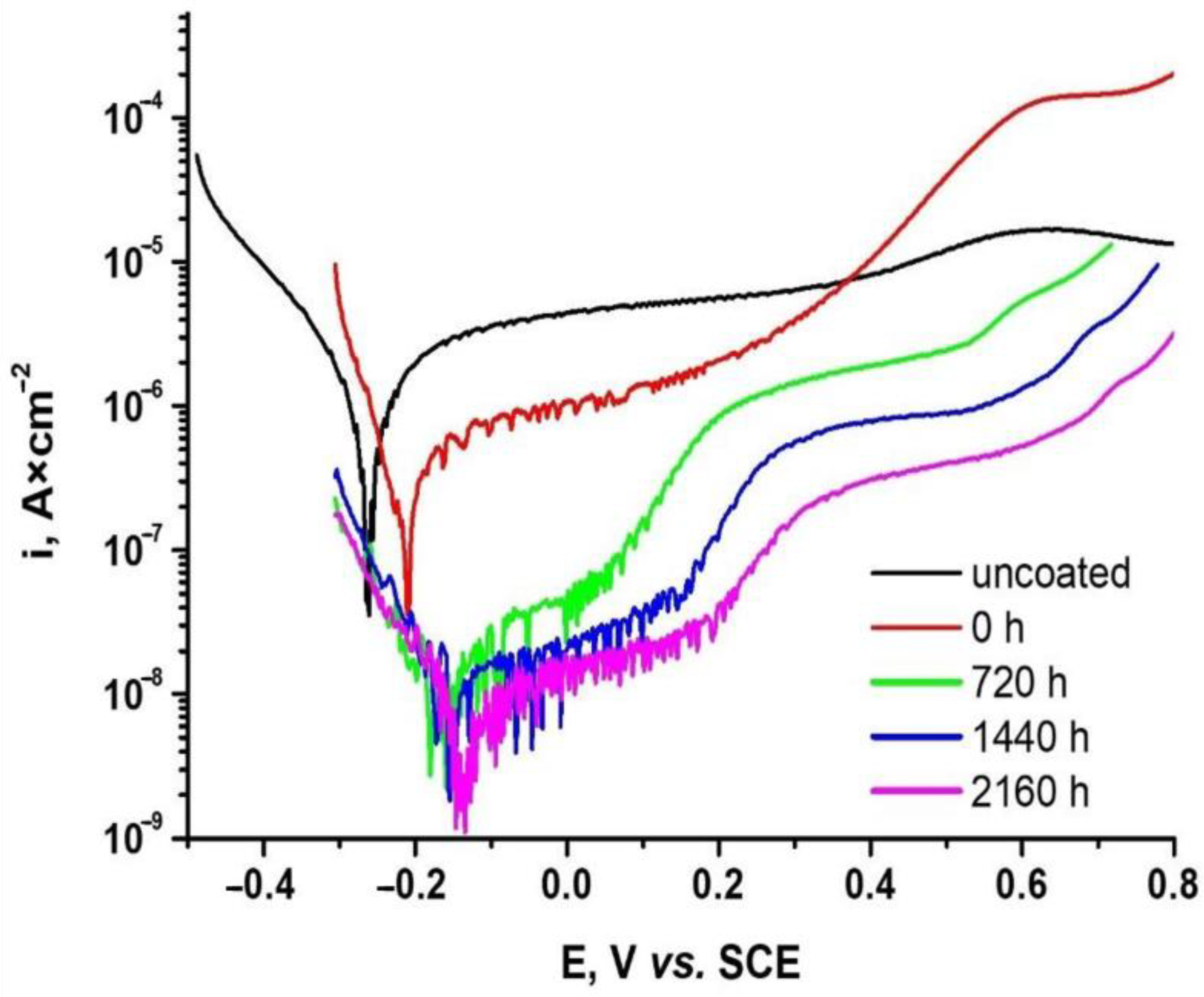
3.3.2. Potentiodynamic Linear Polarization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Economic Commission for Europe. Carbon Neutrality in the UNECE Region: Integrated Life-Cycle Assessment of Electricity Sources; United Nations: Geneva, Switzerland, 2022. [Google Scholar]
- Stanculescu, A. Worldwide status of advanced reactors (GEN IV) research and technology development. In Encyclopedia of Nuclear Energy; Greenspan, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 478–489. ISBN 9780128197325. [Google Scholar]
- Yvon, P. Structural Materials for Generation IV Nuclear Reactors, 1st ed.; Yvon, P., Ed.; Woodhead Publishing: Sawston, UK, 2016; ISBN 9780081009062. [Google Scholar]
- Griffiths, M. Effect of Neutron Irradiation on the Mechanical Properties, Swelling and Creep of Austenitic Stainless Steels. Materials 2021, 14, 2622. [Google Scholar] [CrossRef] [PubMed]
- Maziasz, P.J.; Busby, J.T. Properties of Austenitic Steels for Nuclear Reactor Applications. In Comprehensive Nuclear Materials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 267–283. [Google Scholar]
- Reinhold, E.; Faber, J. Large area electron beam physical vapor deposition (EB-PVD) and plasma activated electron beam (EB) evaporation. Status and prospects. Surf Coat. Tech. 2011, 206, 1653–1659. [Google Scholar] [CrossRef]
- Mikulla, C.; Steinberg, L.; Niemeyer, P.; Schulz, U.; Naraparaju, R. Microstructure Refinement of EB-PVD Gadolinium Zirconate Thermal Barrier Coatings to Improve Their CMAS Resistance. Coatings 2023, 13, 905. [Google Scholar] [CrossRef]
- Feuerstein, A.; Knapp, J.; Taylor, T.; Ashary, A.; Bolcavage, A.; Hitchman, N. Technical and economical aspects of current thermal barrier coating systems for gas turbine engines by thermal spray and EBPVD: A Review. J. Therm. Spray Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Zhen, Z.; Qu, C.; Fu, D. Characterization of the Internal Stress Evolution of an EB-PVD Thermal Barrier Coating during a Long-Term Thermal Cycling. Materials 2023, 16, 2910. [Google Scholar] [CrossRef]
- Lee, G.-W.; Lee, I.-H.; Oh, Y.-S. Oxidation-Induced Changes in the Lattice Structure of YSZ Deposited by EB-PVD in High-Vacuum Conditions. Processes 2023, 11, 2743. [Google Scholar] [CrossRef]
- Ziyuan, X.; Yingying, Y.; Shijie, M.; Weidong, W.; Qiguo, Y. Review on corrosion of alloys for application in supercritical carbon dioxide brayton cycle. Heliyon 2023, 9, e22169. [Google Scholar] [CrossRef]
- Van Nieuwenhove, R.; Balak, J.; Ejenstam, J.; Szakalos, P. Investigations of CrN and AlCrN coatings, applied by PVD, for the corrosion protection of metals in liquid lead at 550 °C. In Proceedings of the The Nuclear Materials Conference (NuMat), Clearwater, FL, USA, 27–30 October 2014. [Google Scholar]
- Gui, Y.; Liang, Z.; Zhao, Q. Corrosion and carburization behavior of heat-resistant steels in a high-temperature supercritical carbon dioxide environment. Oxid. Met. 2019, 92, 123–136. [Google Scholar] [CrossRef]
- Yae Kina, A.; Tavares, S.S.M.; Pardal, J.M.; Souza, J.A. Microstructure and intergranular corrosion resistance evaluation of AISI 304 steel for high temperature service. Mater. Charact. 2008, 59, 651–655. [Google Scholar] [CrossRef]
- Yang, L.; Qian, H.; Kuang, W. Corrosion Behaviors of Heat-Resisting Alloys in High Temperature Carbon Dioxide. Materials 2022, 15, 1331. [Google Scholar] [CrossRef] [PubMed]
- Tavares, S.S.M.; Moura, V.; da Costa, V.C.; Ferreira, M.L.R.; Pardal, J.M. Microstructural Changes and Corrosion Resistance of AISI 310S Steel Exposed to 600–800 °C. Mater. Charact. 2009, 60, 573–578. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, V.K.; Bansal, A.; Sharma, Y. High temperature oxidation behaviour of NiCrAlY clad developed on SS-304 through microwave irradiation. Results Surf. Interfaces 2024, 14, 100179. [Google Scholar] [CrossRef]
- Schulz, U.; Leyens, C.; Fritscher, K.; Peters, M.; Saruhan-Brings, B.; Lavigne, O.; Dorvaux, J.M.; Poulain, M.; Mevrel, R.; Caliez, M. Some recent trends in research and technology of advanced thermal barrier coatings. Aero. Sci. Technol. 2003, 7, 73–80. [Google Scholar] [CrossRef]
- Hebbale, A.J.; Ramesh, M.R.; Petru, J.; Chandramouli, T.V.; Srinath, M.S.; Shetty, R.K. A microstructural study and high-temperature oxidation behaviour of plasma sprayed NiCrAlY based composite coatings. Results Eng. 2025, 25, 103926. [Google Scholar] [CrossRef]
- IAEA-TECDOC-1869; Status of Research and Technology Development for Supercritical Water Cooled Reactors. International Atomic Energy Agency: Vienna, Austria, 2019.
- Pennefather, R.C.; Boone, D.H. Mechanical degradation of coating systems in high-temperature cyclic oxidation. Surf. Coat. Technol. 1995, 76–77, 47–52. [Google Scholar] [CrossRef]
- Tudose, A.; Golgovici, F.; Anghel, A.; Fulger, M.; Demetrescu, I. Corrosion Testing of CrNx-Coated 310 H Stainless Steel under Simulated Supercritical Water Conditions. Materials 2022, 15, 5489. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, D.; Nițu, A.; Golgovici, F.; Demetrescu, I.; Corban, M. Behaviour Aspects of an EB-PVD Alumina (Al2O3) Film with an Interlayer (NiCrAlY) Deposited on AISI 316L Steel Investigated in Liquid Lead. Metals 2023, 13, 616. [Google Scholar] [CrossRef]
- Wang, X.; Yan, H.; Li, N.; Jiang, Z. High-temperature oxidation behavior of 301 stainless steel containing Cu. Mater. Today Commun. 2021, 35, 106121. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, K.; Fang, X.; Xiong, Z.; Wei, L.; Zhu, P.; Li, X. Oxidation behavior of 316L austenitic stainless steel in high temperature air with long-term exposure. Mater. Res. Express 2020, 7, 066517. [Google Scholar] [CrossRef]
- Gurtaran, M.; Zhang, Z.; Li, X.; Dong, H. High-Temperature Oxidation Behaviour of CrSi Coatings on 316 Austenitic Stainless Steel. Materials 2023, 16, 3533. [Google Scholar] [CrossRef]
- Tudose, A.E.; Golgovici, F.; Demetrescu, I.; Fulger, M.; Anghel, A.; Brincoveanu, O. Influence of chromium nitride ceramic layers thicknesses developed onto 310 H stainless steel on the corrosion resistance. U.P.B. Sci. Bull. Ser. B 2022, 84, 191–202. [Google Scholar]
- Gong, X.; Chen, R.R.; Wang, Y.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and Oxidation Behavior of NiCoCrAlY Coating with Different Sm2O3 Concentration on TiAl Alloy. Front. Mater. Sec. Polym. Compos. Mater. 2021, 8, 710431. [Google Scholar] [CrossRef]
- Outokumpu Stainless AB Company. Inspection Certificate No. 1293516/ 18.05.2007-1.4484; Outokumpu Stainless AB Company: Dalarna, Sweden, 2007. [Google Scholar]
- Huang, X.; Yang, Q.; Guzonas, D. Performance of Chemical Vapor Deposition and Plasma Spray-Coated Stainless Steel 310 in Supercritical Water. J. Nuclear Rad. Sci. 2016, 2, 1011-1–1011-8. [Google Scholar] [CrossRef]
- Hӧganӓs, Sweden. Inspection Certificate 3.1 per EN10204/ 07.11.2018; Hӧganӓs: Hoganas, Sweden, 2018. [Google Scholar]
- Piticescu, R.; Urbina, M.; Rinaldi, A. Development of novel material systems and coatings for extreme environments: A Brief Overview. JOM 2019, 71, 683–690. [Google Scholar] [CrossRef]
- Urbina, M.; Rinaldi, A.; Cuesta-Lopez, S.; Sobetkii, A.; Slobozeanu, A.E.; Szakalos, P.; Qin, Y.; Prakasam, M.; Piticescu, R.-R.; Ducros, C.; et al. The methodologies and strategies for the development of novel material systems and coatings for applications in extreme environments—A critical review. Manuf. Rev. 2018, 5, 9. [Google Scholar] [CrossRef]
- Baboian, R. Corrosion Tests and Standards: Application and Interpretation; ASTM Manual Series: MNL 20; ASTM Publication Code Number (PCN): 28-020095-27; ASTM: West Conshohocken, PA, USA, 1995; Volume 1. [Google Scholar]
- Gălan, F.; Ducu, M.; Fulger, M.; Negrea, D. Investigation of oxidation behavior of 304l and 310s steels with potential application in supercritical water-cooled nuclear reactors. Rev. Chim. 2020, 71, 98–105. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Macdonald, D.D. Effects of temperature and pH on the electrochemical behaviour of alloy 600 in simulated pressurized water reactor primary water. J. Nucl. Mater. 2020, 528, 151850. [Google Scholar] [CrossRef]
- Olasunkanmi, J.A. Surface Defects Characterization and Electrochemical Corrosion Studies of TiAlN, TiCN and AlCrN PVD Coatings. Master’s Thesis, Materials and Processes of Sustainable Energetics KAYM09, Tallinn, Estonia, 2016. [Google Scholar]
- Tsegay, M.G.; Gebretinsae, H.G.; Nuru, Z.Y. Structural and optical properties of green synthesized Cr2O3 nanoparticle. Mater. Today Proc. 2021, 36, 587–590. [Google Scholar] [CrossRef]
- Tudose, A.; Demetrescu, I.; Golgovici, F.; Fulger, M. Oxidation behavior of an austenitic steel (Fe, Cr and Ni), the 310 H, in a deaerated supercritical water static system. Metals 2021, 11, 571. [Google Scholar] [CrossRef]
- Peng, B.; Xu, Y.X.; Fan, Z.; Wang, Q. Oxidation behavior at 1100 ◦C of NiCrAlY coatings deposited by various PVD technique. Surf. Coat. Technol. 2024, 477, 130307. [Google Scholar] [CrossRef]
- Peng, B.; Xu, Y.X.; Qimin Wang, Q. High-temperature structural evolution and mechanical properties of oxygen-containing NiCrAlY coatings. Appl. Surf. Sci. 2024, 671, 160745. [Google Scholar] [CrossRef]
- Dai, S.; Li, X.; Yang, S.; Lyu, P.; Ye, Y.; Hua, Y.; Cai, J. Comparison of oxidation behavior of NiCrAlY coatings before and after surface aluminizing modification. Mater. Today Commun. 2024, 38, 108326. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, Z.; Yang, L.; Qiao, Y. The Oxidation Properties of a NiCrAlY Coating Fabricated by Arc Ion Plating. Coatings 2023, 13, 22. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Luo, J.; Li, Q.; Zhang, J.; Yang, L.; Zhou, Y. Investigation on the Oxidation Behavior of the NiCrAlY Bond-Coat with Low Al Content Sprayed by High Velocity Oxygen Fuel Method. J. Therm. Spray. Tech. 2024, 33, 1510–1525. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, T.; Geng, P.; Yu, H.; Wu, Y.; Gao, K.; Pang, X. Research on oxidation kinetics of CoNiCrAlY coatings in pure steam environment. Corros. Sci. 2024, 224, 111532. [Google Scholar] [CrossRef]
- Bojinov, M.; Fabricius, G.; Kinnunen, P.; Sundholm, G. EIS and CER study of the passivity of NiCr Alloys in a neutral solution at 200 °C. In Proceedings of the The International Symposium on Electrochemistry Methods in Corrosion Research, Budapest, Hungary, 28 May–1 June 2000. [Google Scholar]
- Baek, J.S.; Kim, J.G.; Hur, D.H. Anodic film properties determined by EIS and their relationship with caustic SCC of Alloy 600. Corros. Sci. 2003, 45, 983–994. [Google Scholar] [CrossRef]
- Marcus, P.; Mansfeld, F. Analytical Methods in Corrosion Science and Engineering; CRC Press: New York, NY, USA, 2006; ISBN 13: 978-0-8247-5952-0. [Google Scholar]
- Cicek, V. Corrosion Engineering, in In Martin Scrivener; Phillip Carmical: Beverly, MA, USA, 2014. [Google Scholar]
- Mutyala, K.C.E.; Ghanbari, E.; Doll, G.L. Effect of deposition method on tribological performance and corrosion resistance characteristics of CrxN coatings deposited by physical vapor deposition. Thin Solid Films 2017, 636, 232–239. [Google Scholar] [CrossRef]
- Härkönen, E.; Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Vehkamäki, M.; Sajavaara, T.; Fenker, M.; Marcus, P.; Ritala, M. Corrosion protection of steel with oxide nanolaminates grown by atomic layer deposition. J. Electrochem. Soc. 2011, 158, C369. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Wang, Y.; Wang, Z. An EIS analysis on corrosion resistance of anti-abrasion coating. Surf. Interfaces 2017, 6, 33–39. [Google Scholar] [CrossRef]
- Fusco, M.; Ay, Y.; Casey, A.; Bourham, M.; Leigh, A. Winfrey, Corrosion of single layer thin film protective coatings on steel substrates for high level waste containers. Prog. Nuclear Energy 2016, 89, 159–169. [Google Scholar] [CrossRef]
- El-Azazy, M.; Min, M.; Annus, P. Electrochemical Impedance Spectroscopy; WILEY: Hoboken, NJ, USA, 2020. [Google Scholar]
- Feng, Z.; Hurley, B.; Buchheit, R. Corrosion Inhibition Study of Aqueous Vanadate on Mg Alloy AZ31. J. Electrochem. Soc. 2018, 165, C94–C102. [Google Scholar] [CrossRef]
- William Grips, V.K.; Barshilia, H.C.; Selvi, V.E.; Rajam, K.S. Electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive direct current magnetron sputtering. Thin Solid Films 2006, 514, 204–211. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Feng, Y.P.; Chen, S.; Wan, Q.L.; Zhu, J.F. Electrochemical characterization of AlTiN, AlCrN and AlCrSiWN coatings. Int. J. Refract. Met. Hard Mater. 2015, 53, 68–73. [Google Scholar] [CrossRef]
- Yoo, Y.H.; Le, D.P.; Kim, J.G.; Kim, S.K.; Van Vinh, P. Corrosion behavior of TiN, TiAlN, TiAlSiN thin films deposited on tool steel in the 3.5 wt.% NaCl solution. Thin Solid Films 2008, 516, 3544–3548. [Google Scholar] [CrossRef]
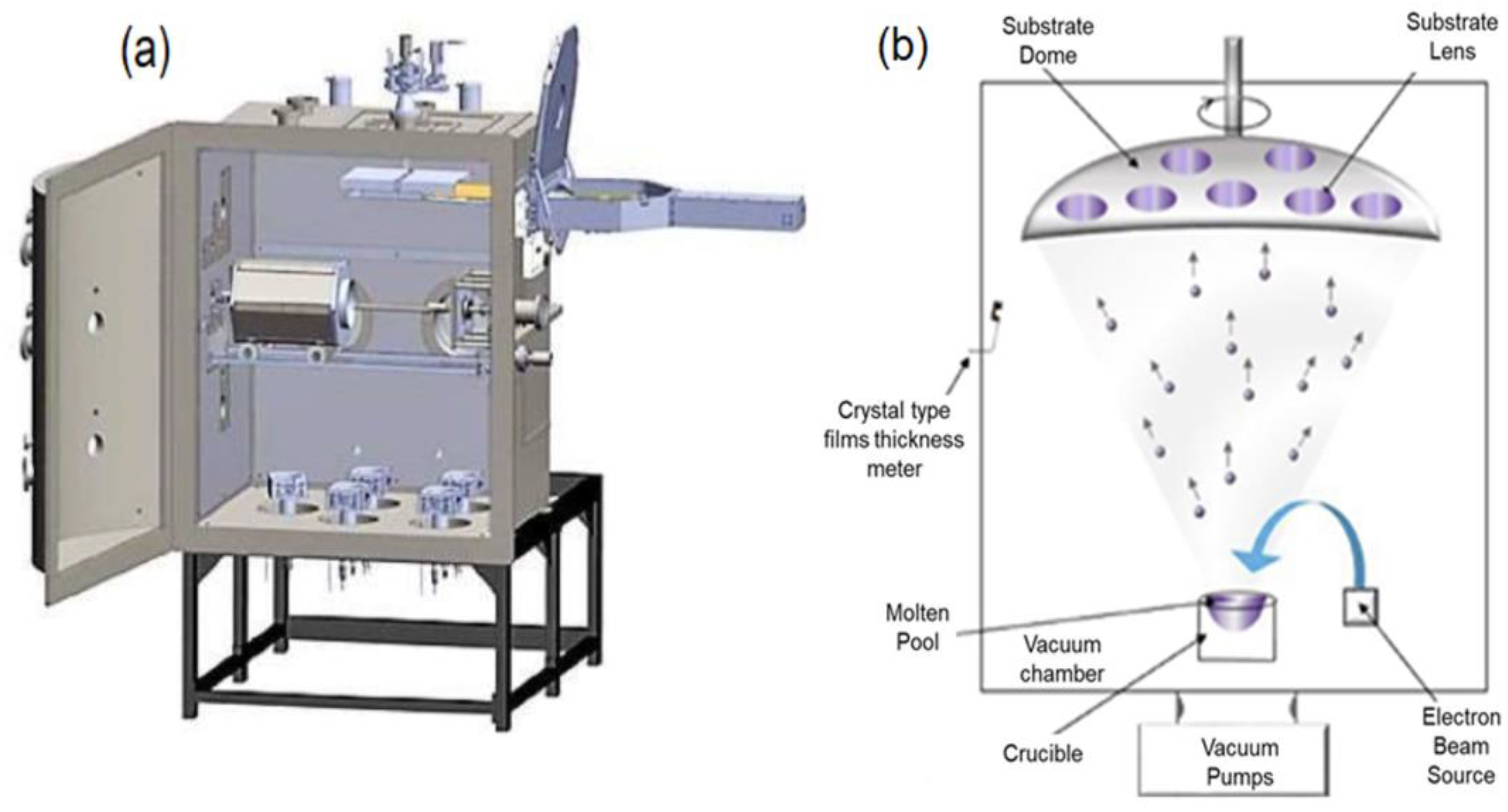



| Alloying Elements, [wt. %] | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cr | Ni | N | Fe |
| 0.063 | 0.71 | 1.61 | 0.016 | 0.001 | 24.13 | 19.03 | 0.04 | 54.34 |
| Powder Type | Particle Size, μm | Element, wt. % | |||
|---|---|---|---|---|---|
| Ni | Cr | Al | Y | ||
| Amperit@413.006 NiCrAlY atomized gas | 125/45 | 67 | 22 | 10 | 0.9 |
| Parameter | Value |
|---|---|
| Starting vacuum | 5.3 × 10−3 Pa |
| Working vacuum | 2.7 × 10−3 Pa |
| Deposit speed | 2 A/s |
| E-Beam beam power | 1–2 KW |
| Distance between the crucible and the substrate | 1.2 m |
| Substrate heater | set to the maximum value of 800 °C |
| Total deposition time | 4 h |
| Kinetics Equation | k | n | R2 |
|---|---|---|---|
| y = 1.3029 × t0.2154 | 1.3029 | 0.2154 | 0.9991 |
| Element Circuit | Uncoated Sample | Coated Sample | |||
|---|---|---|---|---|---|
| Unoxidized | Oxidized Sample | ||||
| 720 h | 1440 h | 2160 h | |||
| Rs, Ω·cm2 | 331.4 | 325.6 | 259.8 | 300.3 | 242.7 |
| Rox, Ω·cm2 | - | - | 1.9 × 104 | 5.8 × 105 | 6.2 × 105 |
| CPEox—T, F·cm−2 | - | - | 1.5 × 10−4 | 1.95 × 10−4 | 5.7 × 10−5 |
| CPEox—P | - | - | 0.68 | 0.53 | 0.54 |
| Rcoat, Ω·cm2 | - | 8291 | 11455 | 2.54 × 106 | 3.1 × 106 |
| CPEcoat—T, F·cm−2 | - | 2.53 × 10−4 | 3.4 × 10−4 | 8.6 × 10−5 | 6.4 × 10−5 |
| CPEcoat—P | - | 0.786 | 0.97 | 0.95 | 0.97 |
| Rct, Ω·cm2 | 8810 | 160.9 | 522.1 | 529.6 | 619 |
| CPEdl—T, F·cm−2 | 2.61 × 10−4 | 5.91 × 10−7 | 2.4 × 10−5 | 3.6 × 10−5 | 3.7 × 10−5 |
| CPEdl—P | 0.856 | 0.52 | 0.68 | 0.65 | 0.79 |
| Chi-squared (χ2) | 3.1 × 10−3 | 1.6 × 10−3 | 4.8 × 10−4 | 2.5 × 10−4 | 6.3 × 10−4 |
| Sample Type | Polarization Resistance (From the Extrapolation of Nyquist Diagrams), (MΩ × cm2) | i0 (Calculated from Polarization Resistance). (nA × cm−2) |
|---|---|---|
| 310H_uncoated | 0.0342 | 370 |
| 310H/NiCrAlY_0 h | 0.039 | 320 |
| 310H/NiCrAlY_720 h | 0.297 | 42.6 |
| 310H/NiCrAlY_1440 h | 0.389 | 32.6 |
| 310H/NiCrAlY_2160 h | 0.514 | 24.6 |
| Sample Type | Electrochemical Parameters | Pi, [%] | P, [%] | ||||
|---|---|---|---|---|---|---|---|
| Tafel Slopes Method | Rp Method | ||||||
| Ecorr, [mV] | icorr, [nA × cm−2] | Vcorr, [mm × year−1] | Rp, [KΩ × cm2] | icorr, [nA × cm−2] | |||
| uncoated | −256 | 1030 | 0.011 | 26.07 | 1190 | - | - |
| 0 h | −219 | 200 | 0.002 | 82.61 | 227 | 80.58 | 0.135 |
| 720 h | −164 | 8.43 | 8.9 × 10−5 | 2.5 × 103 | 8.14 | 99.18 | 1.25 × 10−3 |
| 1440 h | −161 | 7.17 | 7.5 × 10−5 | 3.6 × 103 | 7.25 | 99.30 | 8.01 × 10−4 |
| 2160 h | −123 | 4.62 | 4.8 × 10−5 | 7.3 × 103 | 4.29 | 99.55 | 1.67 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golgovici, F.; Tudose, A.-E.; Mosinoiu, L.F.; Demetrescu, I. Characterization of NiCrAlY Layers Deposited on 310H Alloy Using the EB-PVD Method After Oxidation in Water at High Temperature and Pressure. Appl. Sci. 2025, 15, 2361. https://doi.org/10.3390/app15052361
Golgovici F, Tudose A-E, Mosinoiu LF, Demetrescu I. Characterization of NiCrAlY Layers Deposited on 310H Alloy Using the EB-PVD Method After Oxidation in Water at High Temperature and Pressure. Applied Sciences. 2025; 15(5):2361. https://doi.org/10.3390/app15052361
Chicago/Turabian StyleGolgovici, Florentina, Aurelia-Elena Tudose, Laurențiu Florin Mosinoiu, and Ioana Demetrescu. 2025. "Characterization of NiCrAlY Layers Deposited on 310H Alloy Using the EB-PVD Method After Oxidation in Water at High Temperature and Pressure" Applied Sciences 15, no. 5: 2361. https://doi.org/10.3390/app15052361
APA StyleGolgovici, F., Tudose, A.-E., Mosinoiu, L. F., & Demetrescu, I. (2025). Characterization of NiCrAlY Layers Deposited on 310H Alloy Using the EB-PVD Method After Oxidation in Water at High Temperature and Pressure. Applied Sciences, 15(5), 2361. https://doi.org/10.3390/app15052361









