Pulsed Electric Fields as an Infrared–Convective Drying Pretreatment: Effect on Drying Course, Color, and Chemical Properties of Apple Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pulsed Electric Fields Pretreatment
2.3. Infrared–Convective Drying
2.4. The Analysis of Dry Matter Content
2.5. The Analysis of Color
2.6. The Analysis of Total Phenolic Content
2.7. The Analysis of Antioxidant Capacity
2.8. The Statistical Analysis
3. Results and Discussion
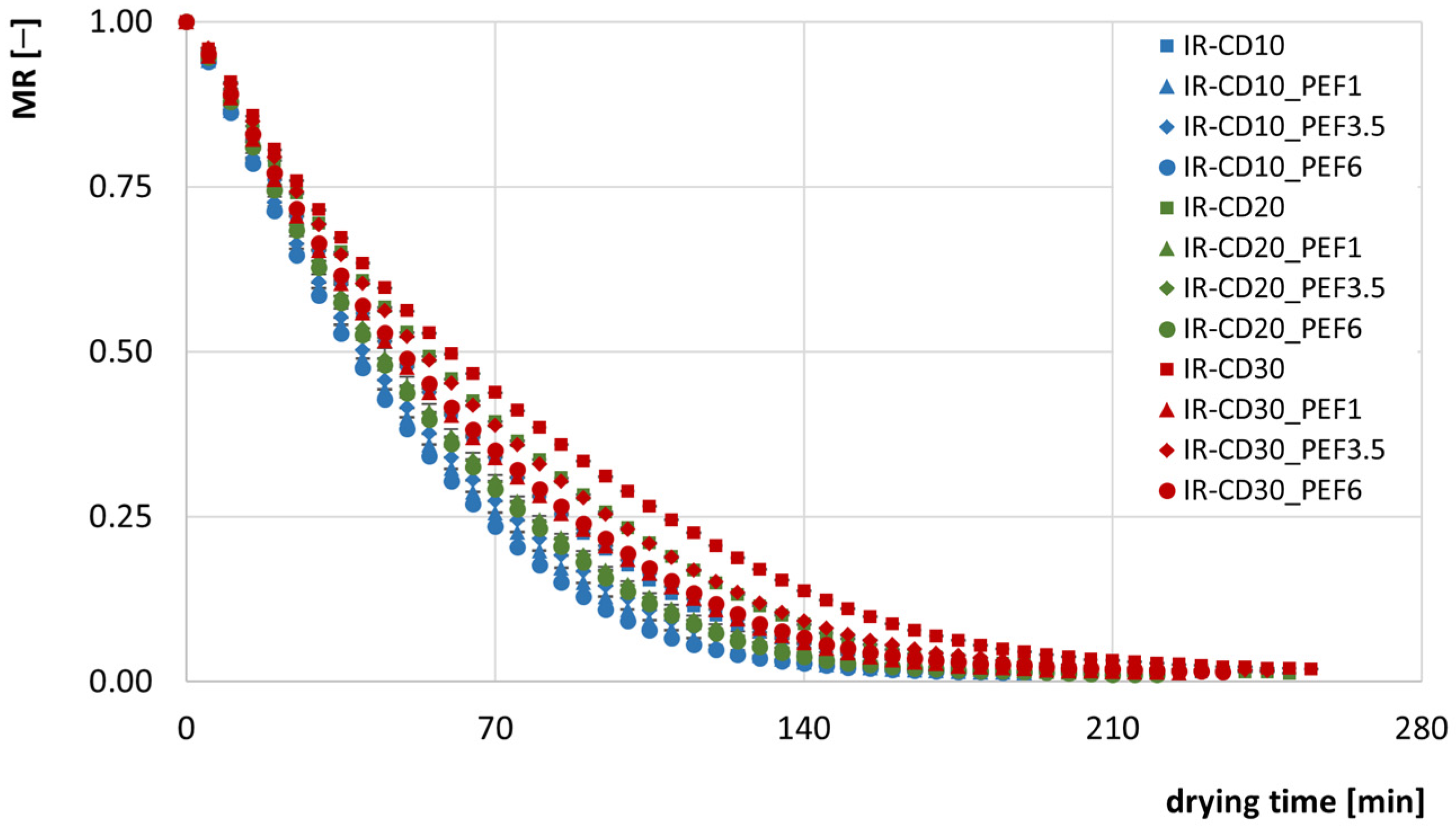
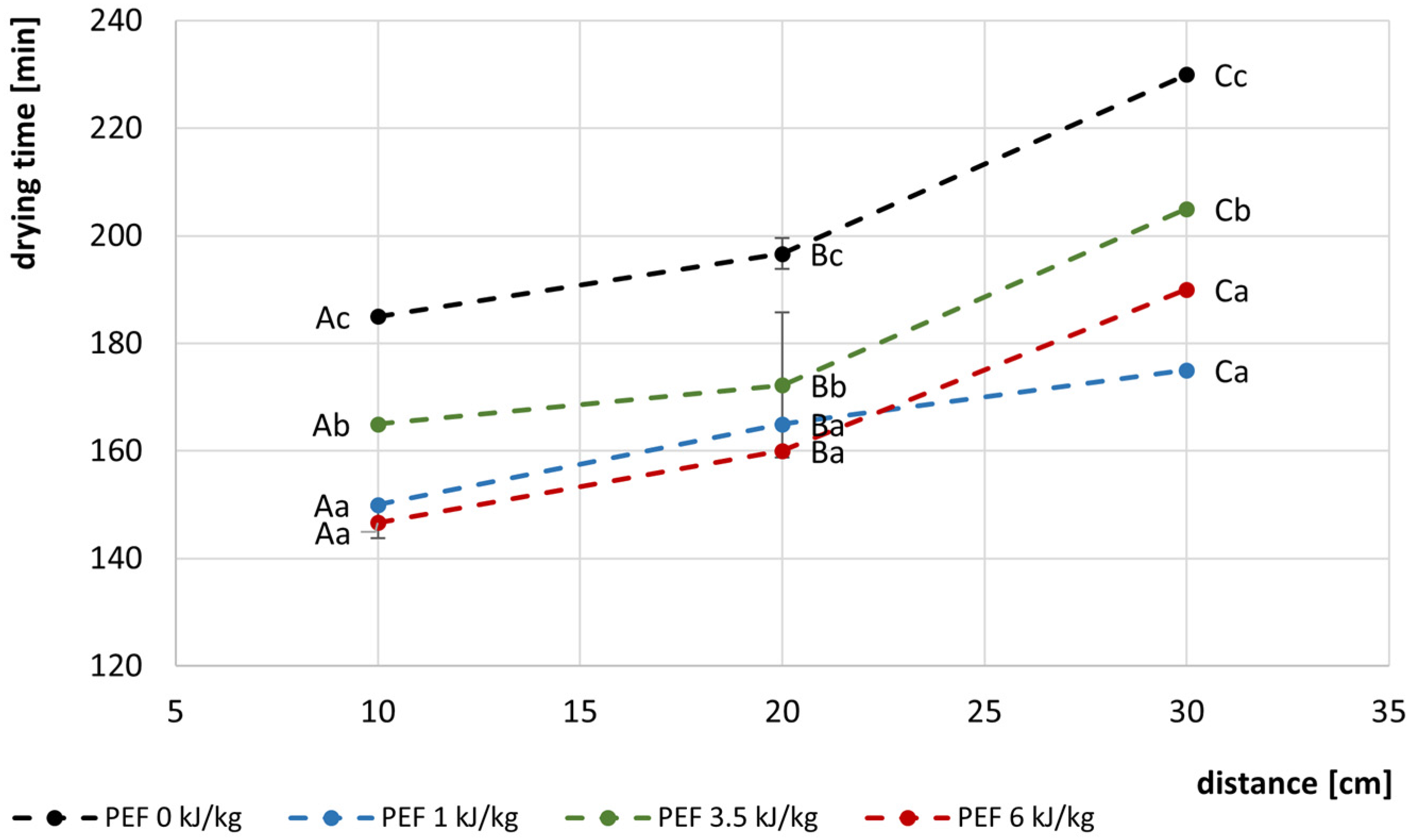
3.1. Drying Kinetics and Drying Times
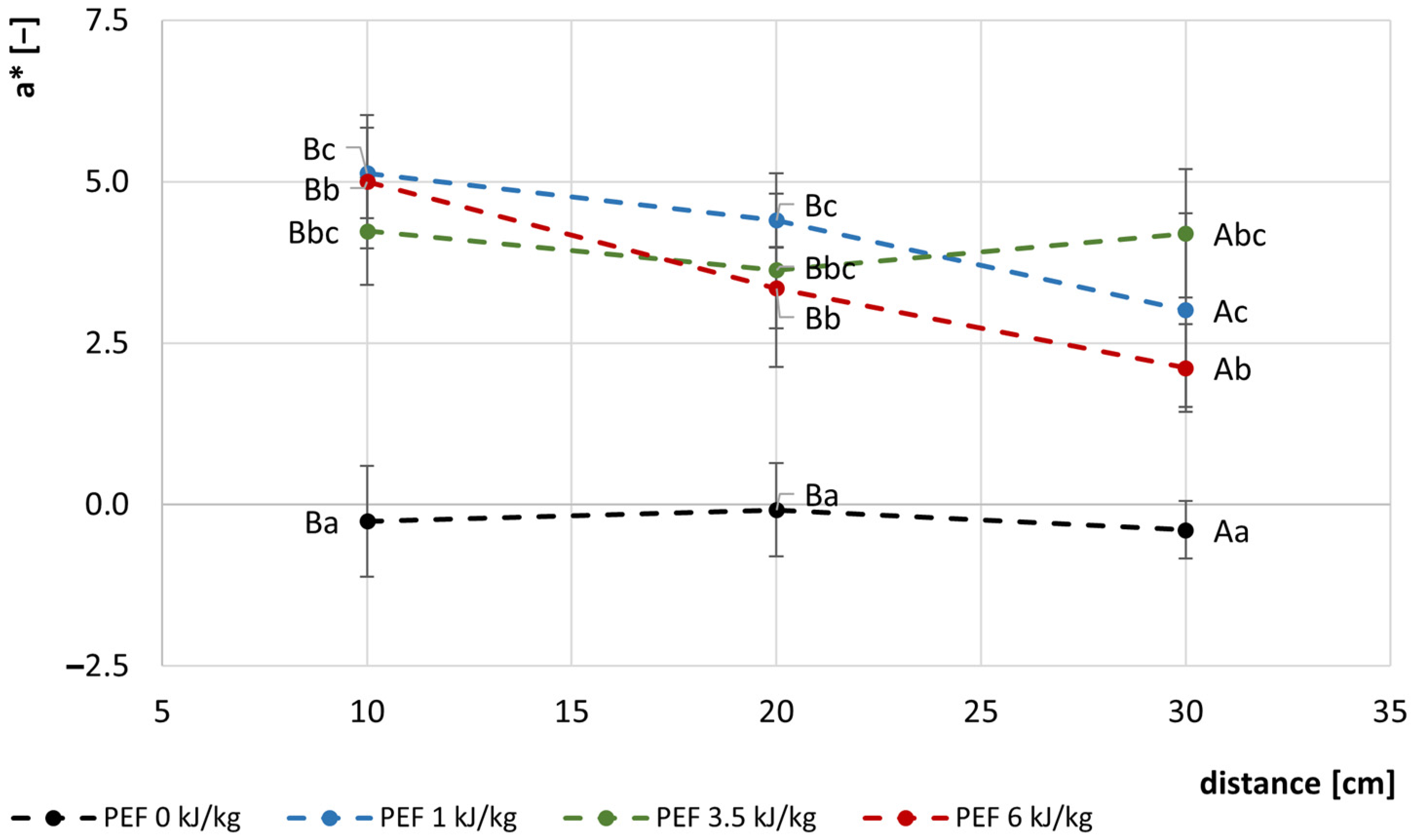
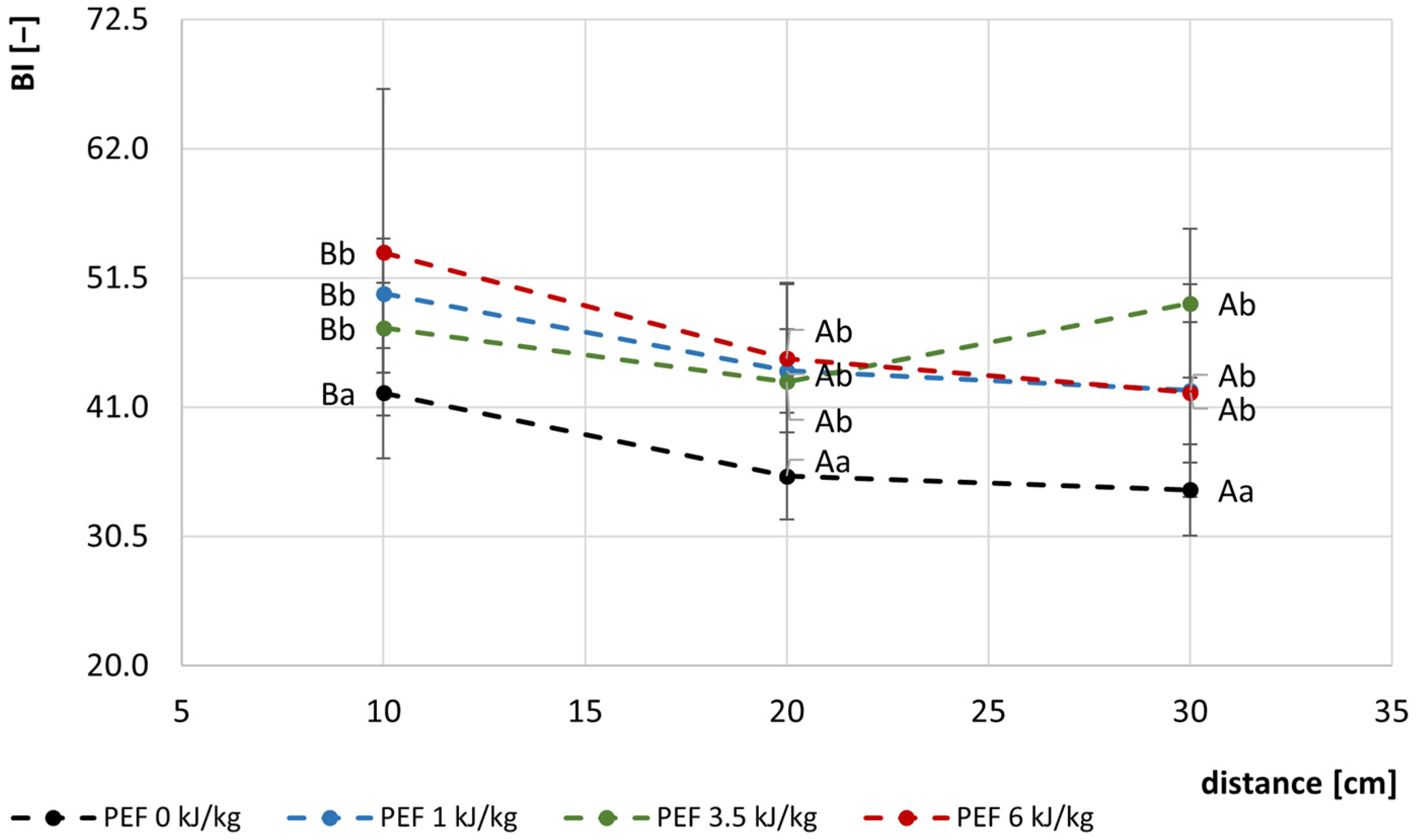
3.2. Color
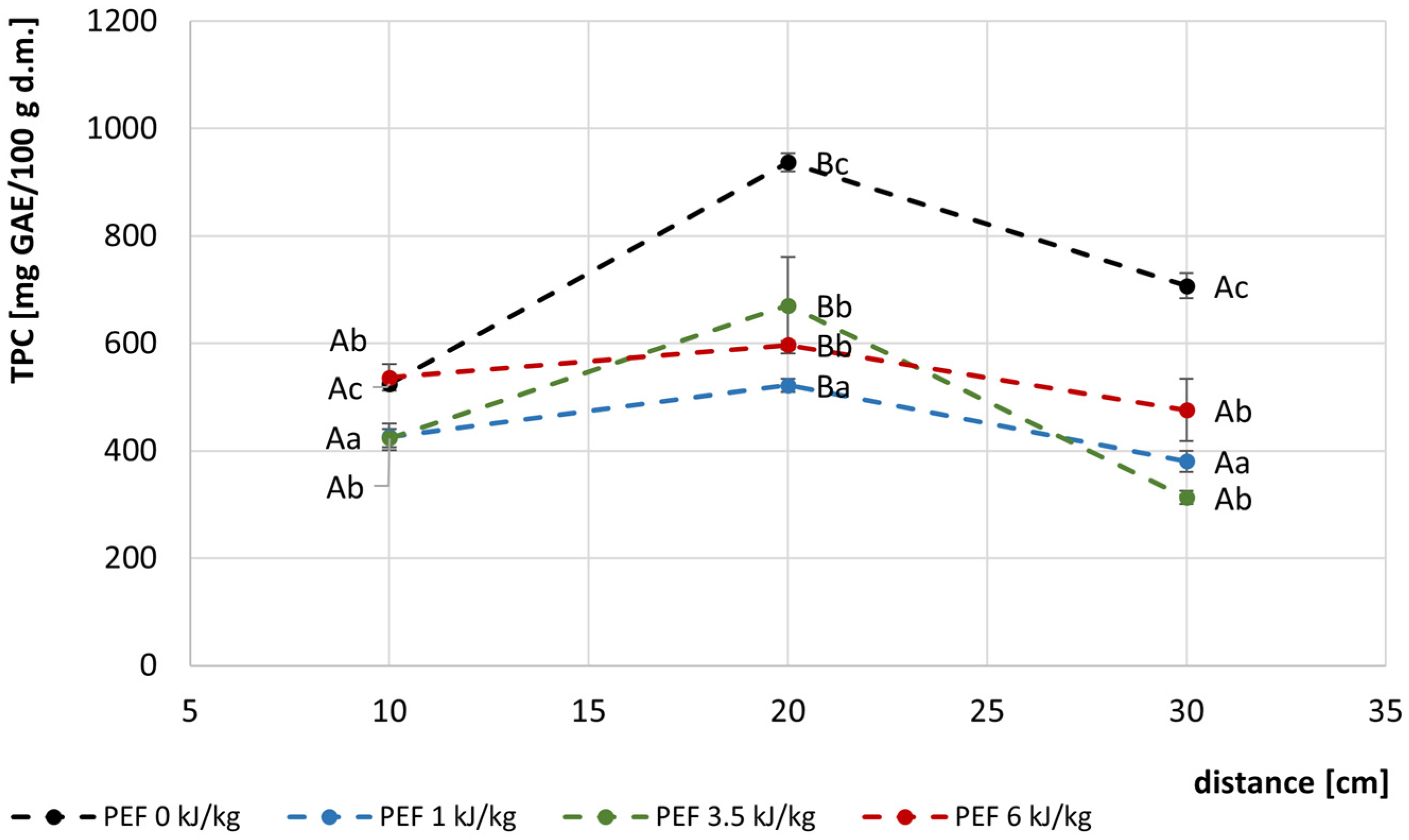
3.3. Chemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riadh, M.H.; Ahmad, S.A.B.; Marhaban, M.H.; Soh, A.C. Infrared Heating in Food Drying: An Overview. Dry. Technol. 2015, 33, 322–335. [Google Scholar] [CrossRef]
- Chua, K.J.; Chou, S.K. Recent Advances in Hybrid Drying Technologies. In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 447–459. ISBN 9780124114791. [Google Scholar]
- Michailidis, P.A.; Krokida, M.K. Drying and Dehydration Processes in Food Preservation and Processing. In Conventional and Advanced Food Processing Technologies; Bhattacharya, S., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 1–32. ISBN 9781118406281. [Google Scholar]
- Joseph Bassey, E.; Cheng, J.-H.; Sun, D.-W. Improving drying kinetics, physicochemical properties and bioactive compounds of red dragon fruit (Hylocereus species) by novel infrared drying. Food Chem. 2022, 375, 131886. [Google Scholar] [CrossRef] [PubMed]
- Inyang, U.; Oboh, I.; Etuk, B. Drying and the different techniques. Int. J. Food Nutr. Saf. 2017, 8, 45–72. [Google Scholar]
- Afolabi, I.S. Moisture Migration and Bulk Nutrients Interaction in a Drying Food Systems: A Review. Food Nutr. Sci. 2014, 5, 692–714. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Sakare, P.; Prasad, N.; Thombare, N.; Singh, R.; Sharma, S.C. Infrared Drying of Food Materials: Recent Advances. Food Eng. Rev. 2020, 12, 381–398. [Google Scholar] [CrossRef]
- Sadin, R.; Chegini, G.-R.; Khodadadi, M. Development and Performance Evaluation of a Combined Infrared and Hot Air Dryer. J. Biol. Environ. Sci. 2014, 8, 11–18. [Google Scholar]
- Huang, D.; Yang, P.; Tang, X.; Luo, L.; Sunden, B. Application of infrared radiation in the drying of food products. Trends Food Sci. Technol. 2021, 110, 765–777. [Google Scholar] [CrossRef]
- Taghinezhad, E.; Kaveh, M.; Khalife, E.; Chen, G. Drying of organic blackberry in combined hot air-infrared dryer with ultrasound pretreatment. Dry. Technol. 2021, 39, 2075–2091. [Google Scholar] [CrossRef]
- Taghinezhad, E.; Kaveh, M.; Szumny, A. Thermodynamic and Quality Performance Studies for Drying Kiwi in Hybrid Hot Air-Infrared Drying with Ultrasound Pretreatment. Appl. Sci. 2021, 11, 1297. [Google Scholar] [CrossRef]
- Jiang, D.-L.; Wang, Q.-H.; Huang, C.; Sutar, P.P.; Lin, Y.-W.; Okaiyeto, S.A.; Lin, Z.-F.; Wu, Y.-T.; Ma, W.-M.; Xiao, H.-W. Effect of various different pretreatment methods on infrared combined hot air impingement drying behavior and physicochemical properties of strawberry slices. Food Chem. X 2024, 22, 101299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Z.; Wang, X.; Yagoub, A.E.A.; Ma, H.; Sun, Y.; Yu, X. Effects of tri-frequency ultrasound-ethanol pretreatment combined with infrared convection drying on the quality properties and drying characteristics of scallion stalk. J. Sci. Food Agric. 2021, 101, 2809–2817. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Aydar, A.Y.; Kutlu, N.; Aslam, R.; Sahni, P.; Mitharwal, S.; Gavahian, M.; Kumar, M.; Raposo, A.; Yoo, S.; et al. Individual and interactive effect of ultrasound pre-treatment on drying kinetics and biochemical qualities of food: A critical review. Ultrason. Sonochemistry 2023, 92, 106261. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, R.; Parniakov, O.; Töpfl, S.; Jäger, H. Applicability of Pulsed Electric Field (PEF) Pre-Treatment for a Convective Two-Step Drying Process. Foods 2020, 9, 512. [Google Scholar] [CrossRef]
- Toepfl, S.; Siemer, C.; Heinz, V. Effect of High-Intensity Electric Field Pulses on Solid Foods. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 147–154. ISBN 9780124114791. [Google Scholar]
- AOAC International Official Methods of Analysis (OMA) of AOAC International, 17th ed., USA. Method Number: 920.15. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&cPath=1&products_id=2685 (accessed on 8 January 2025).
- Matys, A.; Witrowa-Rajchert, D.; Parniakov, O.; Wiktor, A. Assessment of the effect of air humidity and temperature on convective drying of apple with pulsed electric field pretreatment. LWT 2023, 188, 115455. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Matys, A.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. Chemical and Thermal Characteristics of PEF-Pretreated Strawberries Dried by Various Methods. Molecules 2024, 29, 3924. [Google Scholar] [CrossRef] [PubMed]
- Kudra, T. Energy Aspects in Drying. Dry. Technol. 2004, 22, 917–932. [Google Scholar] [CrossRef]
- Delgado, J.M.P.Q.; da Silva, M.V. Food Dehydration: Fundamentals, Modelling and Applications. In Transport Phenomena and Drying of Solids and Particulate Materials; Delgado, J.M.P.Q., Barbosa de Lima, A.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 48, pp. 69–94. ISBN 9783319040530. [Google Scholar]
- Boateng, I.D.; Yang, X.-M. Process optimization of intermediate-wave infrared drying: Screening by Plackett–Burman; comparison of Box-Behnken and central composite design and evaluation: A case study. Ind. Crops Prod. 2021, 162, 113287. [Google Scholar] [CrossRef]
- Eissa, A.S. Development and evaluation of pomegranate seeds drying unit using infrared/hot air dryer. Misr J. Agric. Eng. 2021, 38, 49–64. [Google Scholar] [CrossRef]
- Sadeghi, E.; Haghighi Asl, A.; Movagharnejad, K. Mathematical modelling of infrared-dried kiwifruit slices under natural and forced convection. Food Sci. Nutr. 2019, 7, 3589–3606. [Google Scholar] [CrossRef]
- Bassey, E.J.; Cheng, J.-H.; Sun, D.-W. Novel nonthermal and thermal pretreatments for enhancing drying performance and improving quality of fruits and vegetables. Trends Food Sci. Technol. 2021, 112, 137–148. [Google Scholar] [CrossRef]
- Knorr, D.; Angersbach, A. Impact of high-intensity electric field pulses on plant membrane permeabilization. Trends Food Sci. Technol. 1998, 9, 185–191. [Google Scholar] [CrossRef]
- Matys, A.; Dadan, M.; Witrowa-Rajchert, D.; Parniakov, O.; Wiktor, A. Response Surface Methodology as a Tool for Optimization of Pulsed Electric Field Pretreatment and Microwave-Convective Drying of Apple. Appl. Sci. 2022, 12, 3392. [Google Scholar] [CrossRef]
- Tsivas, D.; Vlyssides, A.; Vlysidis, A. Differentiation of the composting stages of green waste using the CIELAB color model. J. Chem. Technol. Biotechnol. 2024, 99, 2198–2208. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Dalla Rosa, M. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Aghilinategh, N.; Rafiee, S.; Hosseinpour, S.; Omid, M.; Mohtasebi, S.S. Real-time color change monitoring of apple slices using image processing during intermittent microwave convective drying. Food Sci. Technol. Int. 2016, 22, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U. Oxidation of vacuolar and apoplastic phenolic substrates by peroxidase: Physiological significance of the oxidation reactions. Phytochem. Rev. 2004, 3, 207–219. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed electric fields affect endogenous enzyme activities, respiration and biosynthesis of phenolic compounds in carrots. Postharvest Biol. Technol. 2020, 168, 111284. [Google Scholar] [CrossRef]
- Durmus, D. CIELAB color space boundaries under theoretical spectra and 99 test color samples. Color Res. Appl. 2020, 45, 796–802. [Google Scholar] [CrossRef]
- Alam, M.R.; Lyng, J.G.; Frontuto, D.; Marra, F.; Cinquanta, L. Effect of Pulsed Electric Field Pretreatment on Drying Kinetics, Color, and Texture of Parsnip and Carrot. J. Food Sci. 2018, 83, 2159–2166. [Google Scholar] [CrossRef]
- Baltacioglu, C.; Yetisen, M.; Baltacioglu, H.; Karacabey, E.; Buzrul, S. Pulsed Electric Fields (PEF) Treatment Prior to Hot-Air and Microwave Drying of Yellow- and Purple-Fleshed Potatoes. Potato Res. 2024. [Google Scholar] [CrossRef]
- Geršak, J. Quality requirement for clothing materials. In Design of Clothing Manufacturing Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 283–333. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochemistry 2010, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Dadali, G.; Demirhan, E.; Özbek, B. Color Change Kinetics of Spinach Undergoing Microwave Drying. Dry. Technol. 2007, 25, 1713–1723. [Google Scholar] [CrossRef]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–323. ISBN 9780128135723. [Google Scholar]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Fruits: A Source of Polyphenols and Health Benefits. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 189–228. ISBN 9780128115183. [Google Scholar]
- Chen, C.-S.; Zhang, D.; Wang, Y.-Q.; Li, P.-M.; Ma, F.-W. Effects of fruit bagging on the contents of phenolic compounds in the peel and flesh of ‘Golden Delicious’, ‘Red Delicious’, and ‘Royal Gala’ apples. Sci. Hortic. 2012, 142, 68–73. [Google Scholar] [CrossRef]
- Bushra Sultana, B.S.; Farooq Anwar, F.A.; Muhammad Ashraf, M.A.; Nazamid Saari, N.S. Effect of drying techniques on the total phenolic contents and antioxidant activity of selected fruits. J. Med. Plants Res. 2012, 6, 161–167. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, Z.; Bi, J.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Degradation kinetics of total phenolic compounds, capsaicinoids and antioxidant activity in red pepper during hot air and infrared drying process. Int. J. Food Sci. Technol. 2016, 51, 842–853. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarżyńska, A.; Gondek, E.; Nowacka, M.; Wiktor, A. Pulsed Electric Fields as an Infrared–Convective Drying Pretreatment: Effect on Drying Course, Color, and Chemical Properties of Apple Tissue. Appl. Sci. 2025, 15, 2348. https://doi.org/10.3390/app15052348
Skarżyńska A, Gondek E, Nowacka M, Wiktor A. Pulsed Electric Fields as an Infrared–Convective Drying Pretreatment: Effect on Drying Course, Color, and Chemical Properties of Apple Tissue. Applied Sciences. 2025; 15(5):2348. https://doi.org/10.3390/app15052348
Chicago/Turabian StyleSkarżyńska, Aleksandra, Ewa Gondek, Małgorzata Nowacka, and Artur Wiktor. 2025. "Pulsed Electric Fields as an Infrared–Convective Drying Pretreatment: Effect on Drying Course, Color, and Chemical Properties of Apple Tissue" Applied Sciences 15, no. 5: 2348. https://doi.org/10.3390/app15052348
APA StyleSkarżyńska, A., Gondek, E., Nowacka, M., & Wiktor, A. (2025). Pulsed Electric Fields as an Infrared–Convective Drying Pretreatment: Effect on Drying Course, Color, and Chemical Properties of Apple Tissue. Applied Sciences, 15(5), 2348. https://doi.org/10.3390/app15052348








